Effects of Potassium Propyl Xanthate Collector and Sodium Sulfite Depressant on the Floatability of Chalcopyrite in Seawater and KCl Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Microflotation in Saline Solutions
2.2. Electrokinetics of Chalcopyrite
3. Results and Discussions
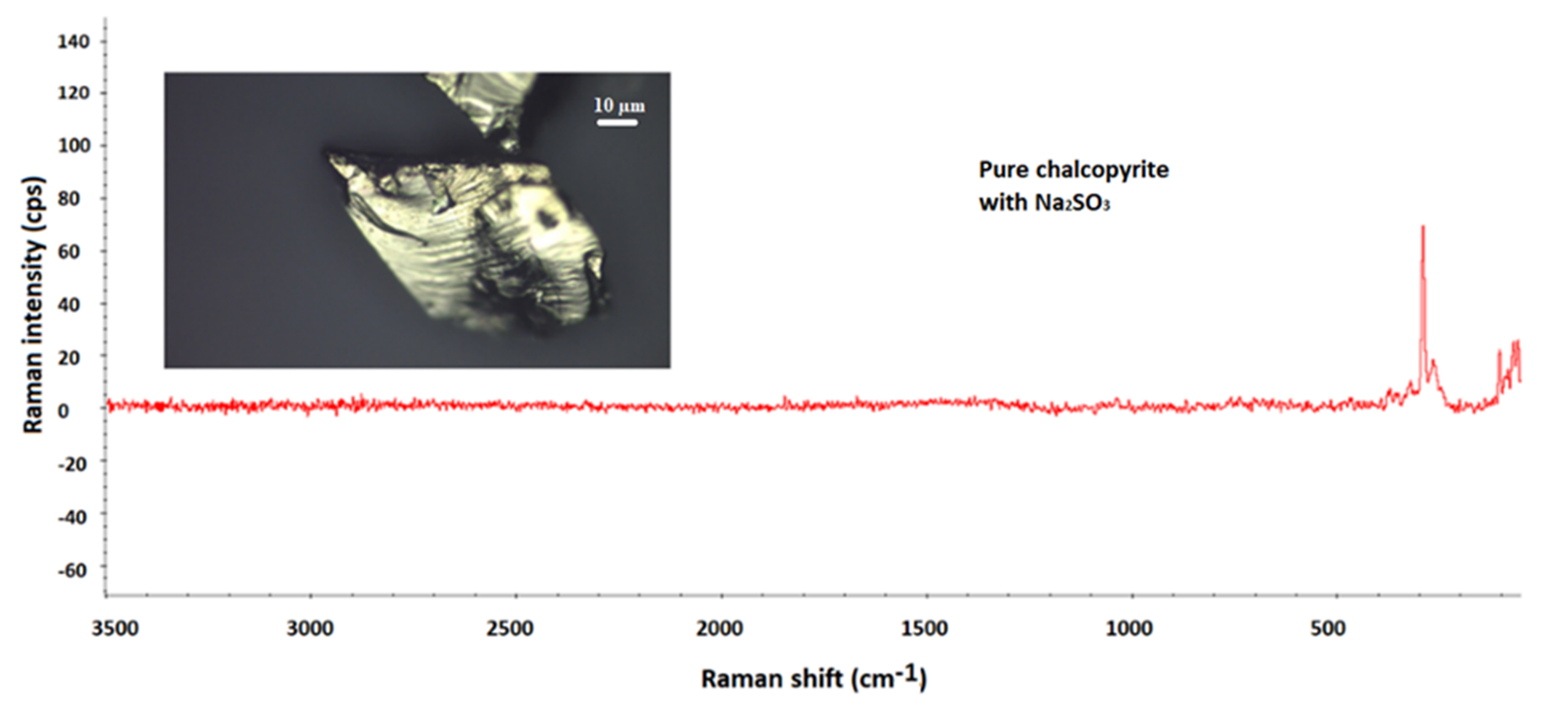
3.1. Mineralogical Characterization of Chalcopyrite by SEM and Raman
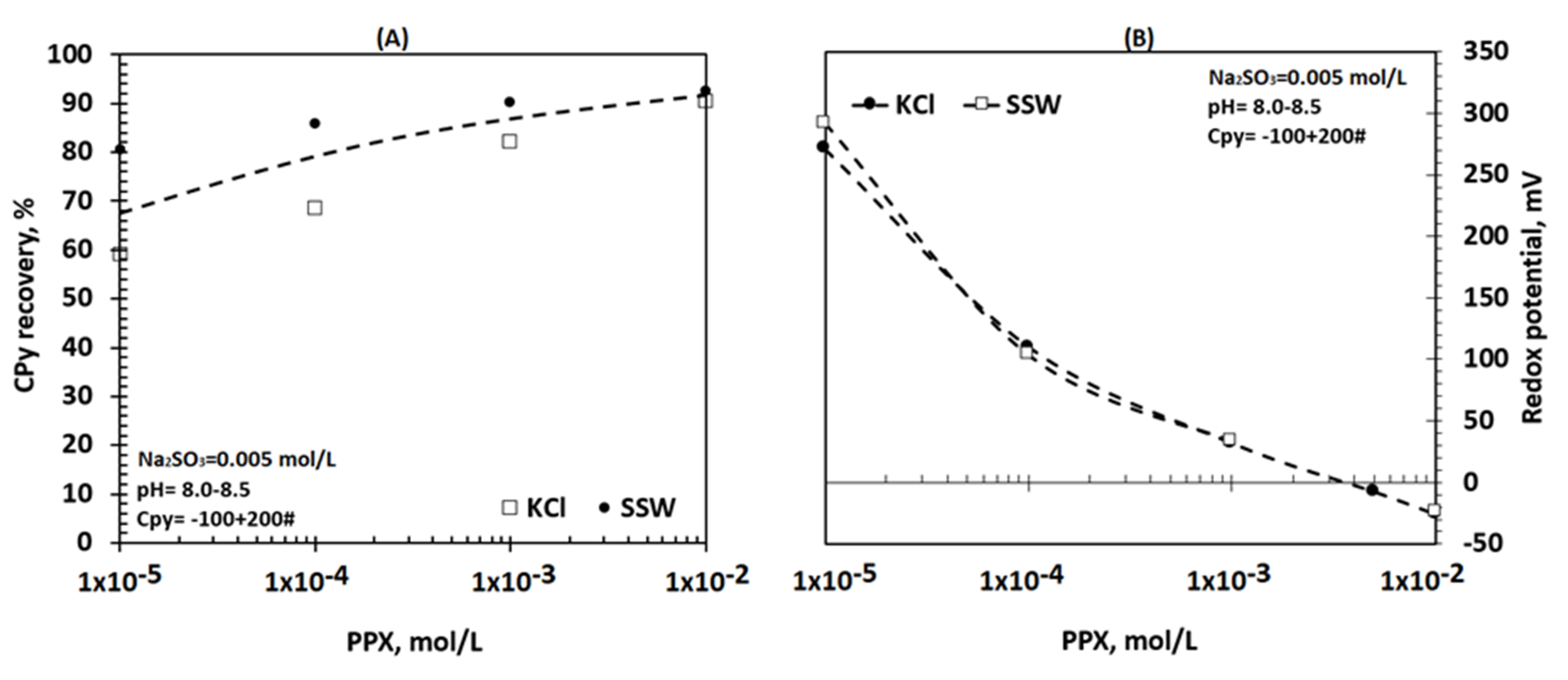
3.2. Effects of Salinity on Chalcopyrite Flotation Using PPX
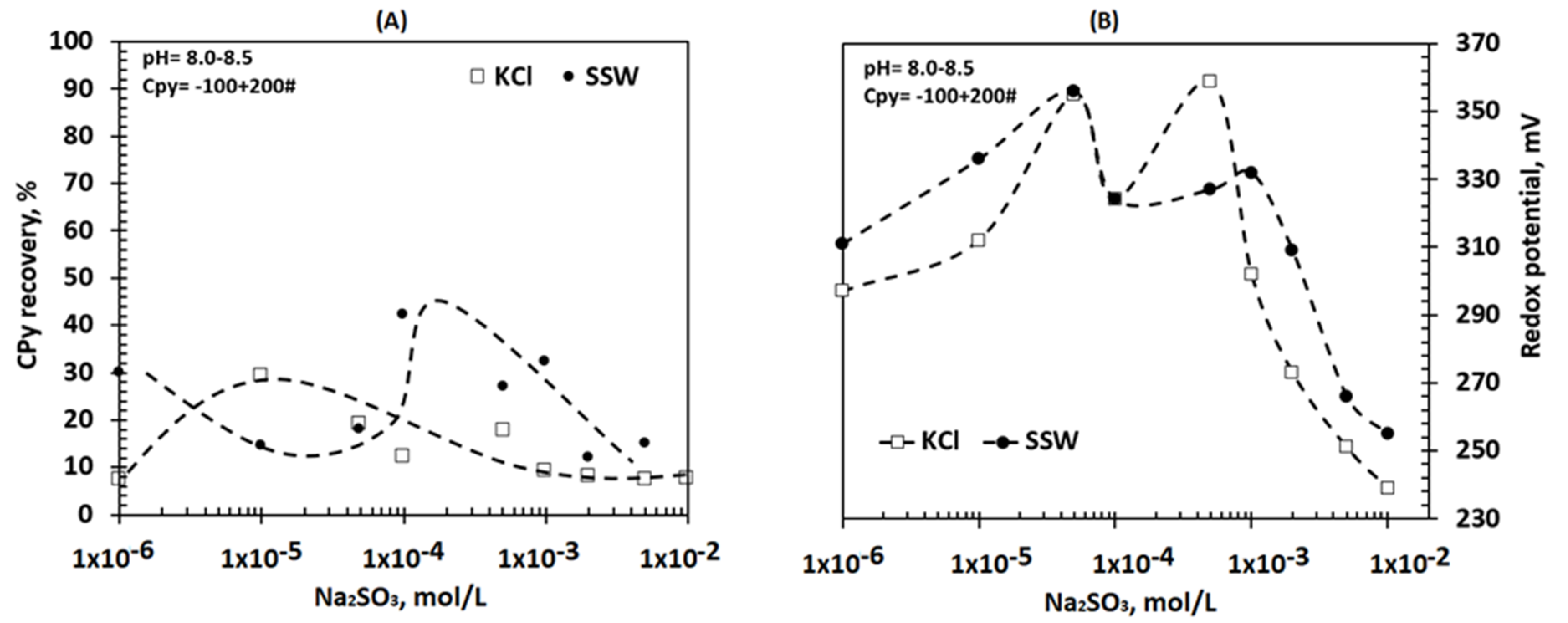
3.3. Effect of Sodium Sulfite Concentration on Flotation without the Chalcopyrite Collector in Saline Solutions
4. Discussion
4.1. Chalcopyrite Floatability in the Presence of PPX and Na2SO3 in Saline Solutions
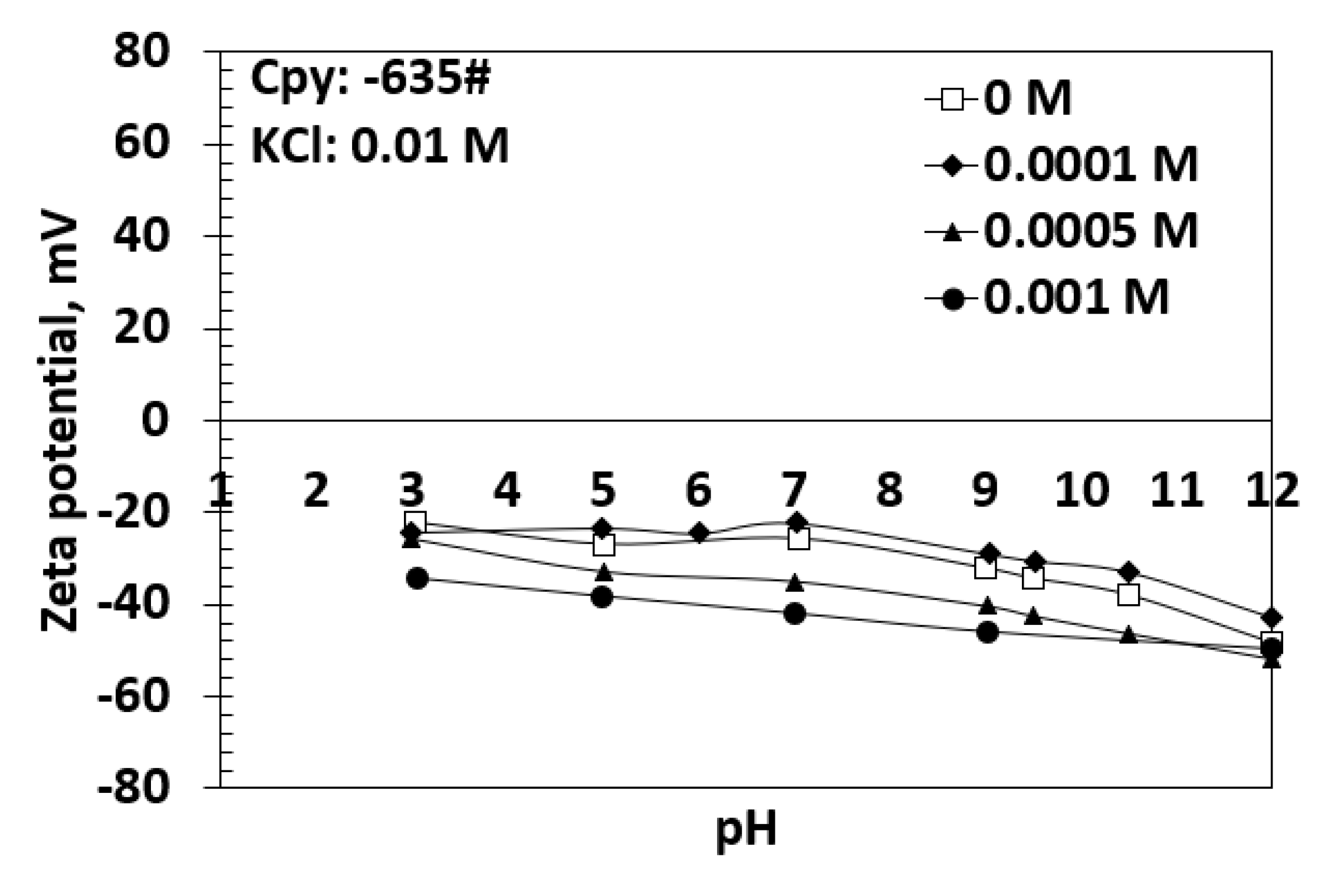
4.2. Effects of Potassium Propyl Xanthate and Sodium Sulfite on Chalcopyrite Zeta Potential Using KCl
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2017, 39, 1–16. [Google Scholar] [CrossRef]
- Herrera-León, S.; Lucay, F.A.; Cisternas, L.A.; Kraslawski, A. Applying a multi-objective optimization approach in designing water supply systems for mining industries. The case of Chile. J. Clean. Prod. 2019, 210, 994–1004. [Google Scholar] [CrossRef]
- Gálvez, E.D.; Cruz, R.; Robles, P.A.; Cisternas, L.A. Optimization of dewatering systems for mineral processing. Miner. Eng. 2014, 63, 110–117. [Google Scholar] [CrossRef]
- Ordóñez, J.I.; Moreno, L.; González, J.F.; Cisternas, L.A. Use of discharged brine from reverse osmosis plant in heap leaching: Opportunity for caliche mining industry. Hydrometallurgy 2015, 155, 61–68. [Google Scholar] [CrossRef]
- Ordóñez, J.I.; Moreno, L.; Gálvez, E.D.; Cisternas, L.A. Seawater leaching of caliche mineral in column experiments. Hydrometallurgy 2013, 139, 79–87. [Google Scholar] [CrossRef]
