Clayey Soil Strength Improvement by Using Alkali Activated Slag Reinforcing
Abstract
:1. Introduction
2. Methodology
2.1. The Experimental Techniques
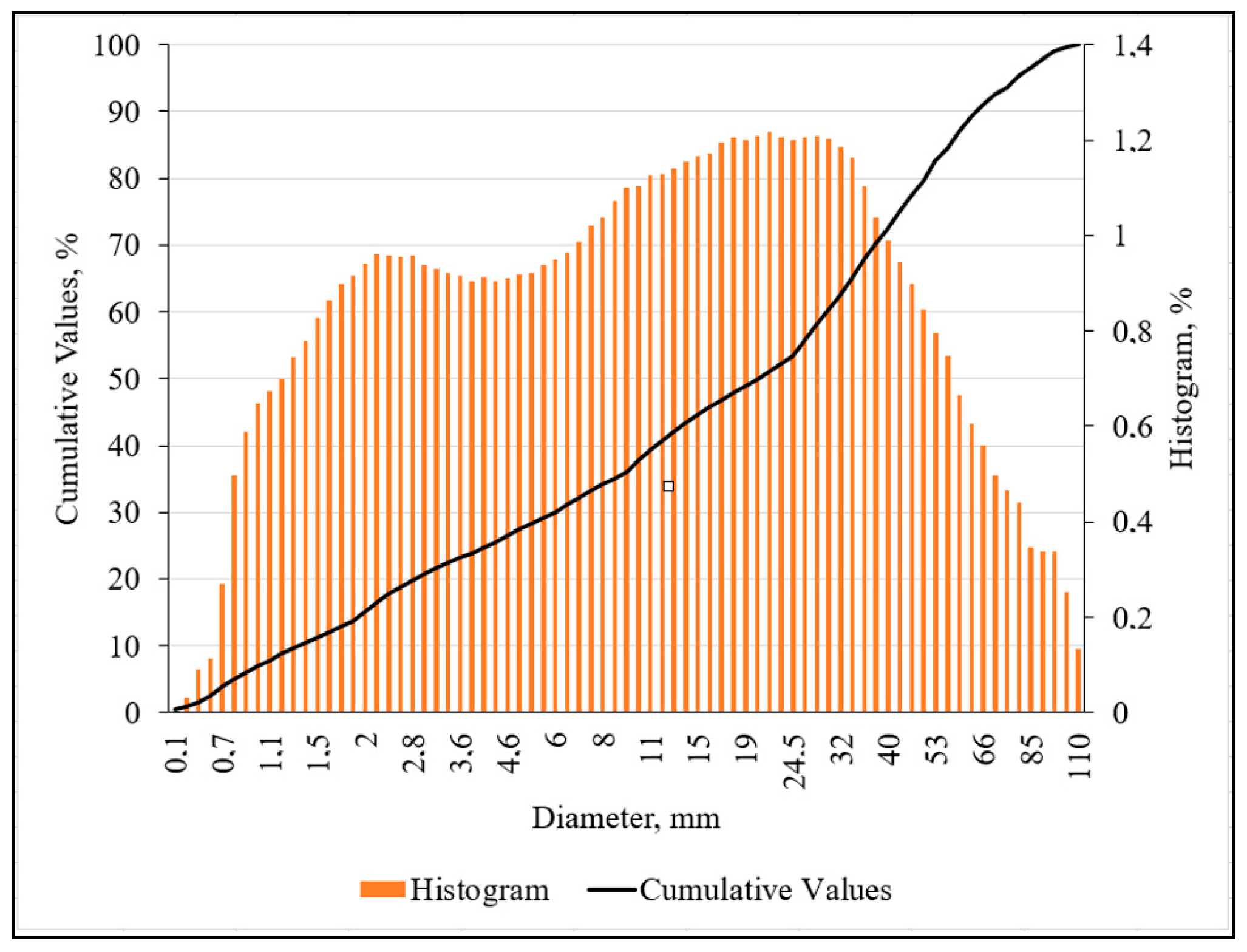
2.2. The Characterization of Raw Clay
