Analysis of the Distribution of Some Potentially Harmful Elements (PHEs) in the Krugersdorp Game Reserve, Gauteng, South Africa
Abstract
:1. Introduction
Soils
2. Materials and Methods
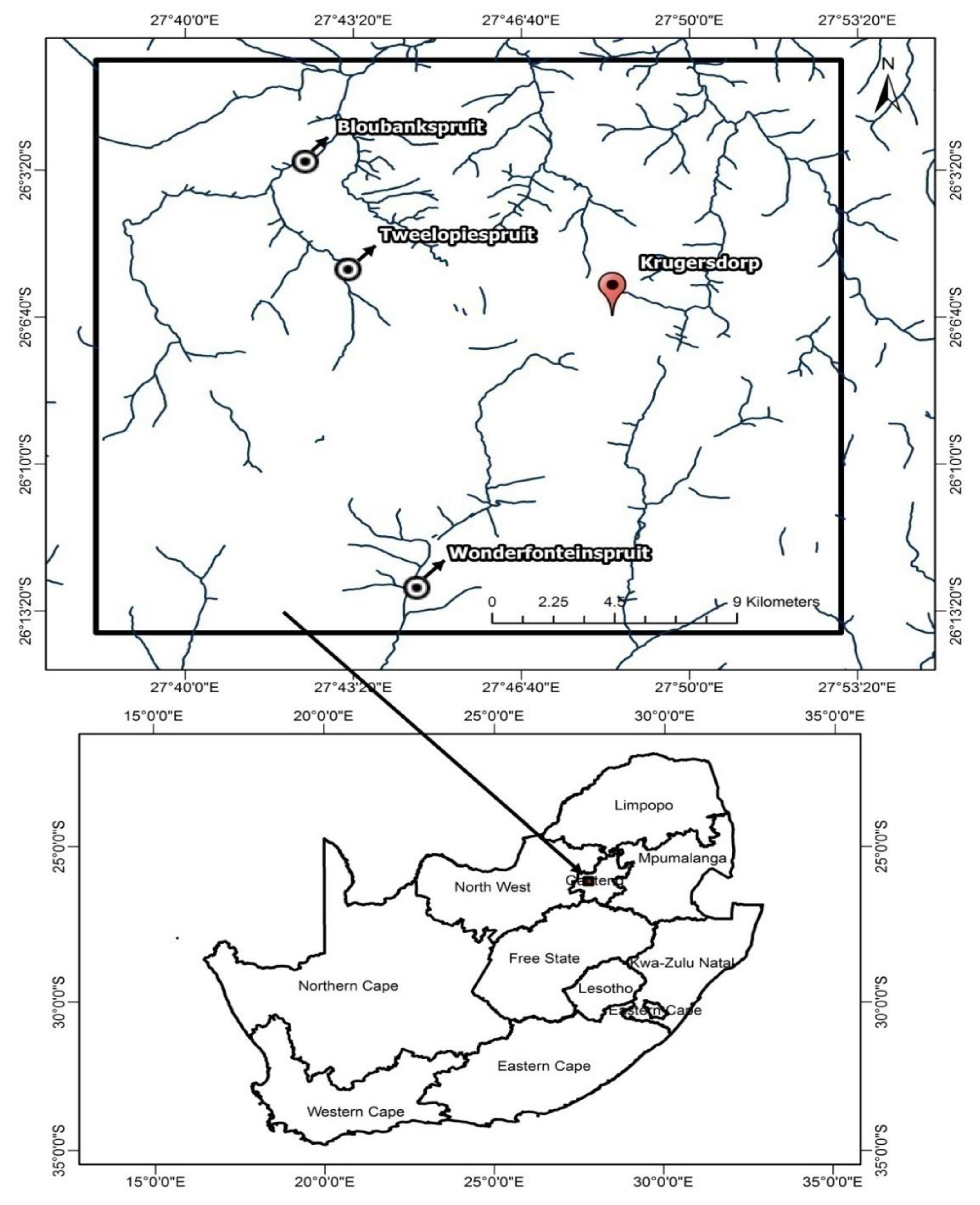
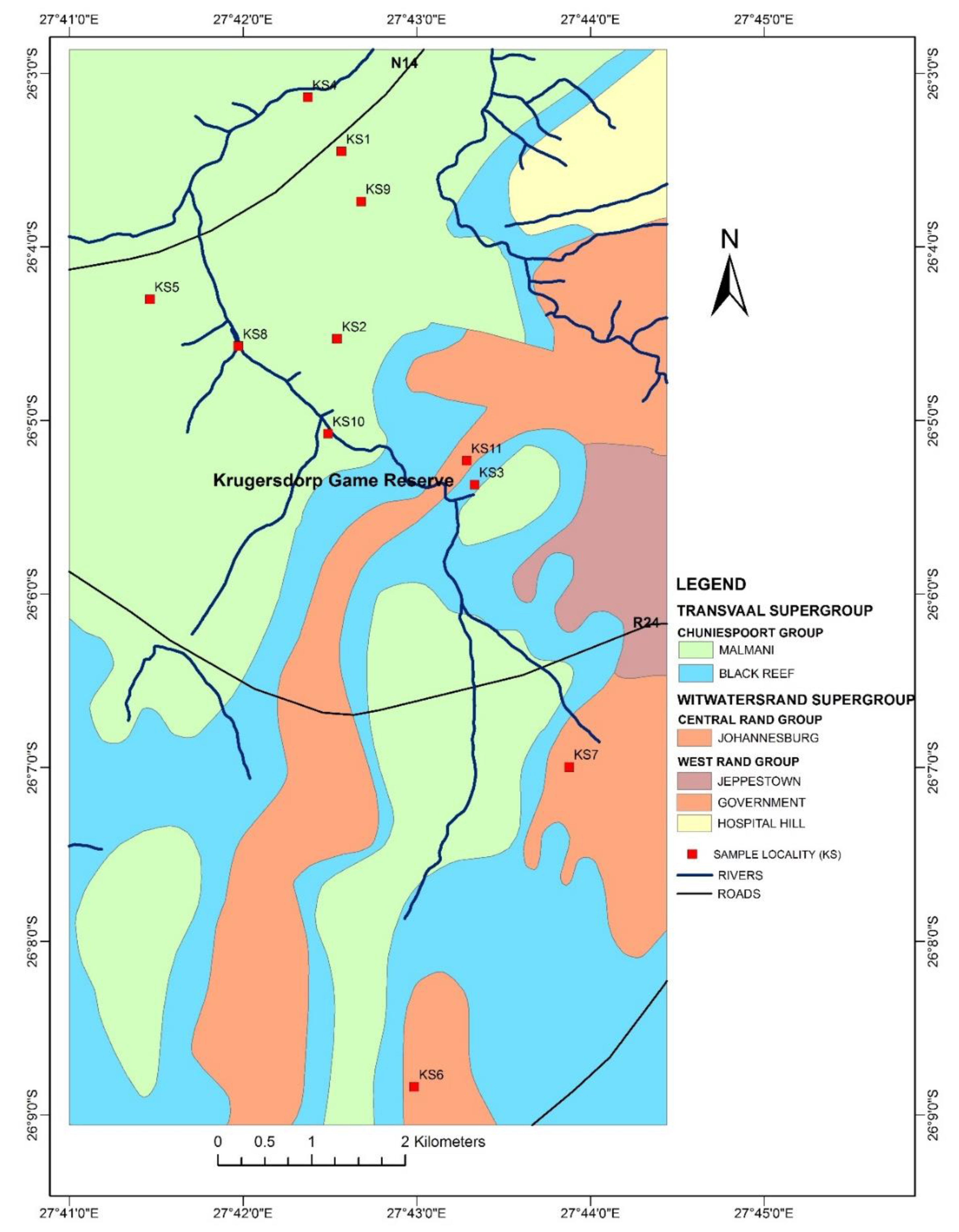
2.1. Sampling Technique and Sample Preparation
2.2. Soil Sample Preparation and Analysis
2.3. Quality Assurance Evaluation
2.4. Soil Fraction Analysed
3. Results and Discussion
3.1. Total Content of Selected Heavy Metals
3.2. Distribution of Soil Geochemical Data
3.3. Sampling Sites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nadasan, D.S.; Davies, T.C.; Shapi, M.; Chirenje, E. The distribution of some potentially harmful elements (PHEs) in the Krugersdorp Game Reserve, Gauteng, South Africa: Implications for wildlife health. In Proceedings of the IGCP/SIDA Projects 594 and 606, Closing Workshop, Prague, Czech Republic, 26–28 May 2014. [Google Scholar]
- Singo, N.K. An Assessment of Heavy Metal Pollution Near an Old Copper Mine Dump in Musina, South Africa. Ph.D. Thesis, University of South Africa, Pretoria, South Africa, 2013. [Google Scholar]
- Pereira, S.I.A.; Lima, A.I.G.; Figueira, E.M.A.P. Heavy metal toxicity in Rhizobium leguminosarum biovar viciae isolated from soils subjected to different sources of heavy metal contamination: Effect on protein expression. Appl. Soil Ecol. 2006, 33, 286–293. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Oves, M.; Wani, P.A. Heavy metal toxicity to legumes. In Heavy Metal Pollution; Samuel, E.B., William, C.W., Eds.; Nova Science: Hauppauge, NY, USA, 2008; pp. 197–225. [Google Scholar]
- Zang, H.; Luo, Y.; Makino, T.; Wu, L.; Nanzyo, M. The heavy metal partition in size fractions of the fine particles in agricultural soils contaminated by waste water and smelter dust. J. Hazard. Mater. 2013, 248, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.K.; KrishnaRaj, S.; Perras, M.R.; Vettakkorumakankav, N.N. Phytoremediation of heavy metal contaminated and polluted soils. In Heavy Metal Stress in Plants; Springer: Berlin, Germany, 1999; pp. 305–329. [Google Scholar]
