Uakitite, VN, a New Mononitride Mineral from Uakit Iron Meteorite (IIAB) †
Abstract
:1. Introduction
2. History of the Uakit Meteorite
3. Analytical Methods
4. General Description of the Uakit Meteorite
5. Morphology, Optical and Physical Properties of Uakitite
6. Chemical Composition of Uakitite
7. Crystal Structural Data for Uakitite
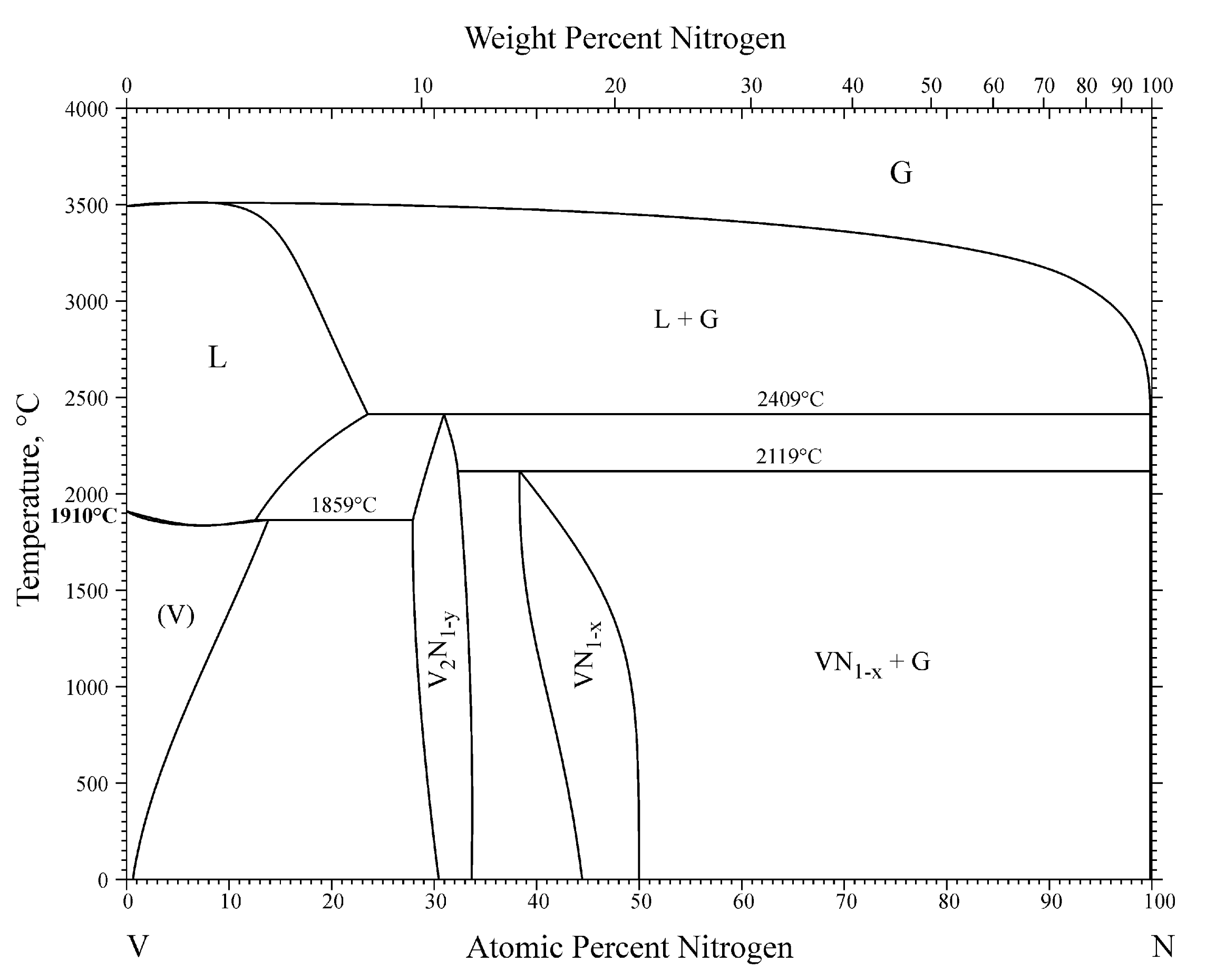
7.1. System V-N
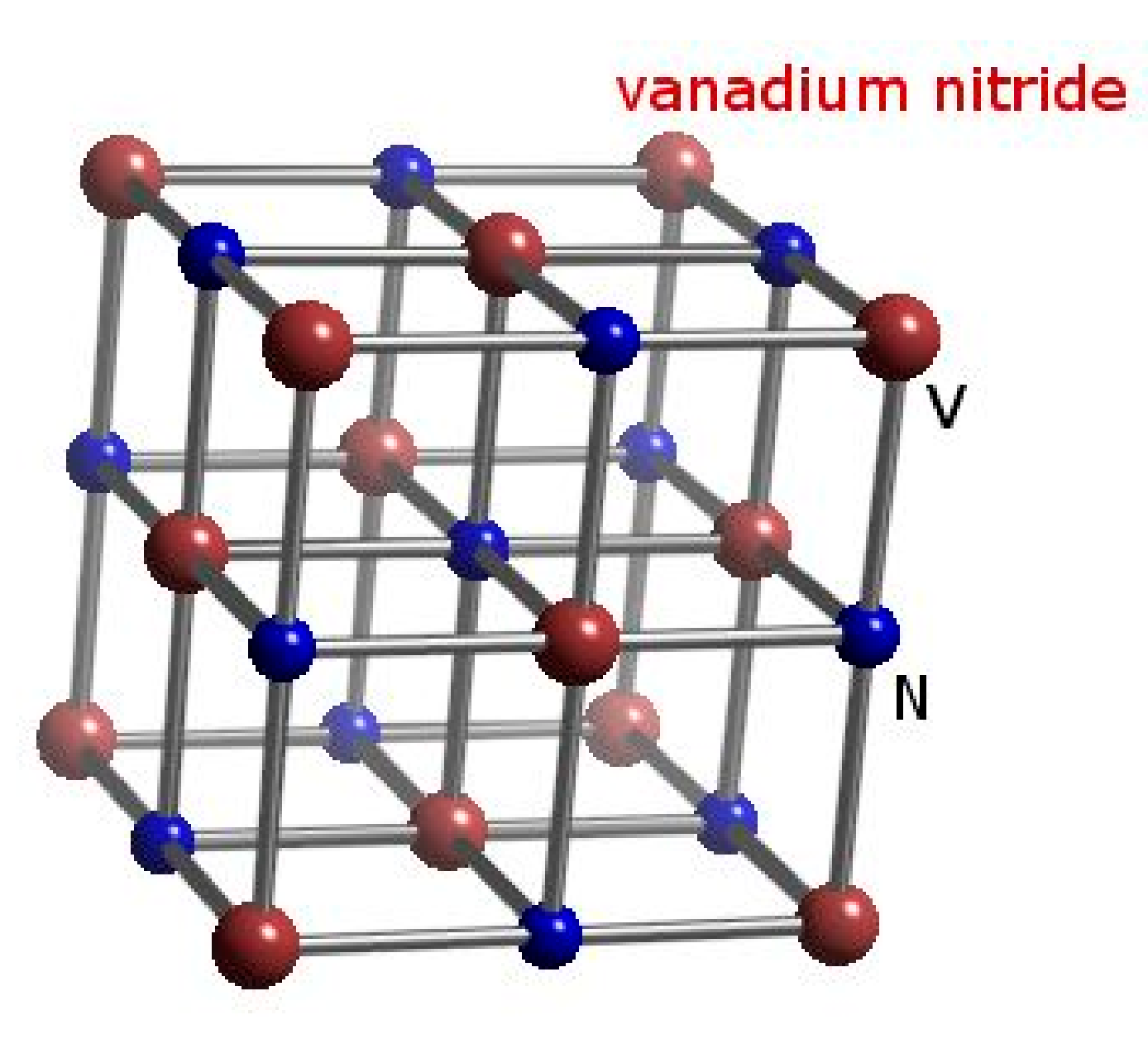
7.2. Crystal Structure for Synthetic VN
7.3. EBSD Data for Uakitite
7.4. Diffraction Data for Uakitite
8. Discussion and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bannister, F.A. Osbornite, meteoritic titanium nitride. Mineral. Mag. 1941, 26, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.A.; Keil, K.; Mason, B. Silicon oxynitride: A meteoritic mineral. Science 1964, 146, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, V.F.; Scott, E.R.D. First nitride (CrN) in iron meteorites. Nat. Phys. Sci. 1971, 233, 113–114. [Google Scholar] [CrossRef]
- Ramdohr, P. The Opaque Minerals in Stony Meteorites; Elsevier Publishing Company: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 1973; 245p. [Google Scholar]
- Buchwald, V.F. Handbook of Iron Meteorites; University California Press: Berkeley, CA, USA, 1975. [Google Scholar]
- Nielsen, H.P.; Buchwald, V.F. Roaldite, a new nitride in iron meteorites. Proc. Lunar Planet. Sci. Conf. 1981, 12, 1343–1348. [Google Scholar]
- Axon, H.J.; Kinder, J.; Haworth, C.W.; Horsfield, J.W. Carlsbergite, CrN, in troilite, FeS, of the Sikhote Alin meteoritic iron. Mineral. Mag. 1981, 44, 107–109. [Google Scholar] [CrossRef]
- Khodakovsky, I.L.; Petaev, M.I. Thermodynamic properties and formation conditions for osbornite, sinoite and carlsbergite in meteorites. Geokhimiya 1981, 19, 329–340. (In Russian) [Google Scholar]
- Nolze, G.; Wagner, G.; Saliwan Neumann, R.; Skála, R.; Geist, V. Orientation relationships of carlsbergite in schreibersite and kamacite in the north Chile iron meteorite. Mineral. Mag. 2006, 70, 373–382. [Google Scholar] [CrossRef]
- Casanova, I. Osbornite and the distribution of titanium in enstatite meteorites. Meteoritics 1992, 27, 208–209. [Google Scholar]
- Russell, S.S.; Lee, M.R.; Arden, J.W.; Pillinger, C.T. The isotopic composition and origins of silicon nitride in ordinary and enstatite chondrites. Meteoritics 1995, 30, 399–404. [Google Scholar] [CrossRef]
- Hoppe, P.; Geiss, J.; El Goresy, A. Nitrogen isotopes in sinoite grains of the Yilmia enstatite chondrite. Meteoritics 1989, 24, 278–298. [Google Scholar]
- Weber, D.; Zinner, E.K.; Bishoff, A. An ion microprobe study of an osbornite-bearing inclusion from ALH-85085. Meteoritics 1994, 29, 547–548. [Google Scholar]
- Rubin, A.E. Sinoite (Si2N2O): Crystallization from EL chondrite impact melts. Am. Mineral. 1997, 82, 1001–1006. [Google Scholar] [CrossRef]
- Lee, M.R.; Russell, S.S.; Arden, J.W.; Pillinger, C.T. Nierite (Si3N4), a new mineral from ordinary and enstatite chondrites. Meteoritics 1995, 30, 387–398. [Google Scholar] [CrossRef]