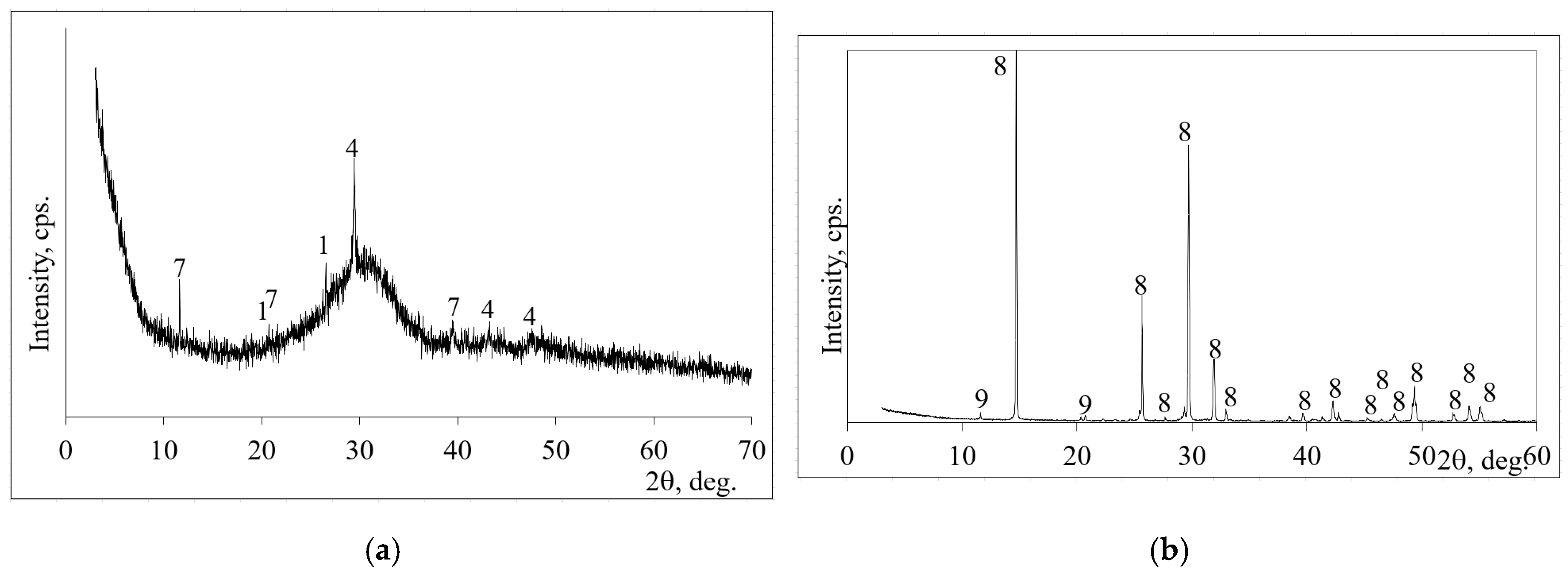
2.3. The Characterization of Initial Materials for the Alkali Activated Binder
2.4. The Procedures for Samples Preparation
3. Results and Discussion
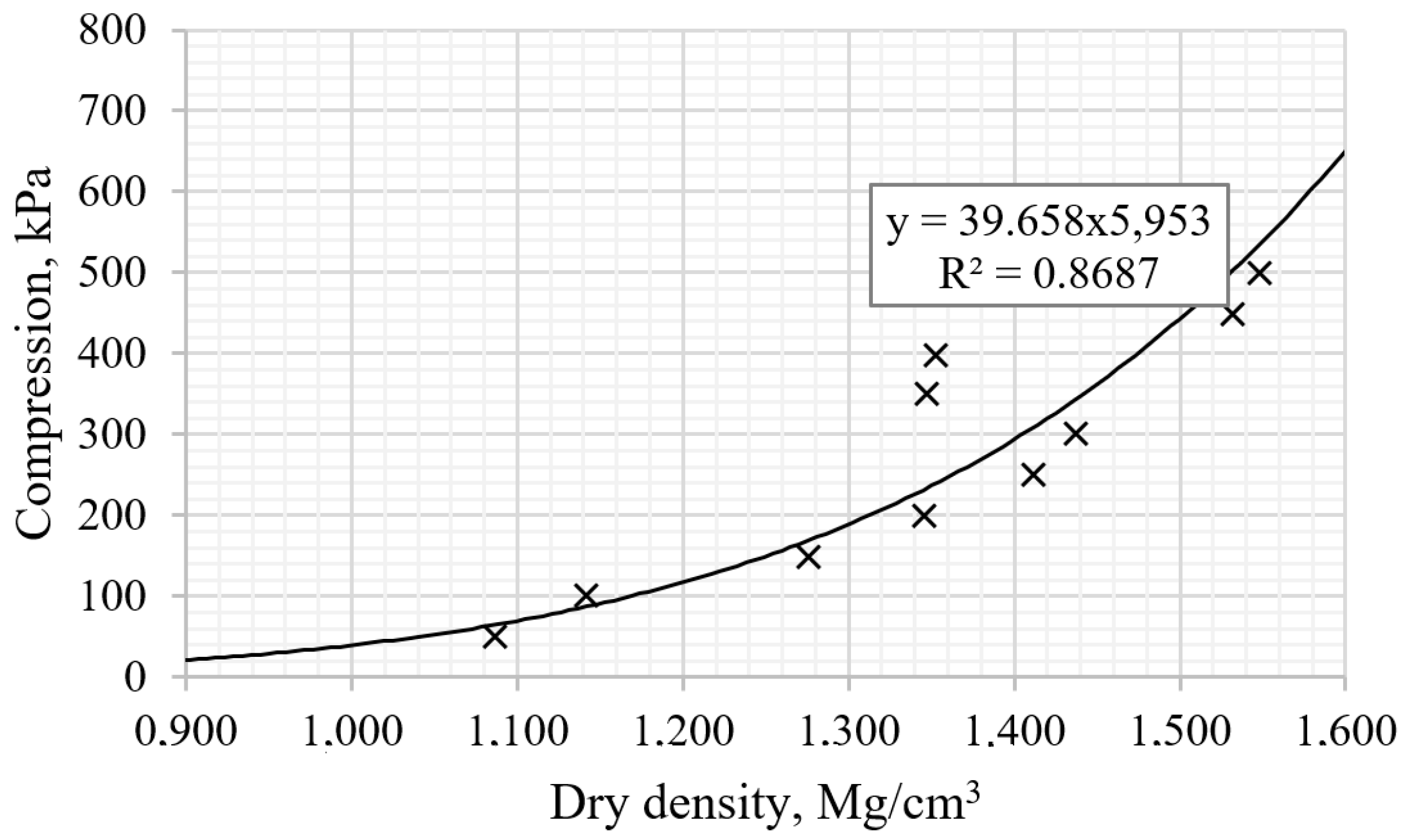
3.1. Standard Proctor Compaction Tests and Consolidation Test of Clay
