Analysis of Ilmenite Slag Using Laser-Induced Breakdown Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
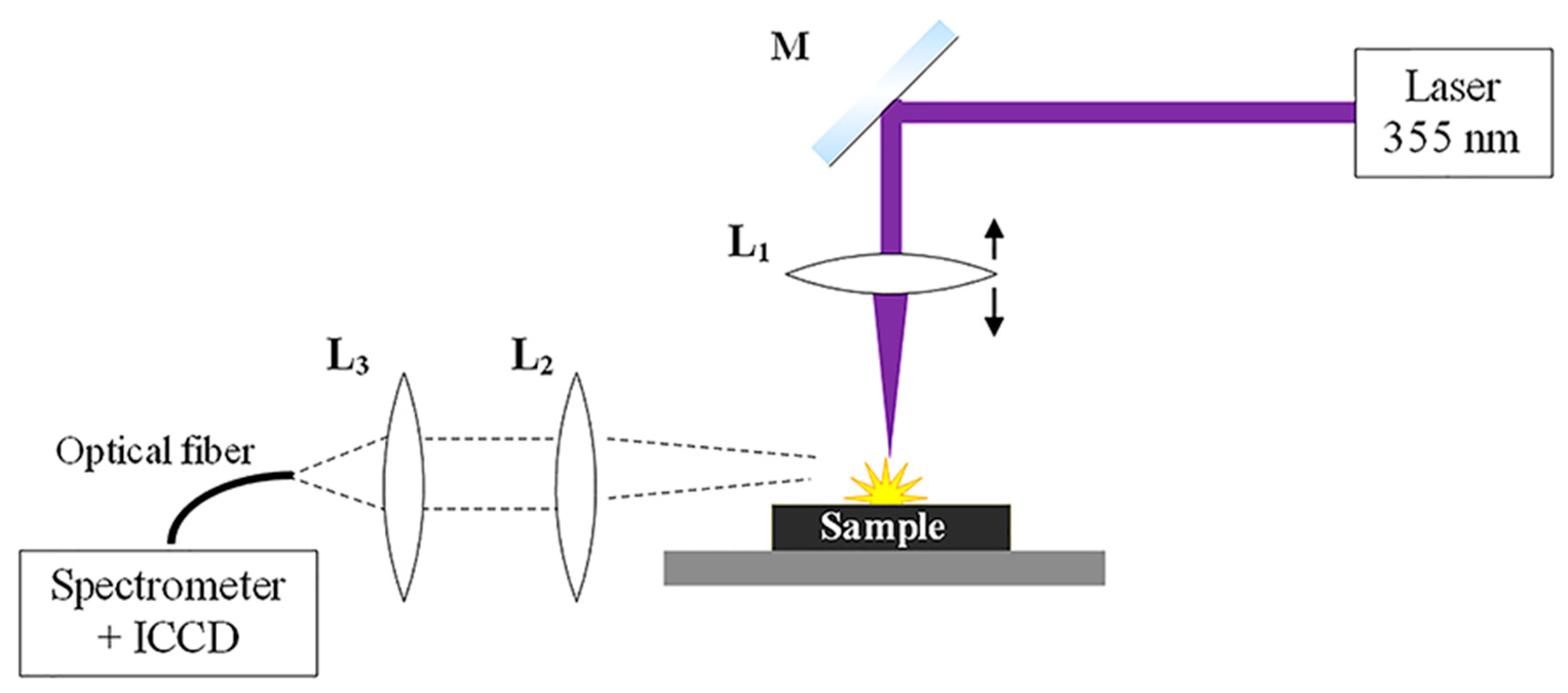
2.1. LIBS Setup
2.2. Sample Materials and LIBS Measurements
3. Results and Discussion
3.1. Plasma Characterization
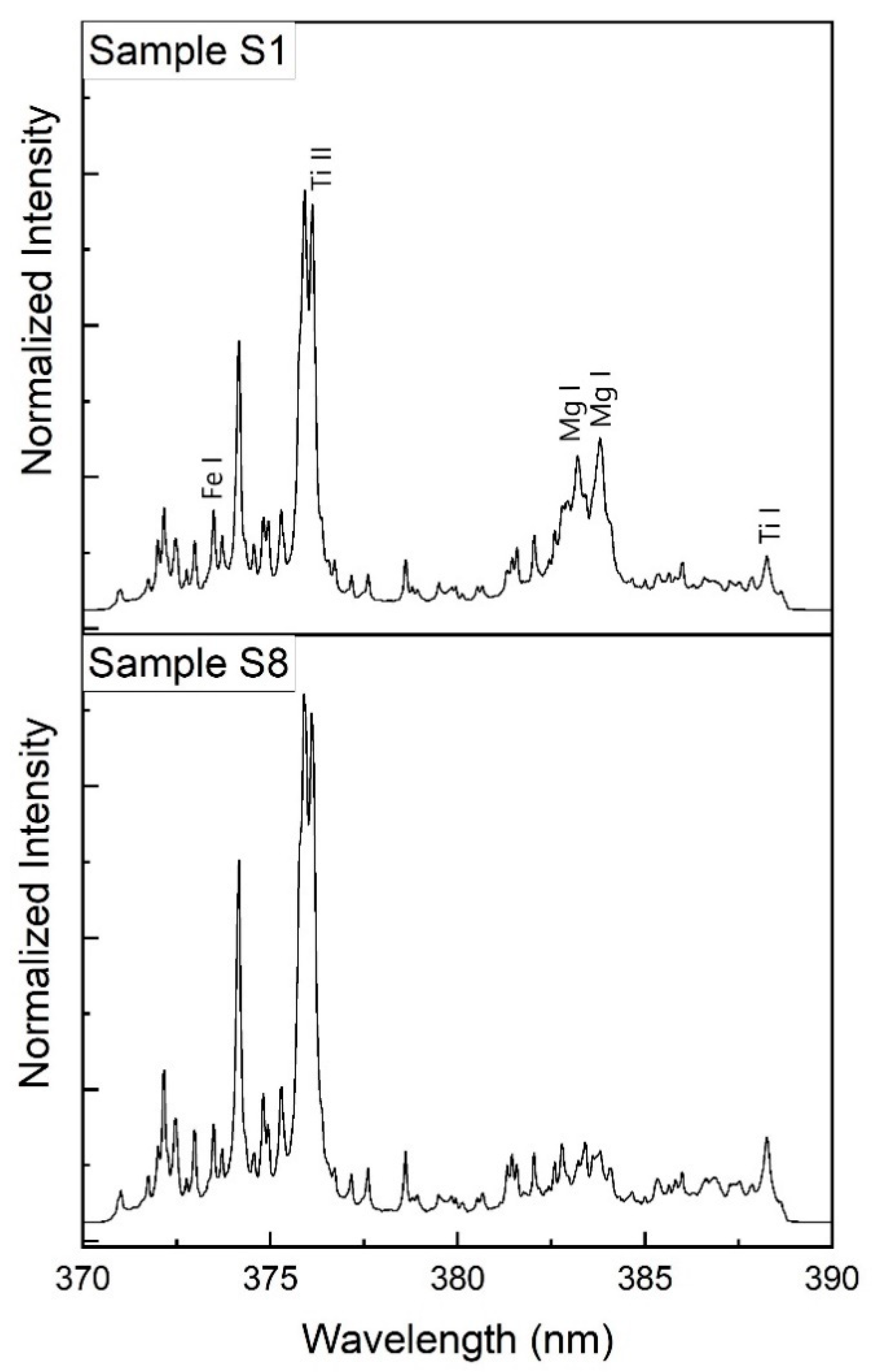
3.2. LIBS Spectra
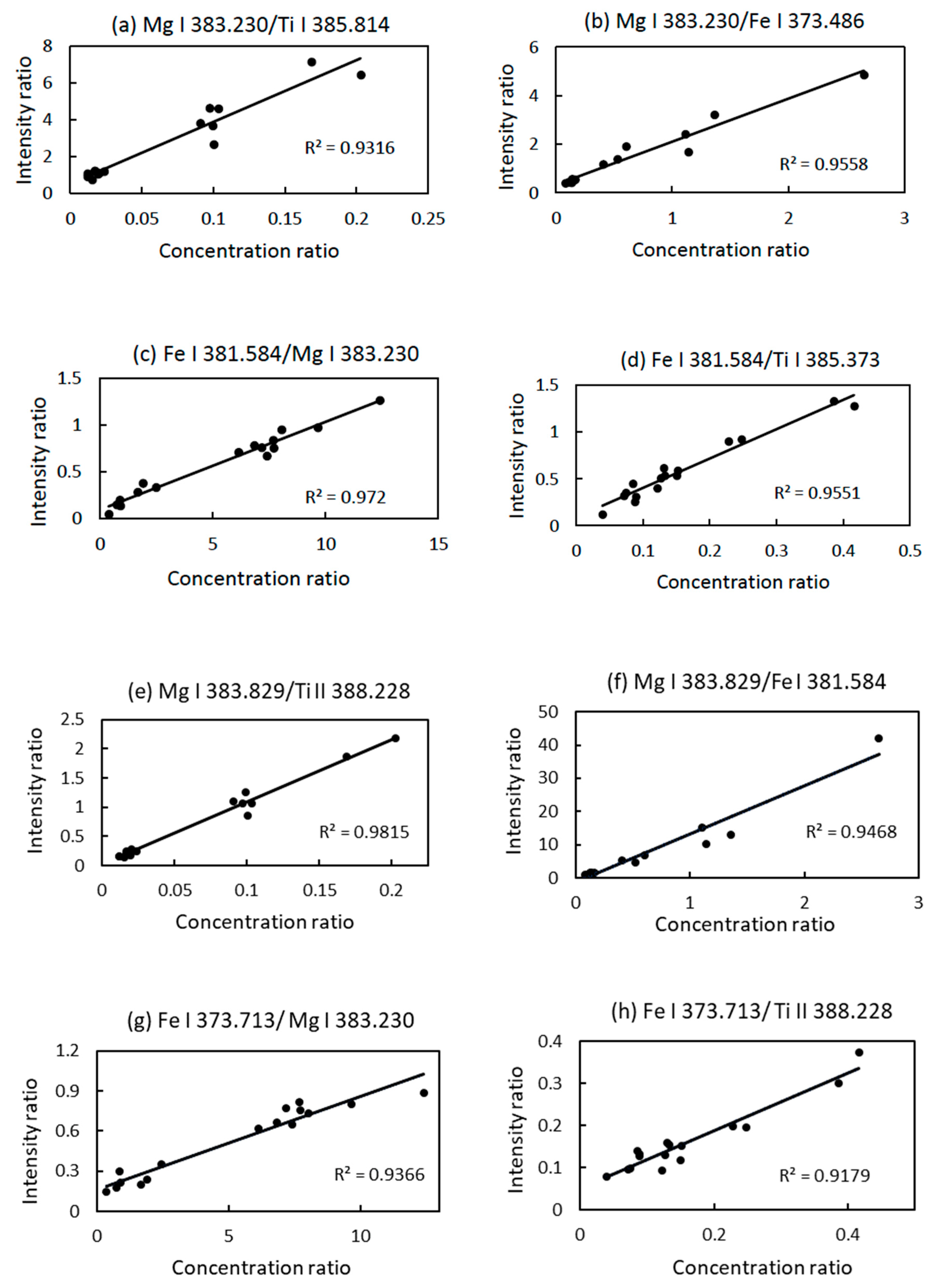
3.3. Univariate Simple Linear Regression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pistorius, P.C. Ilmenite smelting: The basics. J. S. Afr. Inst. Min. Metall. 2008, 108, 35–43. [Google Scholar]
- Gous, M. An overview of the Namakwa Sands ilmenite smelting operations. J. S. Afr. Inst. Min. Metall. 2006, 106, 379–384. [Google Scholar]
- Zietsman, J.H.; Pistorius, P.C. Process mechanisms in ilmenite smelting. SIAMM J. S. Afr. Inst. Min. Metall. 2004, 104, 653–660. [Google Scholar]
- Miziolek, A.; Vincenzo, P.; Schechter, I. Laser-Induced Breakdown Spectroscopy: Fundamentals and Applications; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Adrain, R.S.; Watson, J. Laser microspectral analysis: A review of principles and applications. J. Phys. D Appl. Phys. 1984, 17, 1915–1940. [Google Scholar] [CrossRef]
- Romero, D.; Laserna, J.J. Infrared laser ablation and atomic emission spectrometry of stainless steel at high temperatures. J. Anal. At. Spectrom. 1999, 14, 1883–1887. [Google Scholar]
- Kolmhofer, B.P.; Pedarnig, J.D.; Praher, R.; Huber, N.; Rössler, J.H. Element analysis of complex materials by calibration-free laser-induced breakdown spectroscopy. Appl. Phys. A 2013, 112, 105–111. [Google Scholar] [CrossRef]