- Alexander, C.M.O.; Barber, D.J.; Hutchison, R.H. The microstructure of Semarkona and Bishunpur. Geochim. Cosmochim. Acta 1989, 53, 3045–3057. [Google Scholar] [CrossRef]
- Alexander, C.M.O.D.; Swan, P.; Prombo, C.A. Occurrence and implications of silicon nitride in enstatite chondrites. Meteoritics 1994, 29, 79–85. [Google Scholar] [CrossRef]
- Grokhovsky, V.I. Osbornite in CB/CH-like Carbonaceous Chondrite Isheyevo. Meteorit. Planet. Sci. 2006, 41 (Suppl. 8), A68. [Google Scholar]
- Rubin, A.E.; Ma, C. Meteoritic minerals and their origins. Chem. Erde 2017, 77, 325–385. [Google Scholar] [CrossRef]
- McCoy, T.J.; Keil, K.; Bogard, D.D.; Garrison, D.H.; Casanova, I.; Lindstrom, M.M.; Brearley, A.J.; Kehm, K.; Nichols, R.H.; Hohenberg, C.M. Origin and history of impact-melt rocks of enstatite chondrite parentage. Geochim. Cosmochim. Acta 1995, 59, 161–175. [Google Scholar] [CrossRef]
- Silvestri, O. Das Vorkommen des Stickstoffeisens unter den Fumarolen-Producten des Aetna und Künstliche Darstellung dieser Verbindung. Ann. Phys. Chem. 1876, 157, 165–172. (In German) [Google Scholar] [CrossRef] [Green Version]
- Charles, P.; Berman, H.; Frondel, C. The System of Mineralogy of James Dwight Dana and Edward Salisbury Dana. Yale University 1837–1892, Seventh edition. Science 1944, 1, 126. [Google Scholar]
- Russo, M. I Minerali di Formazione Fumarolica della Grande Eruzione Vesuviana del 1906; Open File Report; Istituto Nazionale di Geofisica e Vulcanologia, sezione Napoli—Osservatorio Vesuviano: Napoli, Italy, 2006; Volume 6, 39p. (In Italian) [Google Scholar]
- Lastochkin, E.I.; Ripp, G.S.; Izbrodin, I.A.; Khromova, E.A.; Sharygin, V.V. Mineral composition of meteorite Uakit (Republic of Buryatia). In Proceedings of the IV Russian Young Scientists Conference; GIN SB RAS, Ulan-Ude, Russia, 30 April–1 June 2017; pp. 146–148. (In Russian). [Google Scholar]
- Ripp, G.S.; Sharygin, V.V.; Izbrodin, I.A.; Ragozin, A.L.; Khromova, E.A. Mineralogy and geochemistry of iron meteorite Uakit (IIAB), Buryatia. In Proceedings of the 200th Anniversary Meeting of the Russian Mineralogical Society, Saint-Petersburg, Russia, 10–13 October 2017; Volume 2, pp. 311–313. (In Russian). [Google Scholar]
- Sharygin, V.V.; Ripp, G.S.; Yakovlev, G.A.; Seryotkin, Y.V.; Karmanov, N.S.; Izbrodin, I.A.; Grokhovsky, V.I.; Khromova, E.A. Uakitite, IMA 2018-003. CNMNC Newsletter No. 43, June 2018, page 781. Mineral. Mag. 2018, 82, 779–785. [Google Scholar]
- Sharygin, V.V.; Ripp, G.S.; Yakovlev, G.A.; Seryotkin, Y.V.; Karmanov, N.S.; Izbrodin, I.A.; Grokhovsky, V.I.; Khromova, E.A. Uakitite VN, a new nitride in iron meteorites. Meteorit. Planet. Sci. 2018, 53, 6052. [Google Scholar]
- Sharygin, V.V.; Ripp, G.S.; Yakovlev, G.A.; Seryotkin, Y.V.; Karmanov, N.S. New mineral species in iron meteorite Uakit (IIAB), Buryatia. In Abstracts of International Conference dedicated to the 110th anniversary of Academician V.S. Sobolev “The Problems of Magmatic and Metamorphic Petrology, Geodynamics and Genesis Of Diamonds”; IGM SD RAS, Publishing House of SB RAS: Novosibirsk, Russia, 2018; p. 167. [Google Scholar]