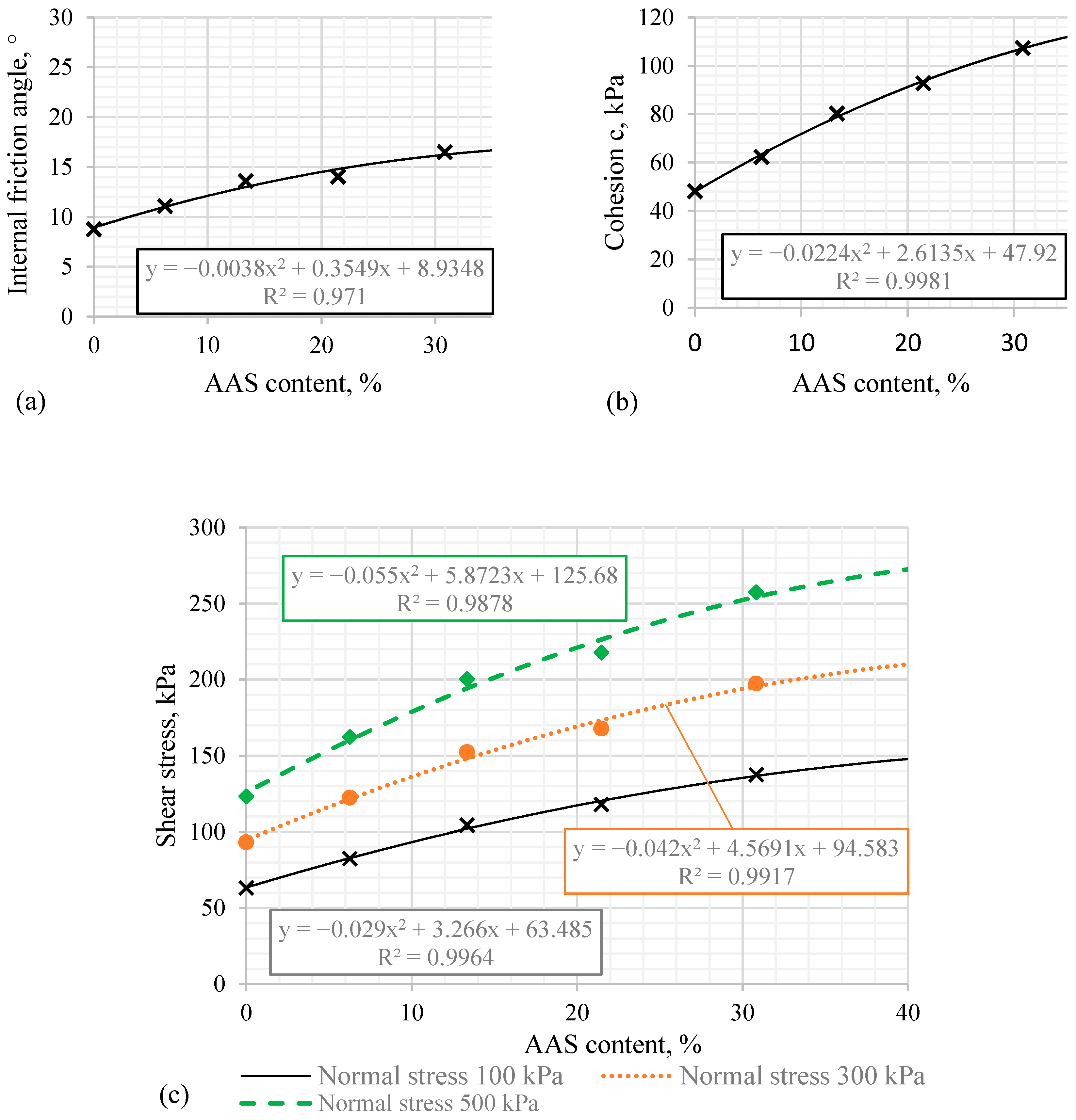
3.2. The Mechanical Properties of AAS Reinforced Clay
- τ—failure shear stress kPa;
- ai—coefficient corresponding to the cohesion, kPa;
- bi—factor corresponding to tanφ.
