A Flow-Through Reaction Cell for Studying Minerals Leaching by In-Situ Synchrotron Powder X-ray Diffraction
Abstract
:1. Introduction
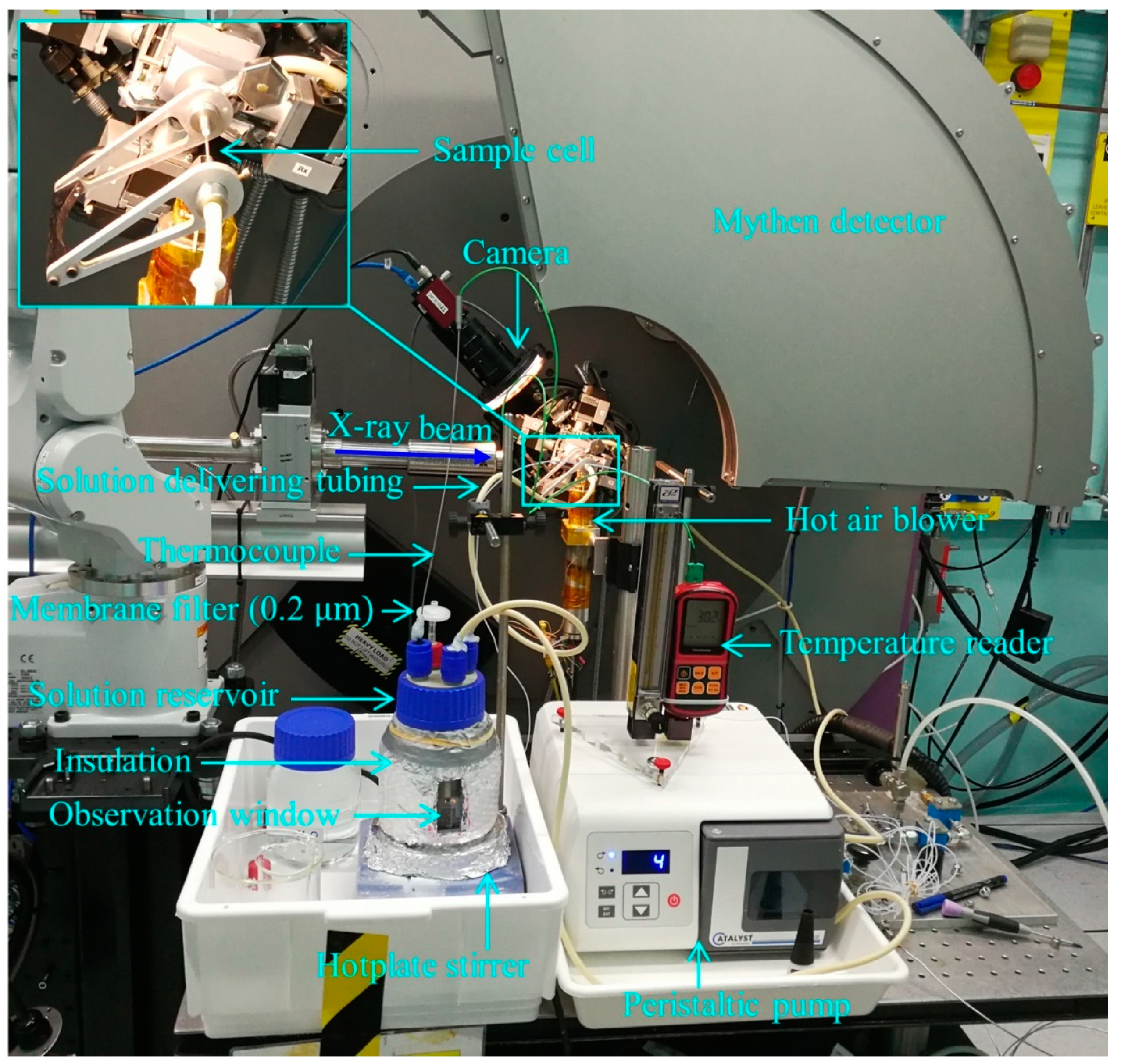
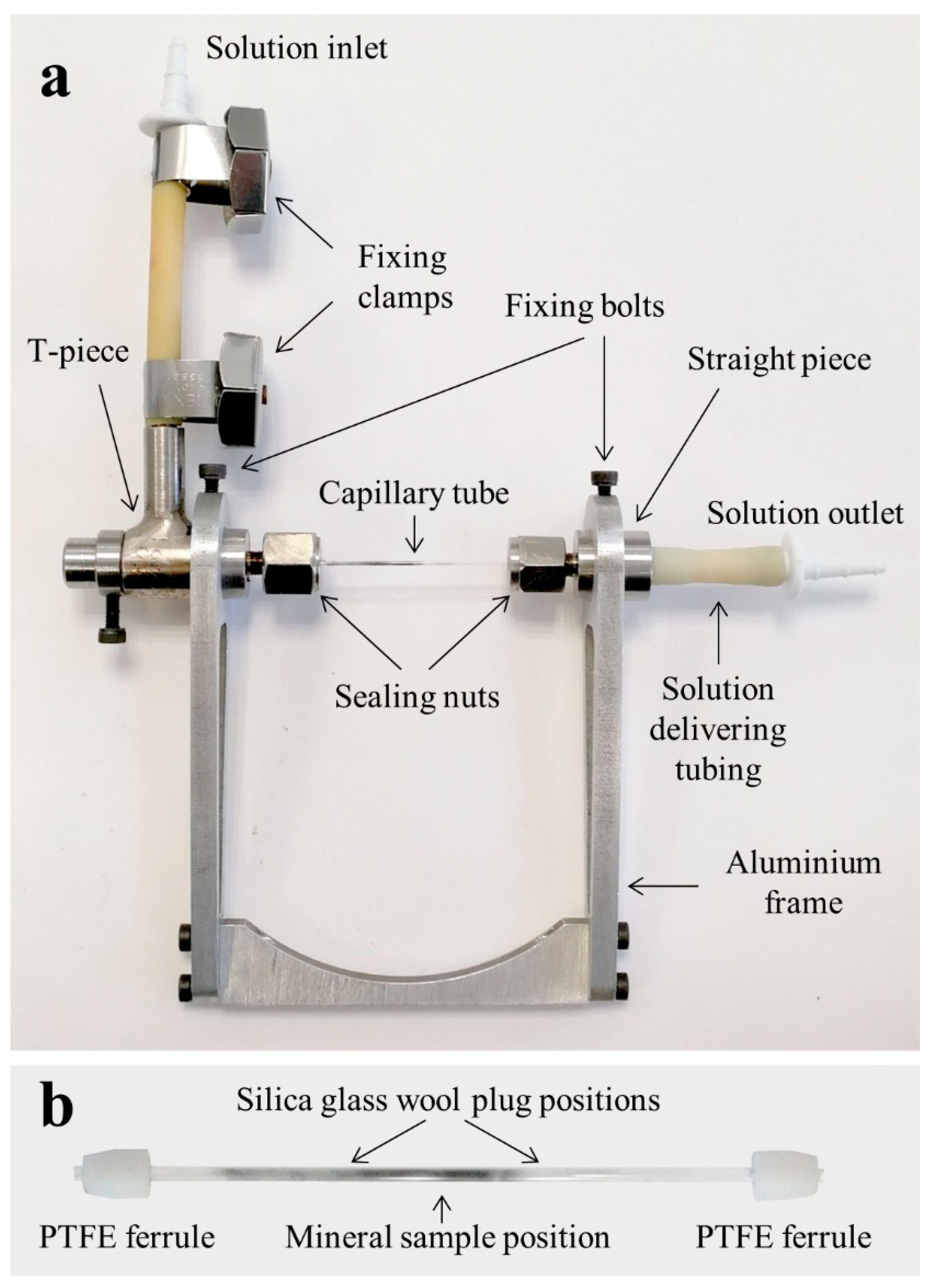
2. The Flow-Through Cell Setup
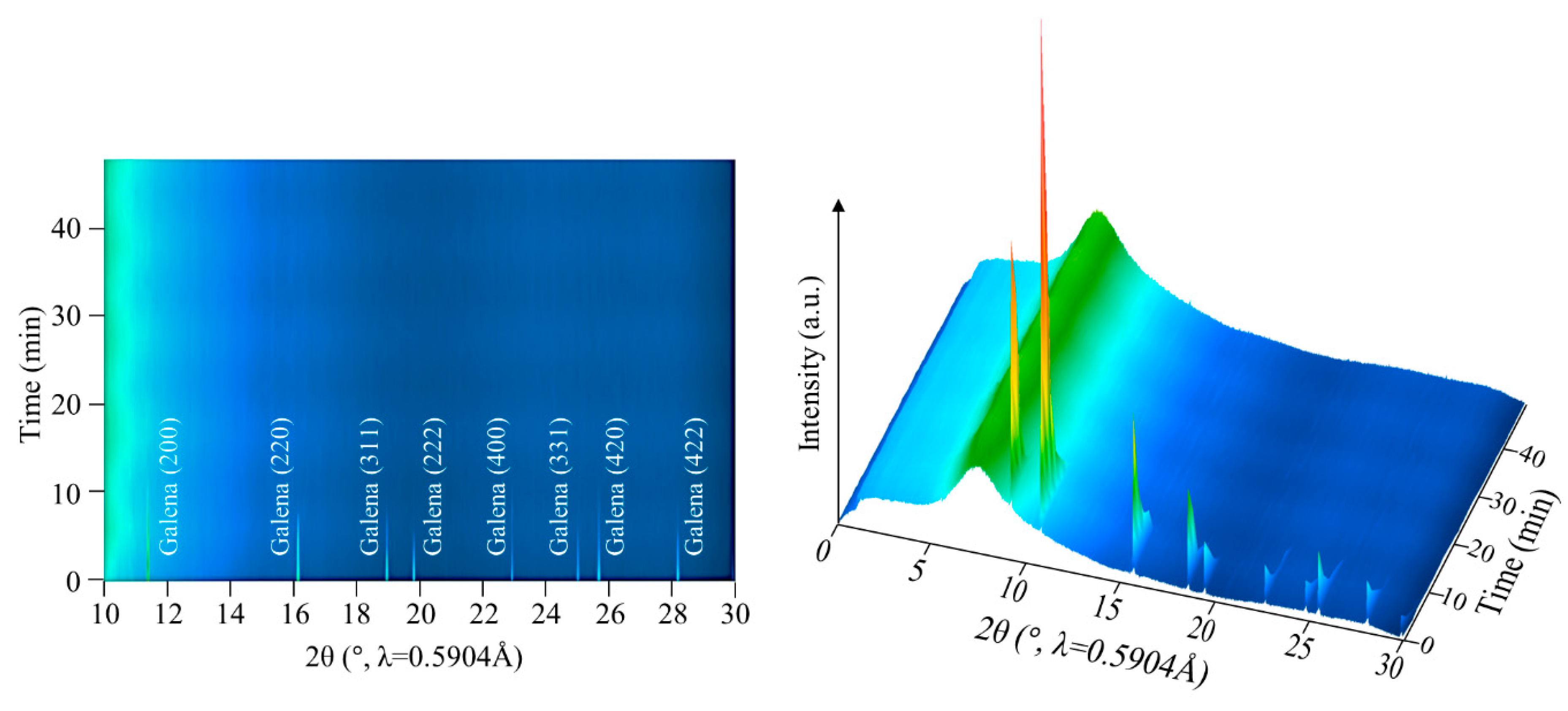
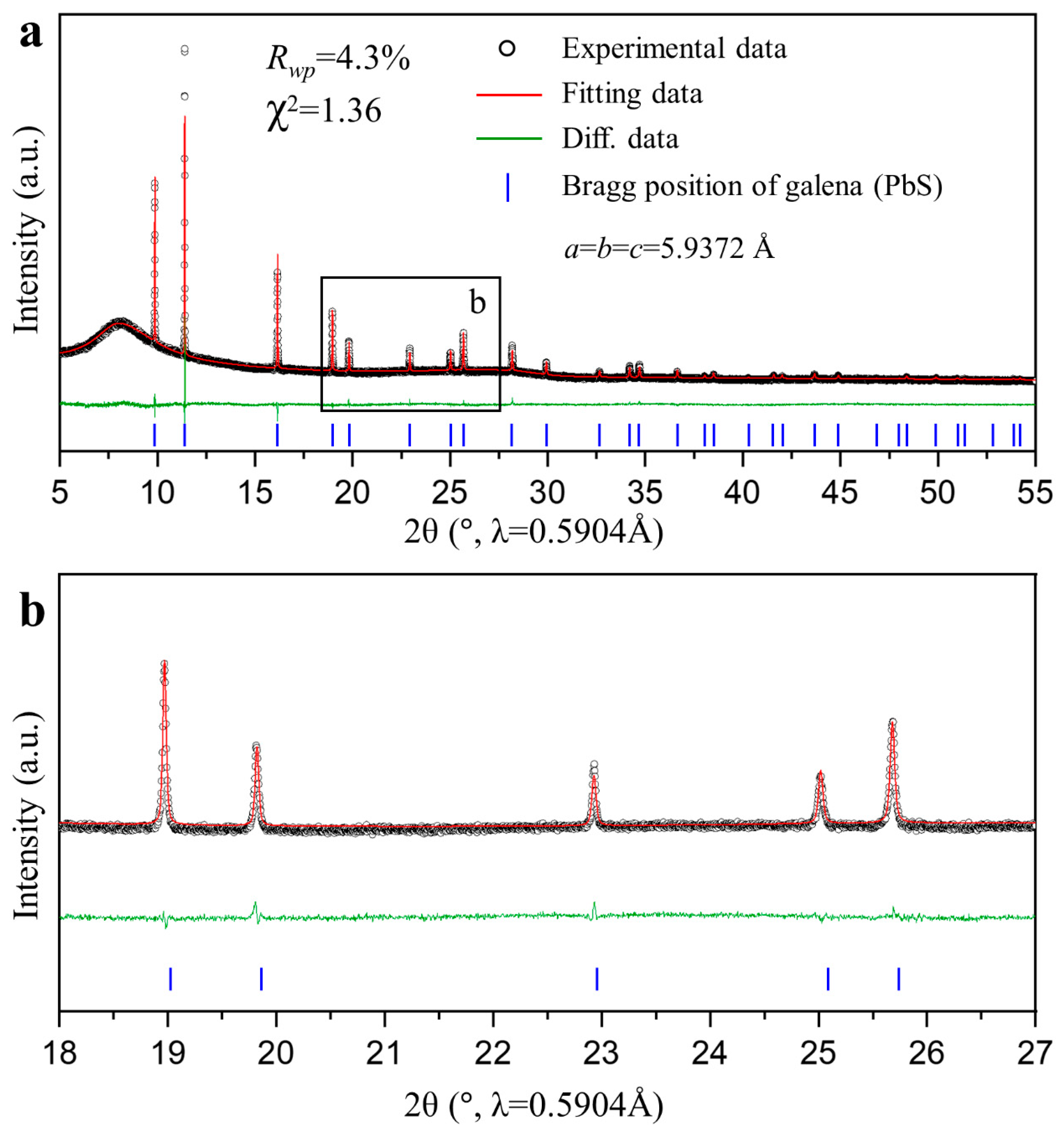
3. In-Situ PXRD of Galena Leaching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruiz, M.; Vera, M.; Padilla, R. Mechanism of enargite pressure leaching in the presence of pyrite. Hydrometallurgy 2011, 105, 290–295. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, F.; Pring, A.; Brugger, J.; Grundler, P.V.; Chen, G. A novel pre-treatment of calaverite by hydrothermal mineral replacement reactions. Miner. Eng. 2010, 23, 451–453. [Google Scholar] [CrossRef]
- Nikkhou, F.; Xia, F.; Deditius, A.P. Variable surface passivation during direct leaching of sphalerite by ferric sulfate, ferric chloride, and ferric nitrate in a citrate medium. Hydrometallurgy 2019, 188, 201–215. [Google Scholar] [CrossRef]
- Kartal, M.; Xia, F.; Ralph, D.; Rickard, W.D.; Renard, F.; Li, W. Enhancing chalcopyrite leaching by tetrachloroethylene-assisted removal of sulphur passivation and the mechanism of jarosite formation. Hydrometallurgy 2019, 191, 105192. [Google Scholar] [CrossRef]
- Dutrizac, J. The dissolution of galena in ferric chloride media. Metall. Trans. B 1986, 17, 5–17. [Google Scholar] [CrossRef]
- Nikkhou, F.; Xia, F.; Deditius, A.P.; Yao, X. Formation mechanisms of surface passivating phases and their impact on the kinetics of galena leaching in ferric chloride, ferric perchlorate, and ferric nitrate solutions. Hydrometallurgy 2020, 197, 105468. [Google Scholar] [CrossRef]