- Becker, K.; Ebert, F. Die Kristallstruktur einiger binärer Carbide und Nitride. Z. Phys. 1925, 31, 268–272. (In German) [Google Scholar] [CrossRef]
- Pessall, N.; Gold, R.E.; Johansen, H.A. A study of superconductivity in interstitial compounds. J. Phys. Chem. Solids 1968, 29, 19–38. [Google Scholar] [CrossRef]
- Pflüger, J.; Fink, J.; Weber, W.; Bohnen, K.P.; Crecelius, G. Dielectric properties of TiCx, TiNx, VCx, and VNx from 1.5 to 40 eV determined by electron-energy-loss spectroscopy. Phys. Rev. B Condens. Matter 1984, 30, 1155–1163. [Google Scholar]
- Zhao, B.R.; Chen, L.; Luo, H.L.; Jack, M.D.; Mullin, D.P. Superconducting and normal-state properties of vanadium nitride. Phys. Rev. B Condens. Matter 1984, 29, 6198–6202. [Google Scholar] [CrossRef]
- Lengauer, W.; Ettmayer, P. Physical and mechanical properties of cubic δ-VN1−x. J. Less Common Met. 1985, 109, 351–359. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chuang, H.C.; Liu, E.W.; Chang, Y.C. Effects of dilution and preheating on SHS of vanadium nitride. Ceram. Int. 2005, 31, 95–104. [Google Scholar] [CrossRef]
- Choi, D.; Blomgren, G.E.; Kumta, P.N. Fast and reversible surface redox reaction in nanocrystalline vanadium nitride supercapacitors. Adv. Mater. 2006, 18, 1178–1182. [Google Scholar] [CrossRef]
- Muñoz Riofano, R.M.; Casteletti, L.; Nascente, P.A.P. Study of the wear behavior of ion nitrided steels with different vanadium contents. Surf. Coat. Tech. 2006, 200, 6101–6110. [Google Scholar] [CrossRef]
- Glaser, A.; Surnev, S.; Netzer, F.P.; Fateh, N.; Fontalvo, G.A.; Mitterer, C. Oxidation of vanadium nitride and titanium nitride coatings. Surf. Sci. 2007, 601, 1153–1159. [Google Scholar] [CrossRef]
- Huang, J.-W.; Peng, H.; Xia, G.-B. Microwave synthesis of vanadium nitride for industrial applications. Ironmak. Steelmak. 2009, 36, 110–114. [Google Scholar] [CrossRef]
- Huang, T.; Mao, S.; Zhou, G.; Wen, Z.; Huang, X.; Cib, S.; Chen, J. Hydrothermal synthesis of vanadium nitride and modulation of its catalytic performance for oxygen reduction reaction. Nanoscale 2014, 6, 9608–9613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Zhang, S.; Li, B.; Wang, Y.; Lee, J.-W.; Li, F.; Zhao, D. Improvement of tribological performance of CrN coating via multilayering with VN. Surf. Coat. Tech. 2013, 231, 357–363. [Google Scholar] [CrossRef]
- Mishra, P.P.; Theerthagiri, J.; Panda, R.N. Mesoporous vanadium nitride synthesized by chemical routes. Adsorpt. Sci. Technol. 2014, 32, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Sen, U. Thermo-reactive diffusion vanadium nitride coatings on AISI 1020 steel. Key Eng. Mat. 2004, 264–268, 577–580. [Google Scholar] [CrossRef]