- Hernández, P.; Taboada, M.; Herreros, O.; Graber, T.; Ghorbani, Y. Leaching of chalcopyrite in acidified nitrate using seawater-based media. Minerals 2018, 8, 238. [Google Scholar] [CrossRef]
- Araya, N.; Cisternas, L.A.; Lucay, F.; Gálvez, E.D. Design of desalinated water distribution networks including energy recovery devices. Comput. Aided Chem. Eng. 2017, 40, 925–930. [Google Scholar]
- Wessman, H.; Salmi, O.; Kohl, J.; Kinnunen, P.; Saarivuori, E.; Mroueh, U.M. Water and society: Mutual challenges for eco-efficient and socially acceptable mining in Finland. J. Clean. Prod. 2014, 84, 289–298. [Google Scholar] [CrossRef]
- Lucay, F.; Cisternas, L.A.; Gálvez, E.; Lopez-Valdivieso, A. Study of the natural floatability of molybdenite fines in saline solutions and effect of gypsum precipitation. Miner. Metall. Process. 2015, 32, 203–208. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Arancibia-Bravo, M.P.; Reyes, A.; Aguirre, C.E.; Cortes, L.; Cisternas, L.A. The impact of seawater with calcium and magnesium removal for the flotation of copper-molybdenum sulphide ores. Miner. Eng. 2017, 109, 10–13. [Google Scholar] [CrossRef]
- Ramos, O.; Castro, S.; Laskowski, J.S. Copper-molybdenum ores flotation in sea water: Floatability and frothability. Miner. Eng. 2013, 53, 108–112. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Calisaya, D.; Cisternas, L.A. An improved flotation test method and pyrite depression by an organic reagent during flotation in seawater. J. S. Afr. Inst. Min. Metall. 2017, 117, 499–504. [Google Scholar] [CrossRef]
- Quinn, J.J.; Sovechles, J.M.; Finch, J.A.; Waters, K.E. Critical coalescence concentration of inorganic salt solutions. Miner. Eng. 2014, 58, 1–6. [Google Scholar] [CrossRef]