- Battaglia, A.; Ghidini, S.; Campanini, G.; Spaggiari, R. Heavy metal contamination in little owl (Athene noctua) and common buzzard (Buteo buteo) from northern Italy. Ecotoxicol Environ. Saf. 2005, 60, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Madejón, E.; Pérez de Mora, A.; Felipe, E.; Burgos, P.; Cabrera, F. Soil amendments reduce trace element solubility in a contaminated soil and allow regrowth of natural vegetation. Environ. Pollut. 2006, 139, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001; Volume 867, pp. 219–796. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Abdullah, S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 1–31. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Winde, F. Uranium pollution of the Wonderfonteinspruit, 1997–2008 Part 1: Uranium toxicity, regional background and mining-related sources of uranium pollution. SA J. Radiol. 2010, 36, 13. [Google Scholar]
- Paterson, D.G. Soils and Agricultural Potential Along the Proposed Westgate-Tarlton-Kromdraai Power Line, Gauteng Province, South Africa. Report for Arcus Gibb; Report No. GW/A/2008/10; Institute for Soil Climate and Water of the Agricultural Research Council. 2008. Available online: http://projects.gibb.co.za/Portals/3/projects/200902%20Tarlton%20Kromdraai%20power/Environmental%20Impact%20Phase/Appendix%20H%20%20Agricultural%20Potential%20and%20Soils%20Study.pdf (accessed on 9 March 2016).
- Darnley, A.G.; Björklund, A.; Bølviken, B.; Gustavsson, N.; Koval, P.V.; Plant, J.A.; Steenfelt, A.; Tauchid, M.; Xie, X. A Global Geochemical Database for Environmental and Resource Management: Recommendations for International Geochemical Mapping; Final Report of IGCP Project 259; UNESCO Publishing: Paris, France, 1995. [Google Scholar]
- Leduc, C.; Itard, Y. Low sampling density exploration geochemistry for gold in arid and tropical climates: Comparison between conventional geochemistry and BLEG. Geochem. Explor. Environ. Anal. 2003, 3, 121–131. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Jensen, P.E. Electro-remediation of heavy-metal contaminated soil. In Soil and Sediment Remediation; IWA publishing: London, UK, 2005; pp. 264–288. [Google Scholar]
- Jung, M.C.; Thornton, I. Heavy metal contamination of soils and plants in the vicinity of a lead-zinc mine, Korea. Appl. Geochem. 1996, 11, 53–59. [Google Scholar] [CrossRef]
- Wang, H.-H.; Li, L.-Q.; Wu, X.M.; Pan, G.-X. Distribution of Cu and Pb in particle size fractions of urban soils from different city zones of Nanjing, China. J. Environ. Sci. 2006, 18, 482–487. [Google Scholar]
- ECOMED Eastern Europe SRL. Contaminated Soil Remediation. Available online: http://www.ecomed-intl.com/contaminated-soil-remediation.html (accessed on 5 March 2016).