- Gyger, F.; Bockstaller, P.; Gerthsen, D.; Feldmann, C. Liquid-crystalline phases with liquid ammonia: Synthesis of porous Si3N4, TiN, VN, and H2−sorption of Si3N4 and Pd@Si3N4. Chem. Mater. 2016, 28, 7816–7824. [Google Scholar] [CrossRef] [Green Version]
- Morel, A.; Borjon-Piron, Y.; Porto, R.L.; Brousse, T.; Bélangerc, D. Suitable conditions for the use of vanadium nitride as an electrode for electrochemical capacitor. J. Electrochem. Soc. 2016, 163, A1077–A1082. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Y.; Liu, T.; Huang, J.; Xue, N.; Hu, P. Preparation of vanadium nitride using a thermally processed precursor with coating structure. Metals 2017, 7, 360. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.E.; Gan, B.K.; McKenzie, D.R.; Bilek, M.M.M.; Taylor, M.B.; McCulloch, D.G.; Latella, B.A. Correlation between stress and hardness in pulsed cathodic arc deposited titanium/vanadium nitride alloys. J. Phys. Condens. Matter 2004, 16, 7947–7954. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.; Zhang, J.; Wang, L.; Leinenweber, K.; He, D.; Zhao, Y. Synthesis, hardness, and electronic properties of stoichiometric VN and CrN. Cryst. Growth Des. 2016, 16, 351–358. [Google Scholar] [CrossRef]
- Sharygin, V.V. Phase CuCrS2 in Uakit iron meteorite (IIAB), Buryatia, Russia: Preliminary data. In Spri. Proceed. in Earth, Environ. Sci., Book “Minerals: Structure, Properties, Methods of Investigation”, 9th Geoscience Conference for Young Scientists, Ekaterinburg, Russia, February 5–8, 2018; Votyakov, S., Kiseleva, D., Grokhovsky, V., Shchapova, Y., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 229–236. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Yakovlev, G.A.; Seryotkin, Y.V.; Karmanov, N.S.; Novoselov, K.A.; Karabanalov, M.S. Grokhovskyite, IMA 2019-065. CNMNC Newsletter No. 52; December 2019, page 890. Mineral. Mag. 2019, 83, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Pouchou, I.L.; Pichoir, F. “PaP” (phi-rho-z) procedure for improved quantitative microanalysis. In Microbeam Analysis; Armstrong, I.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Arbuzov, M.P.; Khaenko, B.V.; Frenkel, O.A. X-ray diffraction investigation of the phases in the system V-N. Neorg. Mater. 1975, 11, 196–200. (In Russian) [Google Scholar]
- Khaenko, B.V. X-ray examination of phase equilibria in V-N system. Dopovidi Akademii Nauk Ukr. RSR Seriya A Fiz. Mat. Tekhnichni Nauk. 1977, 39, 275–279. (In Ukrainian) [Google Scholar]
- Onozuka, T. Vacancy ordering in VN1−x. J. Appl. Crystallogr. 1978, 11, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Onozuka, T. Nitrogen ordering in V9N4 and V32N16 studied by neutron and electron diffraction. T. Jpn. I. Met. 1982, 23, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Carlson, O.N.; Smith, J.F.; Nafziger, R.H. N-V (Nitrogen-Vanadium). In Binary Alloy Phase Diagrams, 2nd ed.; Massalski, T.B., Ed.; ASM International: Materials Park, OH, USA, 1990; Volume 3, pp. 2709–2712. [Google Scholar]