- Quinn, J.J.; Kracht, W.; Gomez, C.O.; Gagnon, C.; Finch, J.A. Comparing the effect of salts and frother (MIBC) on gas dispersion and froth properties. Miner. Eng. 2007, 20, 1296–1302. [Google Scholar] [CrossRef]
- Arancibia-Bravo, M.P.; Lucay, F.A.; López, J.; Cisternas, L.A. Modeling the effect of air flow, impeller speed, frother dosages, and salt concentrations on the bubbles size using response surface methodology. Miner. Eng. 2019, 132, 142–148. [Google Scholar] [CrossRef]
- Maree, W.; Kloppers, L.; Hangone, G.; Oyekola, O. The effects of mixtures of potassium amyl xanthate (PAX) and isopropyl ethyl thionocarbamate (IPETC) collectors on grade and recovery in the froth flotation of a nickel sulfide ore. S. Afr. J. Chem. Eng. 2017, 24, 116–121. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of pyrite in selective flotation by different reagent systems—A Literature review. Miner. Eng. 2016, 96–97, 143–156. [Google Scholar] [CrossRef]
- Houot, R.; Duhamet, D. Floatability of chalcopyrite in the presence of dialkyl-thionocarbamate and sodium sulfite. Int. J. Miner. Process. 1993, 37, 273–282. [Google Scholar] [CrossRef]
- Grano, S.R.; Prestidge, C.A.; Ralston, J. Solution interaction of ethyl xanthate and sulphite and its effect on galena flotation and xanthate adsorption. Int. J. Miner. Process. 2002, 52, 161–186. [Google Scholar] [CrossRef]
- Janetski, N.D.; Woodburn, S.I.; Woods, R. An electrochemical investigation of pyrite flotation and depression. Int. J. Miner. Process. 1977, 4, 227–239. [Google Scholar] [CrossRef]
- Mustafa, S.; Hamid, A.; Naeem, A. Xanthate adsorption studies on chalcopyrite ore. Int. J. Miner. Process. 2004, 74, 317–325. [Google Scholar] [CrossRef]
- Shen, W.Z.; Fornasiero, D.; Ralston, J. Flotation of sphalerite and pyrite in the presence of sodium sulfite. Int. J. Miner. Process. 2001, 63, 17–28. [Google Scholar] [CrossRef]