- Zárate-Gutiérrez, R.; Lapidus, G.; Morales, R. Pressure leaching of a lead–zinc–silver concentrate with nitric acid at moderate temperatures between 130 and 170 °C. Hydrometallurgy 2010, 104, 8–13. [Google Scholar] [CrossRef]
- Webster, N.A.; Madsen, I.C.; Loan, M.J.; Scarlett, N.V.; Wallwork, K.S. A flow cell for in situ synchrotron x-ray diffraction studies of scale formation under Bayer processing conditions. Rev. Sci. Instrum. 2009, 80, 084102. [Google Scholar] [CrossRef]
- Scarlett, N.V.; Grey, I.E.; Brand, H.E. In situ synchrotron diffraction studies on the formation kinetics of jarosites. J. Synchrotron Radiat. 2013, 20, 366–375. [Google Scholar] [CrossRef]
- Majuste, D.; Ciminelli, V.; Eng, P.; Osseo-Asare, K. Applications of in situ synchrotron XRD in hydrometallurgy: Literature review and investigation of chalcopyrite dissolution. Hydrometallurgy 2013, 131, 54–66. [Google Scholar] [CrossRef]
- Madsen, I.C.; Scarlett, N.V.; Whittington, B.I. Pressure acid leaching of nickel laterite ores: An in situ diffraction study of the mechanism and rate of reaction. J. Appl. Crystallogr. 2005, 38, 927–933. [Google Scholar] [CrossRef]
- Testemale, D.; Argoud, R.; Geaymond, O.; Hazemann, J.-L. High pressure/high temperature cell for x-ray absorption and scattering techniques. Rev. Sci. Instrum. 2005, 76, 043905. [Google Scholar] [CrossRef]