- Ohtani, H.; Hillert, M. A thermodynamic assessment of the V-N system. Calphad 1991, 15, 11–24. [Google Scholar] [CrossRef]
- Ohtani, H.; Hillert, M. A thermodynamic assessment of the Fe-N-V system. Calphad 1991, 15, 25–39. [Google Scholar] [CrossRef]
- Okamoto, H. Comment on N-V (Nitrogen-Vanadium). J. Phase Equilib. 1994, 15, 454. [Google Scholar] [CrossRef]
- Okamoto, H. N-V (Nitrogen-Vanadium). J. Phase Equilib. 2001, 22, 362. [Google Scholar] [CrossRef]
- Du, Y.; Schmid-Fetzer, R.; Ohtani, H. Phase Diagram V-N. Z. Metallkd. 1997, 88, 545–556. [Google Scholar]
- Ravi, C.; Sahu, H.K.; Valsakumar, M.C.; van de Walle, A. Cluster expansion Monte Carlo study of phase stability of vanadium nitrides. Phys. Rev. B Condens. Matter 2010, 81, 104111. [Google Scholar] [CrossRef] [Green Version]
- Kubel, F.; Lengauer, W.; Yvon, K.; Knorr, K.; Junod, A. Structural phase transition at 205 K in stoichiometric vanadium nitride. Phys. Rev. B Condens. Matter 1988, 38, 12908–12912. [Google Scholar] [CrossRef]
- Ivashchenko, V.I.; Turchi, P.E.A. Phonon softening and the phase transition in VN. Phys. Rev. B Condens. Matter 2008, 78, 224113. [Google Scholar] [CrossRef]
- Mei, A.B.; Hellman, O.; Wireklint, N.; Schlepütz, C.M.; Sangiovanni, D.G.; Alling, B.; Rockett, A.; Hultman, L.; Petrov, I.; Greene, J.E. Dynamic and structural stability of cubic vanadium nitride. Phys. Rev. B Condens. Matter 2015, 91, 054101. [Google Scholar] [CrossRef] [Green Version]
- Duwez, P.E.; Odell, F. Phase Relationships in the Binary Systems of Nitrides and Carbides of Zirconium, Columbium, Titanium, and Vanadium. J. Electrochem. Soc. 1950, 97, 299–304. [Google Scholar] [CrossRef]
- Schönberg, N. An X-ray investigation on ternary phases in the Ta-Me-N systems (Me = Ti, Cr, Mn, Fe, Co, Ni). Acta Chem. Scandinavica 1954, 8, 213–220. [Google Scholar] [CrossRef]
- Nowotny, H.; Benesovsky, F.; Rudy, E. Hochschmelzende Systeme mit Hafniumkarbid und -nitrid. Monat. Chem. Verw. Tl. 1960, 91, 348–356. (In German) [Google Scholar] [CrossRef]
- Brauer, G.; Schnell, W.D. Zur Kenntnis des Systems Vanadium-Stickstoff und des reinen Vanadiums. J. Less Common Met. 1964, 6, 326–332. (In German) [Google Scholar] [CrossRef]
- Yen, C.M.; Toth, L.E.; Shy, Y.M.; Anderson, D.E.; Rosner, L.G. Superconducting Hc-Jc and Tc measurements in the Nb-Ti-N, Nb-Hf-N, and Nb-V-N ternary systems. J. Appl. Phys. 1967, 38, 2268–2271. [Google Scholar] [CrossRef]
- Hosoya, S.; Yamagishi, T.; Tokonami, M. Study of electron state in vanadium nitride by intensity measurements of X-ray diffraction. J. Phys. Soc. Jpn. 1968, 24, 363–367. [Google Scholar] [CrossRef]
- Spear, K.E.; Leitnaker, J.M. Equilibrium investigations of carbon-rich V(C,N) solutions. High Temp. Sci. 1969, 1, 401–411. [Google Scholar]
- Kieffer, R.; Nowotny, H.; Ettmayer, P.; Dufek, G. Neue Untersuchungen über die Mischbarkeit von Elbergangsmetallnitriden und -karbiden. Met. (Berl.) (Met. (Heidelb.)) 1972, 26, 701–708. (In German) [Google Scholar]