- Rani, A.; Gupta, C.S. Synthesis and characterization of various xanthates and their effects on germination and early seedling growth in wheat (Triticum aestivum L.). Asian J. Chem. 2013, 25, 6995–6996. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef]
- Dávila-Pulido, G.I.; Uribe-Salas, A.; Espinosa-Gómez, R. Comparison of the depressant action of sulfite and metabisulfite for Cu-activated sphalerite. Int. J. Miner. Process. 2011, 101, 71–74. [Google Scholar] [CrossRef]
- Doi, S. One-Step Microwave-Assisted Aqueous Synthesis of Silver-Based Nanoparticles Functionalized by Glutathione. MRS Online Proc. Libr. Arch. 2014, 1, 163–168. [Google Scholar]
- Lucas, M.S. Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation. Dyes Pigment. 2006, 71, 236–244. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, J.; Xu, R. Chemosphere Removal of Cr(VI) from aqueous solutions by Na2SO3/FeSO4 combined with peanut straw biochar. Chemosphere 2014, 101, 71–76. [Google Scholar] [CrossRef]
- Miki, H.; Hirajima, T.; Muta, Y.; Suyantara, G.P.W.; Sasaki, K. Effect of sodium sulfite on floatability of chalcopyrite and molybdenite. Minerals 2018, 8, 172. [Google Scholar] [CrossRef]
- Klimpel, R.R. Selection of Chemical Reagents for Flotation. In Mineral Processing Plant Design, 2nd ed.; Mular, A., Bhappu, R., Eds.; AIME: New York, NY, USA, 1980; pp. 907–934. [Google Scholar]
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale. Deep. Res. Part I Oceanogr. Res. Pap. 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Farrokhrouz, M.; Haghi, H. The Application of Hallimond Tube for Floatability Study of Pure Galena. In Proceedings of the 13th Conference on Environment and Mineral Processing, Ostrava, Czech Republic, 4–5 June 2009; Volume 1, pp. 89–96. [Google Scholar]
