Domain Specificity in Human Symmetry Preferences: Symmetry is Most Pleasant When Looking at Human Faces
Abstract
:1. Introduction
2. Methods
2.1. Participants
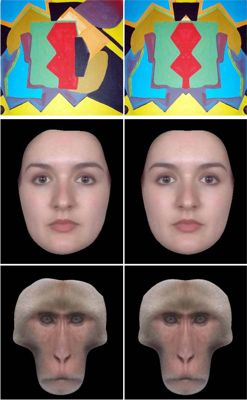
2.2. Asymmetric and Symmetric Stimuli
2.3. Procedure
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Møller, A.P.; Thornhill, R. Bilateral symmetry and sexual selection: A meta-analysis. Am. Nat. 1998, 151, 174–192. [Google Scholar]
- Grammer, K.; Thornhill, R. Human (Homo sapiens) facial attractiveness and sexual selection: The role of symmetry and averageness. J. Comp. Psychol. 1994, 108, 233–242. [Google Scholar]
- Penton-Voak, I.S.; Jones, B.C.; Little, A.C.; Baker, S.; Tiddeman, B.; Burt, D.M.; Perrett, D.I. Symmetry, sexual dimorphism in facial proportions, and male facial attractiveness. Proc. Biol. Sci. R. Soc. 2001, 268, 1617–1623. [Google Scholar]
- Scheib, J.E.; Gangestad, S.W.; Thornhill, R. Facial attractiveness, symmetry, and cues to good genes. Proc. Biol. Sci. R. Soc. 1999, 266, 1913–1917. [Google Scholar]
- Kowner, R. Facial asymmetry and attractiveness judgment in developmental perspective. J. Exp. Psychol. Hum. Percept. Perform. 1996, 22, 662–675. [Google Scholar]
- Perrett, D.I.; Burt, D.M.; Penton-Voak, I.S.; Lee, K.J.; Rowland, D.A.; Edwards, R. Symmetry and human facial attractiveness. Evol. Hum. Behav. 1999, 20, 295–307. [Google Scholar]
- Rhodes, G.; Proffitt, F.; Grady, J.; Sumich, A. Facial symmetry and the perception of beauty. Psychon. Bull. Rev. 1998, 5, 659–669. [Google Scholar]
- Jones, B.C.; Little, A.C.; Burt, D.M.; Perrett, D.I. When facial attractiveness is only skin deep. Perception 2004, 33, 569–576. [Google Scholar]
- Jones, B.C.; Little, A.C.; Penton-Voak, I.S.; Tiddeman, B.P.; Burt, D.M.; Perrett, D.I. Facial symmetry and judgements of apparent health—Support for a “good genes” explanation of the attractiveness-symmetry relationship. Evol. Hum. Behav. 2001, 22, 417–429. [Google Scholar]
- Mealey, L.; Bridgestock, R.; Townsend, G. Symmetry and perceived facial attractiveness. J. Pers. Soc. Psychol. 1999, 76, 151–158. [Google Scholar]
- Little, A.C.; Jones, B.C. Evidence against perceptual bias views for symmetry preferences in human faces. Proc. Biol. Sci. R. Soc. 2003, 270, 1759–1763. [Google Scholar]
- Little, A.C.; Jones, B.C. Attraction independent of detection suggests special mechanisms for symmetry preferences in human face perception. Proc. Biol. Sci. R. Soc. 2006, 273, 3093–3099. [Google Scholar]
- Little, A.C.; Apicella, C.L.; Marlowe, F.W. Preferences for symmetry in human faces in two cultures: Data from the UK and the Hadza, an isolated group of hunter-gatherers. Proc. Biol. Sci. R. Soc. 2007, 274, 3113–3117. [Google Scholar]
- Waitt, C.; Little, A.C. Preferences for symmetry in conspecific facial shape among Macaca mulatta. Int. J. Primatol. 2006, 27, 133–145. [Google Scholar]
- Rhodes, G.; Yoshikawa, S.; Clark, A.; Lee, K.; McKay, R.; Akamatsu, S. Attractiveness of facial averageness and symmetry in non-Western populations: In search of biologically based standards of beauty. Perception 2001, 30, 611–625. [Google Scholar]
- Van Dongen, S. Associations between asymmetry and human attractiveness: Possible direct effects of asymmetry and signatures of publication bias. Ann. Hum. Biol. 2011, 38, 317–323. [Google Scholar]