- Li, Q.; Ji, H.; Qin, F.; Tang, L.; Guo, X.; Feng, J. Sources and the distribution of heavy metals in the particle size of soil polluted by gold mining upstream of Miyun Reservoir, Beijing: Implications for assessing the potential risks. Environ. Monit. Assess. 2014, 186, 6605–6626. Available online: http://dx.doi.org/10.1007/s10661-014-3877-4 (accessed on 5 March 2016). [CrossRef] [PubMed]
- Shuman, L.M. Chemical forms of micronutrients in soil. In Micronutrients in Agriculture; Mortvedt, J.J., Cox, F.R., Shuman, L.M., Welch, R.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1991. [Google Scholar]
- Farmaki, S.; Karakasi, O.; Moutsatsu, A. Pb2+ and Ni2+ Adsorption on Limestone and Dolomite Tailings. Inżynieria Mineralna - Lipiec-Grudzień, Journal of the Polish Mineral Engineering Society, July–December, 2014. Available online: http://www.potopk.com.pl/Full_text/2014_full/2014_2_35.pdf (accessed on 24 February 2016).
- Krishnamurti, G.S.R. Chemical methods for assessing contaminant bioavailability in soils. In Chemical Bioavailability in Terrestrial Environments, 1; Naidu, R., Ed.; Elsevier: Oxford, UK, 2008; pp. 495–520. ISBN 978-0-444-52169. [Google Scholar]
- Du Toit, S. Practical applications−effects of mine water drainage on the Krugersdorp Game Reserve. In Proceedings of the Conference on Mine Water Decant, Randfontein, South Africa, 14−15 October 2006; Mine Water Division, Water Institute of South Africa: Midrand, South Africa, 2006. [Google Scholar]
- Johnson, C.C.; Cave, M.R.; Palumbo-Roe, B.; Nathanail, C.P.; Lark, R.M. Methodology for the determination of normal background concentrations of contaminants in English soil. Sci. Total Environ. 2013, 454, 604–618. [Google Scholar]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Stream Destroyed by Acid Mine Water. Available online: http://earthlife.org.za/stream-destroyed-by-acid-mine-water/ (accessed on 12 June 2016).
- Ure, A.M.; Davidson, C.M. (Eds.) Chemical speciation in soils and related materials by selective chemical extraction. In Chemical Speciation in the Environment, 2nd ed.; Blackwell Science Ltd.: Oxford, UK, 2002. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Perel’man, A.I. Geochemistry of Epigenesis; Springer: Berlin, Germany, 1967; p. 266. [Google Scholar]
- DEAT (Department of Environmental Affairs and Tourism of South Africa). Mine Water Pollution—Acid mine Decant, Effluent and Treatment: A Consideration of Key Emerging Issues That May Impact the State of the Environment. Emerging Issues Paper: Mine Water Pollution 2008. Available online: http://www.hsph.harvard.edu/mining/files/South_Africa.pdf (accessed on 1 March 2016).

| Variables | Directive | August, 2006 | September, 2006 |
|---|---|---|---|
| pH | 6.5–8.4 | 5.1 * | 9.2 * |
| Conductivity (EC) (ms/m) | 70 | 493 * | 438 * |
| Sulphate (SO4) (mg/L) | 200 | 3230 * ** *** | 3000 * ** *** |
| Chloride (Cl) (mg/L) | 36 | 50 * | 43 * |
| Aluminium (Al) (mg/L) | 0.06 | 0.70 * | 0.88 * |
| Managnese (Mn) (mg/L) | 0.07 | 85.18 * ** *** | 18.28 * ** *** |
| Iron (Fe) (mg/L) | 0.1 | 74.67 * ** *** | 12.47 * ** *** |
| Analyte | Mo | Cu | Pb | Zn | Ag | Ni | Co | Mn | Fe | As | Au | Th | |||
| Unit | PPM | PPM | PPM | PPM | PPM | PPM | PPM | PPM | % | PPM | PPB | PPM | |||
