Quantification of Foot Drop Stimulator Effects on Post-Stroke Hemiplegic Gait: A Cyclogram-Based Evaluation of Inter-Limb Gait Symmetry
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention
2.3. Data Collection and Processing
- Spatio-temporal parameters (i.e., gait speed, step length, cadence, stance, swing, and double support phase duration);
- Hip, knee, and ankle kinematics in the sagittal plane (i.e., flexion–extension for hip and knee, dorsi-plantar flexion during the gait cycle). All the graphs derived from 3D-GA were normalized as % of the gait cycle;
- Dynamic range of motion (ROM), calculated as the difference between the maximum and the minimum flexion–extension angles for the hip and knee, and dorsi-plantar flexion angle for the ankle throughout the gait cycle.
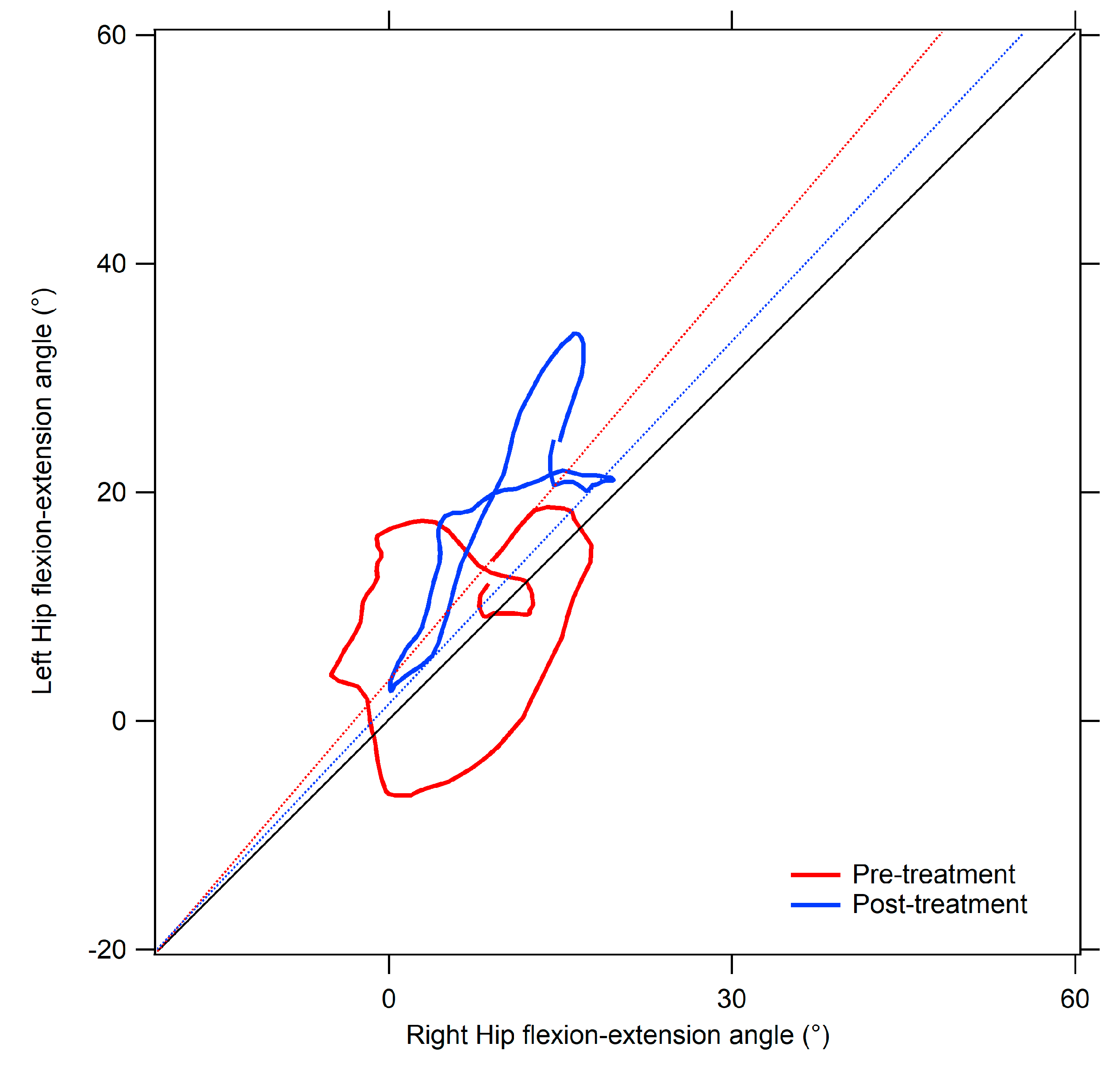
- Area (degrees2), representing the area of the cyclogram. In symmetric gait, the left and right joints are characterized by identical angular positions during each phase of the gait cycle, thus resulting in a null area. Deviations from null value indicate increased gait asymmetry [35].
