Primary Amino Acid Lithium Salt-Catalyzed Asymmetric Michael Addition of Carbon Nucleophiles to Enones
Abstract
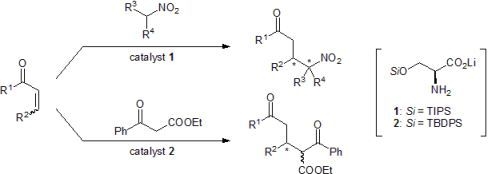
:1. Introduction
2. Results and Discussion

3. Experimental Section
3.1. General
3.2. Typical Procedure for the Michael Addition of Nitroalkanes to Enones
3.3. (S)-3-(2-Nitropropan-2-yl)cyclopentanone (4b)
3.4. (S)-3-(2-Nitropropan-2-yl)cycloheptanone (4c)
3.5. (S)-5-Methyl-5-nitro-4-phenylhexan-2-one (4d)
3.6. (1’R,3S)-3-(1’-Nitroethyl)cyclohexanone and (1’S,3S)-3-(1’-nitroethyl)cyclohexanone (4f)
3.7. (S)-3-Nitromethylcyclohexanone (4g)
3.8. Michael Addition of Ethyl Benzoylacetate (5) to 2-cyclohexen-1-one (3a)
4. Conclusions
Acknowledgements
References and Notes
- MacMillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Asymmetric organocatalysis. Tetrahedron 2007, 63, 9267–9331. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef] [PubMed]
- Houk, K.N.; List, B. A special issue on asymmetric organocatalysis. Acc. Chem. Res. 2004, 37, 487–631. [Google Scholar] [CrossRef]
- Berkessel, A.; Gröger, H. Asymmetric Organocatalysis; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Berner, O.M.; Tedeschi, L.; Enders, D. Asymmetric michael additions to nitroalkenes. Eur. J. Org. Chem. 2002, 2002, 1877–1894. [Google Scholar] [CrossRef]
- Tsogoeva, S.B. Recent advances in asymmetric organocatalytic 1,4-conjugate additions. Eur. J. Org. Chem. 2007, 2007, 1701–1716. [Google Scholar] [CrossRef]
- Almaşi, D.; Alonso, D.A.; Nájera, C. Organocatalytic asymmetric conjugate additions. Tetrahedron: Asymmetry 2007, 18, 299–365. [Google Scholar]
- Yamaguchi, M.; Shiraishi, T.; Hirama, M. Asymmetric michael addition of malonate anions to prochiral acceptors catalyzed by l-proline rubidium salt. J. Org. Chem. 1996, 61, 3520–3530. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Shiraishi, T.; Hirama, M. A catalytic enantioselective michael addition of a simple malonate to prochiral α,β-unsaturated ketoses and aldehydes. Angew. Chem. Int. Ed. Engl. 1993, 32, 1176–1178. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yokota, N.; Minami, T. The michael addition of dimethyl malonate to α,β-unsaturated aldehydes catalysed by proline lithium salt. J. Chem. Soc. Chem. Commun. 1991, 16, 1088–1089. [Google Scholar] [CrossRef]
- Kawara, A.; Taguchi, T. An enantioselective michael addition of soft nucleophiles to prochiral enone catalyzed by (2-pyrrolidyl)alkyl ammonium hydroxide. Tetrahedron Lett. 1994, 35, 8805–8808. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Igarashi, Y.; Reddy, R.S.; Shiraishi, T.; Hirama, M. Asymmetric michael addition of nitroalkanes to prochiral acceptors catalyzed by proline rubidium salts. Tetrahedron 1997, 53, 11223–11236. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Igarashi, Y.; Hirama, M. Catalytic asymmetric michael addition of nitroalkane to enone and enal. Tetrahedron Lett. 1994, 35, 8233–8236. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Zheng, Z.; Ni, B. Highly active water-soluble and recyclable organocatalyst for the asymmetric 1,4-conjugate addition of nitroalkanes to α,β-unsaturated aldehydes. Adv. Synth. Catal. 2010, 352, 2378–2382. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Highly enantioselective michael addition of nitroalkanes to chalcones using chiral squaramides as hydrogen bonding organocatalysts. Org. Lett. 2010, 12, 5450–5453. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Chen, X.K.; Xiao, H.; Liu, W.; Zhao, G. Organocatalyzed enantioselective Michael additions of nitroalkanes to enones by using primary—secondary diamine catalysts. Chem. Commun. 2010, 46, 4130–4132. [Google Scholar] [CrossRef]