| MDL | 0.1 | 0.1 | 0.1 | 1 | 0.1 | 0.1 | 0.1 | 1 | 0.01 | 0.5 | 0.5 | 0.1 | |||
| Sample | Type | ||||||||||||||
| 1 | KS1A | Soil | 0.9 | 15.8 | 5 | 23 | <0.1 | 29.5 | 11 | 2679 | 3.69 | 6.8 | 2.6 | 2.7 | |
| 2 | KS1B | Soil | 1 | 11.8 | 4.8 | 15 | <0.1 | 24 | 11.2 | 3128 | 2.74 | 5.2 | 3.6 | 2.2 | |
| 3 | KS1C | Soil | 1 | 16 | 6.4 | 18 | <0.1 | 26.3 | 11.6 | 2648 | 3.4 | 6.1 | 2.9 | 3.6 | |
| 4 | KS1D | Soil | 0.9 | 10.1 | 4.6 | 15 | <0.1 | 16.4 | 9.3 | 1801 | 2.14 | 5.2 | 3.8 | 2.2 | |
| 5 | KS2A | Soil | 0.5 | 16 | 7.5 | 25 | <0.1 | 38.5 | 10.5 | 5056 | 2.38 | 4.1 | 4.9 | 3 | |
| 6 | KS2B | Soil | 1.1 | 29.5 | 15.1 | 54 | <0.1 | 91.1 | 18 | >10,000 | 4.24 | 5.5 | 7.9 | 4 | |
| 7 | KS2C | Soil | 0.8 | 27.9 | 15.8 | 79 | <0.1 | 97.1 | 14.4 | >10,000 | 4.92 | 6.6 | 12.4 | 6.1 | |
| 8 | KS2C2 | Soil | 0.8 | 28.7 | 16.4 | 84 | <0.1 | 97.2 | 14.9 | >10,000 | 4.74 | 6.7 | 11.8 | 5.7 | |
| 9 | KS2D | Soil | 1.6 | 42 | 13.5 | 216 | <0.1 | 444.8 | 15.3 | >10,000 | 8.23 | 8.6 | 8.8 | 4.4 | |
| 10 | KS3A1 | Soil | 0.9 | 40.6 | 6.3 | 25 | <0.1 | 49.3 | 11.1 | 3625 | 4.15 | 6.5 | 15.7 | 4 | |
| 11 | KS3A2 | Soil | 0.7 | 41.1 | 6.5 | 27 | <0.1 | 50.3 | 11.8 | 3960 | 4.23 | 6.4 | 15.5 | 4.2 | |
| 13 | KS3B | Soil | 0.9 | 43.1 | 7.1 | 30 | <0.1 | 61.4 | 11 | 9233 | 4.32 | 7.4 | 13.1 | 4.1 | |
| 14 | KS3C | Soil | 0.8 | 43.4 | 10.2 | 54 | <0.1 | 54.5 | 10.8 | 2956 | 4.85 | 7.3 | 14.6 | 5 | |
| 15 | KS3D | Soil | 0.9 | 49 | 10.6 | 54 | <0.1 | 59.9 | 14.1 | 3401 | 4.85 | 7.6 | 11.1 | 4.5 | |
| 16 | KS4A | Soil | 1 | 29.8 | 12.4 | 202 | <0.1 | 273.1 | 93 | 7644 | 2.86 | 6.4 | 442.2 | 2.8 | |
| 17 | KS4B | Soil | 0.9 | 13.5 | 7 | 12 | <0.1 | 23.2 | 10.4 | 3226 | 2.59 | 3.6 | 4.7 | 3 | |
| 18 | KS5A | Soil | 0.7 | 20.1 | 9.2 | 13 | <0.1 | 21.7 | 11.4 | 1558 | 2.95 | 4 | 4 | 4.8 | |
| 19 | KS5B | Soil | 0.5 | 16.1 | 5.9 | 12 | <0.1 | 19.6 | 6.4 | 1082 | 2.47 | 3.3 | 3.8 | 4.3 | |
| 20 | KS6A | Soil | 0.7 | 18.5 | 12.4 | 15 | <0.1 | 29.7 | 16.2 | 812 | 3.23 | 5 | 5.5 | 4.9 | |
| 21 | KS6B | Soil | 0.9 | 13.7 | 6.3 | 32 | <0.1 | 61.5 | 46 | 451 | 2.1 | 2.9 | 3.4 | 3.6 | |
| 22 | KS7A | Soil | 1.9 | 122.9 | 72.7 | 153 | 0.2 | 86.8 | 48.4 | 7086 | 3.74 | 140.7 | 272.8 | 4 | |
| 23 | KS7B1 | Soil | 1.7 | 115.3 | 92.3 | 1129 | 0.2 | 97.6 | 48.3 | 3011 | 4.31 | 170.3 | 407.7 | 7.1 | |
| 24 | KS7B2 | Soil | 1 | 61 | 50 | 690 | 0.1 | 59.6 | 26.9 | 1717 | 3.55 | 79.2 | 329.4 | 6.7 | |
| 25 | KS8A | Soil | 0.7 | 80 | 18.4 | 736 | <0.1 | 110.2 | 48.7 | 9290 | 4.01 | 16.8 | 354.9 | 3.2 | |
| 26 | KS8B | Soil | 0.8 | 91.4 | 14.6 | 281 | <0.1 | 77.4 | 102.3 | 7866 | 3.87 | 14.1 | 257.2 | 3.2 | |
| 27 | KS9A | Soil | 0.9 | 18.6 | 7 | 19 | <0.1 | 29.5 | 13.7 | 2366 | 2.71 | 4.7 | 15.4 | 3.7 | |
| 28 | KS9B | Soil | 0.9 | 17.6 | 8.2 | 21 | <0.1 | 27.5 | 13.3 | 2628 | 2.44 | 4.7 | 16.3 | 3.6 | |
| 29 | KS9C | Soil | 0.6 | 20 | 5.2 | 16 | <0.1 | 30.4 | 10.3 | 1807 | 2.82 | 3.9 | 21.1 | 4.3 | |
| 30 | KS9D | Soil | 0.7 | 24 | 3.6 | 17 | <0.1 | 31.6 | 9 | 1122 | 3.4 | 3.2 | 13.2 | 4.7 | |
| 31 | KS 10 A | Soil | 1 | 93.2 | 79.6 | 446 | 4.7 | 117.6 | 39.4 | 8990 | 4.27 | 15.9 | 488.7 | 2.2 | |
| 32 | KS 11 Anthill sample | Soil | 1 | 47.3 | 13.2 | 33 | <0.1 | 39.3 | 11.8 | 527 | 6.39 | 7.5 | 60 | 2.9 | |