- Trend Symmetry Index (dimensionless), computed through an eigenvector analysis as described by Crenshaw et al. [53]. It assesses the similarity between the angular trends of the right and left limbs across the gait cycle. Increased Trend Symmetry values are indicative of higher degrees of asymmetry.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Alawieh, A.; Zhao, J.; Feng, W. Factors Affecting Post-Stroke Motor Recovery: Implications on Neurotherapy after Brain Injury. Behav. Brain Res. 2018, 340, 94–101. [Google Scholar] [CrossRef]
- Einstad, M.S.; Saltvedt, I.; Lydersen, S.; Ursin, M.H.; Munthe-Kaas, R.; Ihle-Hansen, H.; Knapskog, A.-B.; Askim, T.; Beyer, M.K.; Næss, H.; et al. Associations between Post-Stroke Motor and Cognitive Function: A Cross-Sectional Study. BMC Geriatr. 2021, 21, 103. [Google Scholar] [CrossRef]
- Dimyan, M.A.; Cohen, L.G. Neuroplasticity in the Context of Motor Rehabilitation after Stroke. Nat. Rev. Neurol. 2011, 7, 76–85. [Google Scholar] [CrossRef]
- Verstraeten, S.; Mark, R.E.; Dieleman, J.; Van Rijsbergen, M.; De Kort, P.; Sitskoorn, M.M. Motor Impairment Three Months Post Stroke Implies A Corresponding Cognitive Deficit. J. Stroke Cerebrovasc. Dis. 2020, 29, 105119. [Google Scholar] [CrossRef]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait Differences between Individuals with Post-Stroke Hemiparesis and Non-Disabled Controls at Matched Speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef]
- Verma, R.; Arya, K.N.; Sharma, P.; Garg, R.K. Understanding Gait Control in Post-Stroke: Implications for Management. J. Bodyw. Mov. Ther. 2012, 16, 14–21. [Google Scholar] [CrossRef]
- Abe, H.; Michimata, A.; Sugawara, K.; Sugaya, N.; Izumi, S.-I. Improving Gait Stability in Stroke Hemiplegic Patients with a Plastic Ankle-Foot Orthosis. Tohoku J. Exp. Med. 2009, 218, 193–199. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.J. Small Directional Treadmill Perturbations Induce Differential Gait Stability Adaptation. J. Neurophysiol. 2022, 127, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Beyaert, C.; Vasa, R.; Frykberg, G.E. Gait Post-Stroke: Pathophysiology and Rehabilitation Strategies. Neurophysiol. Clin. 2015, 45, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of Gait Symmetry after Stroke: A Comparison of Current Methods and Recommendations for Standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef]
- Carda, S.; Cisari, C.; Invernizzi, M.; Bevilacqua, M. Osteoporosis after Stroke: A Review of the Causes and Potential Treatments. Cerebrovasc. Dis. 2009, 28, 191–200. [Google Scholar] [CrossRef]
- Brown, L.A.; Sleik, R.J.; Winder, T.R. Attentional Demands for Static Postural Control after Stroke. Arch. Phys. Med. Rehabil. 2002, 83, 1732–1735. [Google Scholar] [CrossRef]
- Pomeroy, V.M.; King, L.M.; Pollock, A.; Baily-Hallam, A.; Langhorne, P. Electrostimulation for Promoting Recovery of Movement or Functional Ability after Stroke. Cochrane Database Syst. Rev. 2006, 2006, CD003241. [Google Scholar] [CrossRef]
- Da Cunha, J.M.; Rech, K.D.; Salazar, A.P.; Pagnussat, A.S. Functional Electrical Stimulation of the Peroneal Nerve Improves Post-Stroke Gait Speed When Combined with Physiotherapy. A Systematic Review and Meta-Analysis. Ann. Phys. Rehabil. Med. 2021, 64, 101388. [Google Scholar] [CrossRef]
