Agate Genesis: A Continuing Enigma
Abstract
1. Introduction
2. Scientific Contributions over the Years 1770–1980
2.1. 1770–1900
2.2. 1900–1945
- (1)
- Some samples of agates are found with a hollow space. This demonstrates that the vesicle is not always filled with a gel;
- (2)
- The vesicular infill occurs by the layering of the first generation and can be accompanied by metasomatic substitution of crystals, which have been previously precipitated;
- (3)
- The hypothesis does not explain why, in the same sample, colourless banded chalcedony varies with the pigmented chalcedony;
- (4)
- The role of pigmentation is not clear with (a) well-formed amethyst crystal centres and (b) brown pigments found in various zones with the disappearance of strong pigment zones;
- (5)
- In artificial preparations, the deposits are torn and split while in nature this phenomenon is rare.
2.3. 1945–1955
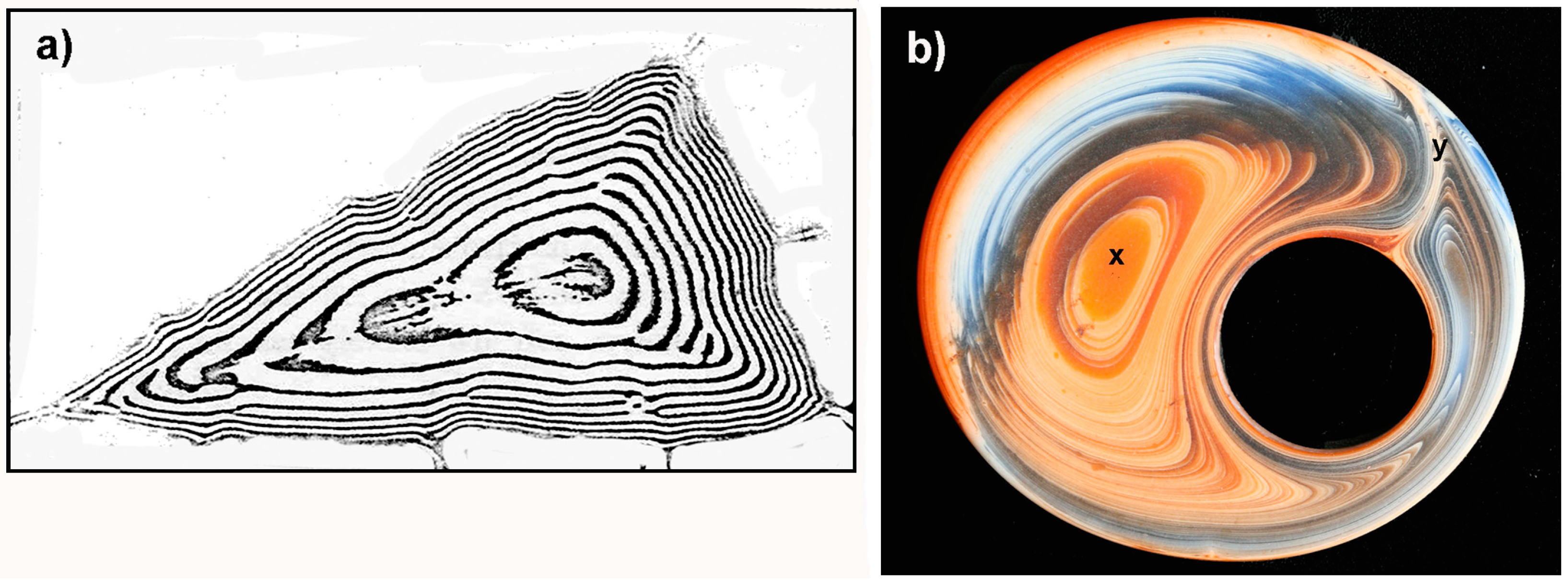
2.4. 1955–1982
3. Scientific Contributions over the Years 1980–2020
3.1. Genesis Contributions in the Early Years
3.2. Chalcedony from Silica Glass
3.3. Agate Formation Temperature
- (1)
- Loosely bound water molecules are lost at <200 °C.
- (2)
- Tightly bound water and various forms of silanol water are lost up to 800 °C.
- (3)