- Soler, C.; Kekalainen, J.; Nunez, M.; Sancho, M.; Nunez, J.; Yaber, I.; Gutierrez, R. Male facial anthropometry and attractiveness. Perception 2012, 41, 1234–1245. [Google Scholar]
- Thornhill, R.; Gangestad, S.W. Facial attractiveness. Trends Cogn. Sci. 1999, 3, 452–460. [Google Scholar]
- Wade, T.J. The Relationships between Symmetry and Attractiveness and Mating Relevant Decisions and Behavior: A Review. Symmetry 2010, 2, 1081–1098. [Google Scholar]
- Møller, A.P. Developmental stability and fitness: A review. Am. Nat. 1997, 149, 916–932. [Google Scholar]
- Dufour, K.W.; Weatherhead, P.J. Bilateral symmetry and social dominance in captive male red-winged blackbirds. Behav. Ecol. Sociobiol. 1998, 42, 71–76. [Google Scholar]
- Møller, A.P.; Swaddle, J.P. Asymmetry, Developmental Stability, and Evolution; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Manning, J.T.; Scutt, D.; Lewis-Jones, D.I. Developmental stability, ejaculate size, and sperm quality in men. Evol. Hum. Behav. 1998, 19, 273–282. [Google Scholar]
- Manning, J.T.; Scutt, D.; Whitehouse, G.H.; Leinster, S.J. Breast asymmetry and phenotypic quality in women. Evol. Hum. Behav. 1997, 18, 223–236. [Google Scholar]
- Møller, A.P.; Soler, M.; Thornhill, R. Breast asymmetry, sexual selection, and human reproductive success. Ethol. Sociobiol. 1995, 16, 207–219. [Google Scholar]
- Thornhill, R.; Gangestad, S.W. Facial sexual dimorphism, developmental stability, and susceptibility to disease in men and women. Evol. Hum. Behav. 2006, 27, 131–144. [Google Scholar]
- Gangestad, S.W.; Thornhill, R. Facial masculinity and fluctuating asymmetry. Evol. Hum. Behav. 2003, 24, 231–241. [Google Scholar]
- Little, A.C.; Jones, B.C.; Waitt, C.; Tiddeman, B.P.; Feinberg, D.R.; Perrett, D.I.; Apicella, C.L.; Marlowe, F.W. Symmetry is related to sexual dimorphism in faces: Data across culture and species. PLoS One 2008, 3, e2106. [Google Scholar] [CrossRef] [Green Version]
- Rensch, B. Vesuche uber menschliche Auslosermerkmale beider Geschlecter. Z. Morphol. Anthropol. 1963, 53, 139–164. [Google Scholar]
- Gombrich, E.H. The Sense of Order: A Study in the Psychology of Decorative Art; Phaidon: London, UK, 1984. [Google Scholar]
- Hodgson, D. The First Appearance of Symmetry in the Human Lineage: Where Perception Meets Art. Symmetry 2011, 3, 37–53. [Google Scholar]
- Herbert, A.M.; Humphrey, G.K. Bilateral symmetry detection: testing a “callosal” hypothesis. Perception 1996, 25, 463–480. [Google Scholar]
- Mach, E. Contributions to the Analysis of the Sensations; Open Court: LaSalle, IL, USA, 1897. [Google Scholar]
- Jansson, L.; Forkman, B.; Enquist, M. Experimental evidence of receiver bias for symmetry. Anim. Behav. 2002, 63, 617–621. [Google Scholar]
- Enquist, M.; Arak, A. Symmetry, Beauty and evolution. Nature 1994, 372, 169–172. [Google Scholar]
- Enquist, M.; Ghirlanda, S. The secrets of faces. Nature 1998, 394, 826–827. [Google Scholar]
- Enquist, M.; Johnstone, R.A. Generalization and the evolution of symmetry preferences. Proc. R. Soc. Lond. B 1997, 264, 1345–1348. [Google Scholar]
