Cytoskeletal Symmetry Breaking and Chirality: From Reconstituted Systems to Animal Development
Abstract
:1. Introduction
| System | Polarizing Cue | Physical Effect | Effector Molecule | Developmental Outcome |
|---|---|---|---|---|
| S. cerevisiae | Cdc42 activation | transport of Cdc42 to plasma membrane | F-actin | polar cap formation followed by budding [11,12] |
| C. elegans | Sperm entry | A/P polarized cortical actomyosin flows | cortical actomyosin | A/P symmetry breaking by segregation of PAR domains [13,14,15,16] |
| D. melanogaster | Toll | planar polarized actomyosin contractility | non-muscle myosin II | convergent extension during gastrulation [17] |
| Danio rerio | fluctuating adhesion, myosin contraction at cell rear | large-scale actin network disassembly by myosin II | cortical actomyosin | symmetry breaking and polarized migration [18] |
| Mus musculus | non-canonical Wnt signaling | posterior tilt of nodal cilia | nodal cilia | L/R symmetry breaking by asymmetric nodal flow [19,20] |
2. Spontaneous Symmetry Breaking in Vitro and in Single Cells
2.1. Microtubules and Microtubule-Motor Systems in Vitro
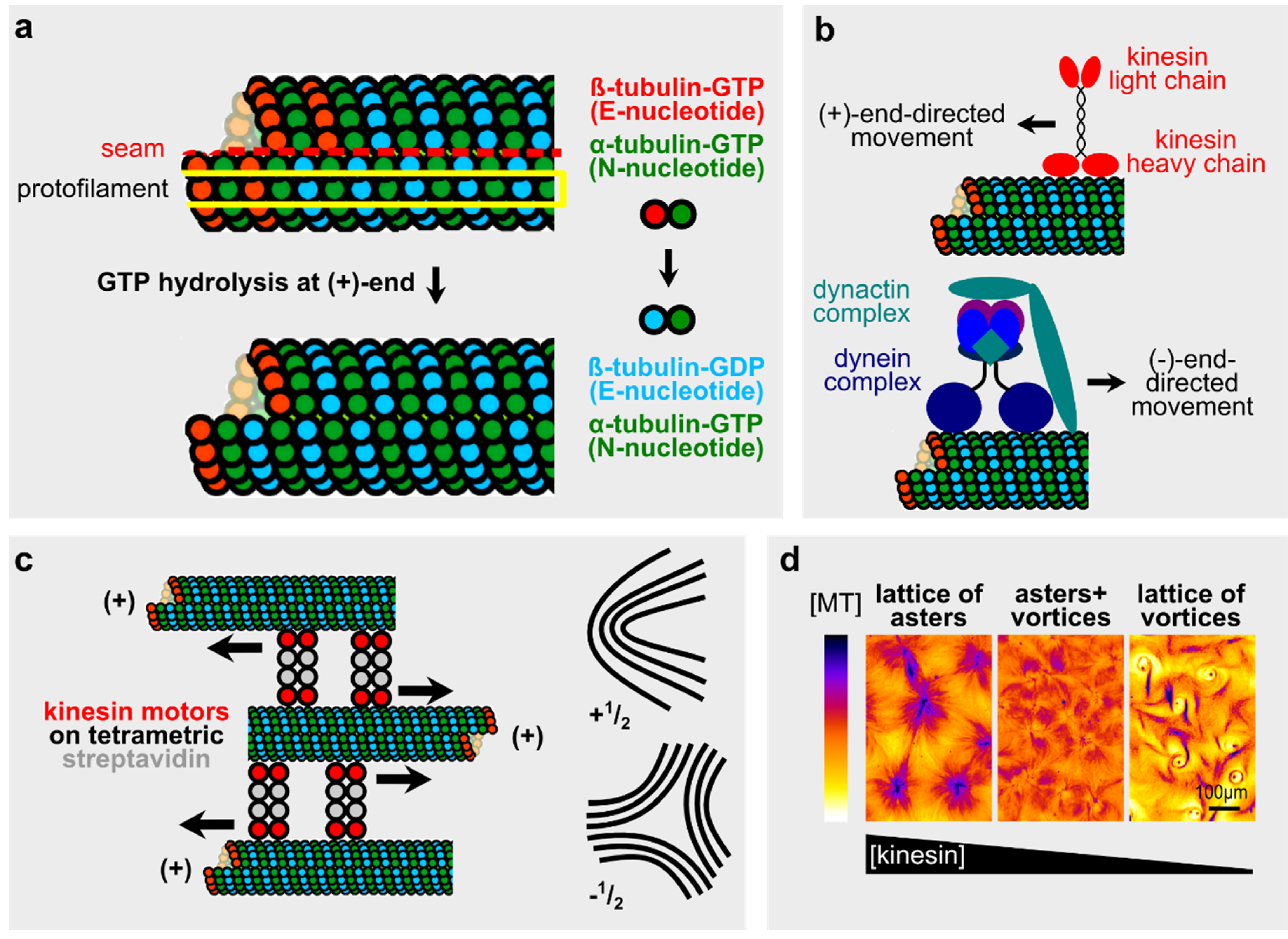
2.2. Microtubules in Neuronal Polarity and Spontaneous Phenomena during Cell Division
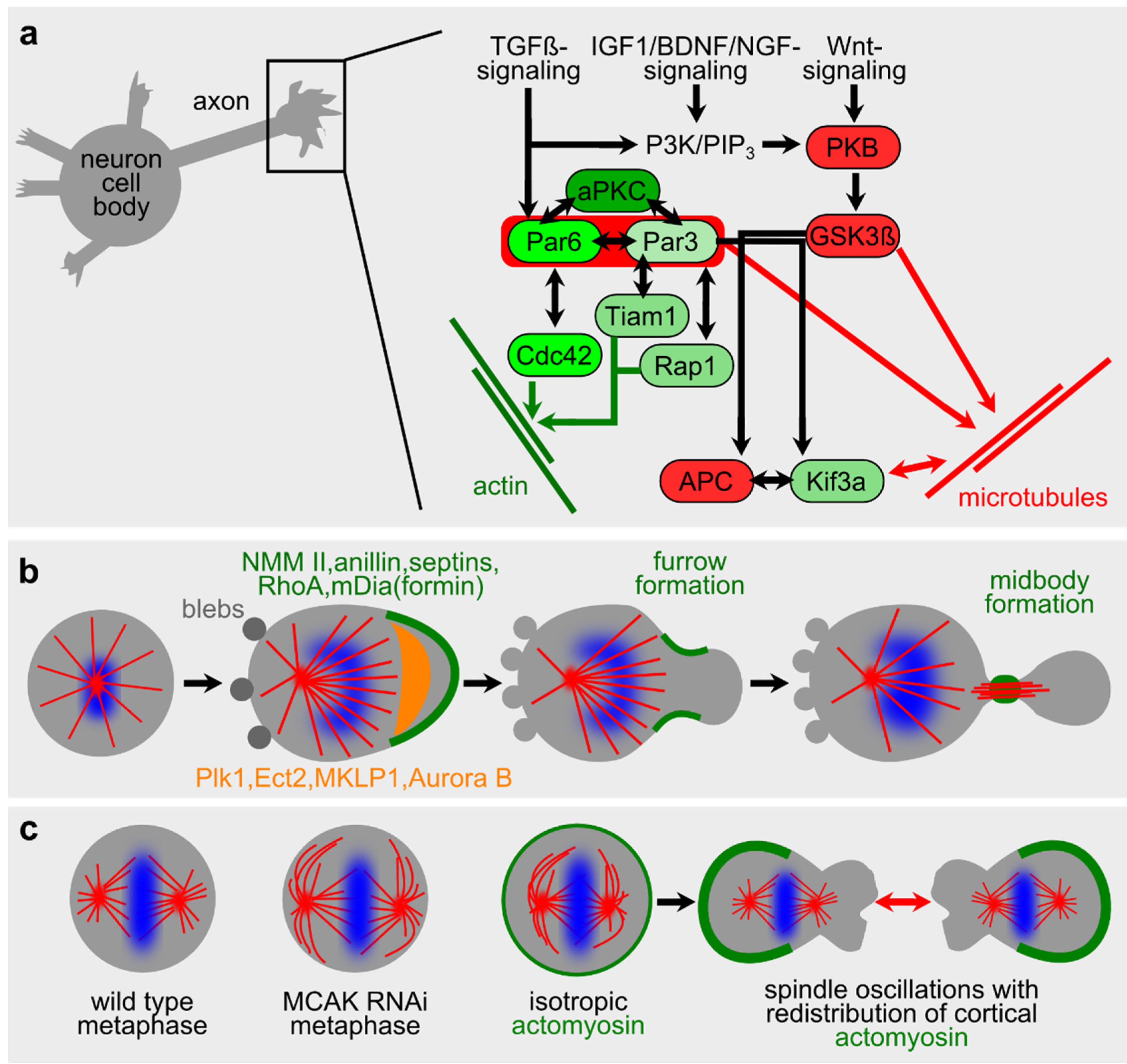
2.3. Actomyosin in Vitro

2.4. Actomyosin in Single Cells
2.4.1. Blebbing and Migration
2.4.2. Defining Singularities in Cells

2.5. Actomyosin-Dependent Chiral Symmetry Breaking
3. Chiral Symmetry Breaking in Vivo
3.1. Left/Right (L/R) Asymmetry and the “Conversion Hypothesis”
3.2. Chiral Symmetry Breaking in Invertebrates
3.2.1. Helobdella
3.2.2. Lymnaea
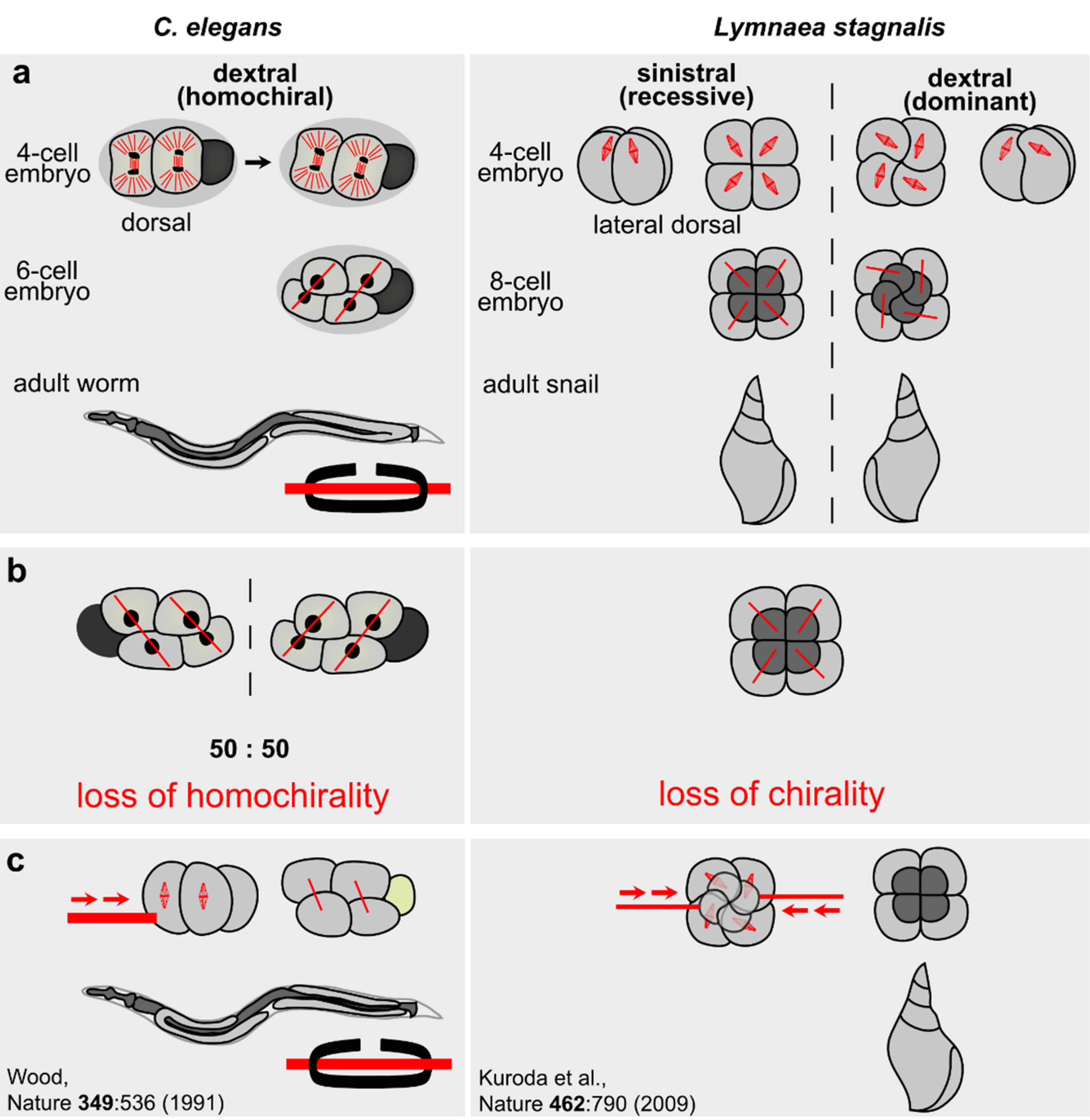
3.2.3. C. elegans
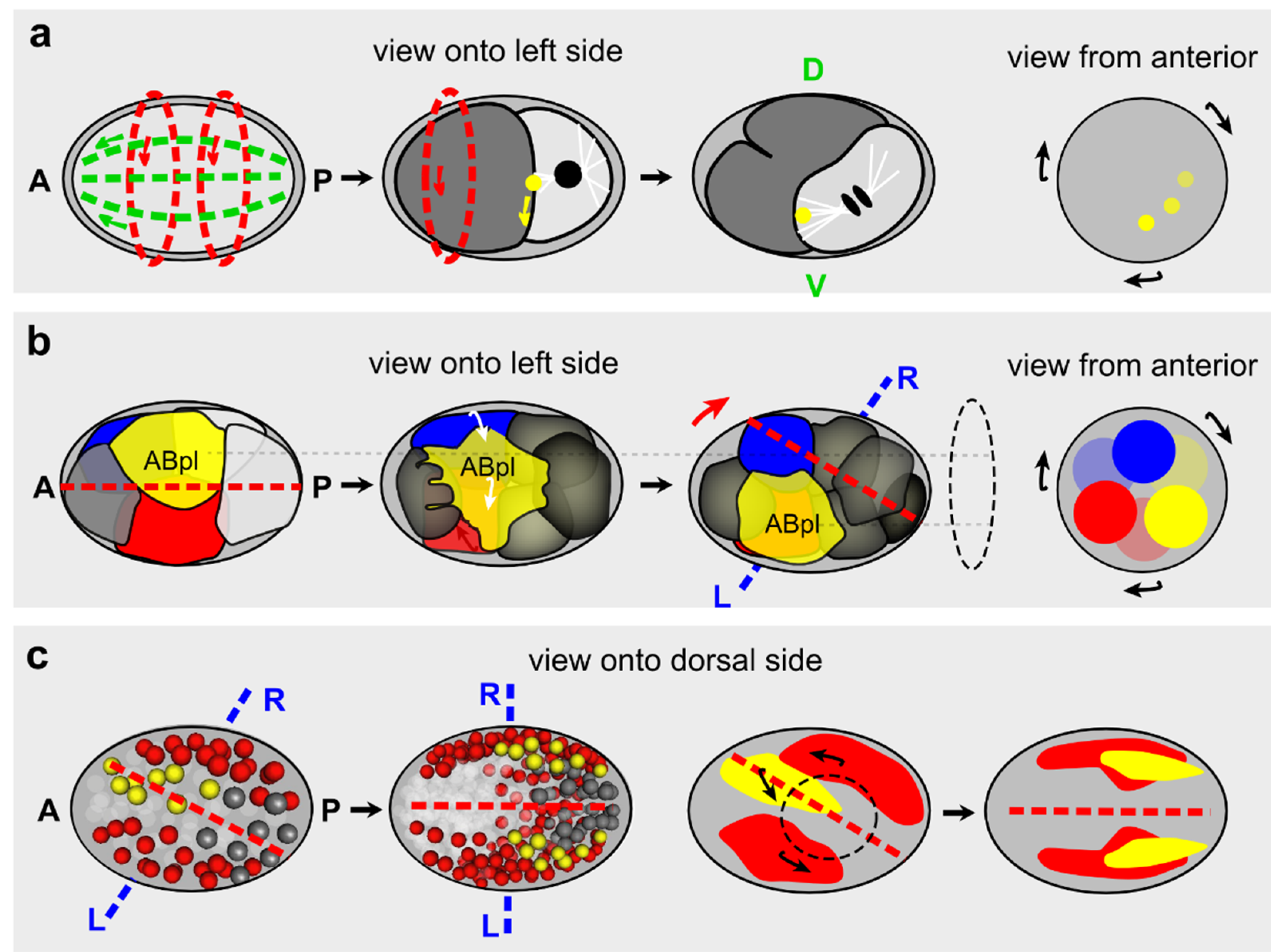
3.2.4. D. melanogaster
3.2.5. Helical Growth Mutants from the Plant A. thaliana and Their Effects on Invertebrate Chirality
3.3. Chiral Symmetry Breaking in Vertebrates
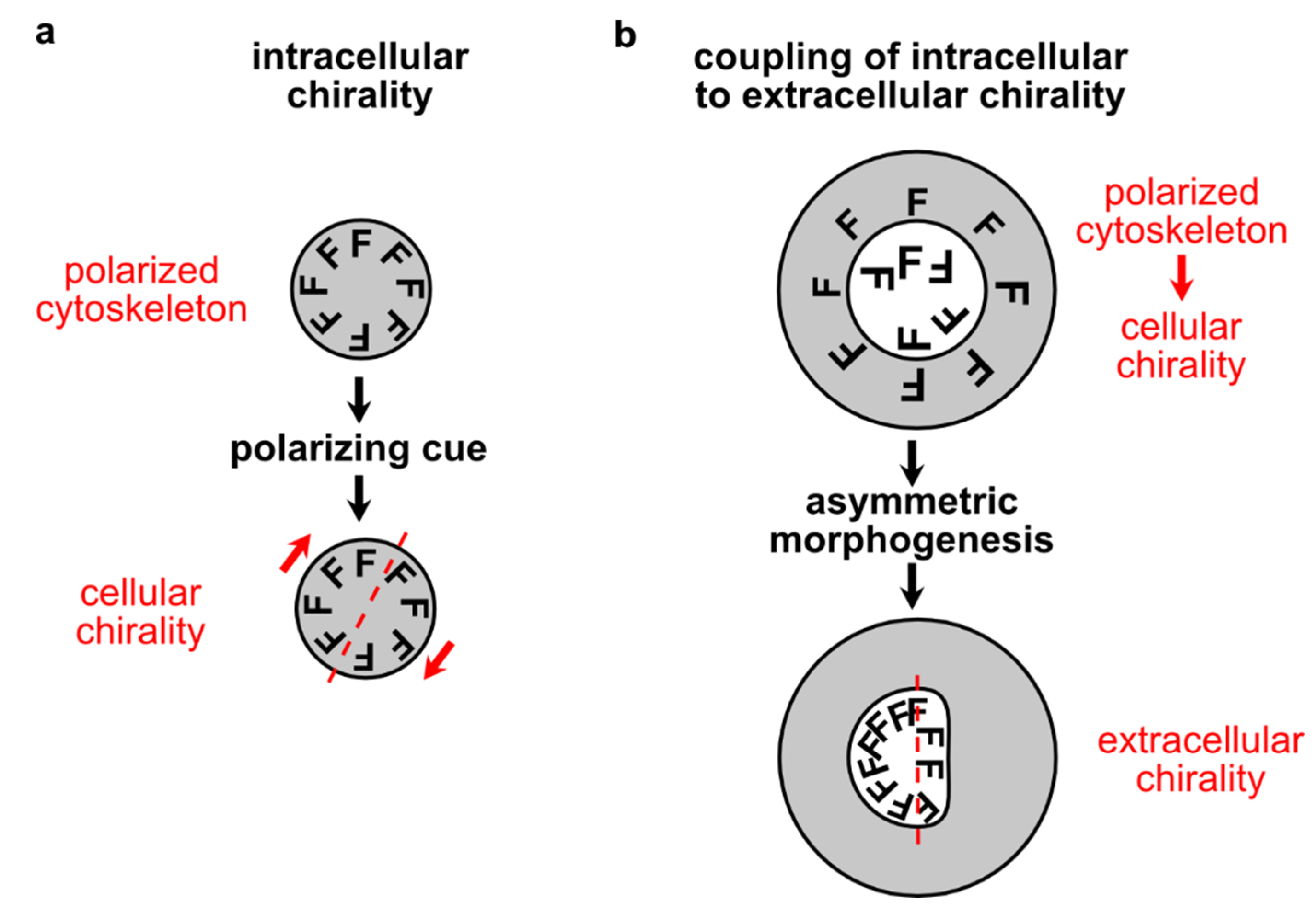
4. Inductive Polarity and Symmetry Breaking
4.1. Actomyosin
4.2. Microtubules: Spindles and Centrosomes
Acknowledgments
Conflicts of Interest
References
- Li, R.; Bowerman, B. Symmetry breaking in biology. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Wodarz, A. Establishing cell polarity in development. Nat. Cell Biol. 2002, 4, E39–E44. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, H. Models for the generation and interpretation of gradients. Cold Spring Harb. Perspect. Biol. 2009, 1. [Google Scholar] [CrossRef]
- Meinhardt, H. Models of biological pattern formation: From elementary steps to the organization of embryonic axes. Curr. Top. Dev. Biol. 2008, 81, 1–63. [Google Scholar] [PubMed]
- Nance, J.; Zallen, J.A. Elaborating polarity: PAR proteins and the cytoskeleton. Development 2011, 138, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.S.; Savage, N.S.; Johnson, S.A.; Bose, I.; Wagner, A.W.; Zyla, T.R.; Nijhout, H.F.; Reed, M.C.; Goryachev, A.B.; Lew, D.J. Singularity in polarization: Rewiring yeast cells to make two buds. Cell 2009, 139, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gundersen, G.G. Beyond polymer polarity: How the cytoskeleton builds a polarized cell. Nat. Rev. Mol. Cell Biol. 2008, 9, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.R.; Hyman, A.A. Acto-myosin reorganization and PAR polarity in C. elegans. Development 2007, 134, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, I. Notch and the awesome power of genetics. Genetics 2012, 191, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Wedlich-Söldner, R.; Altschuler, S.; Wu, L.; Li, R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 2003, 299, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Bi, E.; Park, H.O. Cell polarization and cytokinesis in budding yeast. Genetics 2012, 191, 347–387. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Depken, M.; Bois, J.S.; Jülicher, F.; Grill, S.W. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature 2010, 467, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Munro, E.; Nance, J.; Priess, J.R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 2004, 7, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.; Saam, J.R.; Mango, S.E. CYK-4/GAP provides a localized cue to initiate anteroposterior polarity upon fertilization. Science 2006, 313, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Goehring, N.W.; Trong, P.K.; Bois, J.S.; Chowdhury, D.; Nicola, E.M.; Hyman, A.A.; Grill, S.W. Polarization of PAR proteins by advective triggering of a pattern-forming system. Science 2011, 334, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Paré, A.C.; Vichas, A.; Fincher, C.T.; Mirman, Z.; Farrell, D.L.; Mainieri, A.; Zallen, J.A. A positional Toll receptor code directs convergent extension in Drosophila. Nature 2014, 515, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, V.; Wieser, S.; Callan-Jones, A.; Smutny, M.; Morita, H.; Sako, K.; Barone, V.; Ritsch-Marte, M.; Sixt, M.; Voituriez, R.; et al. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 2015, 160, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Shinohara, K.; Wang, J.; Ikeuchi, S.; Yoshiba, S.; Meno, C.; Nonaka, S.; Takada, S.; Hatta, K.; Wynshaw-Boris, A.; et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat. Cell Biol. 2010, 12, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Borovina, A.; Superina, S.; Voskas, D.; Ciruna, B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat. Cell Biol. 2010, 12, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Van der Gucht, J.; Sykes, C. Physical model of cellular symmetry breaking. Cold Spring Harb. Perspect. Biol. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345. [Google Scholar] [CrossRef] [PubMed]
- Turing, A.M. The chemical basis of morphogenesis. Philosoph. Trans. R. Soc. Lond. 1952, 237, 37–72. [Google Scholar] [CrossRef]
- Ladewig, J.; Koch, P.; Brüstle, O. Leveling Waddington: The emergence of direct programming and the loss of cell fate hierarchies. Nat. Rev. Mol. Cell Biol. 2013, 14, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Fievet, B.T.; Rodriguez, J.; Naganathan, S.; Lee, C.; Zeiser, E.; Ishidate, T.; Shirayama, M.; Grill, S.; Ahringer, J. Systematic genetic interaction screens uncover cell polarity regulators and functional redundancy. Nat. Cell Biol. 2013, 15, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Motegi, F.; Seydoux, G. The PAR network: Redundancy and robustness in a symmetry-breaking system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.; Kirschner, M. Microtubule assembly nucleated by isolated centrosomes. Nature 1984, 312, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M.; Mitchison, T. Beyond self-assembly: From microtubules to morphogenesis. Cell 1986, 45, 329–342. [Google Scholar] [CrossRef]
- Padinhateeri, R.; Kolomeisky, A.B.; Lacoste, D. Random hydrolysis controls the dynamic instability of microtubules. Biophys. J. 2012, 102, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Bayley, P.M.; Schilstra, M.J.; Martin, S.R. A simple formulation of microtubule dynamics: Quantitative implications of the dynamic instability of microtubule populations in vivo and in vitro. J. Cell Sci. 1989, 93, 241–254. [Google Scholar] [PubMed]