- The maximum mass loss is reached at 850 °C.
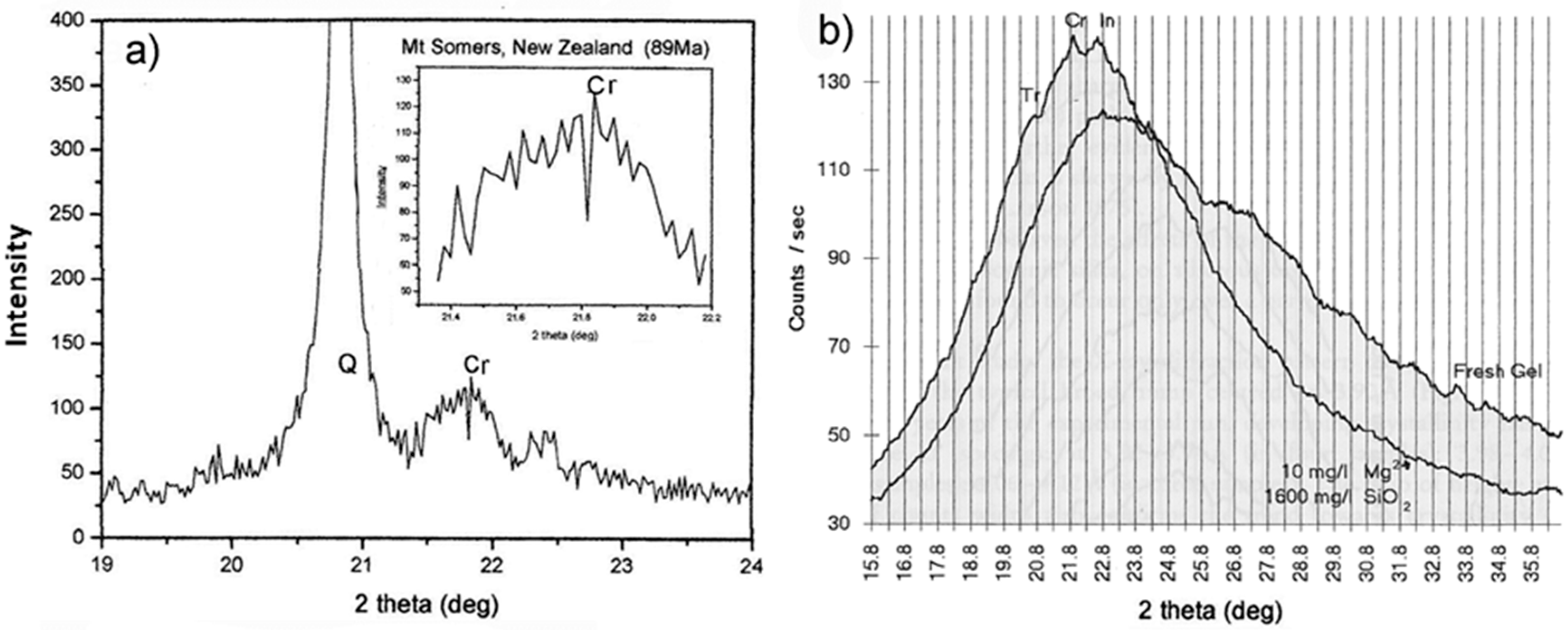
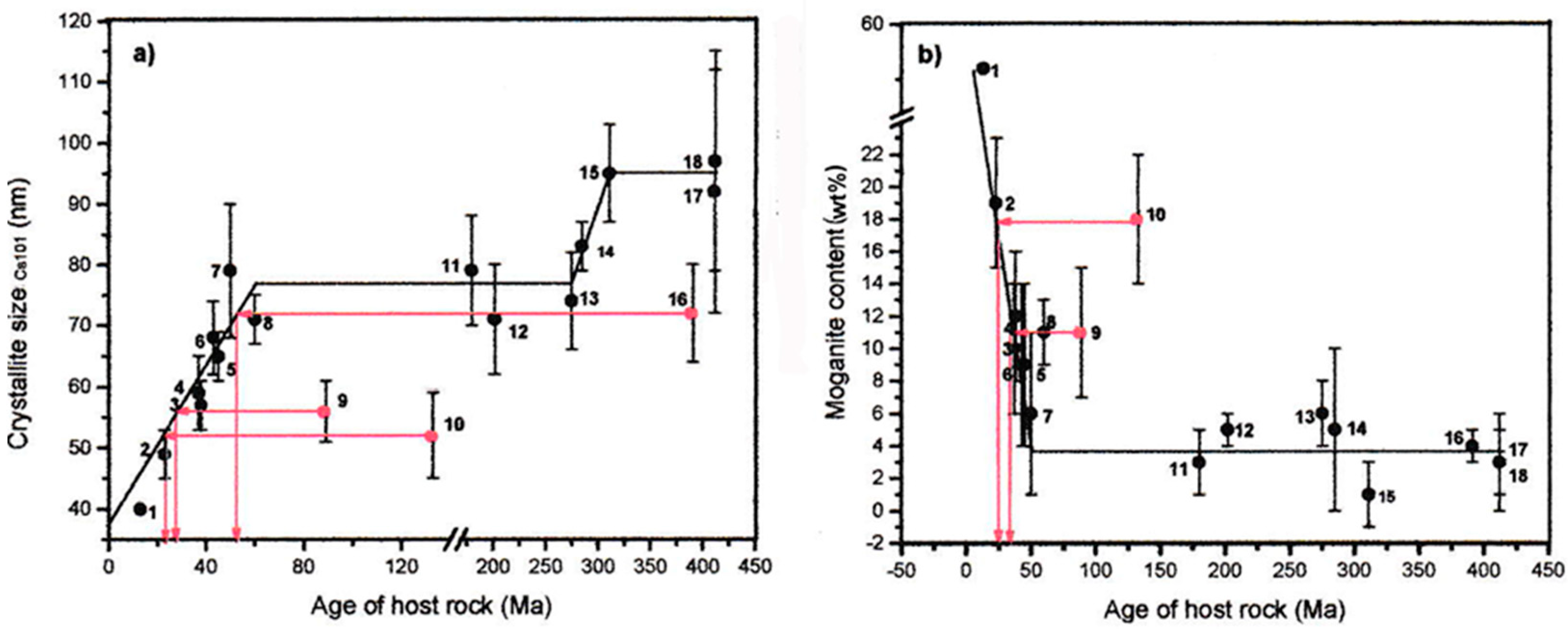
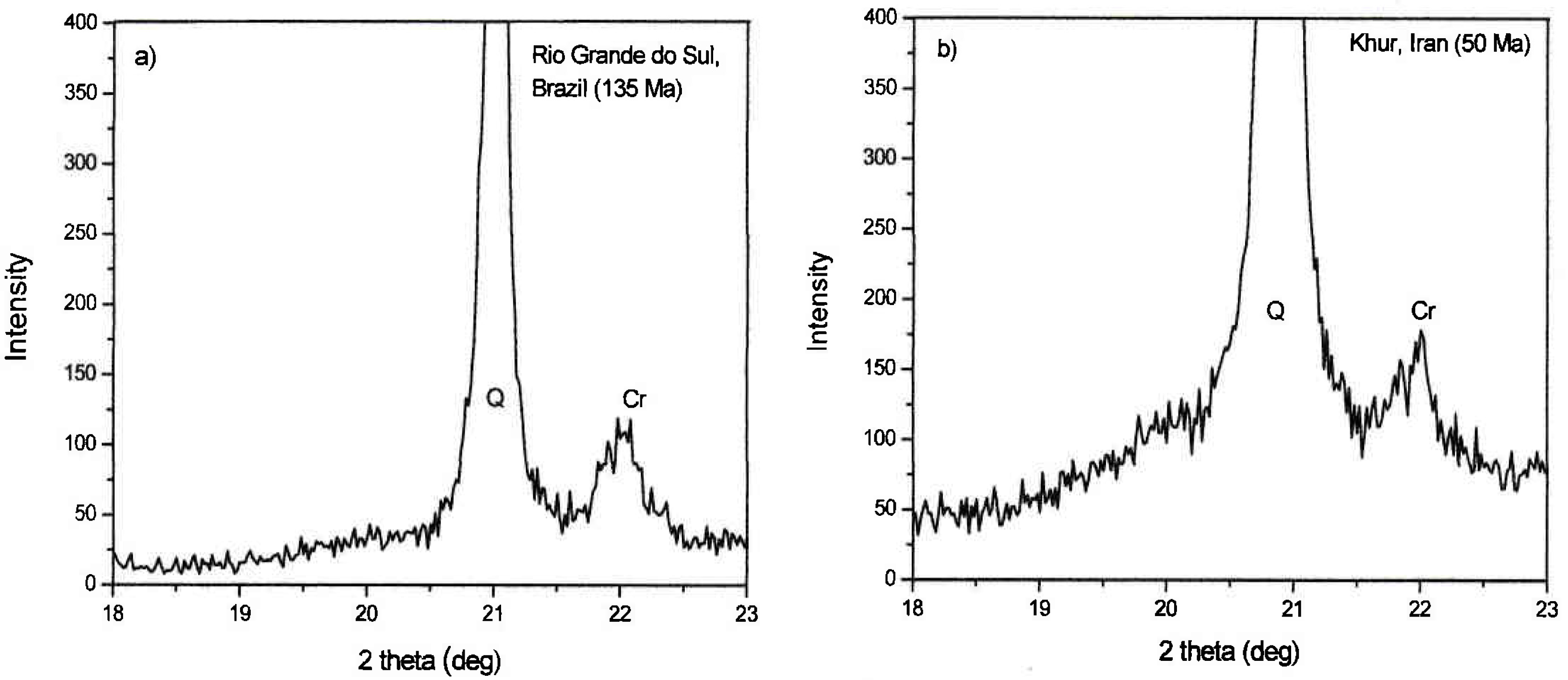
3.4. The Discovery of Moganite
3.5. Cation and Silica Loss from Basalt Host and Their Potential Role in Agate Formation
3.6. Age of Agate and Host
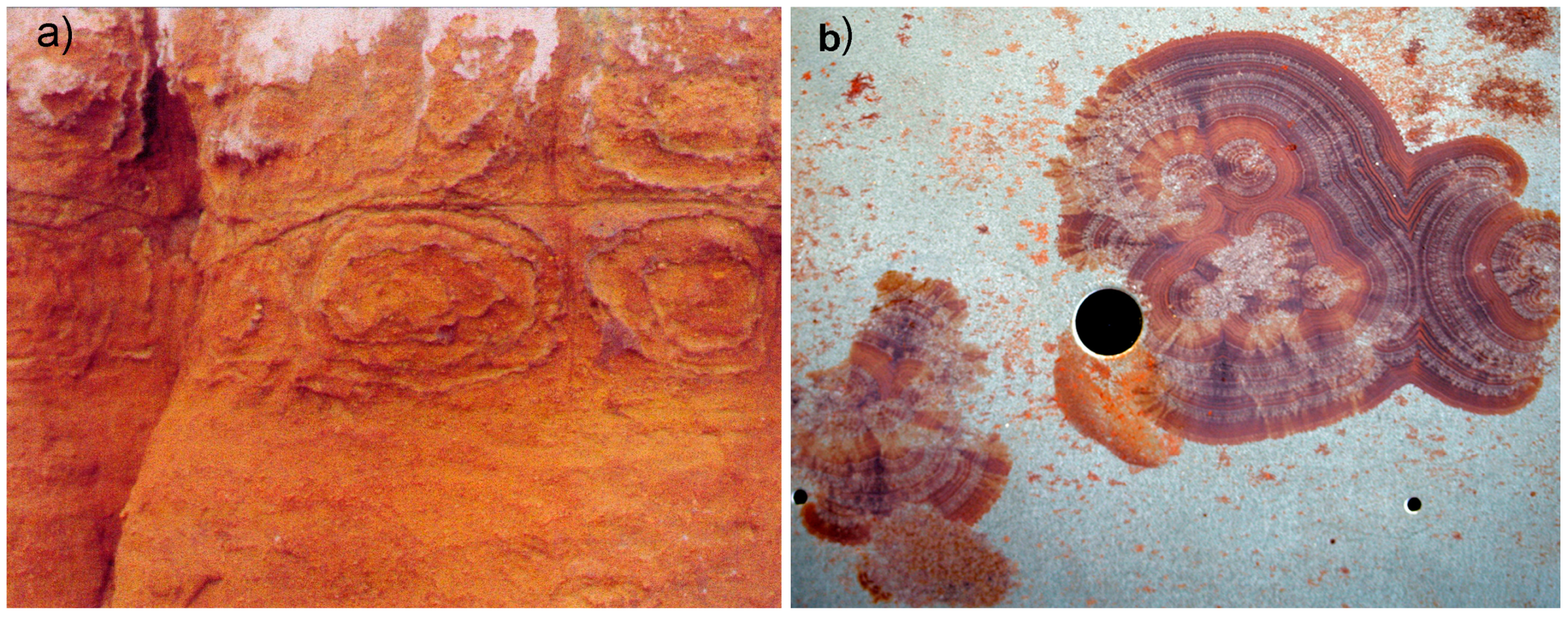
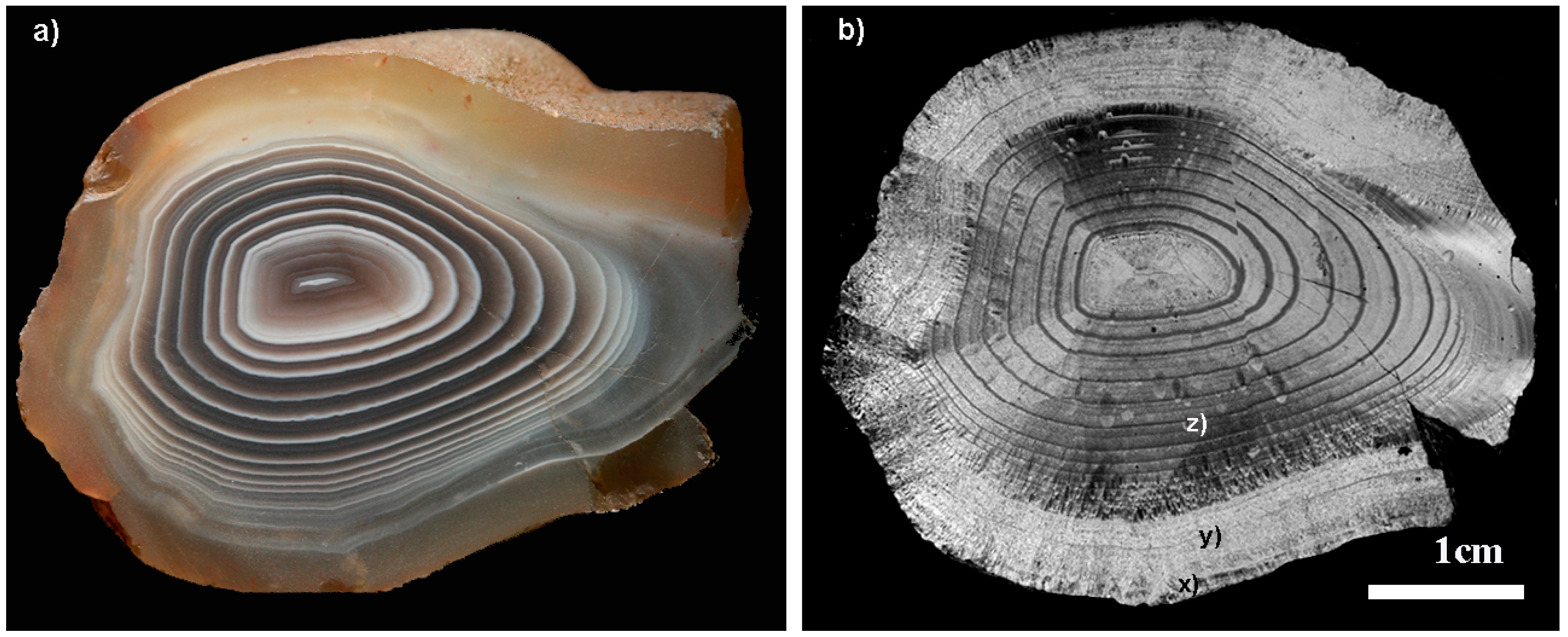
3.7. The Role of the Infiltration Canal