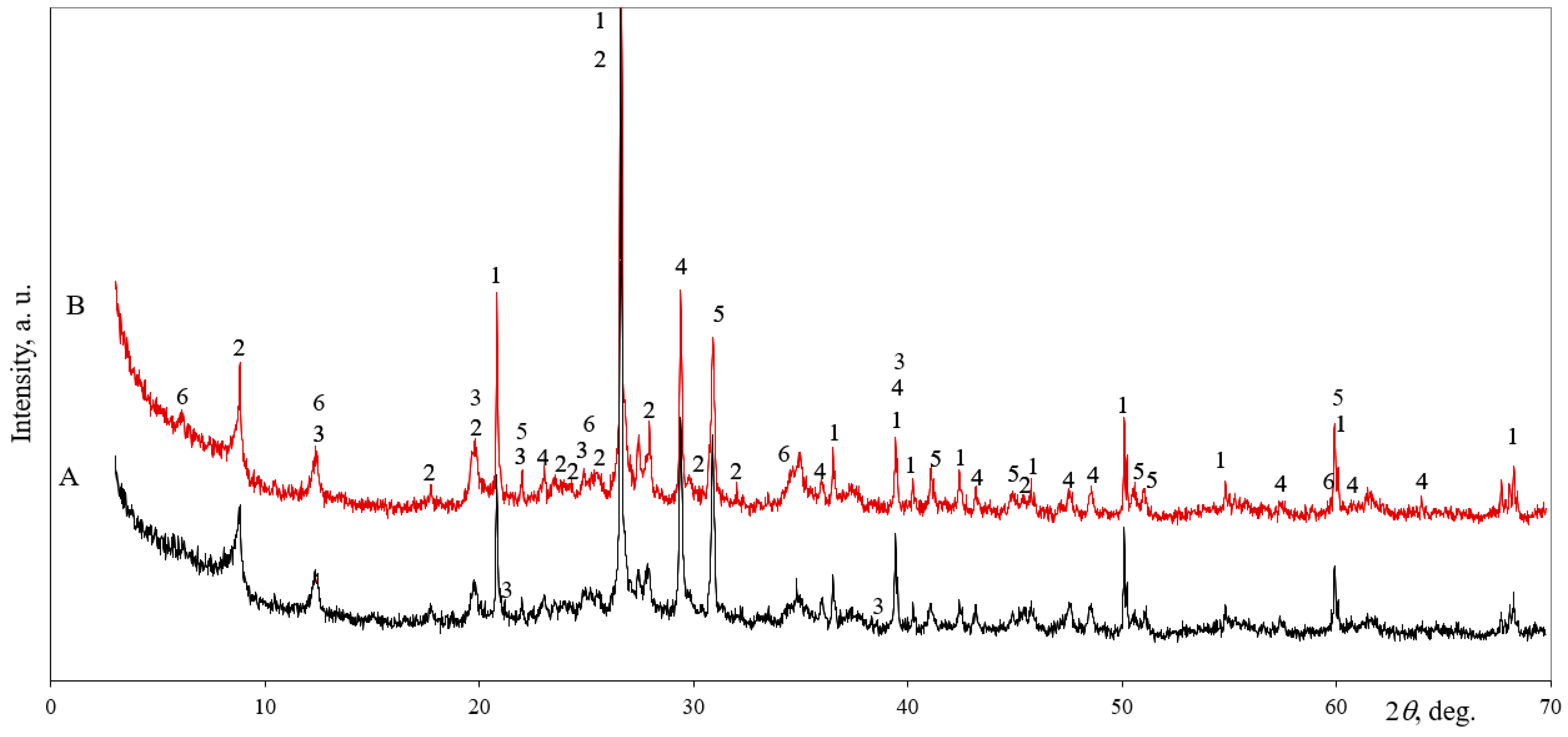
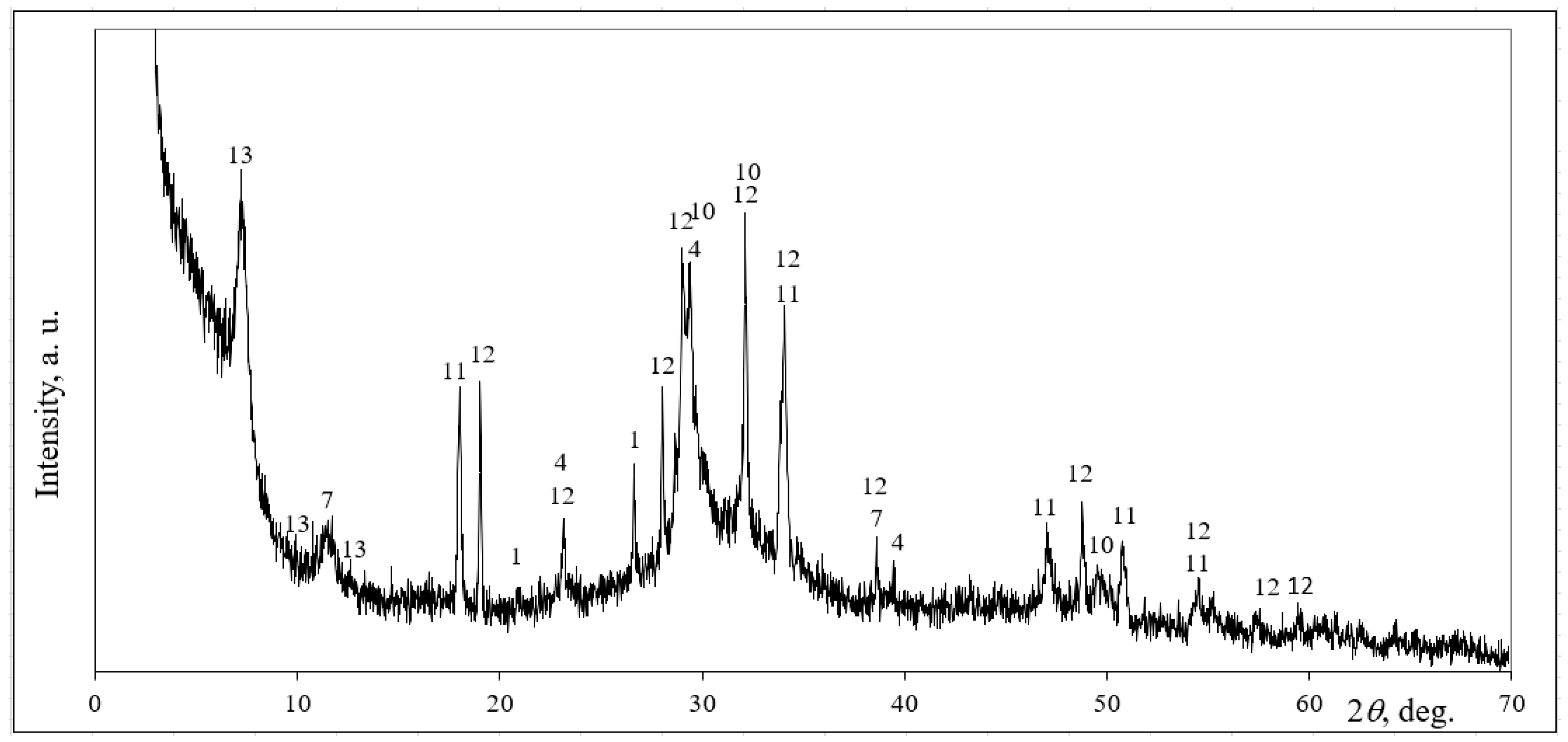
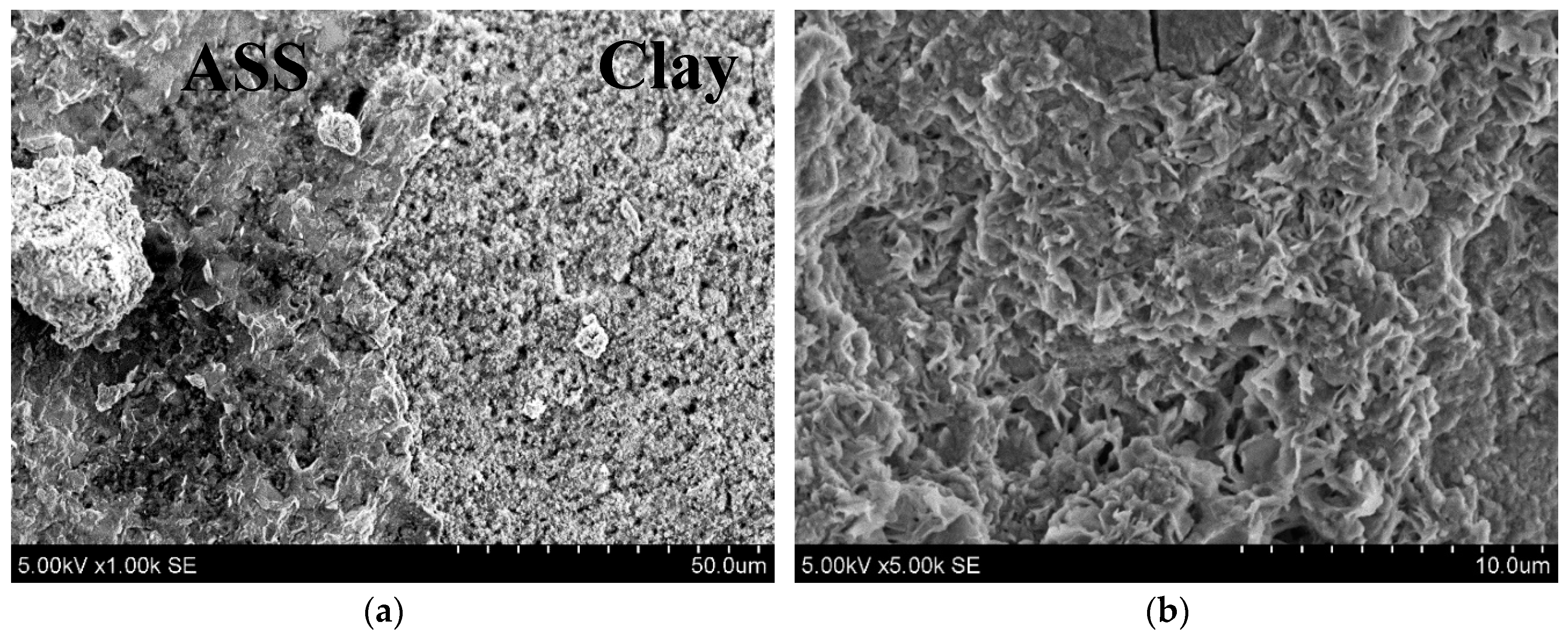
3.3. The Microstructural Development of AAS in Clay Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bose, B. Geo engineering properties of expansive soil stabilized with fly ash. Electron. J. Geotech. Eng. 2012, 17, 1339–1353. [Google Scholar]
- Kampala, A.; Horpibulsuk, S.; Prongmanee, N.; Chinkulkijniwat, A. Influence of wet-dry cycles on compressive strength of calcium carbide residue–fly ash stabilized clay. J. Mater. Civ. Eng. 2014, 26, 633–643. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Phetchuay, C.; Chinkulkijniwat, A. Soil stabilization by calcium carbide residue and fly ash. J. Mater. Civ. Eng. 2012, 24, 184–193. [Google Scholar] [CrossRef]
- Chen, L.; Chuan, S.; Songyu, L. The Effects of GGBS on the Engineering Properties of Stabilized Soil. Chin. J. Undergr. Space Eng. 2013, S2. [Google Scholar]
- Swamy, R.; Sarvade, P.G.; Nayak, D. Utilization of GGBS and Lime to Improve the Compaction and Unconfined Strength Properties of Marine Clay. Asian J. Eng. Technol. 