- Dogterom, M.; Leibler, S. Physical aspects of the growth and regulation of microtubule structures. Phys. Rev. Lett. 1993, 70, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Flyvbjerg, H.; Holy, T.E.; Leibler, S. Stochastic dynamics of microtubules: A model for caps and catastrophes. Phys. Rev. Lett. 1994, 73, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Margolin, G.; Gregoretti, I.V.; Goodson, H.V.; Alber, M.S. Analysis of a mesoscopic stochastic model of microtubule dynamic instability. Phys. Rev. E Stat. Nonliner Soft Matter Phys. 2006, 74. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Lu, T.; Shen, T.; Wolynes, P.G. Nonequilibrium self-assembly of linear fibers: Microscopic treatment of growth, decay, catastrophe and rescue. Phys. Biol. 2006, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Antal, T.; Krapivsky, P.L.; Redner, S.; Mailman, M.; Chakraborty, B. Dynamics of an idealized model of microtubule growth and catastrophe. Phys. Rev. E Stat. Nonliner Soft Matter Phys. 2007, 76. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, P.; Lacoste, D.; Mallick, K.; Joanny, J.F. Nonequilibrium self-assembly of a filament coupled to ATP/GTP hydrolysis. Biophys. J. 2009, 96, 2146–2159. [Google Scholar] [CrossRef] [PubMed]
- Kueh, H.Y.; Mitchison, T.J. Structural plasticity in actin and tubulin polymer dynamics. Science 2009, 325, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Nogales, E. Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly. Nature 2005, 435, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Brouhard, G.J.; Rice, L.M. The contribution of αβ-tubulin curvature to microtubule dynamics. J. Cell Biol. 2014, 207, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Alushin, G.M.; Brown, A.; Nogales, E. Mechanistic origin of microtubule dynamic instability and its modulation by EB proteins. Cell 2015, 162, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.F.; Melki, R.; Pantaloni, D.; Hill, T.L.; Chen, Y. Synchronous oscillations in microtubule polymerization. Proc. Natl. Acad. Sci. USA 1987, 84, 5257–5261. [Google Scholar] [CrossRef] [PubMed]
- Pirollet, F.; Job, D.; Margolis, R.L.; Garel, J.R. An oscillatory mode for microtubule assembly. EMBO J. 1987, 6, 3247–3252. [Google Scholar] [PubMed]
- Tabony, J.; Job, D. Spatial structures in microtubular solutions requiring a sustained energy source. Nature 1990, 346, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.; Mandelkow, E.M.; Hotani, H.; Hess, B.; Müller, S.C. Spatial patterns from oscillating microtubules. Science 1989, 246, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, R.E.; Dennerll, T.; Weiss, S.; Heidemann, S.R. F-actin and microtubule suspensions as indeterminate fluids. Science 1987, 235, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Tabony, J.; Job, D. Gravitational symmetry breaking in microtubular dissipative structures. Proc. Natl. Acad. Sci. USA 1992, 89, 6948–6952. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, C.; Pochon, N.; Tabony, J. Microtubule self-organization is gravity-dependent. Proc. Natl. Acad. Sci. USA 2000, 97, 8364–8368. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, J.A.; Sataric, M.V.; Portet, S.; Dixon, J.M. Gravitational symmetry breaking leads to a polar liquid crystal phase of microtubules in vitro. J. Biol. Phys. 2005, 31, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Neff, A.W.; Malacinski, G.M. Early development of Xenopus embryos is affected by simulated gravity. Adv. Space Res. 1994, 14, 249–255. [Google Scholar] [CrossRef]
- Nouri, C.; Tuszynski, J.A.; Wiebe, M.W.; Gordon, R. Simulation of the effects of microtubules in the cortical rotation of amphibian embryos in normal and zero gravity. Biosystems 2012, 109, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Tabony, J.; Rigotti, N.; Glade, N.; Cortès, S. Effect of weightlessness on colloidal particle transport and segregation in self-organising microtubule preparations. Biophys. Chem. 2007, 127, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Reber, S.B.; Baumgart, J.; Widlund, P.O.; Pozniakovsky, A.; Howard, J.; Hyman, A.A.; Jülicher, F. XMAP215 activity sets spindle length by controlling the total mass of spindle microtubules. Nat. Cell Biol. 2013, 15, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Brugués, J.; Needleman, D. Physical basis of spindle self-organization. Proc. Natl. Acad. Sci. USA 2014, 111, 18496–18500. [Google Scholar] [CrossRef] [PubMed]
- Schliwa, M.; Woehlke, G. Molecular motors. Switching on kinesin. Nature 2001, 411, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Nédélec, F.J.; Surrey, T.; Maggs, A.C.; Leibler, S. Self-organization of microtubules and motors. Nature 1997, 389, 305–308. [Google Scholar] [PubMed]
- Surrey, T.; Nedelec, F.; Leibler, S.; Karsenti, E. Physical properties determining self-organization of motors and microtubules. Science 2001, 292, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E. An electron microscopy journey in the study of microtubule structure and dynamics. Protein Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Millecamps, S.; Julien, J.P. Axonal transport deficits and neurodegenerative disease. Nat. Rev. Neurosci. 2013, 14, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Chen, D.T.; DeCamp, S.J.; Heymann, M.; Dogic, Z. Spontaneous motion in hierarchically assembled active matter. Nature 2012, 491, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Keber, F.C.; Loiseau, E.; Sanchez, T.; de Camp, S.J.; Giomi, L.; Bowick, M.J.; Marchetti, M.C.; Dogic, Z.; Bausch, A.R. Topology and dynamics of active nematic vesicles. Science 2014, 345, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Sumino, Y.; Nagai, K.H.; Shitaka, Y.; Tanaka, D.; Yoshikawa, K.; Chaté, H.; Oiwa, K. Large-scale vortex lattice emerging from collectively moving microtubules. Nature 2012, 483, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Serbus, L.R.; Cha, B.J.; Theurkauf, W.E.; Saxton, W.M. Dynein and the actin cytoskeleton control kinesin-driven cytoplasmic streaming in Drosophila oocytes. Development 2005, 132, 3743–3752. [Google Scholar] [CrossRef] [PubMed]
- Wasteneys, G.O.; Ambrose, J.C. Spatial organization of plant cortical microtubules: Close encounters of the 2D kind. Trends Cell Biol. 2009, 19, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Welch, D.; Nicastro, D.; Dogic, Z. Cilia-like beating of active microtubule bundles. Science 2011, 333, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Dotti, C.G.; Banker, G.A. Experimentally induced alteration in the polarity of developing neurons. Nature 1987, 330, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Forscher, P.; Smith, S.J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 1988, 107, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.S.; Bi, G.Q. Axon formation: A molecular model for the generation of neuronal polarity. Bioessays 2000, 22, 172–179. [Google Scholar] [CrossRef]
- Bradke, F.; Dotti, C.G. The role of local actin instability in axon formation. Science 1999, 283, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.W.; Schoonderwoert, V.T.; Ji, L.; Mederios, N.; Danuser, G.; Forscher, P. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev. Cell 2008, 15, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Burnette, D.T.; Ji, L.; Schaefer, A.W.; Medeiros, N.A.; Danuser, G.; Forscher, P. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev. Cell 2008, 15, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Witte, H.; Neukirchen, D.; Bradke, F. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 2008, 180, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Seetapun, D.; Odde, D.J. Cell-length-dependent microtubule accumulation during polarization. Curr. Biol. 2010, 20, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Macara, I.G. The PAR proteins: Fundamental players in animal cell polarization. Dev. Cell 2007, 13, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Kemphues, K. PARsing embryonic polarity. Cell 2000, 101, 345–348. [Google Scholar] [CrossRef]