- Belfiore, P.; Miele, A.; Gallè, F.; Liguori, G. Adapted Physical Activity and Stroke: A Systematic Review. J. Sports Med. Phys. Fit. 2018, 58, 1867–1875. [Google Scholar] [CrossRef]
- Mazzucchelli, M.; Mazzoleni, D.; Campanini, I.; Merlo, A.; Mazzoli, D.; Melegari, C.; Colombo, V.; Cerulli, S.; Piscitelli, D.; Perin, C.; et al. Evidence-Based Improvement of Gait in Post-Stroke Patients Following Robot-Assisted Training: A Systematic Review. NeuroRehabilitation 2022, 51, 595–608. [Google Scholar] [CrossRef]
- Maggio, M.G.; Latella, D.; Maresca, G.; Sciarrone, F.; Manuli, A.; Naro, A.; De Luca, R.; Calabrò, R.S. Virtual Reality and Cognitive Rehabilitation in People With Stroke: An Overview. J. Neurosci. Nurs. 2019, 51, 101–105. [Google Scholar] [CrossRef]
- Choo, Y.J.; Chang, M.C. Effectiveness of an Ankle–Foot Orthosis on Walking in Patients with Stroke: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 15879. [Google Scholar] [CrossRef] [PubMed]
- Aqueveque, P.; Ortega, P.; Pino, E.; Saavedra, F.; Germany, E.; Gómez, B. After Stroke Movement Impairments: A Review of Current Technologies for Rehabilitation. In Physical Disabilities—Therapeutic Implications; Tan, U., Ed.; InTech: Singapore, 2017; ISBN 978-953-51-3247-9. [Google Scholar]
- Marrone, F.; Pau, M.; Vismara, L.; Porta, M.; Bigoni, M.; Leban, B.; Cerfoglio, S.; Galli, M.; Mauro, A.; Cimolin, V. Synchronized Cyclograms to Assess Inter-Limb Symmetry during Gait in Post-Stroke Patients. Symmetry 2022, 14, 1560. [Google Scholar] [CrossRef]
- Marquez-Chin, C.; Popovic, M.R. Functional Electrical Stimulation Therapy for Restoration of Motor Function after Spinal Cord Injury and Stroke: A Review. BioMed Eng. OnLine 2020, 19, 34. [Google Scholar] [CrossRef]
- Kesar, T.M.; Perumal, R.; Reisman, D.S.; Jancosko, A.; Rudolph, K.S.; Higginson, J.S.; Binder-Macleod, S.A. Functional Electrical Stimulation of Ankle Plantarflexor and Dorsiflexor Muscles: Effects on Poststroke Gait. Stroke 2009, 40, 3821–3827. [Google Scholar] [CrossRef]
- York, G.; Chakrabarty, S. A Survey on Foot Drop and Functional Electrical Stimulation. Int. J. Intell. Robot. Appl. 2019, 3, 4–10. [Google Scholar] [CrossRef]
- Shin, H.E.; Kim, M.; Lee, D.; Jang, J.Y.; Soh, Y.; Yun, D.H.; Kim, S.; Yang, J.; Kim, M.K.; Lee, H.; et al. Therapeutic Effects of Functional Electrical Stimulation on Physical Performance and Muscle Strength in Post-Stroke Older Adults: A Review. Ann. Geriatr. Med. Res. 2022, 26, 16–24. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, G.B.; Shin, S. Association Between Lower Limb Strength Asymmetry and Gait Asymmetry: Implications for Gait Variability in Stroke Survivors. J. Clin. Med. 2025, 14, 380. [Google Scholar] [CrossRef]
- Viteckova, S.; Kutilek, P.; Svoboda, Z.; Krupicka, R.; Kauler, J.; Szabo, Z. Gait Symmetry Measures: A Review of Current and Prospective Methods. Biomed. Signal Process. Control 2018, 42, 89–100. [Google Scholar] [CrossRef]
- Ekman, L.; Lindholm, E.; Brogren, E.; Dahlin, L.B. Normative Values of the Vibration Perception Thresholds at Finger Pulps and Metatarsal Heads in Healthy Adults. PLoS ONE 2021, 16, e0249461. [Google Scholar] [CrossRef]