2015, 3. [Google Scholar]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B. Initial Investigation of Soil Stabilization with Calcined Dolomite-GGBS Blends. In Ground Improvement and Geosynthetics; American Society of Civil Engineers: Reston, VA, USA, 2014; pp. 148–157. [Google Scholar]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L. Geo-mechanical behavior of clay soils stabilized at ambient temperature with fly-ash geopolymer-incorporated granulated slag. Soils Found. 2019, 59, 1906–1920. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, H.; El-Korchi, T.; Zhang, G.; Tao, M. Experimental feasibility study of geopolymer as the next-generation soil stabilizer. Constr. Build. Mater. 2013, 47, 1468–1478. [Google Scholar] [CrossRef]
- Verdolotti, L.; Iannace, S.; Lavorgna, M.; Lamanna, R. Geopolymerization reaction to consolidate incoherent pozzolanic soil. J. Mater. Sci. 2008, 43, 865–873. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Teixeira Pinto, A. Deep soft soil improvement by alkaline activation. Proc. Inst. Civ. Eng. Ground Improv. 2011, 164, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Cristelo, N.; Glendinning, S.; Fernandes, L.; Pinto, A.T. Effect of calcium content on soil stabilisation with alkaline activation. Constr. Build. Mater. 2012, 29, 167–174. [Google Scholar] [CrossRef]