- Boué-Bigne, F. Laser-induced breakdown spectroscopy and multivariate statistics for the rapid identification of oxide inclusions in steel products. Spectrochim. Acta Part B At. Spectrosc. 2016, 119, 25–35. [Google Scholar] [CrossRef]
- Lorenzen, C.J.; Carlhoff, C.; Hahn, U.; Jogwich, M. Applications of Laser-induced Emission Spectral Analysis for Industrial Process and Quality Control. J. Anal. At. Spectrom. 1992, 7, 1029–1035. [Google Scholar] [CrossRef]
- Wang, Z.; Deguchi, Y.; Shiou, F.; Yan, J.; Liu, J. Application of Laser-Induced Breakdown Spectroscopy to Real-Time Elemental Monitoring of Iron and Steel Making Processes. ISIJ Int. 2016, 56, 723–735. [Google Scholar] [CrossRef] [Green Version]
- Kolmhofer, P.J.; Eschlböck-Fuchs, S.; Huber, N.; Rössler, R.; Heitz, J.; Pedarnig, J.D. Calibration-free analysis of steel slag by laser-induced breakdown spectroscopy with combined UV and VIS spectra. Spectrochim. Acta Part B At. Spectrosc. 2015, 106, 67–74. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, S.; Dong, J.; Wang, K.; Tang, H.; Yang, X.; Li, H. Quantitative and classi fi cation analysis of slag samples by laser induced breakdown spectroscopy and partial least square (PLS) methods. J. Anal. At. Spectrom. 2015, 30, 368–374. [Google Scholar] [CrossRef]
- Ciucci, A.; Corsi, M.; Palleschi, V.; Rastelli, S.; Salvetti, A. New Procedure for Quantitative Elemental Analysis by Laser-Induced Plasma Spectroscopy. Appl. Spectrosc. 1999, 53, 960–964. [Google Scholar] [CrossRef]
- Sturm, V.; Schmitz, H.U.; Reuter, T.; Fleige, R.; Noll, R. Fast vacuum slag analysis in a steel works by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1167–1170. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, L.; Wang, K.; Tang, H.; Yang, X.; Duan, Y.; Li, H. A novel approach for the quantitative analysis of multiple elements in steel based on laser-induced breakdown spectroscopy (LIBS) and random forest regression (RFR). J. Anal. At. Spectrom. 2014, 29, 2323–2329. [Google Scholar] [CrossRef]