- Maltsev, O.V.; Kucherenko, A.S.; Beletskaya, I.P.; Tartakovsky, V.A.; Zlotin, S.G. Chiral ionic liquids bearing o-silylated α,α-diphenyl (s)- or (r)-prolinol units: recoverable organocatalysts for asymmetric michael addition of nitroalkanes to α,β-enals. Eur. J. Org. Chem. 2010, 2010, 2927–2933. [Google Scholar] [CrossRef]
- Lu, H.H.; Wang, X.F.; Yao, C.J.; Zhang, J.M.; Wu, H.; Xiao, W.J. Highly enantioselective organocatalytic michael addition of nitroalkanes to 4-oxo-enoates. Chem. Commun. 2009, 4251–4253. [Google Scholar] [CrossRef]
- Malmgren, M.; Granander, J.; Amedjkouh, M. Asymmetric conjugate addition of nitroalkanes to enones with a chiral α-aminophosphonate catalyst. Tetrahedron: Asymmetry 2008, 19, 1934–1940. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Liang, X.; Ye, J. Asymmetric multifunctional organocatalytic Michael addition of nitroalkanes to α,β-unsaturated ketones. Chem. Commun. 2008, 28, 3302–3304. [Google Scholar] [CrossRef]
- Ballini, R.; Barboni, L.; Castrica, L.; Fringuelli, F.; Lanari, D.; Pizzo, F.; Vaccaro, L. Polystyryl-BEMP as an efficient recyclable catalyst for the nucleophilic addition of nitroalkanes to α,β-unsaturated carbonyl compounds under solvent-free conditions. Adv. Synth. Catal. 2008, 350, 1218–1224. [Google Scholar] [CrossRef]
- Vakulya, B.; Varga, S.; Soós, T. Epi-Cinchona based thiourea organocatalyst family as an efficient asymmetric michael addition promoter: enantioselective conjugate addition of nitroalkanes to chalcones and α,β-unsaturated n-acylpyrroles. J. Org. Chem. 2008, 73, 3475–3480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Liang, X.; Zhang, T.Y.; Ye, J. An efficient enantioselective method for asymmetric Michael addition of nitroalkanes to α,β-unsaturated aldehydes. Chem. Commun. 2008, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, H.; Ishikawa, H.; Hayashi, Y. Diphenylprolinol Silyl Ether as catalyst of an asymmetric, catalytic, and direct michael reaction of nitroalkanes with α,β-unsaturated aldehydes. Org. Lett. 2007, 9, 5307–5309. [Google Scholar] [CrossRef] [PubMed]
- Hojabri, L.; Hartikka, A.; Moghaddam, F.M.; Arvidsson, P.I. A new imidazole-containing imidazolidinone catalyst for organocatalyzed asymmetric conjugate addition of nitroalkanes to aldehydes. Adv. Synth. Catal. 2007, 349, 740–748. [Google Scholar] [CrossRef]
- Liang, Y.; Dong, D.; Lu, Y.; Wang, Y.; Pan, W.; Chai, Y.; Liu, Q. One-pot synthesis of substituted δ1-pyrrolines through the michael addition of nitroalkanes to chalcones and subsequent reductive cyclization in aqueous media. Synthesis 2006, 19, 3301–3304. [Google Scholar] [CrossRef]
- Tsogoeva, S.B.; Jagtap, S.B.; Ardemasova, Z.A. 4-trans-amino-proline based di- and tetrapeptides as organic catalysts for asymmetric C–C bond formation reactions. Tetrahedron: Asymmetry 2006, 17, 989–992. [Google Scholar] [CrossRef]
- Hanessian, S.; Govindan, S.; Warrier, J.S. Probing the “additive effect” in the proline and proline hydroxamic acid catalyzed asymmetric addition of nitroalkanes to Cyclic Enones. Chirality 2005, 17, 540–543. [Google Scholar] [CrossRef]
- Prieto, A.; Halland, N.; Jørgensen, K.A. Novel imidazolidine-tetrazole organocatalyst for asymmetric conjugate addition of nitroalkanes. Org. Lett. 2005, 7, 3897–3900. [Google Scholar] [CrossRef]
- Tsogoeva, S.B.; Jagtap, S.B. Dual Catalyst control in the chiral diamine-dipeptide-catalyzed asymmetric michael addition. Synlett 2004, 14, 2624–2626. [Google Scholar] [CrossRef]