- Shi, S.H.; Jan, L.Y.; Jan, Y.N. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 2003, 112, 63–75. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Shi, H.; Wei, M.; Castaneda-Castellanos, D.R.; Bultje, R.S.; Pei, X.; Kriegstein, A.R.; Zhang, M.; Shi, S.H. Regulation of microtubule stability and organization by mammalian Par3 in specifying neuronal polarity. Dev. Cell 2013, 24, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.K.; Coughlin, M.; Field, C.M.; Mitchison, T.J. Cell polarization during monopolar cytokinesis. J. Cell Biol. 2008, 181, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Rankin, K.E.; Wordeman, L. Long astral microtubules uncouple mitotic spindles from the cytokinetic furrow. J. Cell Biol. 2010, 190, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Motegi, F.; Zonies, S.; Hao, Y.; Cuenca, A.A.; Griffin, E.; Seydoux, G. Microtubules induce self-organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nat. Cell Biol. 2011, 13, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Insolera, R.; Chen, S.; Shi, S.H. Par proteins and neuronal polarity. Dev. Neurobiol. 2011, 71, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Salbreux, G.; Charras, G.; Paluch, E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012, 22, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Heisenberg, C.P.; Bellaïche, Y. Forces in tissue morphogenesis and patterning. Cell 2013, 23, 948–962. [Google Scholar] [CrossRef] [PubMed]
- Murrell, M.; Oakes, P.W.; Lenz, M.; Gardel, M.L. Forcing cells into shape: The mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 2015, 16, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Goehring, N.W.; Grill, S.W. Cell polarity: Mechanochemical patterning. Trends Cell Biol. 2013, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Grill, S.W. Growing up is stressful: Biophysical laws of morphogenesis. Curr. Opin. Genet. Dev. 2011, 21, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Gardel, M.L.; Shin, J.H.; MacKintosh, F.C.; Mahadevan, L.; Matsudaira, P.; Weitz, D.A. Elastic behavior of cross-linked and bundled actin networks. Science 2004, 304, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Salbreux, G.; Prost, J.; Joanny, J.F. Hydrodynamics of cellular cortical flows and the formation of contractile rings. Phys. Rev. Lett. 2009, 103. [Google Scholar] [CrossRef] [PubMed]
- Dalhaimer, P.; Discher, D.E.; Lubensky, T.C. Crosslinked actin networks show liquid crystal elastomer behaviour, including soft-mode elasticity. Nat. Phys. 2007, 3, 354–360. [Google Scholar] [CrossRef]
- Wagner, B.; Tharmann, R.; Haase, I.; Fischer, M.; Bausch, A.R. Cytoskeletal polymer networks: The molecule structure of cross-linkers determines macroscopic properties. Proc. Natl. Acad. Sci. USA 2006, 103, 13974–13978. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.K.; Schwille, P. Minimal systems to study membrane-cytoskeleton interactions. Curr. Opin. Biotechnol. 2012, 23, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.K.; Heinemann, F.; Chwastek, G.; Schwille, P. The design of MACs (minimal actin cortices). Cytoskeleton 2013, 70, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Schaller, V.; Weber, C.; Semmrich, C.; Frey, E.; Bausch, A.R. Polar patterns of driven filaments. Nature 2010, 467, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Charras, G.T.; Yarrow, J.C.; Horton, M.A.; Mahadevan, L.; Mitchison, T.J. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 2005, 435, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Sedzinski, J.; Biro, M.; Oswald, A.; Tinevez, J.Y.; Salbreux, G.; Paluch, E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 2011, 476, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Purvanov, V.; Holst, M.; Khan, J.; Baarlink, C.; Grosse, R. G-protein-coupled receptor signaling and polarized actin dynamics drive cell-in-cell invasion. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.T.; Wilson, C.A.; Ji, L.; Hebert, B.; Barnhart, E.L.; Dye, N.A.; Wiseman, P.W.; Danuser, G.; Theriot, J.A. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J. Cell Biol. 2007, 178, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, E.; Lee, K.C.; Allen, G.M.; Theriot, J.A.; Mogilner, A. Balance between cell-substrate adhesion and myosin contraction determines the frequency of motility initiation in fish keratocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 5045–5050. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.K.; Petrasek, Z.; Heinemann, F.; Schwille, P. Myosin motors fragment and compact membrane-bound actin filaments. Elife 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.; Tsai, F.C.; Lees, E.; Voituriez, R.; Koenderink, G.H.; Sykes, C. Cell-sized liposomes reveal how actomyosin cortical tension drives shape change. Proc. Natl. Acad. Sci. USA 2013, 110, 16456–16461. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.; Lemière, J.; Faqir, F.; Manzi, J.; Blanchoin, L.; Plastino, J.; Betz, T.; Sykes, C. Actin polymerization or myosin contraction: Two ways to build up cortical tension for symmetry breaking. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Oudenaarden, A.; Theriot, J.A. Cooperative symmetry-breaking by actin polymerization in a model for cell motility. Nat. Cell Biol. 1999, 1, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Van der Gucht, J.; Paluch, E.; Plastino, J.; Sykes, C. Stress release drives symmetry breaking for actin-based movement. Proc. Natl. Acad. Sci. USA 2005, 102, 7847–7852. [Google Scholar] [CrossRef] [PubMed]
- Sekimoto, K.; Prost, J.; Jülicher, F.; Boukellal, H.; Bernheim-Grosswasser, A. Role of tensile stress in actin gels and a symmetry-breaking instability. Eur. Phys. J. E Soft Matter 2004, 13, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Abu Shah, E.; Keren, K. Symmetry breaking in reconstituted actin cortices. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- John, K.; Peyla, P.; Kassner, K.; Prost, J.; Misbah, C. Nonlinear study of symmetry breaking in actin gels: Implications for cellular motility. Phys. Rev. Lett. 2008, 100. [Google Scholar] [CrossRef] [PubMed]
- Lewis, O.L.; Guy, R.D.; Allard, J.F. Actin-myosin spatial patterns from a simplified isotropic viscoelastic model. Biophys. J. 2014, 107, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Pinot, M.; Steiner, V.; Dehapiot, B.; Yoo, B.K.; Chesnel, F.; Blanchoin, L.; Kervrann, C.; Gueroui, Z. Confinement induces actin flow in a meiotic cytoplasm. Proc. Natl. Acad. Sci. USA 2012, 109, 11705–11710. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.C. Actin polymerization and intracellular solvent flow in cell surface blebbing. J. Cell Biol. 1995, 129, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.C.; Stone, N.; Erhardt, J.; Pittman, R.N. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J. Cell Biol. 1998, 140, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Umeshita, K.; Sakon, M.; Imajoh-Ohmi, S.; Fujitani, K.; Gotoh, M.; Oiki, E.; Kambayashi, J.; Monden, M. Calpain activation in plasma membrane bleb formation during tert-butyl hydroperoxide-induced rat hepatocyte injury. Gastroenterology 1996, 110, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Marshall, C.J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signaling and extracellular proteolysis. Nat. Cell Biol. 2003, 5, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Sykes, C.; Prost, J.; Bornens, M. Dynamic modes of the cortical actomyosin gel during cell locomotion and division. Trends Cell Biol. 2006, 16, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Soldati, T. Dissection of amoeboid movement into two mechanically distinct modes. J. Cell Sci. 2006, 119, 3833–3844. [Google Scholar] [CrossRef] [PubMed]
