Concise Review: Asymmetric Cell Divisions in Stem Cell Biology
Abstract
:1. The Fate of Somatic Stem Cells is Tightly Controlled
2. The Process of Asymmetric Cell Division
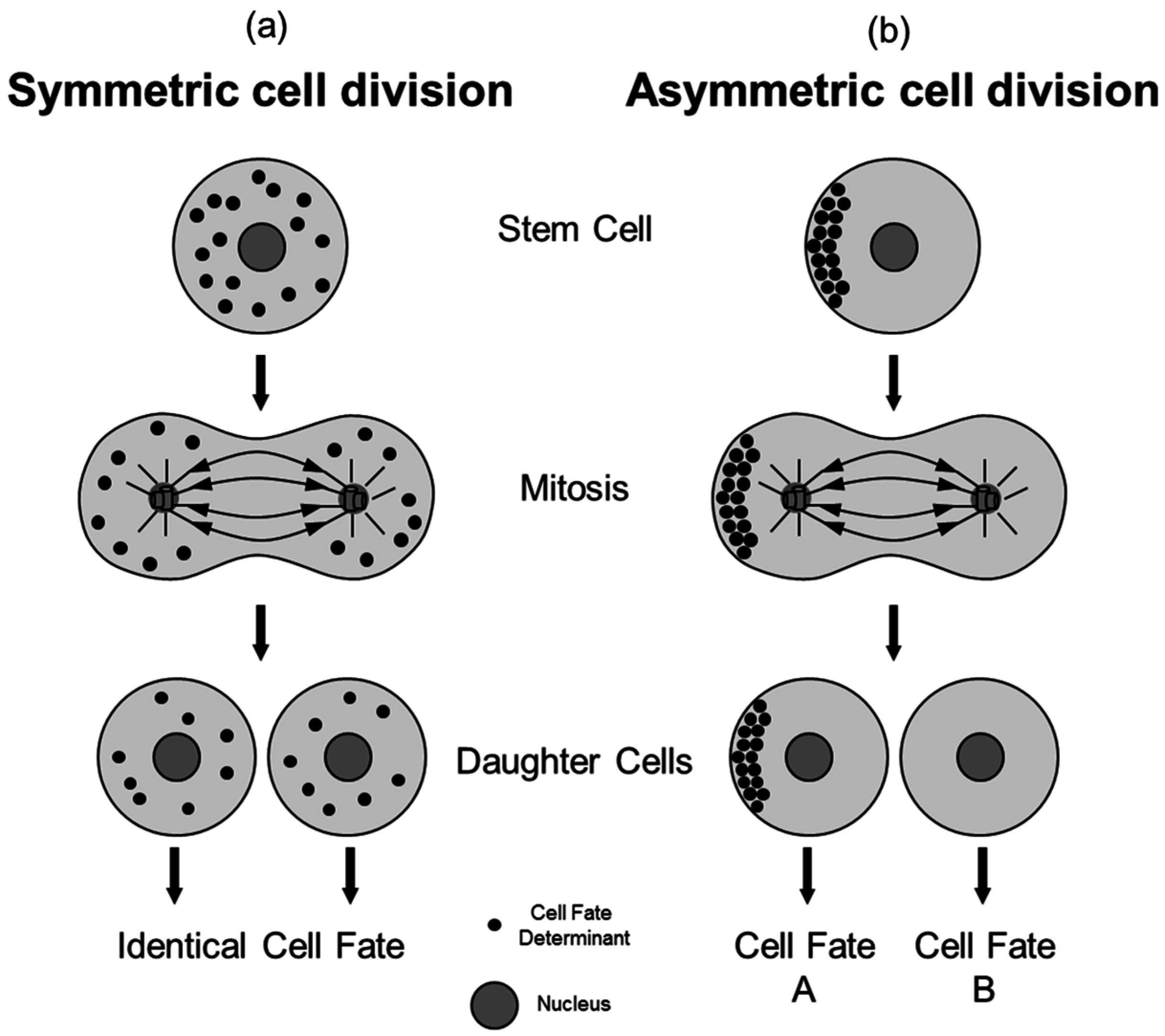
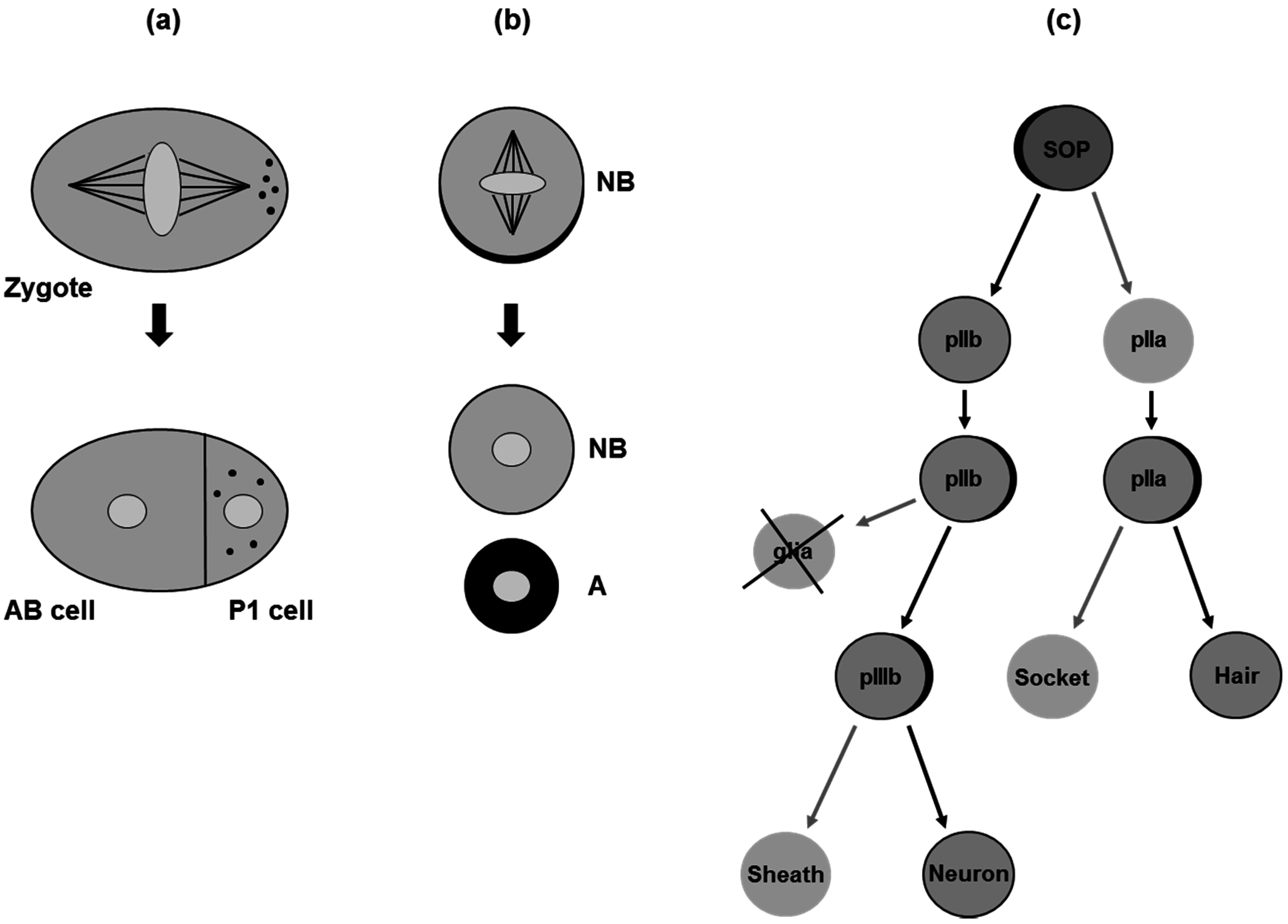
3. Asymmetric Cell Division in Model Organisms
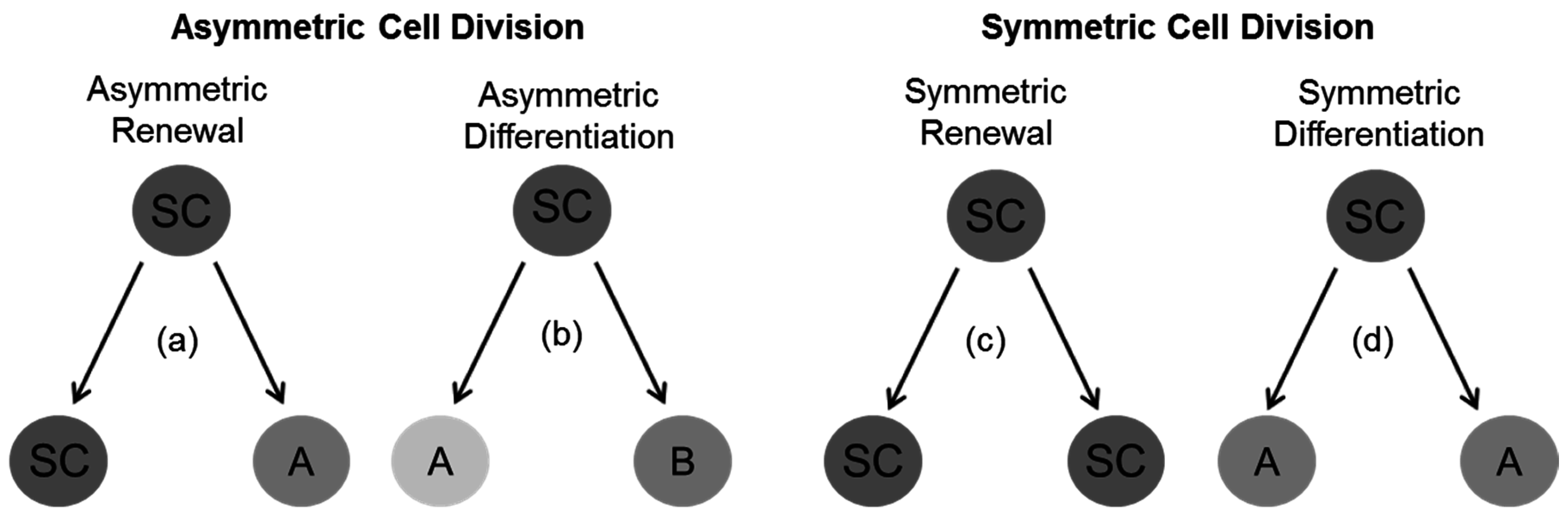
4. General Concepts for Divisional Modes in Stem and Progenitor Cells
5. Cell Polarity as a Prerequisite for ACD
6. ACD in Human Hematopoiesis
7. Identification of ACD Markers in Human Hematopoiesis

8. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Görgens, A.; Giebel, B. Self-renewal of primitive hematopoietic cells: A focus on asymmetric cell division. In Umbilical Cord Blood: A Future for Regenerative Medicine? Kadereit, S., Udolph, G., Eds.; World Scientific Publishing Company, Incorporated: Singapore, 2010; pp. 51–75. [Google Scholar]
- Al-Hajj, M. Cancer stem cells and oncology therapeutics. Curr. Opin. Oncol. 2007, 19, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Savona, M.; Talpaz, M. Getting to the stem of chronic myeloid leukaemia. Nat. Rev. Cancer 2008, 8, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nature Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.J.; Jamieson, C.H.M.; Weissman, I.L. Stems cells and the pathways to aging and cancer. Cell 2008, 132, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Ruzankina, Y.; Brown, E.J. Relationships between stem cell exhaustion, tumour suppression and ageing. Br. J. Cancer 2007, 97, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E.; DePinho, R.A. How stem cells age and why this makes us grow old. Nature Rev. Mol. Cell Biol. 2007, 8, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Boulais, P.E.; Frenette, P.S. Making sense of hematopoietic stem cell niches. Blood 2015, 125, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Mesa, K.R.; Rompolas, P.; Greco, V. The dynamic duo: Niche/stem cell interdependency. Stem Cell Rep. 2015, 4, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Scadden, D.T.; Sanchez-Aguilera, A. Bone marrow stem cells: Current and emerging concepts. Ann. NY Acad. Sci. 2015, 1335, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Kusek, G.; Campbell, M.; Doyle, F.; Tenenbaum, S.A.; Kiebler, M.; Temple, S. Asymmetric segregation of the double-stranded RNA binding protein staufen2 during mammalian neural stem cell divisions promotes lineage progression. Cell Stem Cell 2012, 11, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Vessey, J.P.; Amadei, G.; Burns, S.E.; Kiebler, M.A.; Kaplan, D.R.; Miller, F.D. An asymmetrically localized staufen2-dependent RNA complex regulates maintenance of mammalian neural stem cells. Cell Stem Cell 2012, 11, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Katajisto, P.; Döhla, J.; Chaffer, C.L.; Pentinmikko, N.; Marjanovic, N.; Iqbal, S.; Zoncu, R.; Chen, W.; Weinberg, R.A.; Sabatini, D.M. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science 2015, 348, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, A. Stem cells: Asymmetric rejuvenation. Nature 2015, 521, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Strome, S.; Wood, W.B. Immunofluorescence visualization of germ-line-specific cytoplasmic granules in embryos, larvae, and adults of caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1982, 79, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Hird, S.N.; Paulsen, J.E.; Strome, S. Segregation of germ granules in living caenorhabditis elegans embryos: Cell-type-specific mechanisms for cytoplasmic localisation. Development 1996, 122, 1303–1312. [Google Scholar] [PubMed]
- Priess, J.R.; Thomson, J.N. Cellular interactions in early C. elegans embryos. Cell 1987, 48, 241–250. [Google Scholar] [CrossRef]
- Sulston, J.E.; Schierenberg, E.; White, J.G.; Thomson, J.N. The embryonic cell lineage of the nematode caenorhabditis elegans. Dev. Biol. 1983, 100, 64–119. [Google Scholar] [CrossRef]
- Kemphues, K.J.; Priess, J.R.; Morton, D.G.; Cheng, N.S. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 1988, 52, 311–320. [Google Scholar] [CrossRef]
- Hirata, J.; Nakagoshi, H.; Nabeshima, Y.; Matsuzaki, F. Asymmetric segregation of the homeodomain protein prospero during drosophila development. Nature 1995, 377, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Spana, E.P.; Doe, C.Q. The prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in drosophila. Development 1995, 121, 3187–3195. [Google Scholar] [PubMed]
- Kaltschmidt, J.A.; Davidson, C.M.; Brown, N.H.; Brand, A.H. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nature Cell Biol. 2000, 2, 7–12. [Google Scholar] [PubMed]
- Kraut, R.; Campos-Ortega, J.A. Inscuteable, a neural precursor gene of drosophila, encodes a candidate for a cytoskeleton adaptor protein. Dev. Biol. 1996, 174, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Kraut, R.; Chia, W.; Jan, L.Y.; Jan, Y.N.; Knoblich, J.A. Role of inscuteable in orienting asymmetric cell divisions in drosophila. Nature 1996, 383, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Schober, M.; Schaefer, M.; Knoblich, J.A. Bazooka recruits inscuteable to orient asymmetric cell divisions in drosophila neuroblasts. Nature 1999, 402, 548–551. [Google Scholar] [PubMed]