- Lauzière, S.; Betschart, M.; Aissaou, R.; Nadeau, S. Understanding Spatial and Temporal Gait Asymmetries in Individuals Post Stroke. Int. J. Phys. Med. Rehabil. 2014, 2. [Google Scholar] [CrossRef]
- Zifchock, R.A.; Davis, I.; Higginson, J.; Royer, T. The Symmetry Angle: A Novel, Robust Method of Quantifying Asymmetry. Gait Posture 2008, 27, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Kutilek, P.; Viteckova, S.; Svoboda, Z.; Smrcka, P. Kinematic Quantification of Gait Asymmetry in Patients with Peroneal Nerve Palsy Based on Bilateral Cyclograms. J. Musculoskelet. Neuronal Interact. 2013, 13, 244–250. [Google Scholar] [PubMed]
- Grieve, D.W. Gait Patterns and the Speed of Walking. Biomed. Eng. 1968, 3, 119–122. [Google Scholar]
- Goswami, A. A New Gait Parameterization Technique by Means of Cyclogram Moments: Application to Human Slope Walking. Gait Posture 1998, 8, 15–36. [Google Scholar] [CrossRef]
- Goswami, A. Kinematic Quantification of Gait Asymmetry Based on Bilateral Cyclograms. United States Patent Application No. US 2005/0004495A1, 6 January 2005. [Google Scholar]
- Hershler, C.; Milner, M. Angle-Angle Diagrams in the Assessment of Locomotion. Am. J. Phys. Med. 1980, 59, 109–125. [Google Scholar]
- Kutílek, P.; Slavka, V.; Zdeněk, S.; Vladimír, S.; Pavel, S. Kinematic Quantification of Gait Asymmetry Based on Characteristics of Angle-Angle Diagrams. Acta Polytech. Hung. 2014, 11, 25–38. [Google Scholar] [CrossRef]
- Pilkar, R.; Ramanujam, A.; Chervin, K.; Forrest, G.F.; Nolan, K.J. Cyclogram-Based Joint Symmetry Assessment After Utilization of a Foot Drop Stimulator During Post-Stroke Hemiplegic Gait. J. Biomech. Eng. 2018, 140, 121005. [Google Scholar] [CrossRef]
- Pau, M.; Leban, B.; Deidda, M.; Putzolu, F.; Porta, M.; Coghe, G.; Cocco, E. Kinematic Analysis of Lower Limb Joint Asymmetry During Gait in People with Multiple Sclerosis. Symmetry 2021, 13, 598. [Google Scholar] [CrossRef]
- Di Giminiani, R.; Di Lorenzo, D.; La Greca, S.; Russo, L.; Masedu, F.; Totaro, R.; Padua, E. Angle-Angle Diagrams in the Assessment of Locomotion in Persons with Multiple Sclerosis: A Preliminary Study. Appl. Sci. 2022, 12, 7223. [Google Scholar] [CrossRef]
- Porta, M.; Pau, M.; Leban, B.; Deidda, M.; Sorrentino, M.; Arippa, F.; Marongiu, G. Lower Limb Kinematics in Individuals with Hip Osteoarthritis during Gait: A Focus on Adaptative Strategies and Interlimb Symmetry. Bioengineering 2021, 8, 47. [Google Scholar] [CrossRef]
- Pau, M.; Cerfoglio, S.; Capodaglio, P.; Marrone, F.; Grugni, G.; Porta, M.; Leban, B.; Galli, M.; Cimolin, V. Cyclogram-Based Evaluation of Inter-Limb Gait Symmetry in Prader-Willi Syndrome. Gait Posture 2024, 112, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Cerfoglio, S.; Capodaglio, P.; Marrone, F.; Mendolicchio, L.; Porta, M.; Leban, B.; Galli, M.; Cimolin, V. Synchronized Cyclograms to Assess Inter-Limb Symmetry during Gait in Women with Anorexia and Bulimia: A Retrospective Study. Symmetry 2023, 15, 2200. [Google Scholar] [CrossRef]