- Lämmermann, T.; Sixt, M. Mechanical modes of “amoeboid” cell migration. Curr. Opin. Cell Biol. 2009, 21, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Renkawitz, J.; Schumann, K.; Weber, M.; Lämmermann, T.; Pflicke, H.; Piel, M.; Polleux, J.; Spatz, J.P.; Sixt, M. Adaptive force transmission in amoeboid cell migration. Nat. Cell Biol. 2009, 11, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Bergert, M.; Erzberger, A.; Desai, R.A.; Aspalter, I.M.; Oates, A.C.; Charras, G.; Salbreux, G.; Paluch, E.K. Force transmission during adhesion-independent migration. Nat. Cell Biol. 2015, 17, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Florey, O.; Krajcovic, M.; Sun, Q.; Overholtzer, M. Entosis. Curr. Biol. 2010, 20, R88–R89. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Luo, T.; Ren, Y.; Florey, O.; Shirasawa, S.; Sasazuki, T.; Robinson, D.N.; Overholtzer, M. Competition between human cells by entosis. Cell Res. 2014, 24, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Cramer, L.P. Forming the cell rear first: Breaking cell symmetry to trigger directed cell migration. Nat. Cell Biol. 2010, 12, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Cramer, L.P. Mechanism of cell rear retraction in migrating cells. Curr. Opin. Cell Biol. 2013, 25, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Mseka, T.; Bamburg, J.R.; Cramer, L.P. ADF/cofilin family proteins control formation of oriented actinfilament bundles in the cell body to trigger fibroblast polarization. J. Cell Sci. 2007, 120, 4332–4344. [Google Scholar] [CrossRef] [PubMed]
- Verkhovsky, A.B.; Svitkina, T.M.; Borisy, G.G. Selfpolarization and directional motility of cytoplasm. Curr. Biol. 1999, 9, 11–20. [Google Scholar] [CrossRef]
- Chi, Q.; Yin, T.; Gregersen, H.; Deng, X.; Fan, Y.; Zhao, J.; Liao, D.; Wang, G. Rear actomyosin contractility-driven directional cell migration in three-dimensional matrices: A mechano-chemical coupling mechanism. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.F.; Sauser, R.; Ambrosi, D.; Meister, J.-J.; Verkhovsky, A.B. Force transmission in migrating cells. J. Cell Biol. 2010, 188, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Nagel, O.; Guven, C.; Theves, M.; Driscoll, M.; Losert, W.; Beta, C. Geometry-driven polarity in motile amoeboid cells. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; le Berre, M.; Lautenschlaeger, F.; Maiuri, P.; Callan-Jones, A.; Heuzé, M.; Takaki, T.; Voituriez, R.; Piel, M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 2015, 160, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Toriyama, M.; Sakumura, Y. Systems biology of symmetry breaking during neuronal polarity formation. Dev. Neurobiol. 2011, 71, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Fivaz, M.; Bandara, S.; Inoue, T.; Meyer, T. Robust neuronal symmetry breaking by Ras-triggered local positive feedback. Curr. Biol. 2008, 18, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Oinuma, I.; Katoh, H.; Negishi, M. R-Ras controls axon specification upstream of glycogen synthase kinase-3β through integrin-linked kinase. J. Biol. Chem. 2007, 282, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, N.; Negishi, M.; Oinuma, I. R-Ras controls axon branching through afadin in cortical neurons. Mol. Biol. Cell 2012, 23, 2793–2804. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.; Kirschner, M. Cytoskeletal dynamics and nerve growth. Neuron 1988, 1, 761–772. [Google Scholar] [CrossRef]
- Shimada, T.; Toriyama, M.; Uemura, K.; Kamiguchi, H.; Sugiura, T.; Watanabe, N.; Inagaki, N. Shootin1 interacts with actin retrograde flow and L1-CAM to promote axon outgrowth. J. Cell Biol. 2008, 181, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Neukirchen, D.; Bradke, F. Neuronal polarization and the cytoskeleton. Semin. Cell Dev. Biol. 2011, 22, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Bendezú, F.O.; Vincenzetti, V.; Vavylonis, D.; Wyss, R.; Vogel, H.; Martin, S.G. Spontaneous Cdc42 polarization independent of GDI-mediated extraction and actin-based trafficking. PLoS Biol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Wedlich-Söldner, R.; Wai, S.C.; Schmidt, T.; Li, R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell Biol. 2004, 166, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Irazoqui, J.E.; Gladfelter, A.S.; Lew, D.J. Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 2003, 5, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Freisinger, T.; Klünder, B.; Johnson, J.; Müller, N.; Pichler, G.; Beck, G.; Costanzo, M.; Boone, C.; Cerione, R.A.; Frey, E.; Wedlich-Söldner, R. Establishment of a robust single axis of cell polarity by coupling multiple positive feedback loops. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Fairn, G.D.; Hermansson, M.; Somerharju, P.; Grinstein, S. Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nat. Cell Biol. 2011, 13, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Haupt, A.; Campetelli, A.; Bonazzi, D.; Piel, M.; Chang, F.; Minc, N. Electrochemical regulation of budding yeast polarity. PLoS Biol. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Blackmond, G. The origin of biological homochirality. Cold Spring Harb. Perspect. Biol. 2010, 2, 2787–2884. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.C.; Popp, D.; Gebhard, W.; Kabsch, W. Atomic model of the actin filament. Nature 1990, 347, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sase, I.; Miyata, H.; Ishiwata, S.; Kinosita, K., Jr. Axial rotation of sliding actin filaments revealed by single-fluorophore imaging. Proc. Natl. Acad. Sci. USA 1997, 94, 5646–5650. [Google Scholar] [CrossRef] [PubMed]
- Beausang, J.F.; Schroeder, H.W., III; Nelson, P.C.; Goldman, Y.E. Twirling of actin by myosins II and V observed via polarized TIRF in a modified gliding assay. Biophys. J. 2008, 95, 5820–5831. [Google Scholar] [CrossRef] [PubMed]
- Nishizaka, T.; Yagi, T.; Tanaka, Y.; Ishiwata, S. Right-handed rotation of an actin filament in an in vitro motile system. Nature 1993, 361, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Vilfan, A. Twirling motion of actin filaments in gliding assays with nonprocessive Myosin motors. Biophys. J. 2009, 97, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Schroeder, H.W., III; Beausang, J.F.; Homma, K.; Ikebe, M.; Goldman, Y.E. Myosin VI walks “wiggly” on actin with large and variable tilting. Mol. Cell 2007, 28, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Heacock, A.M.; Agranoff, B.W. Clockwise growth of neurites from retinal explants. Science 1977, 198, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Agranoff, B.W. Outgrowth and maintenance of neurites from cultured goldfish retinal ganglion cells. Brain Res. 1981, 206, 331–343. [Google Scholar] [CrossRef]
- Romijn, H.J.; Mud, M.T.; Wolters, P.S.; Corner, M.A. Neurite formation in dissociated cerebral cortex in vitro: Evidence for clockwise outgrowth and autotopic contacts. Brain Res. 1980, 192, 575–580. [Google Scholar] [CrossRef]
- Tamada, A.; Kawase, S.; Murakami, F.; Kamiguchi, H. Autonomous right-screw rotation of growth cone filopodia drives neurite turning. J. Cell Biol. 2010, 188, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Kondo, S. Rotating pigment cells exhibit an intrinsic chirality. Genes Cells 2015, 20, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Tanner, K.; Mori, H.; Mroue, R.; Bruni-Cardoso, A.; Bissell, M.J. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc. Natl. Acad. Sci. USA 2012, 109, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; van Keymeulen, A.; Wakida, N.M.; Carlton, P.; Berns, M.W.; Bourne, H.R. Polarity reveals intrinsic cell chirality. Proc. Natl. Acad. Sci. USA 2007, 104, 9296–9300. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Q.; Ronaldson, K.; Park, M.; Taylor, G.; Zhang, Y.; Gimble, J.M.; Vunjak-Novakovic, G. Micropatterned mammalian cells exhibit phenotype-specific left-right asymmetry. Proc. Natl. Acad. Sci. USA 2011, 108, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Q.; Ronaldson, K.; Guirguis, M.; Vunjak-Novakovic, G. Micropatterning of cells reveals chiral morphogenesis. Stem Cell Res. Ther. 2013, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Hsu, J.J.; Zhao, X.; Guo, C.; Wong, M.N.; Huang, Y.; Li, Z.; Garfinkel, A.; Ho, C.M.; Tintut, Y.; et al. Left-right symmetry breaking in tissue morphogenesis via cytoskeletal mechanics. Circ. Res. 2012, 110, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Rørth, P. Fellow travellers: Emergent properties of collective cell migration. EMBO Rep. 2012, 13, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Doxzen, K.; Vedula, S.R.K.; Leong, M.C.; Hirata, H.; Gov, N.; Kabla, A.J.; Ladoux, B.; Lim, C.T. Guidance of collective cell migration by substrate geometry. Integr. Biol. 2013, 5, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Rappel, W.-J.; Nicol, A.; Sarkissian, A.; Levine, H.; Loomis, W.F. Self-organized vortex state in two-dimensional Dictyostelium dynamics. Phys. Rev. Lett. 1999, 83, 1247–1250. [Google Scholar] [CrossRef]