| 33 | KS 11 A1 | Soil | 0.8 | 18.8 | 6.3 | 16 | <0.1 | 19.8 | 4.8 | 256 | 2.83 | 11.5 | 26.5 | 2 | |
| 34 | KS 11 A2 | Soil | 1 | 18.5 | 6.1 | 15 | <0.1 | 18.6 | 4.5 | 212 | 2.62 | 11.6 | 30.7 | 1.9 | |
| 35 | KS 11 B | Soil | 0.7 | 11.1 | 3.6 | 10 | <0.1 | 13.9 | 2.8 | 115 | 1.67 | 7.6 | 19.6 | 1.5 | |
| 36 | KS 11 C | Soil | 0.8 | 36 | 6.6 | 26 | <0.1 | 39.6 | 12.9 | 630 | 9.22 | 4.2 | 18.7 | 3 | |
| 37 | KS 11 D | Soil | 1.1 | 55.3 | 9.6 | 36 | <0.1 | 51.9 | 20.6 | 1273 | 9.08 | 8.5 | 30 | 3.8 | |
| 38 | * Pulp Duplicates | ||||||||||||||
| 39 | KS 11 D | Soil | 1.1 | 55.3 | 9.6 | 36 | <0.1 | 51.9 | 20.6 | 1273 | 9.08 | 8.5 | 30 | 3.8 | |
| 40 | KS 11 D | REP | 1.1 | 57 | 9.5 | 37 | <0.1 | 53.9 | 21.7 | 1241 | 9.4 | 8.6 | 20 | 3.9 | |
| 41 | KS8B | Soil | 0.8 | 91.4 | 14.6 | 281 | <0.1 | 77.4 | 102.3 | 7866 | 3.87 | 14.1 | 257.2 | 3.2 | |
| 42 | KS8B | REP | 0.8 | 90.8 | 14.6 | 282 | <0.1 | 77.4 | 100.3 | 8027 | 3.93 | 14.2 | 258.4 | 3.3 | |
| 42 | Reference Materials | ||||||||||||||
| 44 | STD DS10 | STD | 14 | 153.9 | 152.7 | 360 | 1.9 | 74 | 13.1 | 874 | 2.78 | 44.3 | 89.5 | 7.8 | |
| 45 | STD OXC109 | STD | 1.3 | 34.1 | 11.1 | 40 | <0.1 | 67.3 | 19.1 | 390 | 2.88 | 0.8 | 180.5 | 1.5 | |
| 46 | STD DS10 | STD | 14.4 | 148.6 | 152 | 326 | 2 | 71.6 | 12.7 | 840 | 2.58 | 43.3 | 88.8 | 7.9 | |
| 47 | STD OXC109 | STD | 1.5 | 34.7 | 11.8 | 39 | <0.1 | 69.5 | 19.1 | 385 | 2.71 | 0.7 | 189.1 | 1.6 | |
| 48 | BLK | BLK | <0.1 | <0.1 | <0.1 | <1 | <0.1 | <0.1 | <0.1 | <1 | <0.01 | <0.5 | <0.5 | <0.1 | |
| 50 | Prep Wash | ||||||||||||||
| 51 | G1 | Prep Blank | 0.1 | 3.1 | 3.6 | 45 | <0.1 | 3.5 | 4.1 | 517 | 1.79 | 0.5 | <0.5 | 5.8 | |
| 52 | G1 | Prep Blank | 0.2 | 2.8 | 3.5 | 45 | <0.1 | 3.2 | 3.8 | 523 | 1.82 | <0.5 | 0.7 | 5.6 | |
| Analyte | Sr | Cd | Sb | Bi | V | Ca | P | La | Cr | Mg | Ba | ||||
| Unit | PPM | PPM | PPM | PPM | PPM | % | % | PPM | PPM | % | PPM | ||||
| MDL | 1 | 0.1 | 0.1 | 0.1 | 2 | 0.01 | 0.001 | 1 | 1 | 0.01 | 1 | ||||
| Sample | Type | ||||||||||||||
| 1 | KS1A | Soil | 3 | <0.1 | 0.3 | 0.1 | 42 | 0.02 | 0.014 | 6 | 64 | 0.02 | 54 | ||
| 2 | KS1B | Soil | 1 | <0.1 | 0.2 | 0.1 | 31 | <0.01 | 0.013 | 6 | 47 | 0.02 | 62 | ||
| 3 | KS1C | Soil | 4 | <0.1 | 0.4 | 0.2 | 46 | 0.02 | 0.014 | 7 | 67 | 0.02 | 74 | ||
| 4 | KS1D | Soil | <1 | <0.1 | 0.2 | 0.1 | 25 | <0.01 | 0.008 | 5 | 38 | 0.01 | 40 | ||
| 5 | KS2A | Soil | 3 | <0.1 | 0.3 | 0.1 | 39 | 0.01 | 0.011 | 6 | 52 | 0.03 | 113 | ||
| 6 | KS2B | Soil | 24 | 0.1 | 0.4 | 0.2 | 55 | 0.04 | 0.023 | 16 | 71 | 0.05 | 319 | ||
| 7 | KS2C | Soil | 52 | 0.2 | 0.5 | 0.4 | 56 | 0.02 | 0.015 | 15 | 65 | 0.03 | 245 | ||
| 8 | KS2C2 | Soil | 52 | 0.1 | 0.5 | 0.4 | 53 | 0.03 | 0.015 | 14 | 60 | 0.03 | 239 | ||
| 9 | KS2D | Soil | 387 | 0.5 | 0.5 | 0.2 | 54 | 0.07 | 0.017 | 21 | 57 | 0.07 | 226 | ||
| 10 | KS3A1 | Soil | 4 | <0.1 | 0.2 | 0.1 | 85 | 0.02 | 0.02 | 10 | 107 | 0.02 | 67 | ||
| 11 | KS3A2 | Soil | 4 | <0.1 | 0.2 | 0.1 | 86 | 0.02 | 0.022 | 11 | 114 | 0.03 | 72 | ||
| 13 | KS3B | Soil | 22 | <0.1 | 0.3 | 0.1 | 88 | 0.02 | 0.021 | 11 | 100 | 0.03 | 113 | ||
| 14 | KS3C | Soil | 9 | <0.1 | 0.3 | 0.2 | 93 | 0.1 | 0.033 | 13 | 123 | 0.04 | 76 | ||