- Geisler, T.; Dohmen, L.; Lenting, C.; Fritzsche, M.B. Real-time in situ observations of reaction and transport phenomena during silicate glass corrosion by fluid-cell Raman spectroscopy. Nat. Mater. 2019, 18, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Chen, C.-Y.; Wu, W.-W.; Hong, J.-I.; Yun, B.K.; Zhou, Y.; Lee, N.; Jo, W.; Chen, L.-J.; Chou, L.-J. In situ observation of dehydration-induced phase transformation from Na2Nb2O6–H2O to NaNbO3. J. Phys. Chem. C 2012, 116, 22261–22265. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Vaughan, J.; Gillespie, A. In situ AFM investigation of gibbsite growth in high ionic strength, highly alkaline, aqueous media. Hydrometallurgy 2016, 161, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Xia, F.; Brugger, J.; Qian, G.; Ngothai, Y.; O’Neill, B.; Zhao, J.; Pullen, S.; Olsen, S.; Pring, A. Single-pass flow-through reaction cell for high-temperature and high-pressure in situ neutron diffraction studies of hydrothermal crystallization processes. J. Appl. Cryst. 2012, 45, 166–173. [Google Scholar] [CrossRef]
- Xia, F.; Qian, G.; Brugger, J.; Studer, A.; Olsen, S.; Pring, A. A large volume cell for in situ neutron diffraction studies of hydrothermal crystallizations. Rev. Sci. Instrum. 2010, 81, 105107. [Google Scholar] [CrossRef]
- Xia, F.; O’Neill, B.; Ngothai, Y.; Peak, J.; Tenailleau, C.; Etschmann, B.; Qian, G.; Brugger, J.; Studer, A.; Olsen, S. A thermosyphon-driven hydrothermal flow-through cell for in situ and time-resolved neutron diffraction studies. J. Appl. Cryst. 2010, 43, 511–519. [Google Scholar] [CrossRef]