- Leong, F.Y. Physical explanation of coupled cell-cell rotational behavior and interfacial morphology: A particle dynamics model. Biophys. J. 2013, 105, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- Tee, Y.H.; Shemesh, T.; Thiagarajan, V.; Hariadi, R.F.; Anderson, K.L.; Page, C.; Volkmann, N.; Hanein, D.; Sivaramakrishnan, S.; Kozlov, M.M.; et al. Cellular chirality arising from the self-organization of the actin cytoskeleton. Nat. Cell Biol. 2015, 17, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Ambravaneswaran, V.; Wong, I.Y.; Aranyosi, A.J.; Toner, M.; Irimia, D. Directional decisions during neutrophil chemotaxis inside bifurcating channels. Integr. Biol. 2010, 2, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C. Left-right patterning in the C. elegans embryo: Unique mechanisms and common principles. Commun. Integr. Biol. 2011, 4, 1–7. [Google Scholar] [CrossRef]
- Wood, W.B. Evidence from reversal of handedness in C. elegans embryos for early cell interactions determining cell fates. Nature 1991, 349, 536–538. [Google Scholar] [CrossRef] [PubMed]
- McManus, C. Reversed bodies, reversed brains and (some) reversed behaviors: Of zebrafish and men. Dev. Cell 2005, 8, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.J.; Ware, S.M. Disorders of left-right asymmetry: Heterotaxy and situs inversus. Am. J. Med. Genet. C Semin. Med. Genet. 2009, 151, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Yost, H.J. Coordinating the development of bilateral symmetry and left-right asymmetry. Semin. Cell Dev. Biol. 2009, 20, 455. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Wolpert, L. The development of handedness in left/right asymmetry. Development 1990, 109, 1–9. [Google Scholar] [PubMed]
- Weisblat, D.A. Asymmetric cell divisions in the early embryo of the leech Helobdella robusta. Prog. Mol. Subcell Biol. 2007, 45, 79–95. [Google Scholar] [PubMed]
- Crampton, H. Reversal of cleavage in a sinistral gastropod. Ann. N. Y. Acad. Sci. 1894, 8, 167–170. [Google Scholar] [CrossRef]
- Kuroda, R.; Endo, B.; Abe, M.; Shimizu, M. Chiral blastomere arrangement dictates zygotic left-right asymmetry pathway in snails. Nature 2009, 462, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.J.; Hobert, O. Early embryonic programming of neuronal left/right asymmetry in C. elegans. Curr. Biol. 2006, 16, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.; Lundelius, J.W. Evolutionary implications of the mode of D quadrant specification in coelomates with spiral cleavage. J. Evol. Biol. 1992, 5, 205–247. [Google Scholar] [CrossRef]
- Lyons, D.C.; Weisblat, D.A. D quadrant specification in the leech Helobdella: Actomyosin contractility controls the unequal cleavage of the CD blastomere. Dev. Biol. 2009, 334, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Weisblat, D.A. Asymmetrization of first cleavage by transient disassembly of one spindle pole aster in the leech Helobdella robusta. Dev. Biol. 2006, 292, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Luetjens, C.M. Multiple, alternative cleavage patterns precede uniform larval morphology during normal development of Dreissena polymorpha (Mollusca, Lamellibranchia). Wilhelm Roux Arch. Dev. Biol. 1995, 205, 138–149. [Google Scholar] [CrossRef]
- Luetjens, C.M.; Dorresteijn, A.W.C. The site of fertilisation determines dorsoventral polarity but not chirality in the zebra mussel embryo. Zygote 1998, 6, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Boycott, A.E.; Diver, C.; Garstang, S.L.; Hardy, A.C.; Turner, F.M. The inheritance of sinistrality in Lymnaea peregra. Phil. Trans. R. Soc. Lond. B 1930, 219, 51–131. [Google Scholar] [CrossRef]
- Sturtevant, A.H. Inheritance of direction of coiling in Lymnaea. Science 1923, 58, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.; Lundelius, J.W. The developmental genetics of dextrality and sinistrality in the gastropod Lymnaea peregra. Wilhelm Roux Arch. Dev. Biol. 1982, 191, 69–83. [Google Scholar] [CrossRef]
- Hosoiri, Y.; Harada, Y.; Kuroda, R. Construction of a backcross progeny collection of dextral and sinistral individuals of a freshwater gastropod, Lymnaea stagnalis. Dev. Genes Evol. 2003, 213, 193–198. [Google Scholar] [PubMed]
- Shibazaki, Y.; Shimizu, M.; Kuroda, R. Body handedness is directed by genetically determined cytoskeletal dynamics in the early embryo. Curr. Biol. 2004, 14, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Meshcheryakov, V.N.; Beloussov, L.V. Asymmetrical rotations of blastomeres in early cleavage of gastropoda. Wilhelm Roux Arch. Dev. Biol. 1975, 177, 193–203. [Google Scholar] [CrossRef]
- Grande, C.; Patel, N.H. Nodal signalling is involved in left-right asymmetry in snails. Nature 2009, 457, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Fürthauer, S.; Strempel, M.; Grill, S.W.; Jülicher, F. Active chiral fluids. Eur. Phys. J. E Soft Matter 2012, 35, 89. [Google Scholar] [CrossRef] [PubMed]
- Naganathan, S.R.; Fürthauer, S.; Nishikawa, M.; Jülicher, F.; Grill, S.W. Active torque generation by the actomyosin cell cortex drives left-right symmetry breaking. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Schonegg, S.; Hyman, A.A.; Wood, W.B. Timing and mechanism of the initial cue establishing handed left-right asymmetry in Caenorhabditis elegans embryos. Genesis 2014, 52, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, D.C.; Lee, M.; Robertson, B.; Tsou, M.F.; Rose, L.S.; Wood, W.B. Embryonic handedness choice in C. elegans involves the Galpha protein GPA-16. Development 2003, 130, 5731–5740. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.B.; Bergmann, D.; Florance, A. Maternal effect of low temperature on handedness determination in C. elegans embryos. Dev. Genet. 1996, 19, 222–230. [Google Scholar] [CrossRef]
- Pohl, C.; Bao, Z. Chiral forces organize left-right patterning in C. elegans by uncoupling midline and anteroposterior axis. Dev. Cell 2010, 19, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Pohl, C. A function for the midbody remnant in embryonic patterning. Commun. Integr. Biol. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Pohl, C. Coupling of rotational cortical flow, asymmetric midbody positioning, and spindle rotation mediates dorsoventral axis formation in C. elegans. Dev. Cell 2014, 28, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, D.; Bellaïche, Y. Making the most of the midbody remnant: Specification of the dorsal-ventral axis. Dev. Cell 2014, 28, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Hutter, H.; Schnabel, R. glp-1 and inductions establishing embryonic axes in C. elegans. Development 1994, 120, 2051–2064. [Google Scholar] [PubMed]