| 15 | KS3D | Soil | 8 | <0.1 | 0.3 | 0.2 | 97 | 0.14 | 0.035 | 18 | 122 | 0.04 | 141 | ||
| 16 | KS4A | Soil | 7 | 0.5 | 0.3 | 0.1 | 47 | 0.1 | 0.026 | 7 | 100 | 0.03 | 125 | ||
| 17 | KS4B | Soil | 3 | <0.1 | 0.2 | <0.1 | 44 | 0.02 | 0.015 | 7 | 84 | 0.02 | 99 | ||
| 18 | KS5A | Soil | 6 | <0.1 | 0.2 | 0.2 | 62 | 0.04 | 0.015 | 15 | 107 | 0.04 | 94 | ||
| 19 | KS5B | Soil | 3 | <0.1 | 0.2 | 0.2 | 53 | 0.02 | 0.013 | 13 | 95 | 0.03 | 73 | ||
| 20 | KS6A | Soil | 3 | <0.1 | 0.2 | <0.1 | 71 | 0.03 | 0.017 | 7 | 99 | 0.02 | 83 | ||
| 21 | KS6B | Soil | 2 | <0.1 | 0.1 | <0.1 | 46 | 0.02 | 0.016 | 6 | 73 | 0.02 | 29 | ||
| 22 | KS7A | Soil | 64 | 0.3 | 1.4 | 0.6 | 46 | 0.14 | 0.065 | 20 | 88 | 0.04 | 405 | ||
| 23 | KS7B1 | Soil | 53 | 0.8 | 1 | 0.7 | 64 | 0.09 | 0.077 | 23 | 120 | 0.03 | 290 | ||
| 24 | KS7B2 | Soil | 29 | 0.4 | 0.5 | 0.4 | 61 | 0.06 | 0.051 | 18 | 99 | 0.02 | 162 | ||
| 25 | KS8A | Soil | 11 | 0.7 | 0.4 | 0.2 | 70 | 0.16 | 0.025 | 17 | 96 | 0.09 | 150 | ||
| 26 | KS8B | Soil | 11 | 0.4 | 0.3 | 0.1 | 59 | 0.08 | 0.032 | 11 | 80 | 0.06 | 169 | ||
| 27 | KS9A | Soil | 1 | <0.1 | 0.3 | 0.2 | 48 | <0.01 | 0.016 | 10 | 69 | 0.02 | 78 | ||
| 28 | KS9B | Soil | 2 | <0.1 | 0.3 | 0.2 | 40 | 0.01 | 0.016 | 9 | 64 | 0.02 | 89 | ||
| 29 | KS9C | Soil | 2 | <0.1 | 0.3 | 0.2 | 51 | 0.02 | 0.016 | 10 | 73 | 0.02 | 84 | ||
| 30 | KS9D | Soil | 2 | <0.1 | 0.3 | 0.2 | 63 | 0.02 | 0.016 | 13 | 91 | 0.02 | 73 | ||
| 31 | KS 10 A | Soil | 21 | 0.7 | 1.5 | 5.3 | 59 | 0.27 | 0.145 | 15 | 113 | 0.06 | 258 | ||
| 32 | KS 11 Anthill sample | Soil | 4 | <0.1 | 0.4 | 0.2 | 70 | 0.04 | 0.046 | 12 | 176 | 0.03 | 32 | ||
| 33 | KS 11 A1 | Soil | 2 | <0.1 | 0.5 | 0.2 | 42 | 0.01 | 0.019 | 6 | 79 | 0.02 | 15 | ||
| 34 | KS 11 A2 | Soil | 2 | <0.1 | 0.7 | 0.2 | 40 | <0.01 | 0.018 | 6 | 76 | 0.02 | 14 | ||
| 35 | KS 11 B | Soil | 2 | <0.1 | 0.2 | 0.1 | 24 | <0.01 | 0.013 | 5 | 49 | 0.01 | 9 | ||
| 36 | KS 11 C | Soil | 4 | <0.1 | 0.3 | 0.2 | 74 | 0.06 | 0.029 | 11 | 227 | 0.03 | 26 | ||
| 37 | KS 11 D | Soil | 3 | <0.1 | 0.5 | 0.2 | 82 | 0.01 | 0.042 | 15 | 233 | 0.03 | 38 | ||
| 38 | *Pulp Duplicates | ||||||||||||||
| 39 | KS 11 D | Soil | 3 | <0.1 | 0.5 | 0.2 | 82 | 0.01 | 0.042 | 15 | 233 | 0.03 | 38 | ||
| 40 | KS 11 D | REP | 3 | <0.1 | 0.5 | 0.2 | 86 | 0.01 | 0.046 | 15 | 232 | 0.04 | 38 | ||
| 41 | KS8B | Soil | 11 | 0.4 | 0.3 | 0.1 | 59 | 0.08 | 0.032 | 11 | 80 | 0.06 | 169 | ||
| 42 | KS8B | REP | 11 | 0.4 | 0.4 | 0.1 | 60 | 0.1 | 0.034 | 12 | 87 | 0.06 | 174 | ||
| 42 | Reference Materials | ||||||||||||||
| 44 | STD DS10 | STD | 66 | 2.6 | 8.6 | 12.7 | 42 | 1.07 | 0.075 | 18 | 54 | 0.74 | 315 | ||
| 45 | STD OXC109 | STD | 130 | <0.1 | <0.1 | <0.1 | 44 | 0.62 | 0.095 | 12 | 55 | 1.36 | 54 | ||
| 46 | STD DS10 | STD | 68 | 2.7 | 9.4 | 12.6 | 40 | 1.02 | 0.075 | 18 | 52 | 0.75 | 348 | ||
| 47 | STD OXC109 | STD | 138 | <0.1 | <0.1 | <0.1 | 46 | 0.68 | 0.104 | 12 | 56 | 1.43 | 57 | ||
| 48 | BLK | BLK | <1 | <0.1 | <0.1 | <0.1 | <2 | <0.01 | <0.001 | <1 | <1 | <0.01 | <1 | ||
| 50 | Prep Wash | ||||||||||||||
| 51 | G1 | Prep Blank | 45 | <0.1 | <0.1 | <0.1 | 38 | 0.45 | 0.073 | 10 | 7 | 0.5 | 158 | ||
| 52 | G1 | Prep Blank | 47 | <0.1 | <0.1 | <0.1 | 37 | 0.45 | 0.066 | 10 | 7 | 0.5 | 155 | ||