3.8. The Wall-Contact Layer
→ granular quartz
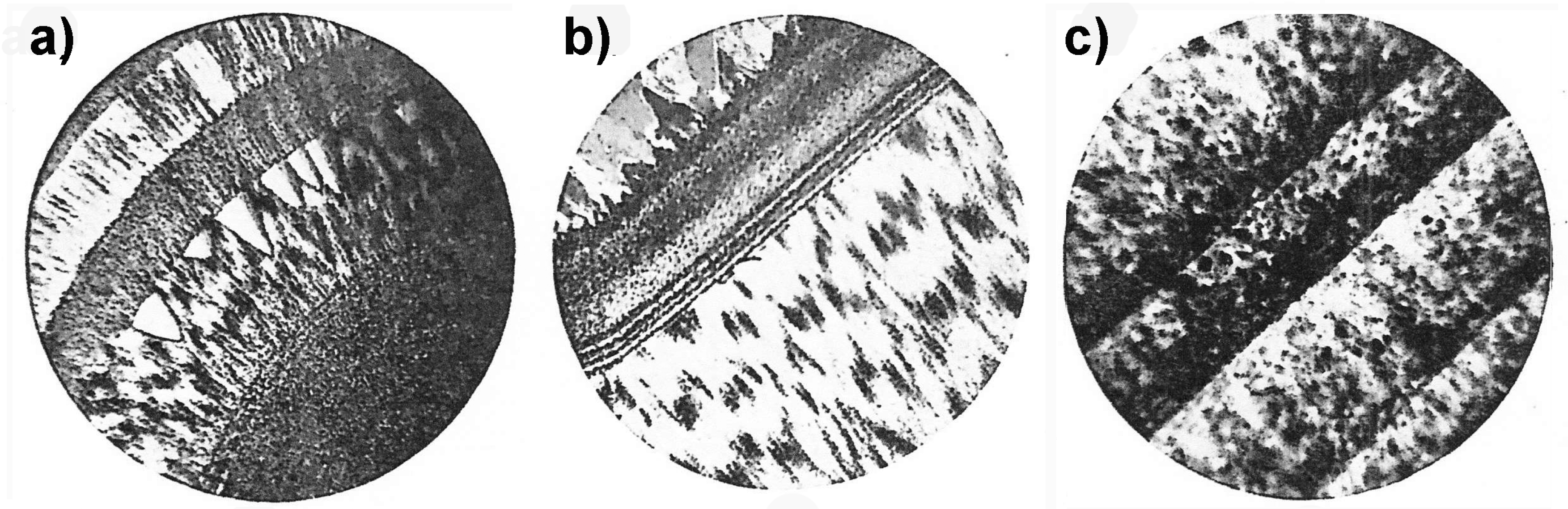
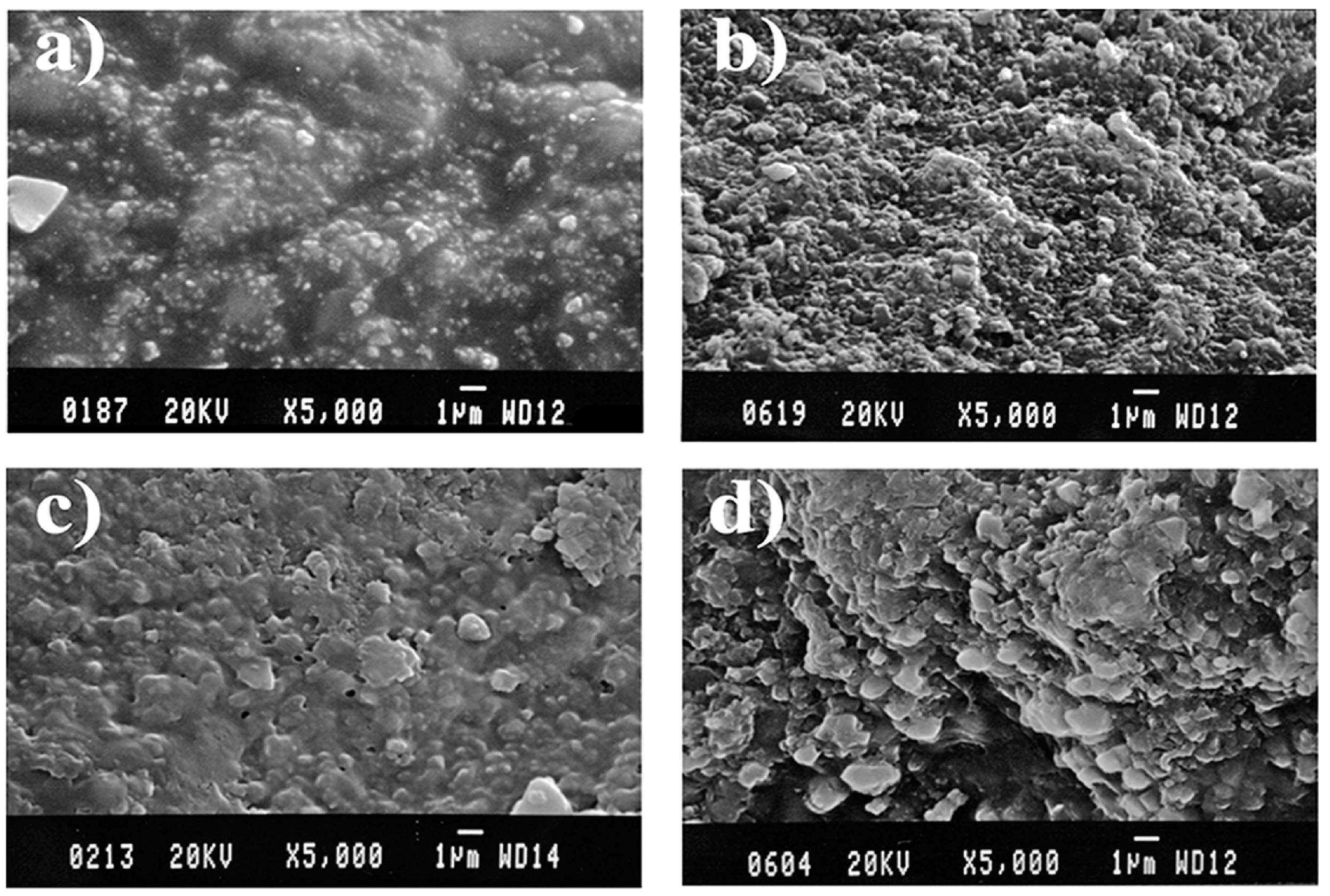
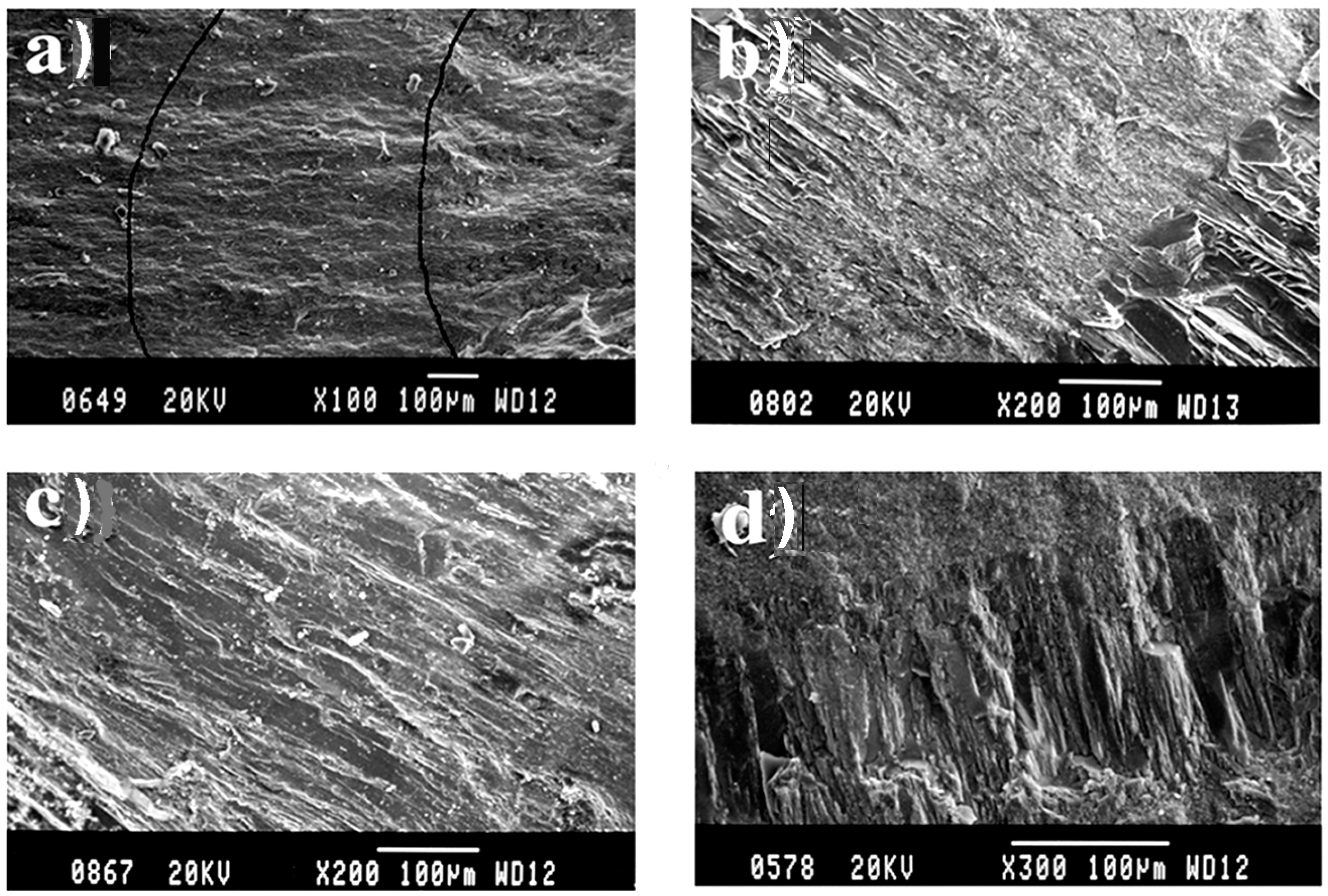
3.9. Agate under the Scanning Electron Microscope
3.10. Hydrocarbon Inclusions in Agate
4. Discussion
- (a)
- What is the formation temperature?
- (b)
- What is the source of the silica?
- (c)
- What is the nature of the silica deposit?