- Gatterer, J.; Dufek, G.; Ettmayer, P.; Kieffer, R. Das kubische Tantalmononitrid (B1-Typ) und seine Mischbarkeit mit den isotypen Übergangsmetall-nitriden und -carbiden. Monat. Chem. Verw. Tl. 1975, 106, 1137–1147. (In German) [Google Scholar] [CrossRef]
- Eddine, M.N.; Bertaut, E.F.; Roubin, M.; Paris, J. Etude cristallographique de Cr1−xVxN a basse température. Acta Crystall. B Stru 1977, 33, 3010–3013. (In French) [Google Scholar] [CrossRef] [Green Version]
- Ettmayer, P.; Schebesta, W.; Vendl, A.; Kieffer, R. Beitrag zur Kenntnis des Systems Vanadin-Chrom-Stickstoff. Monat. Chem. Verw. Tl. 1978, 109, 929–941. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Yang, B.; Wang, W.; Qian, Y. Thermal nitridation synthesis of MN (M = Ti, V and Cr) nanocrystals from metals and NH4Cl. Mater. Res. Bull. 2004, 39, 957–962. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yagi, T. Systematic study of formation and crystal structure of 3d-transition metal nitrides synthesized in a supercritical nitrogen fluid under 10 GPa and 1800 K using diamond anvill cell and YAG laser heating. J. Alloy Compd. 2005, 403, 131–142. [Google Scholar] [CrossRef]
- Gajbhiye, N.S.; Ningthoujam, R.S. Low temperature synthesis, crystal structure and thermal stability studies of nanocrystalline VN particles. Mater. Res. Bull. 2006, 41, 1612–1621. [Google Scholar] [CrossRef]
- Zhao, H.; Lei, M.; Chen, X.; Tang, W. Facile route to metal nitrides through melamine and metal oxides. J. Mater. Chem. 2006, 16, 4407–4412. [Google Scholar] [CrossRef]
- Fokin, V.N.; Fokina, E.E.; Tarasov, B.P. Reaction of the intermetallide ZrV2 with ammonia. Russ. J. Inorg. Chem. 2012, 57, 21–23. [Google Scholar] [CrossRef]
- Lei, L.; Yin, W.; Jiang, X.; Lin, S.; He, D. Synthetic route to metal nitrides: High-pressure solid-state metathesis reaction. Inorg. Chem. 2013, 52, 13356–13362. [Google Scholar] [CrossRef] [PubMed]
- Saravanakannan, V.; Radhakrishnan, T. Influence of oxygen and titanium substitution on vanadium nitride nanostructures—ADFT study. Int. J. Chem. Tech. Res. 2014, 6, 5745–5750. [Google Scholar]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- El Goresy, A.; Kullerud, G. Phase Relations in the System Cr-Fe-S. In Meteorite Research: Proceedings of a Symposium on Meteorite Research Held in Vienna, Austria, 7–13 August 1968; Millman, P.M., Ed.; D. Reidel Publishing Co.: Dordrecht, The Netherlands, 1969; pp. 638–656. [Google Scholar]
- Ma, C.; Beckett, J.R.; Rossman, G.R. Allendeite (Sc4Zr3O12) and hexamolybdenum (Mo,Ru,Fe), two new minerals from an ultrarefractory inclusion from the Allende meteorite. Am. Mineral. 2014, 99, 654–666. [Google Scholar] [CrossRef]
- Ma, C.; Krot, A.N. Hutcheonite, Ca3Ti2(SiAl2)O12, a new garnet mineral from the Allende meteorite: An alteration phase in a Ca-Al-rich inclusion. Am. Mineral. 2014, 99, 667–670. [Google Scholar] [CrossRef]
