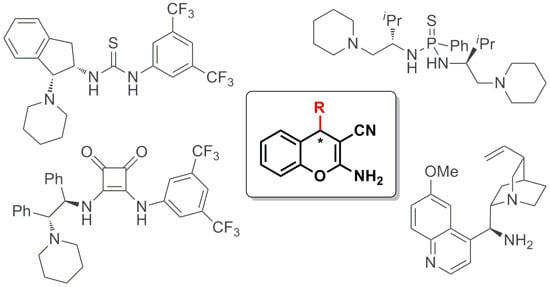
Enantioselective Organocatalyzed Synthesis of 2-Amino-3-cyano-4H-chromene Derivatives
Abstract
:1. Introduction


2. Addition of Naphthol Compounds to Substituted α,α-dicyanoolefins



3. Addition of Malononitrile to Substituted o-vinylphenols








4. Addition of Malononitrile to N-protected o-iminophenols


5. Michael Addition of Nucleophiles to 2-iminochromene Derivatives








6. Organocatalyzed Formal [4+2] Cycloaddition Reaction

7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pratap, R.; Ram, V.J. Natural and synthetic chromenes, fused chromenes and versatility of dihydrobenzo[h]chromenes in organic synthesis. Chem. Rev. 2014, 144, 10476–10526. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Zachariah, S.M. Pharmacological activities of chromene derivatives: An overview. Asian J. Pharm. Clin. Res. 2013, 6, 11–15. [Google Scholar]
- Patil, S.A.; Patil, R.; Pfeffer, L.M.; Miller, D.D. Chromenes: Potential new chemotherapeutic agents for cancer. Future Med. Chem. 2013, 5, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Szczurowska, E.; Mareš, P. Positive allosteric modulator of mGluR4 PHCCC exhibits proconvulsant action in three models of epileptic seizures in immature rats. Physiol. Res. 2012, 61, 619–628. [Google Scholar] [PubMed]
- Liu, X.; Hong, S.I.; Park, S.J.; dela Peña, J.B.; Che, H.; Yoon, S.Y.; Kim, D.H.; Kim, J.M.; Cai, M.; Risbrough, V.; et al. The ameliorating effects of 5,7-dihydroxy-6-methoxy-2(4-phenoxyphenyl)-4H-chromene-4-one, an oroxylin A derivative, against memory impairment and sensorimotor gating deficit in mice. Arch. Pharm. Res. 2013, 36, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.; Saxena, S.; Khan, M.K.R.; Thukral, S.S. Aqua mediated synthesis of substituted 2-amino-4H-chromenes and in vitro study as antibacterial agents. Bioorg. Med. Chem. Lett. 2005, 15, 4295–4298. [Google Scholar] [CrossRef] [PubMed]
- Thumar, N.J.; Patel, M.P. Synthesis and in vitro antimicrobial evaluation of 4H-pyrazolopyran, benzopyran and naphthopyran derivatives of 1H-pyrazole. Arkivoc 2009, 13, 363–380. [Google Scholar]
- Sabry, N.M.; Mohamed, H.M.; Khattab, E.S.A.E.H.; Motlaq, S.S.; El-Agrody, A.M. Synthesis of 4H-chromene, coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur. J. Med. Chem. 2011, 46, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Shanthi, G.; Perumal, P.T.; Rao, U.; Sehgal, P.K. Synthesis and antioxidant activity of indolyl chromenes. Indian J. Chem. 2009, 48B, 1319–1323. [Google Scholar]
- Kemnitzer, W.; Drewe, J.; Jiang, S.; Zhang, H.; Wang, Y.; Zhao, J.; Jia, S.; Herich, J.; Labreque, D.; Storer, R.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 1. Structure-activity relationships of the 4-aryl group. J. Med. Chem. 2004, 47, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Kasibhatla, S.; Gourdeau, H.; Meerovitch, K.; Drewe, J.; Reddy, S.; Qiu, L.; Zhang, H.; Bergeron, F.; Bouffard, D.; Yang, Q.; et al. Discovery and mechanism of action of a novel series of apoptosis inducers with potential vascular targeting activity. Mol. Cancer Ther. 2004, 3, 1365–1373. [Google Scholar] [PubMed]