- Kim, D.Y.; Huh, S.C. Enantioselective Michael reaction of nitroalkanes and chalcones by phase-transfer catalysis using chiral quaternary ammonium salts. Tetrahedron 2001, 57, 8933–8938. [Google Scholar] [CrossRef]
- Yoshida, M.; Narita, M.; Hirama, K.; Hara, S. Asymmetric michael addition of malonates to enones catalyzed by a siloxy amino acid lithium salt. Tetrahedron Lett. 2009, 50, 7297–7299. [Google Scholar] [CrossRef]
- Teo, Y.C.; Lau, J.J.; Wu, M.C. Direct asymmetric three-component mannich reactions catalyzed by a siloxy serine organocatalyst in water. Tetrahedron: Asymmetry 2008, 19, 186–190. [Google Scholar] [CrossRef]
- Teo, Y.C.; Chua, G.L. A recyclable non-immobilized siloxy serine organocatalyst for the asymmetric direct aldol reaction. Tetrahedron Lett. 2008, 49, 4235–4238. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, Z.; Shen, H.M.; Lu, Y. Highly efficient threonine-derived organocatalysts for direct asymmetric aldol reactions in water. Adv. Synth. Catal. 2007, 349, 812–816. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, X.; Lu, Y. Direct asymmetric three-component organocatalytic anti-selective Mannich reactions in a purely aqueous system. Org. Biomol. Chem. 2007, 5, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Aratake, S.; Itoh, T.; Okano, T.; Nagae, N.; Sumiya, T.; Shoji, M.; Hayashi, Y. Highly diastereo- and enantioselective direct aldol reactions of aldehydes and ketones catalyzed by siloxyproline in the presence of water. Chem. Eur. J. 2007, 13, 10246–10256. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, N.; Kimura, M.; Sugahara, T.; Iwabuchi, Y. Organocatalytic entry to chiral bicyclo[3.n.1]alkanones via direct asymmetric intramolecular aldolization. Org. Lett. 2005, 7, 4185–4188. [Google Scholar] [CrossRef]
- Yoshida, M.; Ohno, Y.; Hara, S. Organocatalytic asymmetric thio-michael addition of arylmethyl mercaptans to cyclic enones by a primary amino acid. Tetrahedron Lett. 2010, 51, 5134–5136. [Google Scholar] [CrossRef]
- Yoshida, M.; Kitamikado, N.; Ikehara, H.; Hara, S. One-pot asymmetric synthesis of γ-nitroaldehydes from aldehydes and nitroalkanes through a catalytic tandem reaction using an amino acid lithium salt. J. Org. Chem. 2011, 76, 2305–2309. [Google Scholar] [CrossRef]
- Yoshida, M.; Sato, A.; Hara, S. Asymmetric michael addition of aldehydes to nitroalkenes using a primary amino acid lithium salt. Org. Biomol. Chem. 2010, 8, 3031–3036. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Yoshida, M.; Hara, S. Primary amino acidlithium salt as a catalyst for asymmetric Michael addition of isobutyraldehyde with β-nitroalkenes. Chem. Commun. 2008, 46, 6242–6244. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Barbas, C.F., III. Anti-selective asymmetric michael reactions of aldehydes and nitroolefins catalyzed by a primary amine/thiourea. Angew. Chem. Int. Ed. 2009, 48, 9848–9852. [Google Scholar] [CrossRef] [PubMed]
- Imine-based catalytic asymmetric Michael addition of nitroalkanes to cyclopentenone 3b usually resulted in lower yields and selectivity than those in the case of cyclohexenone 3a. Organocatalytic Enantioselective Michael Additions of Malonates to 2-Cyclopentenone. (Mase, N.; Fukasawa, M.; Kitagawa, N.; Shibagaki, F.; Noshiro, N.; Takabe, K. Synlett 2010, 2340.) See also References 13, 14 and 20, 21, 22.
- It is likely that the reaction mechanism is similar to that of the Michael addition of malonates to enoens. See Reference 33.
- TLC tracing of the reactions indicated the generation of high-polarity compounds.