- Wodarz, A.; Ramrath, A.; Grimm, A.; Knust, E. Drosophila atypical protein kinase C associates with bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 2000, 150, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Wodarz, A.; Ramrath, A.; Kuchinke, U.; Knust, E. Bazooka provides an apical cue for inscuteable localization in drosophila neuroblasts. Nature 1999, 402, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Frise, E.; Knoblich, J.A.; Younger-Shepherd, S.; Jan, L.Y.; Jan, Y.N. The drosophila numb protein inhibits signaling of the notch receptor during cell-cell interaction in sensory organ lineage. Proc. Natl. Acad. Sci. USA 1996, 93, 11925–11932. [Google Scholar] [CrossRef] [PubMed]
- Gho, M.; Bellaïche, Y.; Schweisguth, F. Revisiting the drosophila microchaete lineage: A novel intrinsically asymmetric cell division generates a glial cell. Development 1999, 126, 3573–3584. [Google Scholar] [PubMed]
- Guo, M.; Jan, L.Y.; Jan, Y.N. Control of daughter cell fates during asymmetric division: Interaction of numb and notch. Neuron 1996, 17, 27–41. [Google Scholar] [CrossRef]
- Uemura, T.; Shepherd, S.; Ackerman, L.; Jan, L.Y.; Jan, Y.N. Numb, a gene required in determination of cell fate during sensory organ formation in drosophila embryos. Cell 1989, 58, 349–360. [Google Scholar] [CrossRef]
- Couturier, L.; Vodovar, N.; Schweisguth, F. Endocytosis by numb breaks notch symmetry at cytokinesis. Nat. Cell. Biol. 2012, 14, 131–139. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.A.; Dho, S.E.; Weinmaster, G.; McGlade, C.J. Numb regulates post-endocytic trafficking and degradation of notch1. J. Biol. Chem. 2009, 284, 26427–26438. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, M.S.; Jan, L.Y.; Jan, Y.N. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 1994, 76, 477–491. [Google Scholar] [CrossRef]
- Furman, D.P.; Bukharina, T.A. Drosophila mechanoreceptors as a model for studying asymmetric cell division. Int. J. Dev. Biol. 2011, 55, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Giebel, B.; Wodarz, A. Notch signaling: Numb makes the difference. Curr. Biol. 2012, 22, R133–R135. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.D.; Wagner, W. The beauty of asymmetry: Asymmetric divisions and self-renewal in the haematopoietic system. Curr. Opin. Hematol. 2007, 14, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Pina, C.; Enver, T. Differential contributions of haematopoietic stem cells to foetal and adult haematopoiesis: Insights from functional analysis of transcriptional regulators. Oncogene 2007, 26, 6750–6765. [Google Scholar] [CrossRef] [PubMed]