- Fullston, D.; Fornasiero, D.; Ralston, J. Zeta potential study of the oxidation of copper sulfide minerals. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 146, 113–121. [Google Scholar] [CrossRef]
- Adamson, A.; Gast, A. Physical Chemistry of Surfaces; Adamson, G., Ed.; Wiley: Hoboken, NJ, USA, 1997; p. 190. [Google Scholar]
- Process, I.J.M.; Bafghi, M.S.; Emami, A.H.; Khaki, J.V.; Zakeri, A. Development of a mathematical expression for the variation of amorphization phenomenon during intensive milling of minerals. Int. J. Miner. Process. 2009, 93, 149–154. [Google Scholar]
- Pan, G.; Zhang, G.; Shi, Q.; Chen, W. The effect of sodium alginate on chlorite and serpentine in chalcopyrite flotation. Minerals 2019, 9, 196. [Google Scholar] [CrossRef]
- Andreev, G.N.; Barzev, A. Raman spectroscopic study of some chalcopyrite-xanthate flotation products. J. Mol. Struct. 2003, 661–662, 325–332. [Google Scholar] [CrossRef]
- Jehlička, J.; Vítek, P.; Edwards, H.G.M.; Heagraves, M.; Čapoun, T. Application of portable Raman instruments for fast and non-destructive detection of minerals on outcrops. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 410–419. [Google Scholar] [CrossRef]
- Vazquez-Sanchez, E.E.; Cabrera-Robledo, A.; Tong, X.; Lopez-Valdivieso, A. Raman spectroscopy characterization of some Cu, Fe and Zn sulfides and their relevant surface chemical species for flotation. Physicochem. Probl. Miner. Process. 2020, 56, 483–492. [Google Scholar] [CrossRef]
- Fuerstenau, M.C. On the natural floatability of sulfides. Int. J. 1981, 8, 79–84. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y. The effect of saline water on copper activation of pyrite in chalcopyrite flotation. Miner. Eng. 2019, 131, 336–341. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Xiao, Q.; He, N.; Ren, Z.; Lartey, C.; Gerson, A. The influence of common monovalent and divalent chlorides on chalcopyrite flotation. Minerals 2017, 7, 111. [Google Scholar] [CrossRef]