- Gourdeau, H.; Leblond, L.; Hamelin, B.; Desputeau, C.; Dong, K.; Kianicka, I.; Custeau, D.; Boudreau, C.; Geerts, L.; Cai, S.-X.; et al. Antivascular and antitumor evaluation of 2-amino-4-(3-bromo-4,5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a novel series of anticancer agents. Mol. Cancer Ther. 2004, 3, 1375–1383. [Google Scholar] [PubMed]
- Kemnitzer, W.; Kasibhatla, S.; Jiang, S.; Zhang, H.; Zhao, J.; Jia, S.; Xu, L.; Crogan-Grundy, C.; Denis, R.; Barriault, N.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure-activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg. Med. Chem. Lett. 2005, 15, 4745–4751. [Google Scholar] [CrossRef] [PubMed]
- Skommer, J.; Wlodkowic, D.; Mättö, M.; Eray, M.; Pelkonen, J. HA14–1, a small molecule Bcl-2 antagonist, induces apoptosis and modulates action of selected anticancer drugs in follicular lymphoma B cells. Leukemia Res. 2006, 30, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.X.; Drewe, J.A.; Kasibhatla, S.; Kemnitzer, W.D.; Tseng, B.Y.; Blais, C.; Labrecque, D.; Gourdeau, H. Substituted 4-aryl-chromene as activator of caspases and inducer of apoptosis and as antivascular agent and the use thereof. US Patent 7,968,595 B2, 2011. [Google Scholar]
- Shestopalov, A.M.; Litvinov, Y.M.; Rodinovskaya, L.A.; Malyshev, O.R.; Semenova, M.N.; Semenov, V.V. Polyalkoxy substituted 4H-chromenes: Synthesis by domino reaction and anticancer activity. ACS Comb. Sci. 2012, 14, 484–490. [Google Scholar] [CrossRef] [PubMed]
- A phase I/II trial of Crolibulin (EPC2407) plus cisplatin in adults with solid tumors with a focus on anaplastic thyroid cancer (ATC). Available online: https://clinicaltrials.gov (accessed on 18 May 2015).
- Berkessel, A.; Gröger, H. Asymmetric Organocatalysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2005. [Google Scholar]
- Dalko, P.I. Enantioselective Organocatalysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007. [Google Scholar]
- Dalko, P.I. Comprehensive Enantioselective Organocatalysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2013. [Google Scholar]
- Herrera, R.P. Fundamentals in Organocatalysis. Past, Present and Future. Curr. Org. Chem. 2011, 15(13), 2082–2082. [Google Scholar] [CrossRef]
- Murthy, S.N.; Madhav, B.; Reddy, V.P.; Nageswar, Y.V.D. One-pot synthesis of 2-amino-4H-chromen-4-yl phosphonate derivatives using β-cyclodextrin as reusable catalyst in water. Tetrahedron Lett. 2010, 51, 3649–3653. [Google Scholar] [CrossRef]
- Moafi, L.; Ahadi, S.; Bazgir, A. New HA 14-1 analogues: synthesis of 2-amino-4-cyano-4H-chromenes. Tetrahedron Lett. 2010, 51, 6270–6274. [Google Scholar] [CrossRef]
- Kolla, S.R.; Lee, Y.R. Efficient one-pot synthesis of β-phosphono malonates and 2-amino-4H-chromene-4-ylphosphonate derivatives by ethylenediamine diacetate-catalyzed three-component reactions. Tetrahedron 2012, 68, 226–237. [Google Scholar] [CrossRef]
- Mondal, J.; Modak, A.; Nandi, M.; Uyama, H.; Bhaumik, A. Triazine functionalized ordered mesoporous organosilica as a novel organocatalyst for the facile one-pot synthesis of 2-amino-4H-chromenes under solvent-free conditions. RSC Adv. 2012, 2, 11306–11317. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Eslami, M.; Maleki, A. Potassium phthalimide-N-oxyl: A novel, efficient, and simple organocatalyst for the one-pot three-component synthesis of various 2-amino-4H-chromene derivatives in water. Tetrahedron 2013, 69, 1074–1085. [Google Scholar] [CrossRef]