- Gorgens, A.; Beckmann, J.; Ludwig, A.K.; Mollmann, M.; Durig, J.; Horn, P.A.; Rajendran, L.; Giebel, B. Lipid raft redistribution and morphological cell polarization are separable processes providing a basis for hematopoietic stem and progenitor cell migration. Int. J. Biochem. Cell Biol. 2012, 44, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Tabuse, Y.; Izumi, Y.; Piano, F.; Kemphues, K.J.; Miwa, J.; Ohno, S. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in caenorhabditis elegans. Development 1998, 125, 3607–3614. [Google Scholar] [PubMed]
- Watts, J.L.; Etemad-Moghadam, B.; Guo, S.; Boyd, L.; Draper, B.W.; Mello, C.C.; Priess, J.R.; Kemphues, K.J. PAR-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development 1996, 122, 3133–3140. [Google Scholar] [PubMed]
- Suzuki, A.; Ohno, S. The PAR-aPKC system: Lessons in polarity. J. Cell Sci. 2006, 119, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Petronczki, M.; Knoblich, J.A. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in drosophila. Nature Cell Biol. 2001, 3, 43–49. [Google Scholar] [PubMed]
- Roegiers, F.; Younger-Shepherd, S.; Jan, L.Y.; Jan, Y.N. Bazooka is required for localization of determinants and controlling proliferation in the sensory organ precursor cell lineage in drosophila. Proc. Natl. Acad. Sci. USA 2001, 98, 14469–14474. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niu, C.; Ye, L.; Huang, H.; He, X.; Tong, W.G.; Ross, J.; Haug, J.; Johnson, T.; Feng, J.Q.; et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.J.; Yilmaz, O.H.; Iwashita, T.; Terhorst, C.; Morrison, S.J. Slam family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Hsu, Y.M.; Day, R.B.; Schuettpelz, L.G.; Christopher, M.J.; Borgerding, J.N.; Nagasawa, T.; Link, D.C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013, 495, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Saunders, T.L.; Enikolopov, G.; Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012, 481, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Hanoun, M.; Frenette, P.S. This niche is a maze; an amazing niche. Cell Stem Cell 2013, 12, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, Y.; Scadden, D.T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 2015, 16, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Roeder, I.; Lorenz, R. Asymmetry of stem cell fate and the potential impact of the niche: Observations, simulations, and interpretations. Stem Cell Rev. 2006, 2, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Brummendorf, T.H.; Dragowska, W.; Zijlmans, J.; Thornbury, G.; Lansdorp, P.M. Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells. J. Exp. Med. 1998, 188, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Giebel, B.; Zhang, T.; Beckmann, J.; Spanholtz, J.; Wernet, P.; Ho, A.D.; Punzel, M. Primitive human hematopoietic cells give rise to differentially specified daughter cells upon their initial cell division. Blood 2006, 107, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Leary, A.G.; Strauss, L.C.; Civin, C.I.; Ogawa, M. Disparate differentiation in hemopoietic colonies derived from human paired progenitors. Blood 1985, 66, 327–332. [Google Scholar] [PubMed]
- Punzel, M.; Zhang, T.; Liu, D.; Eckstein, V.; Ho, A.D. Functional analysis of initial cell divisions defines the subsequent fate of individual human CD34+CD38–cells. Exp. Hematol. 2002, 30, 464–472. [Google Scholar] [CrossRef]
- Giebel, B.; Corbeil, D.; Beckmann, J.; Hohn, J.; Freund, D.; Giesen, K.; Fischer, J.; Kogler, G.; Wernet, P. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood 2004, 104, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.; Scheitza, S.; Wernet, P.; Fischer, J.C.; Giebel, B. Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: Identification of asymmetrically segregating proteins. Blood 2007, 109, 5494–5501. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, S.; Godfrey, W.; Yin, A.H.; Atkins, K.; Warnke, R.; Holden, J.T.; Bray, R.A.; Waller, E.K.; Buck, D.W. A novel five-transmembrane hematopoietic stem cell antigen: Isolation, characterization, and molecular cloning. Blood 1997, 90, 5013–5021. [Google Scholar] [PubMed]
- Yin, A.H.; Miraglia, S.; Zanjani, E.D.; Almeida-Porada, G.; Ogawa, M.; Leary, A.G.; Olweus, J.; Kearney, J.; Buck, D.W. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997, 90, 5002–5012. [Google Scholar] [PubMed]
- Andrews, R.; Ahringer, J. Asymmetry of early endosome distribution in C. elegans embryos. PLoS One 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Giebel, B.; Beckmann, J. Asymmetric cell divisions of human hematopoietic stem and progenitor cells meet endosomes. Cell Cycle 2007, 6, 2201–2204. [Google Scholar] [CrossRef] [PubMed]