- Ma, C.; Beckett, J.R. Nuwaite (Ni6GeS2) and butianite (Ni6SnS2), two new minerals from the Allende meteorite: Alteration products in the early solar system. Am. Mineral. 2018, 103, 1918–1924. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Yakovlev, G.A.; Wirth, R.; Seryotkin, Y.V.; Sokol, E.V.; Nigmatulina, E.N.; Karmanov, N.S.; Pautov, L.A. Nataliakulikite, Ca4Ti2(Fe3+, Fe2+)(Si, Fe3+, Al)O11, a new perovskite-supergroup mineral from Hatrurim Basin, Negev Desert, Israel. Minerals 2019, 9, 700. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Lee, S.; Xu, H. Luogufengite: A new nano-mineral of Fe2O3 polymorph with giant coercive field. Am. Mineral. 2017, 102, 711–719. [Google Scholar] [CrossRef]










| Mineral | Fe | Mn | Ni | Co | Zn | Cu | Cr | V | P | S | Sum | Formula | Fe | Mn | Ni | Co | Zn | Cu | Cr | V | P | S | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| based on | |||||||||||||||||||||||
| Kamacite | n = 34 | 93.46 | n.d. | 6.09 | 0.48 | n.d. | n.d. | n.d. | n.d. | 0.06 | n.d. | 100.09 | 1 ion | 0.94 | 0.06 | 0.00 | 0.00 | ||||||
| sd | 0.56 | 0.51 | 0.05 | 0.04 | |||||||||||||||||||
| Taenite | n = 19 | 67.63 | n.d. | 32.12 | 0.13 | n.d. | 0.23 | n.d. | n.d. | n.d. | n.d. | 100.10 | 1 ion | 0.69 | 0.31 | 0.00 | 0.00 | ||||||
| sd | 6.48 | 6.54 | 0.05 | 0.09 | |||||||||||||||||||
| Tetrataenite | n = 14 | 42.66 | n.d. | 56.96 | 0.01 | n.d. | 0.24 | 0.17 | n.d. | n.d. | n.d. | 100.04 | 1 ion | 0.44 | 0.56 | 0.00 | 0.00 | 0.00 | |||||
| sd | 1.08 | 1.22 | 0.02 | 0.10 | 0.15 | ||||||||||||||||||
| Cohenite | n = 18 | 91.64 | n.d. | 1.57 | 0.08 | n.d. | n.d. | 0.12 | n.d. | n.d. | n.d. | 93.41 | 3 ions | 2.95 | 0.05 | 0.00 | 0.00 | ||||||
| sd | 0.29 | 0.06 | 0.02 | 0.05 | |||||||||||||||||||
| Schreibersite | n = 19 | 50.47 | n.d. | 34.25 | 0.09 | n.d. | n.d. | 0.04 | n.d. | 15.16 | 0.01 | 100.03 | 4 ions | 1.83 | 1.18 | 0.00 | 0.00 | 0.99 | 0.00 | ||||
| sd | 3.56 | 3.51 | 0.02 | 0.09 | 0.08 | 0.02 | |||||||||||||||||
| Nickelphosphide | n = 12 | 39.43 | n.d. | 45.02 | 0.05 | n.d. | n.d. | 0.25 | n.d. | 15.17 | 0.05 | 99.97 | 4 ions | 1.43 | 1.56 | 0.00 | 0.01 | 0.99 | 0.00 | ||||
| sd | 2.81 | 2.74 | 0.04 | 0.37 | 0.06 | 0.04 | |||||||||||||||||
| Daubréelite | n = 39 | 19.22 | 0.23 | 0.07 | 0.01 | 0.48 | 0.07 | 35.47 | 0.01 | n.d. | 44.42 | 99.91 | 7 ions | 0.99 | 0.01 | 0.00 | 0.00 | 0.02 | 0.00 | 1.97 | 0.00 | 4.00 | |
| sd | 0.84 | 0.20 | 0.09 | 0.02 | 0.53 | 0.03 | 0.63 | 0.02 | 0.11 | ||||||||||||||
| Kalininite | n = 11 | 10.15 | 0.04 | n.d. | n.d. | 11.50 | n.d. | 34.55 | n.d. | n.d. | 43.68 | 99.92 | 7 ions | 0.53 | 0.00 | 0.52 | 1.95 | 4.00 | |||||
| sd | 0.92 | 0.00 | 0.91 | 0.20 | 0.05 | ||||||||||||||||||
| Troilite | n = 13 | 62.42 | n.d. | 0.16 | n.d. | n.d. | 0.12 | 0.67 | 0.01 | n.d. | 36.53 | 99.92 | 2 ions | 0.98 | 0.00 | 0.00 | 0.01 | 1.00 | |||||
| sd | 0.32 | 0.29 | 0.03 | 0.18 | 0.02 | 0.07 | |||||||||||||||||
| Pentlandite | n = 9 | 32.87 | n.d. | 31.75 | 1.39 | n.d. | 0.01 | 0.68 | 0.02 | n.d. | 33.27 | 99.98 | 17 ions | 4.54 | 4.17 | 0.18 | 0.00 | 0.10 | 0.00 | 8.00 | |||