- Hutter, H.; Schnabel, R. Establishment of left-right asymmetry in the Caenorhabditis elegans embryo: A multistep process involving a series of inductive events. Development 1995, 121, 3417–3424. [Google Scholar] [PubMed]
- Pohl, C.; Tiongson, M.; Moore, J.L.; Santella, A.; Bao, Z. Actomyosin-based self-organization of cell internalization during C. elegans gastrulation. BMC Biol. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Gros, J.; Feistel, K.; Viebahn, C.; Blum, M.; Tabin, C.J. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 2009, 324, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Maeda, R.; Ando, T.; Okumura, T.; Nakazawa, N.; Hatori, R.; Nakamura, M.; Hozumi, S.; Fujiwara, H.; Matsuno, K. Chirality in planar cell shape contributes to left-right asymmetric epithelial morphogenesis. Science 2011, 333, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Schnabel, R. A posterior centre establishes and maintains polarity of the Caenorhabditis elegans embryo by a Wnt-dependent relay mechanism. PLoS Biol. 2006, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, M.; Schnabel, R. Global cell sorting is mediated by local cell-cell interactions in the C. elegans embryo. Dev. Biol. 2006, 294, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.; Bischoff, M.; Hintze, A.; Schulz, A.K.; Hejnol, A.; Meinhardt, H.; Hutter, H. Global cell sorting in the C. elegans embryo defines a new mechanism for pattern formation. Dev. Biol. 2006, 294, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Houthoofd, W.; Uenk, J.; Vangestel, S.; Schierenberg, E. Plectus—A stepping stone in embryonic cell lineage evolution of nematodes. Evodevo 2012, 3. [Google Scholar] [CrossRef]
- Schulze, J.; Schierenberg, E. Evolution of embryonic development in nematodes. Evodevo 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Coutelis, J.B.; González-Morales, N.; Géminard, C.; Noselli, S. Diversity and convergence in the mechanisms establishing L/R asymmetry in metazoa. EMBO Rep. 2014, 15, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, S.; Maeda, R.; Taniguchi, K.; Kanai, M.; Shirakabe, S.; Sasamura, T.; Spéder, P.; Noselli, S.; Aigaki, T.; Murakami, R.; et al. An unconventional myosin in Drosophila reverses the default handedness in visceral organs. Nature 2006, 440, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Spéder, P.; Adám, G.; Noselli, S. Type ID unconventional myosin controls left-right asymmetry in Drosophila. Nature 2006, 440, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Géminard, C.; González-Morales, N.; Coutelis, J.B.; Noselli, S. The myosin ID pathway and left-right asymmetry in Drosophila. Genesis 2014, 52, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.V.; Flavell, R.A. Myosin I: From yeast to human. Cell Mol. Life Sci. 2008, 65, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Pyrpassopoulos, S.; Feeser, E.; Mazerik, J.N.; Tyska, M.J.; Ostap, E.M. Membrane-bound Myo1c powers asymmetric motility of actin filaments. Curr. Biol. 2012, 22, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Sasamura, T.; Inatomi, M.; Hozumi, S.; Nakamura, M.; Hatori, R.; Taniguchi, K.; Nakazawa, N.; Suzuki, E.; Maeda, R.; et al. Class I myosins have overlapping and specialized functions in left-right asymmetric development in Drosophila. Genetics 2015, 199, 1183–1199. [Google Scholar] [CrossRef] [PubMed]
- Petzoldt, A.G.; Coutelis, J.B.; Géminard, C.; Spéder, P.; Suzanne, M.; Cerezo, D.; Noselli, S. DE-Cadherin regulates unconventional Myosin ID and Myosin IC in Drosophila left-right asymmetry establishment. Development 2012, 139, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Brembeck, F.H.; Rosário, M.; Birchmeier, W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr. Opin. Genet. Dev. 2006, 16, 51–59. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, N.; Géminard, C.; Lebreton, G.; Cerezo, D.; Coutelis, J.-B.; Noselli, S. The atypical cadherin dachsous controls left-right asymmetry in Drosophila. Dev. Cell 2015, 33, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Marcinkevicius, E.; Zallen, J.A. Regulation of cytoskeletal organization and junctional remodeling by the atypical cadherin Fat. Development 2013, 140, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Hatori, R.; Ando, T.; Sasamura, T.; Nakazawa, N.; Nakamura, M.; Taniguchi, K.; Hozumi, S.; Kikuta, J.; Ishii, M.; Matsuno, K. Left-right asymmetry is formed in individual cells by intrinsic cell chirality. Mech. Dev. 2014, 133, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Nakamura, M.; Yoshida, M.; Yamamoto, H.; Maeda, T.; Taniguchi, K.; Nakazawa, N.; Hatori, R.; Ishio, A.; Ozaki, A.; et al. Canonical Wnt signaling in the visceral muscle is required for left-right asymmetric development of the Drosophila midgut. Mech. Dev. 2012, 128, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Coutelis, J.B.; Géminard, C.; Spéder, P.; Suzanne, M.; Petzoldt, A.G.; Noselli, S. Drosophila left/right asymmetry establishment is controlled by the Hox gene abdominal-B. Dev. Cell 2013, 24, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Haigo, S.L.; Bilder, D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science 2011, 331, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Viktorinová, I.; Dahmann, C. Microtubule polarity predicts direction of egg chamber rotation in Drosophila. Curr. Biol. 2013, 23, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Cetera, M.; Ramirez-San Juan, G.R.; Oakes, P.W.; Lewellyn, L.; Fairchild, M.J.; Tanentzapf, G.; Gardel, M.L.; Horne-Badovinac, S. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lacoche, S.; Huang, L.; Xue, B.; Muthuswamy, S.K. Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proc. Natl. Acad. Sci. USA 2013, 110, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Thitamadee, S.; Tuchihara, K.; Hashimoto, T. Microtubule basis for left-handed helical growth in Arabidopsis. Nature 2002, 417, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Fabri, C.O.; Hauptmann, M.; Hutzler, P.; Laux, T.; Lloyd, C.W.; Schäffner, A.R. Helical growth of the Arabidopsis mutant tortifolia1 reveals a plant-specific microtubule-associated protein. Curr. Biol. 2004, 14, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Lobikin, M.; Wang, G.; Xu, J.; Hsieh, Y.W.; Chuang, C.F.; Lemire, J.M.; Levin, M. Early, nonciliary role for microtubule proteins in left-right patterning is conserved across kingdoms. Proc. Natl. Acad. Sci. USA 2012, 109, 12586–12591. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Hsieh, Y.W.; Lesch, B.J.; Bargmann, C.I.; Chuang, C.F. Microtubule-based localization of a synaptic calcium-signaling complex is required for left-right neuronal asymmetry in C. elegans. Development 2011, 138, 3509–3518. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Hauptmann, M.; Niessing, D.; Lloyd, C.W.; Schäffner, A.R. Helical growth of the Arabidopsis mutant tortifolia2 does not depend on cell division patterns but involves handed twisting of isolated cells. Plant Cell 2009, 21, 2090–2106. [Google Scholar] [CrossRef] [PubMed]
- Hoson, T.; Matsumoto, S.; Soga, K.; Wakabayashi, K. Cortical microtubules are responsible for gravity resistance in plants. Plant Signal Behav. 2010, 5, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Fukuda, H. Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science 2012, 337, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Afzelius, B. A human syndrome caused by immotile cilia. Science 1976, 193, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.K.; Yost, H.J. Left-right development: The roles of nodal cilia. Curr. Biol. 2000, 10, R149–R151. [Google Scholar] [CrossRef]