- Kalla, R.M.N.; Byeon, S.J.; Heo, M.S.; Kim, I. Synthesis of 2-amino-3-cyano-4H-chromen-4-ylphosphonates and 2-amino-4H-chromenes catalyzed by tetramethylguanidine. Tetrahedron 2013, 69, 10544–10551. [Google Scholar] [CrossRef]
- Khan, Md.N.; Pal, S.; Karamthulla, S.; Choudhury, L.H. Imidazole as organocatalyst for multicomponent reactions: Diversity oriented synthesis of functionalized hetero- and carbocycles using in situ-generated benzylidenemalononitrile derivatives. RSC Adv. 2014, 4, 3732–3741. [Google Scholar] [CrossRef]
- Davarpanah, J.; Kiasat, A.R. Covalently anchored n-propyl-4-aza-1-azoniabicyclo[2.2.2]octane chloride on SBA-15 as a basic nanocatalyst for the synthesis of pyran heterocyclic compounds. RSC Adv. 2014, 4, 4403–4412. [Google Scholar] [CrossRef]
- Azizi, K.; Karimi, M.; Shaterian, H.R.; Heydari, A. Ultrasound irradiation for the green synthesis of chromenes using L-arginine-functionalized magnetic nanoparticles as a recyclable organocatalyst. RSC Adv. 2014, 4, 42220–42225. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Eslami, M. Highly efficient organocatalytic synthesis of diverse and densely functionalized 2-amino-3-cyano-4H-pyrans under mechanochemical ball milling. Green Chem. 2014, 16, 4914–4921. [Google Scholar] [CrossRef]
- Kalla, R.M.N.; Choi, J.-S.; Yoo, J.-W.; Byeon, S.J.; Heo, M.S.; Kim, I. Synthesis of 2-amino-3-cyano-4H-chromen-4-ylphosphonates and their anticancer properties. Eur. J. Med. Chem. 2014, 76, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Khan, Md.N.; Karamthulla, S.; Choudhury, L.H. Synthesis of pyranocoumarin fused spirooxindoles via Knoevenagel/Michael/cyclization sequence: A regioselective organocatalyzed multicomponent reaction. Tetrahedron Lett. 2015, 56, 359–364. [Google Scholar] [CrossRef]
- Chen, W.; Cai, Y.; Fu, X.; Liu, X.; Lin, L.; Feng, X. Enantioselective one-pot synthesis of 2-amino-4-(indol-3-yl)-4H-chromenes. Org. Lett. 2011, 13, 4910–4913. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Yang, G.-S.; Zhao, G. Enantioselective synthesis of naphthopyran derivatives catalyzed by bifunctional thiourea-tertiary amines. Tetrahedron Asymmetry 2008, 19, 709–714. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Yan, J.; Chen, R.; Wang, S.; Ma, Y.; Wang, R. One-pot enantioselective synthesis of functionalized pyranocoumarins and 2-amino-4H-chromenes: Discovery of a type of potent antibacterial agent. J. Org. Chem. 2012, 77, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-W.; Huang, X.; Fan, L.-P.; Xu, D.-C.; Li, X.-S.; Su, H.; Wen, Y.-H. Efficient method for the synthesis of optically active 2-amino-2-chromene derivatives via one-pot tandem reactions. Adv. Synth. Catal. 2009, 351, 3077–3082. [Google Scholar] [CrossRef]
- Du, Z.; Siau, W.-Y.; Wang, J. Enantioselective organocatalytic synthesis of medicinally privileged 2-amino-4H-chromene-3-carbonitriles via a cascade process. Tetrahedron Lett. 2011, 52, 6137–6141. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, W.; Du, D.-M. Efficient organocatalytic asymmetric synthesis of 2-amino-4H-chromene-3-carbonitrile derivatives. Tetrahedron Asymmetry 2012, 23, 339–344. [Google Scholar] [CrossRef]