| sd | 3.48 | 2.79 | 1.13 | 0.02 | 0.16 | 0.02 | 0.05 | ||||||||||||||||
| Heazlewoodite | n = 5 | 5.68 | n.d. | 64.77 | 1.57 | n.d. | n.d. | 0.45 | 0.02 | n.d. | 27.45 | 99.94 | 5 ions | 0.24 | 2.63 | 0.06 | 0.02 | 0.00 | 2.04 | ||||
| sd | 0.70 | 2.20 | 1.27 | 0.29 | 0.02 | 1.02 |
| VN (All Grains) | VN-1 | VN-2 | VN-3 | VN-6 | Ideal-1 | Ideal-2 | CrN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Min | Max | Mean | sd | Mean | sd | Mean | sd | Mean | VN | V0.9Cr0.1N | Mean | sd | |
| n = 54 | n = 34 | n = 9 | n = 10 | n = 1 | n = 47 | ||||||||||
| V | 71.33 | 0.21 | 70.91 | 71.90 | 71.33 | 0.22 | 71.24 | 0.13 | 71.42 | 0.24 | 71.07 | 78.43 | 70.48 | 0.06 | 0.08 |
| Cr | 5.57 | 0.27 | 5.02 | 6.18 | 5.59 | 0.31 | 5.57 | 0.19 | 5.53 | 0.18 | 5.43 | 0.00 | 7.99 | 76.64 | 0.73 |
| Fe | 1.56 | 0.22 | 1.16 | 2.08 | 1.54 | 0.24 | 1.66 | 0.17 | 1.54 | 0.21 | 2.08 | 0.00 | 0.00 | 2.18 | 0.73 |
| N | 21.41 | 0.07 | 21.22 | 21.54 | 21.41 | 0.07 | 21.40 | 0.07 | 21.44 | 0.05 | 21.38 | 21.57 | 21.53 | 21.13 | 0.08 |
| Sum | 99.88 | 99.87 | 99.86 | 99.93 | 99.96 | 100.00 | 100.00 | 100.01 | |||||||
| Formula based on 2 ions | |||||||||||||||
| V | 0.914 | 0.914 | 0.913 | 0.914 | 0.911 | 1.000 | 0.900 | 0.001 | |||||||
| Cr | 0.070 | 0.070 | 0.070 | 0.069 | 0.068 | 0.000 | 0.100 | 0.975 | |||||||
| Fe | 0.018 | 0.018 | 0.019 | 0.018 | 0.024 | 0.000 | 0.000 | 0.026 | |||||||
| N | 0.998 | 0.998 | 0.998 | 0.998 | 0.997 | 1.000 | 1.000 | 0.998 | |||||||
| h | k | l | dcalc, Å | Irel |
| 1 | 1 | 1 | 2.386 | 71.22 |
| 2 | 0 | 0 | 2.066 | 100.00 |
| 2 | 2 | 0 | 1.461 | 61.15 |
| 3 | 1 | 1 | 1.246 | 29.12 |
| 2 | 2 | 2 | 1.193 | 18.92 |
| 4 | 0 | 0 | 1.033 | 8.03 |
| 3 | 3 | 1 | 0.948 | 10.16 |
| 4 | 2 | 0 | 0.924 | 20.55 |
| 4 | 2 | 2 | 0.844 | 14.29 |
| 5 | 1 | 1 | 0.795 | 4.99 |
| 3 | 3 | 3 | 0.795 | 1.66 |
| 4 | 4 | 0 | 0.731 | 4.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharygin, V.V.; Ripp, G.S.; Yakovlev, G.A.; Seryotkin, Y.V.; Karmanov, N.S.; Izbrodin, I.A.; Grokhovsky, V.I.; Khromova, E.A. Uakitite, VN, a New Mononitride Mineral from Uakit Iron Meteorite (IIAB). Minerals 2020, 10, 150. https://doi.org/10.3390/min10020150
Sharygin VV, Ripp GS, Yakovlev GA, Seryotkin YV, Karmanov NS, Izbrodin IA, Grokhovsky VI, Khromova EA. Uakitite, VN, a New Mononitride Mineral from Uakit Iron Meteorite (IIAB). Minerals. 2020; 10(2):150. https://doi.org/10.3390/min10020150
Chicago/Turabian StyleSharygin, Victor V., German S. Ripp, Grigoriy A. Yakovlev, Yurii V. Seryotkin, Nikolai S. Karmanov, Ivan A. Izbrodin, Victor I. Grokhovsky, and Elena A. Khromova. 2020. "Uakitite, VN, a New Mononitride Mineral from Uakit Iron Meteorite (IIAB)" Minerals 10, no. 2: 150. https://doi.org/10.3390/min10020150
APA StyleSharygin, V. V., Ripp, G. S., Yakovlev, G. A., Seryotkin, Y. V., Karmanov, N. S., Izbrodin, I. A., Grokhovsky, V. I., & Khromova, E. A. (2020). Uakitite, VN, a New Mononitride Mineral from Uakit Iron Meteorite (IIAB). Minerals, 10(2), 150. https://doi.org/10.3390/min10020150