- Kutilek, P.; Hejda, J.; Svoboda, Z. Kinematic Quantification of Gait Asymmetry in Patients with Elastic Ankle Wrap Based on Cyclograms. In XIII Mediterranean Conference on Medical and Biological Engineering and Computing 2013; Roa Romero, L.M., Ed.; IFMBE Proceedings; Springer International Publishing: Cham, Switzerland, 2014; Volume 41, pp. 117–120. ISBN 978-3-319-00845-5. [Google Scholar]
- Kutilek, P.; Socha, V.; Viteckova, S.; Svoboda, Z. Quantification of Gait Asymmetry in Patients with Ankle Foot Orthoses Based on Hip–Hip Cyclograms. Biocybern. Biomed. Eng. 2014, 34, 46–52. [Google Scholar] [CrossRef]
- Lee, H.S.; Ryu, H.; Lee, S.-U.; Cho, J.; You, S.; Park, J.H.; Jang, S.-H. Analysis of Gait Characteristics Using Hip-Knee Cyclograms in Patients with Hemiplegic Stroke. Sensors 2021, 21, 7685. [Google Scholar] [CrossRef]
- Damiano, D.L.; Prosser, L.A.; Curatalo, L.A.; Alter, K.E. Muscle Plasticity and Ankle Control After Repetitive Use of a Functional Electrical Stimulation Device for Foot Drop in Cerebral Palsy. Neurorehabil Neural Repair. 2013, 27, 200–207. [Google Scholar] [CrossRef]
- Everaert, D.G.; Stein, R.B.; Abrams, G.M.; Dromerick, A.W.; Francisco, G.E.; Hafner, B.J.; Huskey, T.N.; Munin, M.C.; Nolan, K.J.; Kufta, C.V. Effect of a Foot-Drop Stimulator and Ankle–Foot Orthosis on Walking Performance After Stroke: A Multicenter Randomized Controlled Trial. Neurorehabil Neural Repair. 2013, 27, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Kluding, P.M.; Dunning, K.; O’Dell, M.W.; Wu, S.S.; Ginosian, J.; Feld, J.; McBride, K. Foot Drop Stimulation Versus Ankle Foot Orthosis After Stroke: 30-Week Outcomes. Stroke 2013, 44, 1660–1669. [Google Scholar] [CrossRef]
- Hwang, D.-Y.; Lee, H.-J.; Lee, G.-C.; Lee, S.-M. Treadmill Training with Tilt Sensor Functional Electrical Stimulation for Improving Balance, Gait, and Muscle Architecture of Tibialis Anterior of Survivors with Chronic Stroke: A Randomized Controlled Trial. Technol. Health Care 2015, 23, 443–452. [Google Scholar] [CrossRef]
- Lee, H.-J.; Cho, K.-H.; Lee, W.-H. The Effects of Body Weight Support Treadmill Training with Power-Assisted Functional Electrical Stimulation on Functional Movement and Gait in Stroke Patients. Am. J. Phys. Med. Rehabil. 2013, 92, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S. Locomotor Therapy in Neurorehabilitation. NeuroRehabilitation 2001, 16, 133–139. [Google Scholar] [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A Gait Analysis Data Collection and Reduction Technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Crenshaw, S.J.; Richards, J.G. A Method for Analyzing Joint Symmetry and Normalcy, with an Application to Analyzing Gait. Gait Posture 2006, 24, 515–521. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.; Cho, J.; Kim, I.; Lee, J.; Jang, S.H. Effects of Knee Osteoarthritis Severity on Inter-Joint Coordination and Gait Variability as Measured by Hip-Knee Cyclograms. Sci. Rep. 2021, 11, 1789. [Google Scholar] [CrossRef]