- Rhodes, G.; Sumich, A.; Byatt, G. Are average facial configurations attractive only because of their symmetry? Psychol. Sci. 1999, 10, 52–58. [Google Scholar]
- Little, A.C.; Burt, D.M.; Penton-Voak, I.S.; Perrett, D.I. Self-perceived attractiveness influences human female preferences for sexual dimorphism and symmetry in male faces. Proc. Biol. Sci. R. Soc. 2001, 268, 39–44. [Google Scholar]
- Little, A.C.; Jones, B.C.; DeBruine, L.M.; Feinberg, D.R. Symmetry and sexual dimorphism in human faces: Interrelated preferences suggest both signal quality. Behav. Ecol. 2008, 19, 902–908. [Google Scholar]
- Little, A.C.; Jones, B.C.; DeBruine, L.M. Exposure to visual cues of pathogen contagion changes preferences for masculinity and symmetry in opposite-sex faces. Proc. R. Soc. Lond. B 2011, 278, 2032–2039. [Google Scholar]
- Young, S.G.; Sacco, D.F.; Hugenberg, K. Vulnerability to disease is associated with a domain-specific preference for symmetrical faces relative to symmetrical non-face stimuli. Eur. J. Soc. Psychol. 2011, 41, 558–563. [Google Scholar]
- Evans, C.S.; Wenderoth, P.; Cheng, K. Detection of bilateral symmetry in complex biological images. Perception 2000, 29, 31–42. [Google Scholar]
- Benson, P.J.; Perrett, D.I. Extracting prototypical facial images from exemplars. Perception 1993, 22, 257–262. [Google Scholar]
- Little, A.C.; Hancock, P.J. The role of masculinity and distinctiveness on the perception of attractiveness in human male faces. Br. J. Psychol. 2002, 93, 451–464. [Google Scholar]
- Tiddeman, B.P.; Burt, D.M.; Perrett, D.I. Prototyping and transforming facial texture for perception research. IEEE Comput. Graph. Appl. 2001, 21, 42–50. [Google Scholar]
- Little, A.C.; Jones, B.C.; Burt, D.M.; Perrett, D.I. Preferences for symmetry in faces change across the menstrual cycle. Biol. Psychol. 2007, 76, 209–216. [Google Scholar]
- Category: Abstract paintings. Available online: http://commons.wikimedia.org/wiki/Category:Abstract_paintings (31 March 2014).
- File: Acrilic on canvas 323.jpg. Available online: http://commons.wikimedia.org/wiki/File:Acrilic_on_canvas_323.jpg (31 March 2014).
- Attneave, F. Symmetry, information, and memory for patterns. Am. J. Psychol. 1955, 68, 209–222. [Google Scholar]
- Rhodes, G.; Yoshikawa, S.; Palermo, R.; Simmons, L.W.; Peters, M.; Lee, K.; Halberstadt, J.; Crawford, J.R. Perceived health contributes to the attractiveness of facial symmetry, averageness, and sexual dimorphism. Perception 2007, 36, 1244–1252. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Little, A.C. Domain Specificity in Human Symmetry Preferences: Symmetry is Most Pleasant When Looking at Human Faces. Symmetry 2014, 6, 222-233. https://doi.org/10.3390/sym6020222
Little AC. Domain Specificity in Human Symmetry Preferences: Symmetry is Most Pleasant When Looking at Human Faces. Symmetry. 2014; 6(2):222-233. https://doi.org/10.3390/sym6020222
Chicago/Turabian StyleLittle, Anthony C. 2014. "Domain Specificity in Human Symmetry Preferences: Symmetry is Most Pleasant When Looking at Human Faces" Symmetry 6, no. 2: 222-233. https://doi.org/10.3390/sym6020222
APA StyleLittle, A. C. (2014). Domain Specificity in Human Symmetry Preferences: Symmetry is Most Pleasant When Looking at Human Faces. Symmetry, 6(2), 222-233. https://doi.org/10.3390/sym6020222