| Analyte | Ti | B | Al | Na | K | W | Hg | Sc | Tl | S | Ga | Se | Te | ||
| Unit | % | PPM | % | % | % | PPM | PPM | PPM | PPM | % | PPM | PPM | PPM | ||
| MDL | 0.001 | 1 | 0.01 | 0.001 | 0.01 | 0.1 | 0.01 | 0.1 | 0.1 | 0.05 | 1 | 0.5 | 0.2 | ||
| Sample | Type | ||||||||||||||
| 1 | KS1A | Soil | 0.017 | <1 | 0.86 | 0.002 | 0.03 | <0.1 | 0.03 | 4.7 | <0.1 | <0.05 | 5 | <0.5 | <0.2 |
| 2 | KS1B | Soil | 0.014 | <1 | 0.82 | 0.002 | 0.02 | <0.1 | 0.03 | 3.7 | <0.1 | <0.05 | 4 | <0.5 | <0.2 |
| 3 | KS1C | Soil | 0.017 | <1 | 0.99 | 0.004 | 0.02 | <0.1 | 0.04 | 4.7 | 0.1 | <0.05 | 5 | <0.5 | <0.2 |
| 4 | KS1D | Soil | 0.01 | <1 | 0.57 | 0.003 | 0.02 | <0.1 | 0.02 | 2.6 | <0.1 | <0.05 | 3 | <0.5 | <0.2 |
| 5 | KS2A | Soil | 0.018 | <1 | 1.05 | 0.003 | 0.03 | <0.1 | 0.03 | 4.6 | <0.1 | <0.05 | 5 | <0.5 | <0.2 |
| 6 | KS2B | Soil | 0.029 | <1 | 1.81 | 0.003 | 0.06 | <0.1 | 0.04 | 6.3 | 0.1 | <0.05 | 8 | <0.5 | <0.2 |
| 7 | KS2C | Soil | 0.04 | 1 | 1.43 | 0.004 | 0.06 | <0.1 | 0.07 | 8.6 | 0.1 | <0.05 | 8 | <0.5 | <0.2 |
| 8 | KS2C2 | Soil | 0.038 | 2 | 1.46 | 0.004 | 0.06 | 0.1 | 0.08 | 8.4 | 0.1 | <0.05 | 9 | <0.5 | <0.2 |
| 9 | KS2D | Soil | 0.041 | <1 | 1.73 | 0.006 | 0.07 | 0.1 | 0.5 | 6.9 | 0.1 | <0.05 | 13 | <0.5 | <0.2 |
| 10 | KS3A1 | Soil | 0.033 | <1 | 1.63 | 0.004 | 0.03 | <0.1 | 0.04 | 7.1 | <0.1 | <0.05 | 8 | <0.5 | <0.2 |
| 11 | KS3A2 | Soil | 0.034 | <1 | 1.8 | 0.004 | 0.03 | <0.1 | 0.03 | 7.2 | <0.1 | <0.05 | 8 | <0.5 | <0.2 |
| 13 | KS3B | Soil | 0.036 | <1 | 1.76 | 0.003 | 0.04 | <0.1 | 0.04 | 6.7 | <0.1 | <0.05 | 9 | <0.5 | <0.2 |
| 14 | KS3C | Soil | 0.042 | 2 | 2.15 | 0.008 | 0.06 | <0.1 | 0.03 | 8.8 | <0.1 | <0.05 | 10 | <0.5 | <0.2 |
| 15 | KS3D | Soil | 0.035 | 3 | 2.37 | 0.007 | 0.05 | <0.1 | 0.04 | 8.9 | <0.1 | <0.05 | 10 | <0.5 | <0.2 |
| 16 | KS4A | Soil | 0.023 | 1 | 1.13 | 0.004 | 0.04 | <0.1 | 0.04 | 4.8 | 0.1 | <0.05 | 6 | <0.5 | <0.2 |
| 17 | KS4B | Soil | 0.025 | <1 | 1.12 | 0.002 | 0.03 | <0.1 | 0.03 | 4.4 | <0.1 | <0.05 | 5 | <0.5 | <0.2 |
| 18 | KS5A | Soil | 0.027 | <1 | 1.52 | 0.003 | 0.03 | <0.1 | 0.02 | 7 | 0.1 | <0.05 | 7 | <0.5 | <0.2 |
| 19 | KS5B | Soil | 0.026 | <1 | 1.39 | 0.003 | 0.03 | <0.1 | 0.02 | 5.8 | <0.1 | <0.05 | 6 | <0.5 | <0.2 |
| 20 | KS6A | Soil | 0.027 | <1 | 1.38 | 0.005 | 0.03 | <0.1 | 0.01 | 6.5 | 0.1 | <0.05 | 6 | <0.5 | <0.2 |
| 21 | KS6B | Soil | 0.02 | <1 | 1.39 | 0.004 | 0.03 | <0.1 | 0.02 | 4.9 | <0.1 | <0.05 | 5 | <0.5 | <0.2 |
| 22 | KS7A | Soil | 0.022 | 3 | 1.16 | 0.014 | 0.05 | 0.2 | 0.33 | 5 | 0.1 | 0.15 | 6 | 1 | <0.2 |
| 23 | KS7B1 | Soil | 0.034 | 3 | 1.71 | 0.012 | 0.05 | 0.2 | 0.26 | 6.9 | 0.1 | 0.09 | 9 | 0.6 | <0.2 |
| 24 | KS7B2 | Soil | 0.025 | 1 | 1.79 | 0.007 | 0.04 | 0.1 | 0.15 | 6.8 | <0.1 | 0.05 | 8 | <0.5 | <0.2 |
| 25 | KS8A | Soil | 0.036 | 2 | 1.25 | 0.009 | 0.05 | <0.1 | 0.22 | 5.7 | 0.1 | <0.05 | 6 | <0.5 | <0.2 |
| 26 | KS8B | Soil | 0.033 | 2 | 1.52 | 0.01 | 0.05 | <0.1 | 0.24 | 6.5 | 0.2 | 0.09 | 7 | <0.5 | <0.2 |
| 27 | KS9A | Soil | 0.019 | <1 | 1.28 | 0.002 | 0.02 | <0.1 | 0.03 | 6 | 0.1 | <0.05 | 6 | <0.5 | <0.2 |
| 28 | KS9B | Soil | 0.017 | <1 | 1.16 | 0.004 | 0.02 | <0.1 | 0.04 | 5.4 | 0.1 | <0.05 | 5 | <0.5 | <0.2 |
| 29 | KS9C | Soil | 0.02 | <1 | 1.23 | 0.002 | 0.02 | <0.1 | 0.04 | 6.6 | 0.1 | <0.05 | 6 | <0.5 | <0.2 |