- Capodaglio, P.; Leban, B.; Porta, M.; Galli, M.; Cimolin, V. Kinematics Adaptation and Inter-Limb Symmetry during Gait in Obese Adults. Sensors 2021, 21, 5980. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Grundey, J.; Hewitt, M.; Von Lewinski, F.; Kaus, J.; Schmalz, T.; Rohde, V.; Liebetanz, D. Towards Physiological Ankle Movements with the ActiGait Implantable Drop Foot Stimulator in Chronic Stroke. Restor. Neurol. Neurosci. 2013, 31, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.; O’Dell, M.W.; Kluding, P.; McBride, K. Peroneal Stimulation for Foot Drop After Stroke: A Systematic Review. Am. J. Phys. Med. Rehabil. 2015, 94, 649–664. [Google Scholar] [CrossRef]
- Gök, H.; Küçükdeveci, A.; Altinkaynak, H.; Yavuzer, G.; Ergin, S. Effects of Ankle-Foot Orthoses on Hemiparetic Gait. Clin. Rehabil. 2003, 17, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Routson, R.L.; Clark, D.J.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. The Influence of Locomotor Rehabilitation on Module Quality and Post-Stroke Hemiparetic Walking Performance. Gait Posture 2013, 38, 511–517. [Google Scholar] [CrossRef]
- Pittock, S.J.; Moore, A.P.; Hardiman, O.; Ehler, E.; Kovac, M.; Bojakowski, J.; Al Khawaja, I.; Brozman, M.; Kaňovský, P.; Skorometz, A.; et al. A Double-Blind Randomised Placebo-Controlled Evaluation of Three Doses of Botulinum Toxin Type A (Dysport®) in the Treatment of Spastic Equinovarus Deformity after Stroke. Cerebrovasc. Dis. 2003, 15, 289–300. [Google Scholar] [CrossRef]
- Everaert, D.G.; Thompson, A.K.; Chong, S.L.; Stein, R.B. Does Functional Electrical Stimulation for Foot Drop Strengthen Corticospinal Connections? Neurorehabil. Neural Repair. 2010, 24, 168–177. [Google Scholar] [CrossRef]
- Ring, H.; Treger, I.; Gruendlinger, L.; Hausdorff, J.M. Neuroprosthesis for Footdrop Compared with an Ankle-Foot Orthosis: Effects on Postural Control during Walking. J. Stroke Cerebrovasc. Dis. 2009, 18, 41–47. [Google Scholar] [CrossRef]
- Kottink, A.I.R.; Oostendorp, L.J.M.; Buurke, J.H.; Nene, A.V.; Hermens, H.J.; IJzerman, M.J. The Orthotic Effect of Functional Electrical Stimulation on the Improvement of Walking in Stroke Patients with a Dropped Foot: A Systematic Review. Artif. Organs 2004, 28, 577–586. [Google Scholar] [CrossRef]
- Cunha, M.J.D.; Pinto, C.; Zanfir, B.; Cimolin, V.; Pagnussat, A.S. Combining Foot Drop Stimulation Devices with Gait Training Improves Gait, Active Ankle Movement of Chronic Poststroke Individuals. J. Prosthet. Orthot. 2022, 34, 213–222. [Google Scholar] [CrossRef]
| Group | Stroke (n = 21) | Control Group (n = 48) |
|---|---|---|
| Gender, n (%) | ||
| Male | 12 (57.1%) | 29 (60.4%) |
| Female | 9 (42.9%) | 19 (39.6%) |
| Age (years) | 55.8 (9.30) | 54.4 (12.5) |
| Height (m) | 1.69 (0.08) | 1.68 (0.08) |
| Body mass (kg) | 74.48 (12.27) | 67.92 (11.68) |
| Time since stroke (months), median (min–max) | 35 (6–96) | |
| Stroke type, n (%) | ||
| Ischemic | 16 (76%) | |
| Hemorrhagic | 6 (14%) | |
| Affected hemisphere, n (%) | ||
| Right | 8 (38%) | |
| Left | 13 (62%) | |
| FMA-LL (0–34), median (min–max) | 21 (11–32) | |
| MAS, frequency (0/1/1+/2/3/4) | ||
| Plantiflexors | 0/3/2/2/6/8 | |
| Knee extensors | 5/3/4/2/5/2 | |
| Adductors | 5/3/2/6/5/0 |
| Pre-Treatment | Post-Treatment | Control Group | |||
|---|---|---|---|---|---|
| Affected Limb | Non-Affected Limb | Affected Limb | Non-Affected Limb | ||
| Gait speed (m/s) | 0.56 (0.25) * | 0.58 (0.23) * | 1.23 (0.19) | ||
| Cadence (steps/min) | 84.27 (19.26) * | 85.90 (17.58) * | 111.6 (10.7) | ||