- Hu, K.; Lu, A.; Wang, Y.; Zhou, Z.; Tang, C. Chiral bifunctional squaramide catalyzed asymmetric tandem Michael-cyclization reaction: Efficient synthesis of optically active 2-amino-4H-chromene-3-carbonitrile derivatives. Tetrahedron Asymmetry 2013, 24, 953–957. [Google Scholar] [CrossRef]
- Hu, K.; Wang, Y.; Zhou, Z.; Tang, C. Novel thiophosphonodiamides as efficient hydrogen bond donor catalysts in tandem Michael addition-cyclization of malononitrile and 2-(E)-2-nitrovinylphenols. Tetrahedron 2014, 70, 181–185. [Google Scholar] [CrossRef]
- Ren, Q.; Siau, W.-Y.; Du, Z.; Zang, K.; Wang, J. Expeditious assembly of a 2-amino-4H-chromene skeleton by using an enantioselective Mannich intramolecular ring cyclization-tautomerization cascade sequence. Chem. Eur. J. 2011, 17, 7781–7785. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, H.; Jiang, X.; Wang, J. Enantioselective organocatalytic conjugate addition of nitroalkanes to electrophilic 2-iminochromenes. ACS Catal. 2012, 2, 1535–1538. [Google Scholar] [CrossRef]
- Yang, G.; Luo, C.; Mu, X.; Wang, T.; Liu, X.-Y. Highly efficient enantioselective three-component synthesis of 2-amino-4H-chromenes catalysed by chiral tertiary amine-thioureas. Chem. Commun. 2012, 48, 5880–5882. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Du, D.-M. Facile synthesis of chiral 2-amino-4-(indol-3-yl)-4H-chromene derivatives using thiourea as the catalyst. Tetrahedron Asymmetry 2013, 24, 1312–1317. [Google Scholar] [CrossRef]
- Li, W.; Huang, J.; Wang, J. Organocatalytic conjugate addition promoted by multi-hydrogen-bond cooperation: Access to chiral 2-amino-3-nitrile-chromenes. Org. Biomol. Chem. 2013, 11, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Adili, A.; Tao, Z.-L.; Chen, D.-F.; Han, Z.-Y. Quinine-catalyzed highly enantioselective cycloannulation of o-quinone methides with malononitrile. Org. Biomol. Chem. 2015, 13, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Alden-Danforth, E.; Scerba, M.T.; Lectka, T. Asymmetric cycloadditions of o-quinone methides employing chiral ammonium fluoride precatalysts. Org. Lett. 2008, 10, 4951–4953. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, H.; Takemoto, Y. Discovery and application of asymmetric reaction by multi-functional thioureas. Bull. Chem. Soc. Jpn. 2008, 81, 785–795. [Google Scholar] [CrossRef]
- Connon, S.J. Asymmetric catalysis with bifunctional cinchona alkaloid-based urea and thiourea organocatalysts. Chem. Commun. 2008, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonsona, I.G.; Marqués-López, E.; Herrera, R.P. Enantioselective Organocatalyzed Synthesis of 2-Amino-3-cyano-4H-chromene Derivatives. Symmetry 2015, 7, 1519-1535. https://doi.org/10.3390/sym7031519
Sonsona IG, Marqués-López E, Herrera RP. Enantioselective Organocatalyzed Synthesis of 2-Amino-3-cyano-4H-chromene Derivatives. Symmetry. 2015; 7(3):1519-1535. https://doi.org/10.3390/sym7031519
Chicago/Turabian StyleSonsona, Isaac G., Eugenia Marqués-López, and Raquel P. Herrera. 2015. "Enantioselective Organocatalyzed Synthesis of 2-Amino-3-cyano-4H-chromene Derivatives" Symmetry 7, no. 3: 1519-1535. https://doi.org/10.3390/sym7031519
APA StyleSonsona, I. G., Marqués-López, E., & Herrera, R. P. (2015). Enantioselective Organocatalyzed Synthesis of 2-Amino-3-cyano-4H-chromene Derivatives. Symmetry, 7(3), 1519-1535. https://doi.org/10.3390/sym7031519