- Lee, J.D.; Anderson, K.V. Morphogenesis of the node and notochord: The cellular basis for the establishment and maintenance of left-right asymmetry in the mouse. Dev. Dyn. 2008, 237, 3464–3476. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.; Roy, S. Left-right asymmetry: Cilia stir up new surprises in the node. Open Biol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Mishina, Y. Establishment of left-right asymmetry in vertebrate development: The node in mouse embryos. Cell Mol. Life Sci. 2013, 70, 4659–4666. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Witte, D.P.; Potter, S.S.; Brueckner, M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature 1997, 389, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Tanaka, Y.; Okada, Y.; Takeda, S.; Harada, A.; Kanai, Y.; Kido, M.; Hirokawa, N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998, 95, 829–837. [Google Scholar] [CrossRef]
- Okada, Y.; Nonaka, S.; Tanaka, Y.; Saijoh, Y.; Hamada, H.; Hirokawa, N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol. Cell 1999, 4, 459–468. [Google Scholar] [CrossRef]
- Supp, D.M.; Brueckner, M.; Kuehn, M.R.; Witte, D.P.; Lowe, L.A.; McGrath, J.; Corrales, J.; Potter, S.S. Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Development 1999, 126, 5495–5504. [Google Scholar] [PubMed]
- Essner, J.J.; Amack, J.D.; Nyholm, M.K.; Harris, E.B.; Yost, H.J. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 2005, 132, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Schweickert, A.; Weber, T.; Beyer, T.; Vick, P.; Bogusch, S.; Feistel, K.; Blum, M. Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 2007, 17, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Shiratori, H.; Saijoh, Y.; Hamada, H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 2002, 418, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Johnson, R.L.; Sterna, C.D.; Kuehn, M.; Tabin, C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 1995, 82, 803–814. [Google Scholar] [CrossRef]
- Meno, C.; Saijoh, Y.; Fujii, H.; Ikeda, M.; Yokoyama, T.; Yokoyama, M.; Toyoda, Y.; Hamada, H. Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature 1996, 381, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Collignon, J.; Varlet, I.; Robertson, E.J. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature 1996, 381, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Lowe, L.A.; Supp, D.M.; Sampath, K.; Yokoyama, T.; Wright, C.V.E.; Potter, S.S.; Overbeek, P.; Kuehn, M.R. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature 1996, 381, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Okada, Y.; Hirokawa, N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 2005, 435, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Kawasumi, A.; Takamatsu, A.; Yoshiba, S.; Botilde, Y.; Motoyama, N.; Reith, W.; Durand, B.; Shiratori, H.; Hamada, H. Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nat. Commun. 2012, 3, 622. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Somlo, S.; Makova, S.; Tian, X.; Brueckner, M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 2003, 114, 61–73. [Google Scholar] [CrossRef]
- Noël, E.S.; Verhoeven, M.; Lagendijk, A.K.; Tessadori, F.; Smith, K.; Choorapoikayil, S.; Hertog, D.J.; Bakkers, J. A Nodal-independent and tissue-intrinsic mechanism controls heart-looping chirality. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Stephen, L.A.; Johnson, E.J.; Davis, G.M.; McTeir, L.; Pinkham, J.; Jaberi, N.; Davey, M.G. The chicken left right organizer has nonmotile cilia which are lost in a stage-dependent manner in the talpid3 ciliopathy. Genesis 2014, 52, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Manning, M.L.; Amack, J.D. Regional cell shape changes control form and function of Kupffer’s vesicle in the zebrafish embryo. Dev. Biol. 2012, 370, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Itasaki, N.; Nakamura, H.; Sumida, H.; Yasuda, M. Actin bundles on the right side in the caudal part of the heart tube play a role in dextro-looping in the embryonic chick heart. Anat. Embryol. 1991, 183, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Schweickert, A.; Vick, P.; Wright, C.V.; Danilchik, M.V. Symmetry breakage in the vertebrate embryo: When does it happen and how does it work? Dev. Biol. 2014, 393, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Danilchik, M.V.; Brown, E.E.; Riegert, K. Intrinsic chiral properties of the Xenopus egg cortex: An early indicator of left-right asymmetry? Development 2006, 133, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Bray, D.; White, J.G. Cortical flow in animal cells. Science 1988, 239, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.H. The role of a superficial plasmagel layer in changes of form, locomotion and division of cells in tissue culture. Arch. Exp. Zellforsch. 1939, 23, 1–7. [Google Scholar]
- Strome, S. Asymmetric movements of cytoplasmic components in Caenorhabditis elegans zygotes. J. Embryol. Exp. Morphol. 1986, 97, 15–29. [Google Scholar] [PubMed]
- Hird, S.N.; White, J.G. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J. Cell Biol. 1993, 121, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Hird, S. Cortical actin movements during the first cell cycle of the Caenorhabditis elegans embryo. J. Cell Sci. 1996, 109, 525–533. [Google Scholar] [PubMed]
- Goldstein, B.; Hird, S.N. Specification of the anteroposterior axis in Caenorhabditis elegans. Development 1996, 122, 1467–1474. [Google Scholar] [PubMed]
- Sadler, P.L.; Shakes, D.C. Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior-posterior polarization of the 1-cell embryo. Development 2000, 127, 355–366. [Google Scholar] [PubMed]
- Nance, J.; Priess, J.R. Cell polarity and gastrulation in C. elegans. Development 2002, 129, 387–397. [Google Scholar] [PubMed]
- Lee, J.Y.; Goldstein, B. Mechanisms of cell positioning during C. elegans gastrulation. Development 2003, 130, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Nance, J.; Munro, E.M.; Priess, J.R. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development 2003, 130, 5339–5350. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Marston, D.J.; Walston, T.; Hardin, J.; Halberstadt, A.; Goldstein, B. Wnt/frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr. Biol. 2006, 16, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Rohrschneider, M.R.; Nance, J. Polarity and cell fate specification in the control of Caenorhabditis elegans gastrulation. Dev. Dyn. 2009, 238, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Roh-Johnson, M.; Shemer, G.; Higgins, C.D.; McClellan, J.H.; Werts, A.D.; Tulu, U.S.; Gao, L.; Betzig, E.; Kiehart, D.P.; Goldstein, B. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science 2012, 335, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, F.; Lv, S.; Yi, P.; Zhu, Z.; Yang, Y.; Feng, G.; Li, W.; Ou, G. Transmembrane protein MIG-13 links the Wnt signaling and Hox genes to the cell polarity in neuronal migration. Proc. Natl. Acad. Sci. USA 2013, 110, 11175–11180. [Google Scholar] [CrossRef] [PubMed]