- Norby, P. In-situ XRD as a tool to understanding zeolite crystallization. Curr. Opin. Colloid Interface Sci. 2006, 11, 118–125. [Google Scholar] [CrossRef]
- Chupas, P.J.; Chapman, K.W.; Kurtz, C.; Hanson, J.C.; Lee, P.L.; Grey, C.P. A versatile sample-environment cell for non-ambient X-ray scattering experiments. J. Appl. Crystallogr. 2008, 41, 822–824. [Google Scholar] [CrossRef]
- Bösenberg, U.; Pistidda, C.; Tolkiehn, M.; Busch, N.; Saldan, I.; Suarez-Alcantara, K.; Arendarska, A.; Klassen, T.; Dornheim, M. Characterization of metal hydrides by in-situ XRD. Int. J. Hydrog. Energy 2014, 39, 9899–9903. [Google Scholar] [CrossRef] [Green Version]
- Clausen, B.S.; Steffensen, G.; Fabius, B.; Villadsen, J.; Feidenhans, R.; Topsøe, H. In situ cell for combined XRD and on-line catalysis tests: Studies of Cu-based water gas shift and methanol catalysts. J. Catal. 1991, 132, 524–535. [Google Scholar] [CrossRef]
- Xia, F.; Chen, D.; Scarlett, N.V.; Madsen, I.C.; Lau, D.; Leoni, M.; Ilavsky, J.; Brand, H.E.; Caruso, R.A. Understanding solvothermal crystallization of mesoporous anatase beads by in situ synchrotron PXRD and SAXS. Chem. Mater. 2014, 26, 4563–4571. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, F.; Song, Z.; Webster, N.A.; Luo, H.; Gao, Y. Synthesis and formation mechanism of VO2(A) nanoplates with intrinsic peroxidase-like activity. RSC Adv. 2015, 5, 61371–61379. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Yuan, J.; Xia, F.; Liu, J.; Zhang, Y.; Zhong, Y.L.; Zheng, J.; Liu, Y.; Li, S.; Zhao, M. Large-scale production of bismuth chalcogenide and graphene heterostructure and its application for flexible broadband photodetector. Adv. Electron. Mater. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Rodriguez, E.F.; Xia, F.; Chen, D.; Hollenkamp, A.F.; Caruso, R.A. N-doped Li₄Ti₅O₁₂ nanoflakes derived from 2D protonated titanate for high performing anodes in lithium ion batteries. J. Mater. Chem. A 2016, 4, 7772–7780. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.Z.; Nursam, N.M.; Xia, F.; Sani, M.-A.; Li, W.; Wang, X.; Caruso, R.A. High-performance coral reef-like carbon nitrides: Synthesis and application in photocatalysis and heavy metal ion adsorption. ACS Appl. Mater. Interfaces 2017, 9, 4540–4547. [Google Scholar] [CrossRef]
- Mi, J.-L.; Nørby, P.; Bremholm, M.; Iversen, B.B. Insight into nucleation and growth of Bi2−xSbxTe3 (x = 0−2) nanoplatelets in hydrothermal synthesis. Chem. Mater. 2017, 29, 5070–5079. [Google Scholar] [CrossRef] [Green Version]
- De Marco, R.; Pejcic, B.; Prince, K.; Van Riessen, A. A multi-technique surface study of the mercury (II) chalcogenide ion-selective electrode in saline media. Analyst 2003, 128, 742–749. [Google Scholar] [CrossRef]
- Ferrer, P.; da Silva, I.; Rubio-Zuazo, J.; Alfonso, B.F.; Trobajo, C.; Khainakov, S.; Garcia, J.R.; Garcia-Granda, S.; Castro, G.R. A flow-through reaction cell for in situ X-ray diffraction and absorption studies of heterogeneous powder–liquid reactions and phase transformations. J. Synchrotron Radiat. 2012, 19, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Wall, A.J.; Heaney, P.J.; Mathur, R.; Post, J.E.; Hanson, J.C.; Eng, P.J. A flow-through reaction cell that couples time-resolved X-ray diffraction with stable isotope analysis. J. Appl. Cryst. 2011, 44, 429–432. [Google Scholar] [CrossRef]