- Motto-Ros, V.; Negre, E.; Pelascini, F.; Panczer, G.; Yu, J. Precise alignment of the collection fiber assisted by real-time plasma imaging in laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2014, 92, 60–69. [Google Scholar] [CrossRef]
- Negre, E.; Motto-Ros, V.; Pelascini, F.; Lauper, S.; Denis, D.; Yu, J. On the performance of laser-induced breakdown spectroscopy for quantitative analysis of minor and trace elements in glass. J. Anal. At. Spectrom. 2015, 30, 417–425. [Google Scholar] [CrossRef]
- Jolivet, L.; Leprince, M.; Moncayo, S.; Sorbier, L.; Lienemann, C.P.; Motto-Ros, V. Review of the recent advances and applications of LIBS-based imaging. Spectrochim. Acta Part B At. Spectrosc. 2019, 151, 41–53. [Google Scholar] [CrossRef]
- Pauna, H.; Willms, T.; Aula, M.; Echterhof, T.; Huttula, M.; Fabritius, T. Pilot-scale AC electric arc furnace plasma characterization. Plasma Res. Express 2019, 1, 035007. [Google Scholar] [CrossRef]
- Aragón, C.; Aguilera, J.A. Characterization of laser induced plasmas by optical emission spectroscopy: A review of experiments and methods. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 893–916. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; The NIST ASD Team. NIST Atomic Spectra Database (ver. 5.7); 2019. Available online: http://physics.nist.gov/asd (accessed on 15 December 2019). [CrossRef]

| Sample (wt.%) | Mg | Ti | Fe | Mn | Si | Al |
|---|---|---|---|---|---|---|