- Abdeldjouad, L.; Asadi, A.; Nahazanan, H.; Huat, B.B.; Dheyab, W.; Elkhebu, A.G. Effect of clay content on soil stabilization with alkaline activation. Int. J. Geosynth. Ground Eng. 2019, 5, 4. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S. Alkali-activated ground-granulated blast furnace slag for stabilization of marine soft clay. J. Mater. Civ. Eng. 2015, 27, 04014146. [Google Scholar] [CrossRef]
- Mozumder, R.A.; Laskar, A.I. Prediction of unconfined compressive strength of geopolymer stabilized clayey soil using artificial neural network. Comput. Geotech. 2015, 69, 291–300. [Google Scholar] [CrossRef]
- Salimi, M.; Ghorbani, A. Mechanical and compressibility characteristics of a soft clay stabilized by slag-based mixtures and geopolymers. Appl. Clay Sci. 2020, 184, 105390. [Google Scholar] [CrossRef]
- Singhi, B.; Laskar, A.I.; Ahmed, M.A. Investigation on soil–geopolymer with slag, fly ash and their blending. Arab. J. Sci. Eng. 2016, 41, 393–400. [Google Scholar] [CrossRef]
- Davari Algoo, S.; Akhlaghi, T.; Ranjbarnia, M. Engineering properties of clayey soil stabilised with alkali-activated slag. Proc. Inst. Civ. Eng. Ground Improv. 2019. [Google Scholar] [CrossRef]
- Bruker. D8 Advance Diffractometer (Bruker AXS) Technical Details. Available online: https://www.bruker.com/products/x-ray-difractionand-elemental-analysis/x-ray-difraction/d8-advance.html (accessed on 13 March 2020).