chalcedony/moganite → granular quartz
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collini, C. Tagebuch Einer Reise, Welches Verschiedene Mineralogische Beobachtungen, Besonders über Die Achate und den Basalt Enthält; C.F. Schwan: Mannheim, Germany, 1776; p. 582. [Google Scholar]
- Liesegang, R.E. Die Achate; T. Verlag von Theodor Steinkopff: Dresden/Leipzig, Germany, 1915; p. 122. [Google Scholar]
- Liesegang, R.E. Achat Theorien. Chem Erde 1931, 4, 143–152. [Google Scholar]
- Zenz, J. Fascinating Idar Oberstein in Agates; Bode: Lauenstein, Germany, 2009; pp. 164–185. [Google Scholar]
- Noeggerath, J. Sendschreiben an den K. K. wirklichen Bergrath und Professor Haidinger in Wien, über die Achat-Mandeln in den Melaphyren. Verh. Nat. Ver. Preuss. Rheinl. Westphalens 1849, 6, 243–260. (In German) [Google Scholar]
- Haidinger, W. Versammlungsberichte. In Berichte über die Mittheilungen von Freunden der Naturwissenschaften in Wien; Braumüller u. Seidel: Wien, Germany, 1849; pp. 61–69. (In German) [Google Scholar]
- Heddle, M.F. The Mineralogy of Scotland; David Douglas: Edinburgh, Scotland, 1901; Volume 1, p. 148. [Google Scholar]
- Timofeev, V.M. Chalcedony of Sujsar Island. Proc. Soc. St. Petersburg Nat. 1912, 35, 157–174. (In Russian) [Google Scholar]
- Reis, O.M. Einzelheiten über Bau und Entstehung von Enhydros, Kalzitachat und Achat I. Geognost. Jahresh. 1917, 29, 81–298. (In German) [Google Scholar]
- Reis, O.M. Einzelheiten über Bau und Entstehung von Enhydros, Kalzitachat und Achat II. Geognost. Jahresh. 1918, 31, 1–92. (In German) [Google Scholar]
- Fischer, W. Zum Problem der Achatgenese. N. J. Miner. Abh. 1954, 86, 367–392. (In German) [Google Scholar]
- Jessop, R. Agates and cherts of Derbyshire. Geol. Assoc. 1930, 42, 29–43. [Google Scholar] [CrossRef]
- Pilipenko, P.P. Zur Frage der Achat genese. Bul. Soc. Nat. Mosc. 1934, 12, 279–299, (In German and Russian). [Google Scholar]
- Kuzmin, A.M. Periodic-Rhythmical Phenomena in Mineralogy and Geology; Scientific & Technical Translations: Tomsk, Russia, 2019; p. 336. (In Russian) [Google Scholar]
- Nacken, R. Über die Nachbildung von Chalcedon–Mandeln. Natur Folk 1948, 78, 2–8. [Google Scholar]
- Carr, R.M.; Fyfe, W.S. Some observations on the crystallization of amorphous silica. Am. Mineral. 1958, 43, 908–916. [Google Scholar]
- Keat, P.P. A New Crystalline Silica. Science 1954, 120, 328–330. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Corwin, J.F. Synthesis and origin of chalcedony. Am. Mineral. 1961, 46, 112–119. [Google Scholar]
- Heydemann, A. Untersuchungen über die Bildungsbedingungen von Quarz in Temperaturbereich zwischen 100 °C und 250 °C. Beiträge zur Mineralogie und Petrographie 1964, 10, 242–259. (In German) [Google Scholar] [CrossRef]
- Ernst, W.G.; Calvert, S.E. An experimental study of the recrystallization of porcelanite and its bearing on the origin of some bedded cherts. Am. J. Sci. 1969, 267, 114–133. [Google Scholar]
- Oehler, J.H. Hydrothermal crystallization of silica gel. Geol. Soc. Am. Bul. 1976, 87, 1143–1152. [Google Scholar] [CrossRef]
- Flörke, O.W.; Köhler-Herbetz, B.; Langer, K.; Tönges, I. Water in microcrystalline quartz of volcanic origin: Agates. Contrib. Mineral. Petrol. 1982, 80, 324–333. [Google Scholar] [CrossRef]
- Godovikov, A.A.; Ripinen, O.I.; Motorin, S.G. Agates; Nedra: Moscow, Russia, 1987; p. 368. (In Russian) [Google Scholar]
- Goncharov, V.I.; Gorodinsky, M.E.; Pavlov, G.F.; Savva, N.E.; Fadeev, A.P.; Vartanov, V.V.; Gunchenko, E.V. Chalcedony of North-East. of the USSR; Science: Moscow, Russia, 1987; p. 192. (In Russian) [Google Scholar]
- Landmesser, M. Mobility by metastability: Silica transport and accumulation at low temperatures. Chem. Erde 1995, 55, 149–176. [Google Scholar]
- Landmesser, M. Mobility by metastability in sedimentary and agate petrology: Applications. Chem. Erde 1998, 58, 1–22. [Google Scholar]
- Moxon, T. Agate Microstructure and Possible Origin; Terra Publications: Doncaster, UK, 1996; p. 106. [Google Scholar]
- Götze, J. Agate-fascination between legend and science. In Agates III; Zenz, J., Ed.; Bode-Verlag: Salzhemmendorf, German, 2011; pp. 19–133. [Google Scholar]
- Sorby, H.C. On the microscopical structure of Calcareous Grit of the Yorkshire coast. Quart. J. Geol. Soc. Lond. 1851, 7, 1–6. [Google Scholar] [CrossRef]
- Eberspächer, S.; Lange, J.-M.; Zaun, J.; Kehrer, C.; Heide, G. The Historical Collection of Rock Thin Sections at the Technische Universität Bergakademie Freiberg and Evaluation of Digitization Methods. 2015. Available online: https://www.researchgate.net/publication/273118591e (accessed on 1 September 2020).
- Heinz, H. Die Entstehung Die Entstehung der Achate, ihre Verwitterung und ihre künstliche Färbung. Chem. Erde 1930, 4, 501–525. (In German) [Google Scholar]
- Pabian, R.K.; Zarins, A. Banded Agates—Origins and Inclusions. Educational Circular. No. 12; University of Bebraska: Lincoln, NE, USA, 1994; p. 32. [Google Scholar]
- Belousov, B.P. A periodic reaction and its mechanism. In Sbornik Referatov po Radiatsioanoi Medicine for 1958 Year; Medgiz: Moscow, Russia, 1959; p. 145. (In Russian) [Google Scholar]
- Zhabotinsky, A.M. Periodic liquid phase reactions. Proc. Acad. Sci. USSR 1964, 157, 392–395. (In Russian) [Google Scholar]
- Fallick, A.E.; Jocelyn, J.; Donelly, T.; Guy, M.; Behan, C. Origin of agates in the volcanic rocks of Scotland. Nature 1985, 313, 672–674. [Google Scholar] [CrossRef]
- Harris, C. Oxygen-isotope zonation of agates from Karoo volcanic of the Skeleton Coast, Namibia. Am. Mineral. 1989, 74, 476–481. [Google Scholar]