- Kotrusz, P.; Toma, S. L-proline catalysed michael additions of different methylene active compounds to α-enones in ionic liquid. Arkivoc 2006, 5, 100–109. [Google Scholar]
- Smitha, G.; Patnaik, S.; Reddy, C.S. ZrCl4-catalyzed michael reaction of 1,3-dicarbonyls and enones under solvent-free conditions. Synthesis 2005, 5, 711–713. [Google Scholar] [CrossRef]
- Soriente, A.; Arienzo, R.; Rosa, M.D.; Palombi, L.; Spinella, A.; Scettri, A. K10 montmorillonite catalysis. C−C Bond formation by catalyzed conjugate addition and alkoxyalkylation of 1,3-dicarbonyl compounds. Green Chem. 1999, 3, 157–162. [Google Scholar] [CrossRef]
- Soriente, A.; Spinella, A.; Rosa, M.D.; Giordano, M.; Scettri, A. Solvent free reaction under microwave irradiation: A new procedure for Eu+3—catalyzed michael addition of 1,3-dicarbonyl compounds. Tetrahedron Lett. 1997, 38, 289–290. [Google Scholar] [CrossRef]


| Entry | Solvent | Conv. b (%) | Yield c (%) | ee d (%) |
|---|---|---|---|---|
| 1 | DMSO | 100 | 72 | 9 |
| 2 | MeOH | 100 | 62 | 4 |
| 3 | MeCN | 11 | 11 | 59 |
| 4 | AcOEt | 24 | 24 | 68 |
| 5 | THF | 27 | 22 | 68 |
| 6 | CH2Cl2 | 41 | 28 | 68 |
| 7 | Toluene | 60 | 45 | 65 |
| 8 | Cyclohexane | 94 | 69 | 70 |

| Entry | Catalyst | Conv. b (%) | Yield c (%) | ee d (%) |
|---|---|---|---|---|
| 1 | Ser(O-TBS)-OLi, 1a | 94 | 69 | 70 |
| 2 | Thr(O-TBS)-OLi, 1b | 84 | 34 | 68 |
| 3 | Tyr(O-TBS)-OLi, 1c | 79 | 46 | 38 |
| 4 | Ser(O-TIPS)-OLi, 1d | 91 | 46 | 79 |
| 5 | Ser(O-TBDPS)-OLi, 1e | 96 | 28 | 80 |
| 6 | Ser(O-TIPS)-ONa, 1f | 100 | 76 | 48 |
| 7 | Ser(O-TIPS)-OK, 1g | 100 | 64 | 16 |
| 8 | Ser(O-TIPS)-ORb, 1h | 100 | 65 | 24 |
| 9 | Ser(O-TIPS)-OCs, 1i | 100 | 66 | 45 |
| 10 | Ser(O-TIPS)-OH, 1j | 0 | 0 | n.d. |

| Entry | 2a (equiv.) | Conc. b (M) | Conv. c (%) | Yield d (%) | ee e (%) |
|---|---|---|---|---|---|
| 1 | 1.2 | 0.5 | 91 | 46 | 79 |
| 2 | 3.0 | 0.5 | 97 | 70 | 74 |
| 3 | 5.0 | 0.5 | 98 | 82 | 72 |
| 4 | 5.0 | 0.1 | 76 | 70 | 77 |
| 5 | 5.0 | neat | 59 | 48 | 70 |

| Entry | 2, R1, R2 | 3, R3, R4 | Conv. b (%) | Yield c (%) | Ee d (%) |
|---|---|---|---|---|---|
| 1 | 2a, Me, Me | 3a, –(CH2)3– | 76 | 70, 4a | 77 |
| 2 | 2a, Me, Me | 3b, –(CH2)2– | – e | 53, 4b | 15 |
| 3 | 2a, Me, Me | 3c, –(CH2)4– | 44 | 20, 4c | 79 |
| 4 | 2a, Me, Me | 3d, Me, trans-Ph | 73 | 69, 4d | 51 |
| 5 | 2a, Me, Me | 3e, Ph, trans-Ph | 0 | 0, 4e | n.d. |
| 6 | 2b, Me, H | 3a, –(CH2)3– | 100 | 70, 4f | 50, 38 f |
| 7 | 2c, H, H | 3a, –(CH2)3– | 94 | 50, 4g | 55 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yoshida, M.; Hirama, K.; Narita, M.; Hara, S. Primary Amino Acid Lithium Salt-Catalyzed Asymmetric Michael Addition of Carbon Nucleophiles to Enones. Symmetry 2011, 3, 155-164. https://doi.org/10.3390/sym3020155
Yoshida M, Hirama K, Narita M, Hara S. Primary Amino Acid Lithium Salt-Catalyzed Asymmetric Michael Addition of Carbon Nucleophiles to Enones. Symmetry. 2011; 3(2):155-164. https://doi.org/10.3390/sym3020155
Chicago/Turabian StyleYoshida, Masanori, Keisuke Hirama, Mao Narita, and Shoji Hara. 2011. "Primary Amino Acid Lithium Salt-Catalyzed Asymmetric Michael Addition of Carbon Nucleophiles to Enones" Symmetry 3, no. 2: 155-164. https://doi.org/10.3390/sym3020155