- Bruker. X-ray S8 Tiger WD Series 2 Technical Details. Available online: https://www.bruker.com/products/x-ray-diffraction-andelemental-analysis/x-ray-fuorescence/s8-tiger.html (accessed on 13 March 2020).
- Zeiss. EVO MA and LS Series Scanning Electron Microscopes for Materials Analysis and Life Science. In Operator User Guide; Version 1.0; Zeiss: Cambridge, MA, USA, 2008. [Google Scholar]
- LST EN ISO 17892-12:2018 Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 12: Determination of Liquid and Plastic Limits (ISO 17892-12:2018); EN ISO 17892-12:2018; Lithuanian Standards Board: Vilnius, Lithuania, 2018.
- LST EN ISO 14688-2:2018 Geotechnical Investigation and Testing—Identification and Classification of Soil—Part 2: Principles for a Classification (ISO 14688-2:2017); EN ISO 14688-2:2018; Lithuanian Standards Board: Vilnius, Lithuania, 2018.
- LST EN 13286-2:2010/AC:2013 Unbound and Hydraulically Bound Mixtures—Part 2: Test Methods for Laboratory Reference Density and Water Content—Proctor Compaction; EN 13286-2:2010/AC:2012; Lithuanian Standards Board: Vilnius, Lithuania, 2013.
- LST EN ISO 17892-10:2019 Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 10: Direct Shear Tests (ISO 17892-10:2018); EN ISO 17892-10:2018; Lithuanian Standards Board: Vilnius, Lithuania, 2019.