- Saunders, J.A. Oxygen-isotope zonation of agates from Karoo volcanic of the Skeleton Coast, Namibia: Discussion. Am. Mineral. 1990, 75, 1205–1206. [Google Scholar]
- Götze, J.; Plötze, M.; Tichomirova, M.; Fuchs, H. Aluminium in quartz as an indicator of the temperature of formation of agate. Miner. Mag. 2001, 65, 407–413. [Google Scholar] [CrossRef]
- Yamagishi, H.; Nakashima, S.; Ito, Y. High temperature infrared of hydrous spectra microcrystalline quartz. Phys. Chem. Miner. 1997, 24, 66–74. [Google Scholar] [CrossRef]
- Moxon, T. A re-examination of water in agate and its bearing on the agate genesis enigma. Miner. Mag. 2017, 81, 1223–1244. [Google Scholar] [CrossRef]
- Flörke, O.W.; Jones, J.B.; Schmincke, H.U. A new microcrystalline silica from Gran Canaria. Zeitschrift für Kristallographie-Crystalline Materials 1976, 143, 156–165. [Google Scholar] [CrossRef]
- Heaney, P.J.; Post, J.E. The Widespread Distribution of a Noel Silica Polymorph in Microcrystalline Quartz Varieties. Science 1992, 255, 441–443. [Google Scholar] [CrossRef]
- Zhang, M.; Moxon, T. Infrared absorption spectroscopy of SiO2-moganite. Am. Mineral. 2014, 99, 671–680. [Google Scholar] [CrossRef]
- Götze, J.; Nasdala, L.; Kleeberg, R.; Wenzel, M. Occurrence and distribution of “moganite” in agate/chalcedony: A combined micro-Raman, Rietveld, and cathodoluminescence study. Contrib. Mineral. Petrol. 1998, 133, 96–105. [Google Scholar] [CrossRef]
- Petrovic, I.; Heaney, P.J.; Navrotsky, A. Thermochemistry of the new silica polymorph moganite. Phys. Chem. Miner. 1996, 23, 119–126. [Google Scholar] [CrossRef]
- Krauskopf, K. Dissolution and precipitation of silica at low temperatures. Geochim. Cosmochim. Acta 1956, 10, 1–26. [Google Scholar] [CrossRef]
- Patterson, S.H.; Roberson, C.E. Weathered basalt in the Eastern part of Kauai, Hawaii. Prof. Paper 424 C 219 US Geol. Surv. 1961, 424, 195–198. [Google Scholar]
- Fleischer, R.C.; McIntosh, C.E.; Tarr, C.L. Evolution on a volcanic conveyor belt: Using phylogeographic reconstruction and K-Ar based ages of the Hawaiian Islands to estimate molecular evolutionary rates. Mol. Ecol. 1998, 7, 533–545. [Google Scholar] [CrossRef]
- Götze, J.; Tichomirow, M.; Fuchs, H.; Pilot, J.; Sharp, Z.D. Chemistry of agates: A trace element and stable isotope study. Chem. Geol. 2001, 175, 523–541. [Google Scholar] [CrossRef]
- Moxon, T.; Carpenter, M.A. Crystallite growth kinetics in nanocrystalline quartz (agate and chalcedony). Miner. Mag. 2009, 73, 551–568. [Google Scholar] [CrossRef]
- Jones, H.P.; Handreck, K.A. Effects of iron and aluminium oxides on silica in solution in soils. Nature 1963, 198, 852–853. [Google Scholar] [CrossRef]
- Moxon, T. The co-precipitation of Fe3+ and SiO2 and its role in agate genesis. Neues Jahrb. Fur Mineral. Mon. 1996, 1, 21–36. [Google Scholar]
- File, P.D. JCPDS International Centre for Diffraction Data; ICDD: Newtown Square, PA, USA, 1998. [Google Scholar]
- Neymark, L.A.; Amelin, Y.; Paces, J.B.; Peterman, Z.E. U-Pb ages of secondary silica at Yucca Mountain, Nevada: Implications for the paleohydrology of the unsaturated zone. Appl. Geochem. 2002, 17, 709–734. [Google Scholar] [CrossRef]
- Moxon, T.; Nelson, D.R.; Zhang, M. Agate recrystallisation: Evidence found in the Archaen and Proterozoic host rocks, Western Australia. Aust. J. Earth Sci. 2006, 53, 235–248. [Google Scholar] [CrossRef]
- Moxon, T.; Reed, S.J.B.; Zhang, M. Metamorphic effects on agate found near the Shap granite, Cumbria: As demonstrated by petrography, X-ray diffraction spectroscopic methods. Miner. Mag. 2007, 71, 461–476. [Google Scholar] [CrossRef]
- Moxon, T.; Reed, S.J.B. Agate and chalcedony from igneous and sedimentary hosts aged from 13 to 3480 Ma: A cathodoluminescence study. Miner. Mag. 2006, 70, 485–498. [Google Scholar] [CrossRef]
- Walger, E.; Matthess, G.; von Seckendorf, V.; Liebau, F. The formation of agate structure for silica transport, agate layer accretion, and flow atterns and models flow regimes in infiltration canals. N. Jb. für Geologie und Palāontologie Abh. 2009, 186, 113–152. [Google Scholar]
- Smith, J. Semi-Precious Stones of Carrick; Kilwining: Spean Bridge, UK, 1910; p. 84. [Google Scholar]
- Wang, Y.; Merino, E. Self-organisational origin of agates: Banding fiber twisting, composition and dynamic crystallization model. Geochim. Cosmochim. Acta 1990, 54, 1627–1638. [Google Scholar] [CrossRef]
- Dumeńska-Slowik, M.; Natkaniec-Nowak, L.; Kotarba, M.J.; Sikorska, M.; Rzymełka, J.A.; Łoboda, A.; Gaweł, A. Mineralogical and geochemical characterization of the “bituminous” agates from Nowy Kościol Lower Silesia. J. Mineral. Geochem. 2008, 184, 255–268. [Google Scholar]
- Götze, J.; Möckel, R.; Kempe, U.; Kapitonov, I.; Vennemann, T. Characteristics and origin of agates in sedimentary rocks from the Dryhead area, Montana, USA. Miner. Mag. 2009, 73, 673–690. [Google Scholar] [CrossRef]
- Commin-Fischer, A.; Berger, G.; Polve, M.; Dubois, M.; Sardini, P.; Beaufort, D.; Formoso, M. Petrography and chemistry of SiO2 filling phases in amethyst geodes from Sierra Geral Formation deposit, Rio Grande do Sul, Brazil. J. Am. Earth Sci. 2010, 29, 751–760. [Google Scholar] [CrossRef]
- Moxon, T.; Petrone, C.M.; Reed, S.J.B. Characterisation and genesis of horizontal banding in Brazilian agate: An X-ray diffraction, thermogravimetric and microprobe study. Miner. Mag. 2013, 77, 227–248. [Google Scholar] [CrossRef]
- Lange, P.; Blankenburg, H.-J.; Schron, W. Rasterelectronmikroscopische Untersuchgen an Vulkanachaten. Z. Geol. Wiss. 1984, 12, 669–683. (In German) [Google Scholar]