| Step length (m) | 0.33 (0.14) * | 0.42 (0.12) * | 0.36 (0.14) * | 0.44 (0.10) * | 0.66 (0.06) |
| Stance phase (% GC) | 75.93 (6.76) * | 64.58 (6.69) * | 74.04 (5.88) * | 64.15 (7.53) * | 59.49 (1.73) |
| Swing phase (% GC) | 23.70 (6.87) * | 35.06 (7.14) * | 25.93 (6.00) * | 36.19 (6.10) * | 40.41 (1.46) |
| Double support phase (% GC) | 16.65 (5.03) | 24.18 (9.45) | 15.29 (4.69) | 23.57 (9.32) | 19.59 (1.79) |
| Pre-Treatment | Post-Treatment | Control Group | |||
|---|---|---|---|---|---|
| Affected Limb | Non-Affected Limb | Affected Limb | Non-Affected Limb | ||
| Hip ROM (degrees) | 29.81 (10.14) * | 43.94 (9.47) | 31.34 (10.14) +* | 43.79 (8.78) | 45.88 (4.57) |
| Knee ROM (degrees) | 31.94 (12.31) * | 48.00 (9.93) * | 34.69 (12.99) +* | 49.35 (8.38) * | 59.76 (4.27) |
| Ankle ROM (degrees) | 19.30 (6.95) * | 29.31 (9.17) | 21.30 (10.10) * | 27.86 (9.02) | 28.60 (6.02) |
| Parameter | Joint | Pre-Treatment | Post-Treatment | Control Group |
|---|---|---|---|---|
| Cyclogram area (degrees2) | Hip | 391.58 (237.15) * | 422.09 (278.67) * | 96.79 (84.74) |
| Cyclogram orientation ϕ (degrees) | 13.35 (9–82) * | 10.65 (8.19) +* | 1.63 (1.24) | |
| Trend Symmetry | 19.09 (14.29) * | 15.46 (2.56) +* | 1.66 (1.26) | |
| Cyclogram area (degrees2) | Knee | 436.30 (311.13) * | 496.36 (353.95) * | 273.43 (177.67) |
| Cyclogram orientation ϕ (degrees) | 22.80 (23.19) * | 25.17 (21.16) * | 1.37 (1.39) | |
| Trend Symmetry | 30.23 (33.16) * | 31.79 (31.78) * | 1.35 (1.39) | |
| Cyclogram area (degrees2) | Ankle | 139.02 (87.00) * | 134.91 (87.67) * | 67.84 (49.72) |
| Cyclogram orientation ϕ (degrees) | 25.61 (15.77) * | 26.24 (3.87) * | 3.17 (2.95) | |
| Trend Symmetry | 25.83 (16.21) * | 28.37 (27.19) * | 2.89 (2.67) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrone, F.; da Cunha, M.J.; Cerfoglio, S.; Pau, M.; Porta, M.; Leban, B.; Tarabini, M.; Galli, M.; Souza Pagnussat, A.; Cimolin, V. Quantification of Foot Drop Stimulator Effects on Post-Stroke Hemiplegic Gait: A Cyclogram-Based Evaluation of Inter-Limb Gait Symmetry. Symmetry 2025, 17, 631. https://doi.org/10.3390/sym17050631
Marrone F, da Cunha MJ, Cerfoglio S, Pau M, Porta M, Leban B, Tarabini M, Galli M, Souza Pagnussat A, Cimolin V. Quantification of Foot Drop Stimulator Effects on Post-Stroke Hemiplegic Gait: A Cyclogram-Based Evaluation of Inter-Limb Gait Symmetry. Symmetry. 2025; 17(5):631. https://doi.org/10.3390/sym17050631
Chicago/Turabian StyleMarrone, Flavia, Maira Jaqueline da Cunha, Serena Cerfoglio, Massimiliano Pau, Micaela Porta, Bruno Leban, Marco Tarabini, Manuela Galli, Aline Souza Pagnussat, and Veronica Cimolin. 2025. "Quantification of Foot Drop Stimulator Effects on Post-Stroke Hemiplegic Gait: A Cyclogram-Based Evaluation of Inter-Limb Gait Symmetry" Symmetry 17, no. 5: 631. https://doi.org/10.3390/sym17050631
APA StyleMarrone, F., da Cunha, M. J., Cerfoglio, S., Pau, M., Porta, M., Leban, B., Tarabini, M., Galli, M., Souza Pagnussat, A., & Cimolin, V. (2025). Quantification of Foot Drop Stimulator Effects on Post-Stroke Hemiplegic Gait: A Cyclogram-Based Evaluation of Inter-Limb Gait Symmetry. Symmetry, 17(5), 631. https://doi.org/10.3390/sym17050631