- Kubiliūtė, R.; Kaminskas, R. The pozzolanic activity of calcined clay–silica gel composites. Mater. Sci. 2013, 19, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Rashad, A.M.; Sadek, D.M.; Hassan, H.A. An investigation on blast-furnace stag as fine aggregate in alkali-activated slag mortars subjected to elevated temperatures. J. Clean. Prod. 2016, 112, 1086–1096. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Nizevičienė, D.; Kielė, A.; Janavičius, E.; Pupeikis, D. Effect of phosphogypsum on the stability upon firing treatment of alkali-activated slag. Constr. Build. Mater. 2018, 184, 485–491. [Google Scholar] [CrossRef]
- Nizevičienė, D.; Vaičiukynienė, D.; Vaitkevičius, V.; Rudžionis, Ž. Effects of waste fluid catalytic cracking on the properties of semi-hydrate phosphogypsum. J. Clean. Prod. 2016, 137, 150–156. [Google Scholar] [CrossRef]
- Atiş, C.D.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Dadsetan, S.; Siad, H.; Lachemi, M.; Sahmaran, M. Extensive evaluation on the effect of glass powder on the rheology, strength, and microstructure of metakaolin-based geopolymer binders. Constr. Build. Mater. 2020, 121168. [Google Scholar] [CrossRef]
- Bernal, S.A.; Nicolas, R.S.; van Deventer, J.S.; Provis, J.L. Alkali-activated slag cements produced with a blended sodium carbonate/sodium silicate activator. Adv. Cem. Res. 2016, 28, 262–273. [Google Scholar] [CrossRef]
- Sukmak, P.; Horpibulsuk, S.; Shen, S.L.; Chindaprasirt, P.; Suksiripattanapong, C. Factors influencing strength development in clay–fly ash geopolymer. Constr. Build. Mater. 2013, 47, 1125–1136. [Google Scholar] [CrossRef]
- Sukmak, P.; Horpibulsuk, S.; Shen, S.L. Strength development in clay–fly ash geopolymer. Constr. Build. Mater. 2013, 40, 566–574. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, Y.; Yang, L.; Zhang, Y.; Sun, Z. Hydration products, internal relative humidity and drying shrinkage of alkali activated slag mortar with expansion agents. Constr. Build. Mater. 2018, 158, 198–207. [Google Scholar] [CrossRef]

| Sample No | Sample Moisture Content, % | Average Moisture Content, % | Plasticity Index (PI), % |
|---|---|---|---|
| Plastic limit (PL), % | 21.72 | ||
| 1 | 27.93 | 27.85 | |
| 2 | 27.76 | ||
| Liquid limit (LL), % | |||
| 3 | 49.87 | 49.57 | |
| 4 | 49.26 | ||
| Oxides | CaO | SiO2 | SO3 | Al2O3 | MgO | Na2O | P2O5 | K2O | TiO2 | F | SrO | LOI * | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GGBFS | 45.20 | 37.10 | 1.85 | 6.44 | 5.76 | 1.02 | 0.68 | 0.52 | 0.29 | - | 0.07 | - | 1.07 |
| PG | 39.06 | 0.34 | 52.75 | 0.07 | 0.21 | - | 1.11 | - | - | 0.06 | - | 6.40 | - |
| GGBFS, wt.% | Phosphogypsum, wt.% | NaOH, wt.% | W/S * |
|---|---|---|---|
| 97 | 3 | 10 | 0.27 |
| Samples Group | Area of Cylinder, cm2 | Drilled Holes Number, Units | Total Area of AAS, cm2 | The Area of Clay, cm2 | Total Area of AAS, % |
|---|---|---|---|---|---|
| Clay | 40 | 0 | 0 | 40.00 | 0 |
| G 6% | 40 | 3 | 2.36 | 37.64 | 6.26 |
| G 13% | 40 | 6 | 4.71 | 35.29 | 13.35 |
| G 21% | 40 | 9 | 7.07 | 32.93 | 21.46 |
| G 30% | 40 | 12 | 9.42 | 30.58 | 30.82 |
| Sample Group | Normal Stress, kPa | Shear Stress, kPa | Shear Strength Increment, % | ai, kPa | bi | R2 |
|---|---|---|---|---|---|---|
| Clay | 100 | 63.2 | 0 | 48.20 | 0.15 | 0.95 |
| 300 | 93.2 | 0 | ||||
| 500 | 123.2 | 0 | ||||
| G 6% | 100 | 82.4 | 30.4 | 62.40 | 0.20 | 0.93 |
| 300 | 122.4 | 31.3 | ||||
| 500 | 197.4 | 31.8 | ||||
| G 13% | 100 | 104.3 | 65.0 | 80.30 | 0.24 | 0.87 |
| 300 | 152.3 | 63.4 | ||||
| 500 | 200.3 | 62.6 | ||||
| G 21% | 100 | 117.8 | 86.4 | 92.80 | 0.25 | 0.93 |
| 300 | 167.8 | 80.0 | ||||
| 500 | 217.8 | 76.8 | ||||
| G 30% | 100 | 137.4 | 117.4 | 107.40 | 0.30 | 0.90 |
| 300 | 197.4 | 111.8 | ||||
| 500 | 257.4 | 108.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žurinskas, D.; Vaičiukynienė, D.; Stelmokaitis, G.; Doroševas, V. Clayey Soil Strength Improvement by Using Alkali Activated Slag Reinforcing. Minerals 2020, 10, 1076. https://doi.org/10.3390/min10121076
Žurinskas D, Vaičiukynienė D, Stelmokaitis G, Doroševas V. Clayey Soil Strength Improvement by Using Alkali Activated Slag Reinforcing. Minerals. 2020; 10(12):1076. https://doi.org/10.3390/min10121076
Chicago/Turabian StyleŽurinskas, Darius, Danutė Vaičiukynienė, Gediminas Stelmokaitis, and Viktoras Doroševas. 2020. "Clayey Soil Strength Improvement by Using Alkali Activated Slag Reinforcing" Minerals 10, no. 12: 1076. https://doi.org/10.3390/min10121076