- Holzhey, G. Mikrokristalline SiO2 Minerallisation in rhyolithischen. Rotliegendvulkaniten des Thüringer Waldes (Deutschland) und ihre Genese. Chem. Erde 1999, 59, 183–205. (In German) [Google Scholar]
- Fukuda, J.; Yokoyama, T.; Kirino, Y. Characterization of the states and diffusivity of intergranular water in a chalcedonic quartz by high temperature situ infrared spectroscopy. Miner. Mag. 2009, 73, 825–835. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R.; Vennemann, T.; Muller, A. Origin and geochemistry of agates in Permian volcanic rocks of the Sub-Erzgebirge basin, Saxony (Germany). Chem. Geol. 2016, 428, 77–91. [Google Scholar] [CrossRef]
- Moxon, T. Agate: A study of ageing. Eur. J. Mineral. 2002, 14, 1109–1118. [Google Scholar] [CrossRef]
- Barsanov, G.P.; Yakovleva, M.E. Mineralogy, macro- and micromorphological features of agates. New Data Miner. 1982, 30, 3–26. (In Russian) [Google Scholar]
- Silaev, V.I.; Shanina, S.N.; Ivanovskii, V.S. Inclusions of oil gases in agate-type secretions: Implications for forecast of the oil- and gas-bearing potential of the Polar Urals. Dokl. Earth Sci. 2002, 383, 246–252. (In Russian) [Google Scholar]
- Whelan, J.B.; Paces, J.B.; Peterman, Z.E. Physical and stable-isotope evidence for the formation of secondary calcite in the unsaturated zone, Yucca Mountain, Nevada. Appl. Geochem. 2002, 17, 735–750. [Google Scholar] [CrossRef]
- Harder, H.; Flehmig, W. Quarzsynthese bei tiefen temperature. Geochim. Cosmochim. Acta 1970, 34, 295–305. [Google Scholar] [CrossRef]
- Mackenzie, F.T.; Gees, R. Quartz synthesis at earth surface conditions. Science 1971, 173, 533–535. [Google Scholar] [CrossRef]
- Heaney, P.J. A proposed mechanism for the growth of chalcedony. Contrib. Mineral. Petrol. 1993, 115, 66–74. [Google Scholar] [CrossRef]
- Naboko, S.L.; Silnichenko. The formation of silica gel on the solfataras of the Golovin volcano on Kunashir Island. Geochemistry 1957, 3, 253–256. (In Russian) [Google Scholar]
- Meixiang, Z.; Wei, T. Surface hydrothermal minerals and their distribution in the Tengchon geothermal area, China. Geothermics 1987, 16, 181–195. [Google Scholar] [CrossRef]
- Marcoux, E.; Le Berre, P.; Cocherie, A. The Meillers Autunian hydrothermal chalcedony: First evidence of a 295 Ma auriferous epithermal sinter in the French Massif Central. Ore Geol. Rev. 2004, 25, 69–87. [Google Scholar] [CrossRef]
- Knauth, L.P. Petrogenesis of chert. Rev. Mineral. 1994, 29, 233–258. [Google Scholar]
- Rodgers, K.A.; Browne, P.R.L.; Buddle, T.F.; Cook, K.L.; Greatrez, R.A.; Hampton, W.A.; Herdianita, N.R.; Holland, G.R.; Lynne, B.Y.; Martin, R.; et al. Silica phases in sinters and residues from geothermal fields of New Zealand. Earth Sci. Rev. 2004, 66, 1–61. [Google Scholar] [CrossRef]
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, K.A. Mineralogical and textural changes accompanying ageing of silica sinter. Miner. Depos. 2000, 35, 48–62. [Google Scholar] [CrossRef]
- Lynne, B.Y.; Campbell, K.A.; Perry, R.S.; Browne, P.R.L.; Moore, J.N. Acceleration of sinter diagenesis in an active fumarole, Taupo volcanic zone, New Zealand. Geology 2006, 34, 749–752. [Google Scholar] [CrossRef]
- Rodgers, K.A.; Cressey, G. The occurrence, detection and significance of moganite (SiO2) among some silica sinters. Miner. Mag. 2001, 65, 157–167. [Google Scholar] [CrossRef]
- Heaney, P.J. Moganite as an indicator for vanished evaporites: A testament. J. Sediment. Res. 1995, 65, 633–638. [Google Scholar]
- Hesse, R. Origin of chert: Diagenesis of biogenic siliceous sediments. Geosci. Can. 1988, 15, 171–192. [Google Scholar]
- Gilg, H.A.; Morteani, G.; Kostitsyn, Y.; Preinfalk, C.; Gatter, I.; Streider, A.J. Genesis of amethyst geodes in basaltic rocks of the Serra Geral formation (Ametista do Sul, Rio Grande do Sul, Brazil): A fluid inclusion, REE, oxygen, carbon and Sr isotope study on basalt, quartz and calcite. Miner. Depos. 2003, 38, 1009–1025. [Google Scholar] [CrossRef]




| Analysis of Rock Samples | Water Analysis from the Rock | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hole | Depth | SiO2 | Fe2O3 | Al2O3 | MgO | Na2O | H2O | SiO2 | Fe3+ | Al3+ | Mg2+ | Na+ | pH |
| No | m | (wt%) | (ppm) | ||||||||||
| 1 | 0.6 | 5.7 | 40.2 | 25.6 | 0.65 | 0.04 | 18.6 | 2.9 | 0.00 | 0.11 | 1.9 | 11 | 4.6 |
| 6 | 9.5 | 21.5 | 30.7 | 25.1 | 1.0 | 0.06 | 14.6 | 2.5 | 0.00 | 0.12 | 1.5 | 8.5 | 4.7 |
| 8 | ⁻ | 45.0 | 4.3 | 11.8 | 12.0 | 3.1 | 3.1 | 42 | 0.00 | 0.06 | 11 | 21 | 7.6 |
| Agate Source | Country | Host Rock Type | Age of Host * (Ma) | Fe3+ | Al3+ (ppm) | Mg2+ | Na+ |
|---|---|---|---|---|---|---|---|
| Chihuahua | Mexico | Andesite Agate | 38 - | 6695 215 ** | nd 925 ** | 5460 151 ** | 25,000 320 ** |
| Rio Grande do Sol | Brazil | Basalt Agate | 135 - | 14,700 137 | nd 54 | nd 10 | 19,000 140 |
| Montrose | Scotland | Andesite Agate | 412 - | 33,400 70 | nd 456 | nd 15 | 23,000 230 |
| Ardownie Quarry | Scotland | Andesite Agate | 412 - | 37,300 36 | nd 232 | nd 18 | 27,000 210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moxon, T.; Palyanova, G. Agate Genesis: A Continuing Enigma. Minerals 2020, 10, 953. https://doi.org/10.3390/min10110953
Moxon T, Palyanova G. Agate Genesis: A Continuing Enigma. Minerals. 2020; 10(11):953. https://doi.org/10.3390/min10110953
Chicago/Turabian StyleMoxon, Terry, and Galina Palyanova. 2020. "Agate Genesis: A Continuing Enigma" Minerals 10, no. 11: 953. https://doi.org/10.3390/min10110953
APA StyleMoxon, T., & Palyanova, G. (2020). Agate Genesis: A Continuing Enigma. Minerals, 10(11), 953. https://doi.org/10.3390/min10110953