| S1 | 6.63 | 32.7 | 12.6 | 0.94 | 0.43 | 0.73 |
| S2 | 3.05 | 18.1 | 7.53 | 0.94 | 0.55 | 0.71 |
| S3 | 4.67 | 48 | 3.43 | 1.10 | 0.57 | 1.49 |
| S4 | 4.06 | 44.8 | 6.75 | 1.01 | 0.50 | 1.17 |
| S5 | 1.03 | 43.6 | 9.93 | 1.19 | 0.57 | 0.97 |
| S6 | 0.94 | 47.6 | 7.24 | 1.25 | 0.56 | 0.93 |
| S7 | 0.6 | 39.7 | 4.83 | 1.28 | 0.59 | 0.94 |
| S8 | 0.83 | 48.7 | 6.37 | 1.06 | 0.41 | 0.89 |
| S9 | 0.92 | 46 | 11.4 | 1.10 | 0.40 | 0.83 |
| S10 | 0.89 | 52 | 6.58 | 1.15 | 0.39 | 0.85 |
| S11 | 0.97 | 50 | 6.63 | 1.18 | 0.45 | 0.85 |
| S12 | 4.6 | 46.3 | 4.14 | 1.07 | 1.15 | 2.28 |
| S13 | 4.49 | 44.8 | 3.94 | 1.07 | 1.19 | 2.23 |
| S14 | 4.96 | 48 | 1.87 | 0.99 | 1.06 | 2.32 |
| S15 | 0.61 | 51.4 | 4.37 | 0.93 | 0.51 | 0.90 |
| S16 | 0.64 | 52.7 | 3.92 | 0.85 | 0.51 | 1.06 |
| S17 | 2.66 | 44.5 | 8.35 | 1.00 | 0.53 | 1.09 |
| Serial No. | Sample Name | Electron Temperature (K, Fe) | R2 | Error (%) | Ti Plasma Electron Density (1/cm3) | Ti LTE Density (1/cm3) |
|---|---|---|---|---|---|---|
| 1 | S1 | 13,760.687 | 0.972 | 10.872 | 1.42 × 1018 | 6.8883 × 1015 |
| 2 | S2 | 13,368.814 | 0.982 | 10.563 | 1.562 × 1018 | 6.7895 × 1015 |
| 3 | S3 | 14,806.654 | 0.897 | 11.699 | 2.274 × 1018 | 7.1453 × 1015 |
| 4 | S4 | 14,989.253 | 0.912 | 11.843 | 2.238 × 1018 | 7.1892 × 1015 |
| 5 | S5 | 13,587.076 | 0.952 | 10.735 | 1.403 × 1018 | 6.8447 × 1015 |
| 6 | S6 | 13,518.851 | 0.953 | 10.681 | 1.323 × 1018 | 6.8275 × 1015 |
| 7 | S7 | 14,673.382 | 0.947 | 11.593 | 2.335 × 1018 | 7.113 × 1015 |
| 8 | S8 | 15,028.007 | 0.951 | 11.873 | 2.587 × 1018 | 7.1985 × 1015 |
| 9 | S9 | 15,585.366 | 0.950 | 12.314 | 2.934 × 1018 | 7.3307 × 1015 |
| 10 | S10 | 13,543.478 | 0.951 | 10.701 | 1.084 × 1018 | 6.8337 × 1015 |