- Furthauer, M.; Gonzalez-Gaitan, M. Endocytosis, asymmetric cell division, stem cells and cancer: Unus pro omnibus, omnes pro uno. Mol. Oncol. 2009, 3, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.V.; Bauer, N.; Corbeil, D. The stem cell marker CD133 meets the endosomal compartment—New insights into the cell division of hematopoietic stem cells. Blood Cells Mol. Dis. 2008, 41, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Gorgens, A.; Radtke, S.; Horn, P.A.; Giebel, B. New relationships of human hematopoietic lineages facilitate detection of multipotent hematopoietic stem and progenitor cells. Cell Cycle 2013, 12, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- Gorgens, A.; Radtke, S.; Mollmann, M.; Cross, M.; Durig, J.; Horn, P.A.; Giebel, B. Revision of the human hematopoietic tree: Granulocyte subtypes derive from distinct hematopoietic lineages. Cell Rep. 2013, 3, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Radtke, S.; Görgens, A.; Kordelas, L.; Schmidt, M.; Kimmig, K.R.; Koninger, A.; Horn, P.A.; Giebel, B. CD133 allows elaborated discrimination and quantification of haematopoietic progenitor subsets in human haematopoietic stem cell transplants. Br. J. Haematol 2015, 169, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Gorgens, A.; Ludwig, A.K.; Mollmann, M.; Krawczyk, A.; Durig, J.; Hanenberg, H.; Horn, P.A.; Giebel, B. Multipotent hematopoietic progenitors divide asymmetrically to create progenitors of the lymphomyeloid and erythromyeloid lineages. Stem Cell Rep. 2014, 3, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kong, J.; Brat, D.J. Cancer stem cell division: When the rules of asymmetry are broken. Stem Cells Dev. 2015, 24, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Bidlingmaier, S.; Zhu, X.; Liu, B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J. Mol. Med. Berl. 2008, 86, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.K.; Olin, M.R.; Forster, C.L.; Cruz, K.S.; Panyam, J.; Ohlfest, J.R. Identification of a novel monoclonal antibody recognizing CD133. J. Immunol. Methods 2010, 361, 110–115. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murke, F.; Castro, S.V.C.; Giebel, B.; Görgens, A. Concise Review: Asymmetric Cell Divisions in Stem Cell Biology. Symmetry 2015, 7, 2025-2037. https://doi.org/10.3390/sym7042025
Murke F, Castro SVC, Giebel B, Görgens A. Concise Review: Asymmetric Cell Divisions in Stem Cell Biology. Symmetry. 2015; 7(4):2025-2037. https://doi.org/10.3390/sym7042025
Chicago/Turabian StyleMurke, Florian, Symone Vitorianoda Conceição Castro, Bernd Giebel, and André Görgens. 2015. "Concise Review: Asymmetric Cell Divisions in Stem Cell Biology" Symmetry 7, no. 4: 2025-2037. https://doi.org/10.3390/sym7042025
APA StyleMurke, F., Castro, S. V. C., Giebel, B., & Görgens, A. (2015). Concise Review: Asymmetric Cell Divisions in Stem Cell Biology. Symmetry, 7(4), 2025-2037. https://doi.org/10.3390/sym7042025