- Kal’nyi, D.B.; Kokovkin, V.V.; Mironov, I.V. Sodium sulfite: A promising reagent in the electrochemical oxidation of metallic silver. Russ. J. Gen. Chem. 2011, 81, 793–798. [Google Scholar] [CrossRef]
- Castro, S. Physico-chemical factors in flotation of Cu-Mo-Fe ores with seawater: A critical review. Physicochem. Probl. Miner. Process. 2018, 54, 1223–1236. [Google Scholar]
- Birkmann, J.; Pasel, C.; Luckas, M.; Bathen, D. Development of a measuring method for the determination of bisulfite and sulfite in seawater. Chem. Ing. Tech. 2019, 91, 1563–1574. [Google Scholar] [CrossRef]
- Chander, S.; Khan, A. Effect of sulfur dioxide on flotation of chalcopyrite. Int. J. Miner. Process. 2000, 58, 45–55. [Google Scholar] [CrossRef]
- Hancer, M.; Celik, M.S.; Miller, J.D. The significance of interfacial water structure in soluble salt flotation systems. J. Colloid Interface Sci. 2001, 235, 150–161. [Google Scholar] [CrossRef]
- Damian Risberg, E.; Eriksson, L.; Mink, J.; Pettersson, L.G.M.; Skripkin, M.Y.; Sandström, M. Sulfur X-ray absorption and vibrational spectroscopic study of sulfur dioxide, sulfite, and sulfonate solutions and of the substituted sulfonate ions X 3 CSO 3-(X = H, Cl, F). Inorg. Chem. 2007, 46, 8332–8348. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Property | Dose | Units |
|---|---|---|---|
| Mineral | Chalcopyrite | 1 | g |
| Granulometry | 75–150 | µm | |
| Solution | KCl | 0.01 | mol/L |
| SSW | 0.54 | mol/L | |
| Volume | 150 | mL | |
| Collector type | PPX | 0–0.01 | mol/L |
| Depressant | Na2SO3 | 0–0.01 | mol/L |
| Cell type | Hallimond tube | 150 | mL |