- Kautek, W.; Mirwald, S.; Sahre, M.; Nauer, G.E. In-situ grazing incidence X-ray diffractometry of polycrystalline copper in alkaline chloride and sulphate electrolytes. Electrochim. Acta 1998, 43, 2979–2984. [Google Scholar] [CrossRef]
- Nagy, Z.; You, H.; Yonco, R. Cell design for in situ x-ray scattering studies of metal/solution interfaces under electrochemical control. Rev. Sci. Instrum. 1994, 65, 2199–2205. [Google Scholar] [CrossRef]
- De Marco, R.; Jiang, Z.-T.; Pejcic, B.; Van Riessen, A. In situ synchrotron radiation grazing incidence X-ray diffraction—A powerful technique for the characterization of solid-state ion-selective electrode surfaces. Electrochim. Acta 2006, 51, 4886–4891. [Google Scholar] [CrossRef]
- Clancy, M.; Styles, M.J.; Bettles, C.J.; Birbilis, N.; Chen, M.; Zhang, Y.; Gu, Q.; Kimpton, J.A.; Webster, N.A. In situ synchrotron X-ray diffraction investigation of the evolution of a PbO2/PbSO4 surface layer on a copper electrowinning Pb anode in a novel electrochemical flow cell. J. Synchrotron Radiat. 2015, 22, 366–375. [Google Scholar] [CrossRef]
- Wall, A.J.; Mathur, R.; Post, J.E.; Heaney, P.J. Cu isotope fractionation during bornite dissolution: An in situ X-ray diffraction analysis. Ore Geol. Rev. 2011, 42, 62–70. [Google Scholar] [CrossRef]
- Fleeger, C.R.; Heaney, P.J.; Post, J.E. A time-resolved X-ray diffraction study of Cs exchange into hexagonal H-birnessite. Am. Mineral. 2013, 98, 671–679. [Google Scholar] [CrossRef]