| 11 | S11 | 15,561.469 | 0.943 | 12.295 | 3.288 × 1018 | 7.3251 × 1015 |
| 12 | S12 | 13,164.656 | 0.935 | 10.401 | 9.77 × 1017 | 6.7374 × 1015 |
| 13 | S13 | 15,869.721 | 0.932 | 12.539 | 4.06 × 1018 | 7.3973 × 1015 |
| 14 | S14 | 13,777.532 | 0.892 | 10.886 | 1.267 × 1018 | 6.8925 × 1015 |
| 15 | S15 | 15,330.347 | 0.977 | 12.112 | 3.163 × 1018 | 7.2705 × 1015 |
| 16 | S16 | 15,196.106 | 0.959 | 12.006 | 3.028 × 1018 | 7.2386 × 1015 |
| 17 | S17 | 17,867.997 | 0.950 | 14.117 | 1.227 × 1019 | 7.8492 × 1015 |
| Element | Spectral Line (nm) |
|---|---|
| Mg | 382.935, 383.230, 383.829 |
| Ti | 375.929, 376.132, 381.458, 385.373, 385.814, 386.840, 387.320, 388.228 |
| Fe | 373.486, 373.713, 381.584, 382.042, 385.637, 385.991 |
| Sample Name | ICP-OES | LIBS | Absolute Error | % Error | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | Mg | Fe | Ti | Mg | Fe | Ti | Mg | Fe | Ti | Mg | Fe | |
| S1 | 0.630 | 0.128 | 0.243 | 0.622 | 0.118 | 0.259 | −0.007 | −0.009 | 0.016 | 1.15 | 7.21 | 6.79 |
| S2 | 0.631 | 0.106 | 0.263 | 0.642 | 0.111 | 0.247 | 0.010 | 0.005 | −0.015 | 1.66 | 4.52 | 5.81 |
| S3 | 0.856 | 0.083 | 0.061 | 0.858 | 0.081 | 0.061 | 0.002 | −0.002 | 0.000 | 0.26 | 2.55 | 0.19 |
| S4 | 0.806 | 0.073 | 0.121 | 0.805 | 0.079 | 0.116 | −0.001 | 0.006 | −0.005 | 0.10 | 8.05 | 4.19 |
| S5 | 0.799 | 0.019 | 0.182 | 0.781 | 0.018 | 0.201 | −0.018 | −0.001 | 0.019 | 2.22 | 4.57 | 10.23 |
| S6 | 0.853 | 0.017 | 0.130 | 0.853 | 0.013 | 0.135 | −0.001 | −0.004 | 0.005 | 0.09 | 25.23 | 3.86 |
| S7 | 0.880 | 0.013 | 0.107 | 0.902 | 0.010 | 0.089 | 0.022 | −0.003 | −0.018 | 2.48 | 25.69 | 17.21 |