- Witze, E.S.; Litman, E.S.; Argast, G.M.; Moon, R.T.; Ahn, N.G. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science 2008, 320, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Gon, H.; Fumoto, K.; Ku, Y.; Matsumoto, S.; Kikuchi, A. Wnt5a signaling promotes apical and basolateral polarization of single epithelial cells. Mol. Biol. Cell 2013, 24, 3764–3774. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Chatterjee, B.; Lozito, T.P.; Zhang, Z.; Francis, R.J.; Yagi, H.; Swanhart, L.M.; Sanker, S.; Francis, D.; Yu, Q.; et al. Wdpcp, a PCP protein required for ciliogenesis, regulates directional cell migration and cell polarity by direct modulation of the actin cytoskeleton. PLoS Biol. 2013, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, D.J.; Nelson, W.J.; Weis, W.I. A polarized epithelium organized by β- and α-catenin predates cadherin and metazoan origins. Science 2011, 331, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- St Johnston, D.; Ahringer, J. Cell polarity in eggs and epithelia: Parallels and diversity. Cell 2010, 141, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Lynch, J.A. Symmetry breaking during Drosophila oogenesis. Cold Spring Harb. Perspect. Biol. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Doerflinger, H.; Benton, R.; Torres, I.L.; Zwart, M.F.; St Johnston, D. Drosophila anterior-posterior polarity requires actin-dependent PAR-1 recruitment to the oocyte posterior. Curr. Biol. 2006, 16, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Leibfried, A.; Müller, S.; Ephrussi, A. A Cdc42-regulated actin cytoskeleton mediates Drosophila oocyte polarization. Development 2013, 140, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Kiehart, D.P.; Lutz, M.S.; Chan, D.; Ketchum, A.S.; Laymon, R.A.; Nguyen, B.; Goldstein, L.S. Identification of the gene for fly non-muscle myosin heavy chain: Drosophila myosin heavy chains are encoded by a gene family. EMBO J. 1989, 8, 913–922. [Google Scholar] [PubMed]
- Young, P.E.; Richman, A.M.; Ketchum, A.S.; Kiehart, D.P. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 1993, 7, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, J.T.; Wieschaus, E. Two new roles for the Drosophila AP patterning system in early morphogenesis. Development 2001, 128, 5129–5138. [Google Scholar] [PubMed]
- Zallen, J.A.; Wieschaus, E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 2004, 6, 343–355. [Google Scholar] [CrossRef]
- Dawes-Hoang, R.E.; Parmar, K.M.; Christiansen, A.E.; Phelps, C.B.; Brand, A.H.; Wieschaus, E.F. Folded gastrulation, cell shape change and the control of myosin localization. Development 2005, 132, 4165–4178. [Google Scholar] [CrossRef] [PubMed]
- Bertet, C.; Sulak, L.; Lecuit, T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 2004, 429, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Rauzi, M.; Verant, P.; Lecuit, T.; Lenne, P.F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 2008, 10, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, R.; Simoes Sde, M.; Röper, J.C.; Eaton, S.; Zallen, J.A. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 2009, 17, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C.; Kaschube, M.; Wieschaus, E.F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 2009, 457, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Rauzi, M.; Lenne, P.F.; Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 2010, 468, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Simões Sde, M.; Mainieri, A.; Zallen, J.A. Rho GTPase and Shroom direct planar polarized actomyosin contractility during convergent extension. J. Cell Biol. 2014, 204, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Kasza, K.E.; Farrell, D.L.; Zallen, J.A. Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc. Natl. Acad. Sci. USA 2014, 111, 11732–11737. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Doubrovinski, K.; Polyakov, O.; Wieschaus, E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 2014, 508, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Munjal, A.; Philippe, J.M.; Munro, E.; Lecuit, T. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 2015. [Google Scholar] [CrossRef] [PubMed]
- Behrndt, M.; Salbreux, G.; Campinho, P.; Hauschild, R.; Oswald, F.; Roensch, J.; Grill, S.W.; Heisenberg, C.P. Forces driving epithelial spreading in zebrafish gastrulation. Science 2012, 338, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Rubinstein, B.; Li, R. Symmetry breaking and polarity establishment during mouse oocyte maturation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Lepage, S.E.; Bruce, A.E.E. Zebrafish epiboly: Mechanics and mechanisms. Int. J. Dev. Biol. 2010, 54, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.P.; Khanal, I.; Gaspar, P.; Fletcher, G.C.; Polesello, C.; Tapon, N.; Thompson, B.J. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 2013, 201, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B. Cell contacts orient some cell division axes in the Caenorhabditis elegans embryo. J. Cell Biol. 1995, 129, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.J.; Schlesinger, A.; Carter, J.C.; Bowerman, B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell 1997, 90, 695–705. [Google Scholar] [CrossRef]
- Schlesinger, A.; Shelton, C.A.; Maloof, J.N.; Meneghini, M.; Bowerman, B. Wnt pathway components orient a mitotic spindle in the early Caenorhabditis elegans embryo without requiring gene transcription in the responding cell. Genes Dev. 1999, 13, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Hogan, J.; Berkowitz, L.A.; Soto, M.; Rocheleau, C.E.; Pang, K.M.; Collins, J.; Mello, C.C. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev. Cell 2002, 3, 113–125. [Google Scholar] [CrossRef]
- Goldstein, B.; Takeshita, H.; Mizumoto, K.; Sawa, H. Wnt signals can function as positional cues in establishing cell polarity. Dev. Cell 2006, 10, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Arata, Y.; Lee, J.Y.; Goldstein, B.; Sawa, H. Extracellular control of PAR protein localization during asymmetric cell division in the C. elegans embryo. Development 2010, 137, 3337–3345. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.J.; Chen, B.C.; Tsai, F.C.; Anastassiadis, K.; Meyer, T.; Betzig, E.; Nusse, R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science 2013, 339, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, K.; Mizumoto, K.; Sawa, H. Wnt regulates spindle asymmetry to generate asymmetric nuclear β-catenin in C. elegans. Cell 2011, 146, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Knoblich, J.A. Asymmetric cell division: Recent developments and their implications for tumour biology. Nat. Rev. Mol. Cell Biol. 2010, 11, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, L.; Yamashita, Y.M. Centrosome asymmetry and inheritance during animal development. Curr. Opin. Cell Biol. 2012, 24, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.S.; Johnston, C.A. Molecular pathways regulating mitotic spindle orientation in animal cells. Development 2013, 140, 1843–1856. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; O’Bryan, M.K. Microtubules and spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Segalen, M.; Bellaïche, Y. Cell division orientation and planar cell polarity pathways. Semin. Cell Dev. Biol. 2009, 20, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, L.V.; Strutt, D. Principles of planar polarity in animal development. Development 2011, 138, 1877–1892. [Google Scholar] [CrossRef] [PubMed]
- Matis, M.; Axelrod, J.D. Regulation of PCP by the Fat signaling pathway. Genes Dev. 2013, 27, 2207–2220. [Google Scholar] [CrossRef] [PubMed]
- Guirao, B.; Meunier, A.; Mortaud, S.; Aguilar, A.; Corsi, J.M.; Strehl, L.; Hirota, Y.; Desoeuvre, A.; Boutin, C.; Han, Y.G.; et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 2010, 12, 341–350. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohl, C. Cytoskeletal Symmetry Breaking and Chirality: From Reconstituted Systems to Animal Development. Symmetry 2015, 7, 2062-2107. https://doi.org/10.3390/sym7042062
Pohl C. Cytoskeletal Symmetry Breaking and Chirality: From Reconstituted Systems to Animal Development. Symmetry. 2015; 7(4):2062-2107. https://doi.org/10.3390/sym7042062
Chicago/Turabian StylePohl, Christian. 2015. "Cytoskeletal Symmetry Breaking and Chirality: From Reconstituted Systems to Animal Development" Symmetry 7, no. 4: 2062-2107. https://doi.org/10.3390/sym7042062
APA StylePohl, C. (2015). Cytoskeletal Symmetry Breaking and Chirality: From Reconstituted Systems to Animal Development. Symmetry, 7(4), 2062-2107. https://doi.org/10.3390/sym7042062