| pH | HCl/NaOH | 8.0–8.5 | |
| Air type | Nitrogen | 20 | mL/min |
| Agitation | Magnetic | 700 | rpm |
| Flotation | Time | 1 | minutes |
| Parameter | Property | Doses | Units |
|---|---|---|---|
| Mineral | Chalcopyrite | 500 | mg |
| Granulometry | 20 | µm | |
| Solution | KCl | 0.01 | mol/L |
| 50 | mL | ||
| Collector type | PPX | 0–0.01 | mol/L |
| Depressant | Na2SO3 | 0–0.01 | mol/L |
| Cell type | Riddick Zeta D model | 150 | mL |
| pH | HCl/NaOH | 03-dic | |
| Agitation | Magnetic | 800 | rpm |
| Energy | Voltage | 100 | mV |
| Conditioning | Time | 15 | min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arancibia-Bravo, M.P.; López-Valdivieso, A.; Flores, L.F.; Cisternas, L.A. Effects of Potassium Propyl Xanthate Collector and Sodium Sulfite Depressant on the Floatability of Chalcopyrite in Seawater and KCl Solutions. Minerals 2020, 10, 991. https://doi.org/10.3390/min10110991
Arancibia-Bravo MP, López-Valdivieso A, Flores LF, Cisternas LA. Effects of Potassium Propyl Xanthate Collector and Sodium Sulfite Depressant on the Floatability of Chalcopyrite in Seawater and KCl Solutions. Minerals. 2020; 10(11):991. https://doi.org/10.3390/min10110991
Chicago/Turabian StyleArancibia-Bravo, María P., Alejandro López-Valdivieso, Luís F. Flores, and Luís A. Cisternas. 2020. "Effects of Potassium Propyl Xanthate Collector and Sodium Sulfite Depressant on the Floatability of Chalcopyrite in Seawater and KCl Solutions" Minerals 10, no. 11: 991. https://doi.org/10.3390/min10110991
APA StyleArancibia-Bravo, M. P., López-Valdivieso, A., Flores, L. F., & Cisternas, L. A. (2020). Effects of Potassium Propyl Xanthate Collector and Sodium Sulfite Depressant on the Floatability of Chalcopyrite in Seawater and KCl Solutions. Minerals, 10(11), 991. https://doi.org/10.3390/min10110991