| S8 | 0.871 | 0.015 | 0.114 | 0.841 | 0.019 | 0.140 | −0.030 | 0.004 | 0.026 | 3.49 | 27.74 | 23.05 |
| S9 | 0.789 | 0.016 | 0.195 | 0.773 | 0.021 | 0.206 | −0.016 | 0.005 | 0.011 | 1.99 | 30.37 | 5.59 |
| S10 | 0.874 | 0.015 | 0.111 | 0.869 | 0.016 | 0.115 | −0.006 | 0.001 | 0.005 | 0.66 | 7.38 | 4.23 |
| S11 | 0.868 | 0.017 | 0.115 | 0.860 | 0.018 | 0.122 | −0.008 | 0.001 | 0.007 | 0.93 | 5.11 | 6.30 |
| S12 | 0.841 | 0.084 | 0.075 | 0.849 | 0.095 | 0.056 | 0.007 | 0.012 | −0.019 | 0.88 | 14.03 | 25.45 |
| S13 | 0.842 | 0.084 | 0.074 | 0.886 | 0.066 | 0.048 | 0.044 | −0.018 | −0.026 | 5.23 | 21.43 | 35.08 |
| S14 | 0.875 | 0.090 | 0.034 | 0.905 | 0.086 | 0.009 | 0.030 | −0.005 | −0.025 | 3.41 | 5.04 | 74.23 |
| S15 | 0.912 | 0.011 | 0.078 | 0.887 | 0.012 | 0.101 | −0.025 | 0.002 | 0.023 | 2.73 | 15.51 | 29.95 |
| S16 | 0.920 | 0.011 | 0.068 | 0.912 | 0.012 | 0.076 | −0.008 | 0.001 | 0.007 | 0.92 | 10.46 | 10.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.K.; Aula, M.; Negre, E.; Viljanen, J.; Pauna, H.; Mäkelä, P.; Toivonen, J.; Huttula, M.; Fabritius, T. Analysis of Ilmenite Slag Using Laser-Induced Breakdown Spectroscopy. Minerals 2020, 10, 855. https://doi.org/10.3390/min10100855
Gupta AK, Aula M, Negre E, Viljanen J, Pauna H, Mäkelä P, Toivonen J, Huttula M, Fabritius T. Analysis of Ilmenite Slag Using Laser-Induced Breakdown Spectroscopy. Minerals. 2020; 10(10):855. https://doi.org/10.3390/min10100855
Chicago/Turabian StyleGupta, Avishek Kumar, Matti Aula, Erwan Negre, Jan Viljanen, Henri Pauna, Pasi Mäkelä, Juha Toivonen, Marko Huttula, and Timo Fabritius. 2020. "Analysis of Ilmenite Slag Using Laser-Induced Breakdown Spectroscopy" Minerals 10, no. 10: 855. https://doi.org/10.3390/min10100855