- Nikkhou, F.; Xia, F.; Knorsch, M.; Deditius, A.P. Mechanisms of surface passivation during galena leaching by hydrogen peroxide in acetate and citrate solutions at 25–50 °C. ACS Sustain. Chem. Eng. 2020, 8, 14407–14416. [Google Scholar] [CrossRef]
- Margulieux, G.W.; Weidemann, N.; Lacy, D.C.; Moore, C.E.; Rheingold, A.L.; Figueroa, J.S. Isocyano analogues of [Co(CO)4]n: A tetraisocyanide of cobalt isolated in three states of charge. J. Am. Chem. Soc. 2010, 132, 5033–5035. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Mineral | Galena (PbS) |
| Particle size | <38 μm |
| Lixiviant | 0.08 M citric acid + 0.92 M trisodium citrate + 0.75 M H2O2 |
| Solution pH | 6 |
| Flow rate | 0.5 mL min−1 |
| Temperature | 35 °C |
| Leaching time | 48 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikkhou, F.; Xia, F.; Yao, X.; Adegoke, I.A.; Gu, Q.; Kimpton, J.A. A Flow-Through Reaction Cell for Studying Minerals Leaching by In-Situ Synchrotron Powder X-ray Diffraction. Minerals 2020, 10, 990. https://doi.org/10.3390/min10110990
Nikkhou F, Xia F, Yao X, Adegoke IA, Gu Q, Kimpton JA. A Flow-Through Reaction Cell for Studying Minerals Leaching by In-Situ Synchrotron Powder X-ray Diffraction. Minerals. 2020; 10(11):990. https://doi.org/10.3390/min10110990
Chicago/Turabian StyleNikkhou, Fatemeh, Fang Xia, Xizhi Yao, Idowu A. Adegoke, Qinfen Gu, and Justin A. Kimpton. 2020. "A Flow-Through Reaction Cell for Studying Minerals Leaching by In-Situ Synchrotron Powder X-ray Diffraction" Minerals 10, no. 11: 990. https://doi.org/10.3390/min10110990