| 30 | KS9D | Soil | 0.023 | <1 | 1.39 | 0.002 | 0.02 | <0.1 | 0.02 | 7 | <0.1 | <0.05 | 8 | 0.6 | <0.2 |
| 31 | KS 10 A | Soil | 0.028 | 2 | 1.59 | 0.008 | 0.06 | 0.4 | 1.36 | 5.1 | 0.1 | <0.05 | 7 | 0.5 | <0.2 |
| 32 | KS 11 Anthill sample | Soil | 0.025 | 1 | 1.57 | 0.006 | 0.04 | 0.1 | 0.06 | 8.8 | <0.1 | <0.05 | 8 | <0.5 | <0.2 |
| 33 | KS 11 A1 | Soil | 0.014 | <1 | 0.93 | 0.007 | 0.03 | <0.1 | 0.04 | 3.1 | <0.1 | <0.05 | 4 | <0.5 | <0.2 |
| 34 | KS 11 A2 | Soil | 0.013 | <1 | 0.93 | 0.006 | 0.03 | <0.1 | 0.03 | 3 | <0.1 | <0.05 | 4 | <0.5 | <0.2 |
| 35 | KS 11 B | Soil | 0.008 | <1 | 0.65 | 0.007 | 0.03 | <0.1 | 0.03 | 1.8 | <0.1 | <0.05 | 2 | <0.5 | <0.2 |
| 36 | KS 11 C | Soil | 0.025 | <1 | 1.44 | 0.005 | 0.02 | <0.1 | 0.04 | 8.2 | <0.1 | <0.05 | 8 | <0.5 | <0.2 |
| 37 | KS 11 D | Soil | 0.029 | <1 | 2 | 0.005 | 0.04 | 0.2 | 0.05 | 11.5 | 0.1 | <0.05 | 10 | <0.5 | <0.2 |
| 38 | *Pulp Duplicates | ||||||||||||||
| 39 | KS 11 D | Soil | 0.029 | <1 | 2 | 0.005 | 0.04 | 0.2 | 0.05 | 11.5 | 0.1 | <0.05 | 10 | <0.5 | <0.2 |
| 40 | KS 11 D | REP | 0.029 | <1 | 1.93 | 0.005 | 0.04 | 0.3 | 0.06 | 11.1 | 0.1 | <0.05 | 11 | <0.5 | <0.2 |
| 41 | KS8B | Soil | 0.033 | 2 | 1.52 | 0.01 | 0.05 | <0.1 | 0.24 | 6.5 | 0.2 | 0.09 | 7 | <0.5 | <0.2 |
| 42 | KS8B | REP | 0.037 | 3 | 1.67 | 0.01 | 0.06 | <0.1 | 0.26 | 6.8 | 0.2 | 0.1 | 7 | <0.5 | <0.2 |
| 42 | Reference Materials | ||||||||||||||
| 44 | STD DS10 | STD | 0.073 | 6 | 0.99 | 0.062 | 0.32 | 3.2 | 0.29 | 2.8 | 5.2 | 0.26 | 4 | 2.2 | 4.9 |
| 45 | STD OXC109 | STD | 0.332 | 1 | 1.42 | 0.611 | 0.37 | 0.2 | <0.01 | 1 | <0.1 | <0.05 | 5 | <0.5 | <0.2 |
| 46 | STD DS10 | STD | 0.077 | 7 | 0.98 | 0.065 | 0.31 | 3.5 | 0.28 | 2.7 | 4.8 | 0.26 | 5 | 2.2 | 5.3 |
| 47 | STD OXC109 | STD | 0.387 | 2 | 1.46 | 0.654 | 0.4 | 0.2 | <0.01 | 1 | <0.1 | <0.05 | 5 | <0.5 | <0.2 |
| 48 | BLK | BLK | <0.001 | <1 | <0.01 | <0.001 | <0.01 | <0.1 | <0.01 | 0.2 | <0.1 | <0.05 | <1 | <0.5 | <0.2 |
| 50 | Prep Wash | ||||||||||||||
| 51 | G1 | Prep Blank | 0.105 | <1 | 0.86 | 0.066 | 0.46 | <0.1 | <0.01 | 2.3 | 0.2 | <0.05 | 4 | <0.5 | <0.2 |
| 52 | G1 | Prep Blank | 0.099 | <1 | 0.89 | 0.064 | 0.45 | <0.1 | <0.01 | 2.1 | 0.3 | <0.05 | 4 | <0.5 | <0.2 |
| Heavy Metal | Krugersdorp Game Reserve | Rand Uranium Mine | Mintails Mogale Gold | Smallholdings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KS1 (n = 4) | KS2 (n = 5) | KS3 (n = 5) | KS8 (n = 2) | KS9 (n = 4) | KS10 (n = 1) | KS11 (n = 6) | KS6 (n = 2) | KS7 (n = 3) | KS4 (n = 2) | KS5 (n = 2) | ||
| Cr (90.00) * | Mean | 56.25 | 61.00 | 113.20 | 88.00 | 74.25 | 113.00 | 140.00 | 86.00 | 102.33 | 92.00 | 101.00 |
| SD | 17.02 | 7.31 | 9.83 | 11.31 | 11.76 | N/A | 81.99 | 18.39 | 16.26 | 11.31 | 8.49 | |
| Min | 38.00 | 52.00 | 100.00 | 80.00 | 64.00 | N/A | 49.00 | 73.00 | 88.00 | 84.00 | 95.00 | |
| Max | 67.00 | 71.00 | 123.00 | 96.00 | 91.00 | N/A | 233.00 | 99.00 | 120.00 | 100.00 | 107.00 | |
| Mn (850.00) * | Mean | 2564.00 | 9011.20 | 4635.00 | 8578.00 | 1980.75 | 8990.00 | 502.17 | 631.50 | 3938.00 | 5435.00 | 1320.00 |
| SD | 553.94 | 2211.02 | 2596.11 | 1006.92 | 667.08 | N/A | 425.64 | 255.27 | 2801.97 | 3124.00 | 336.58 | |
| Min | 1801.00 | 5056.00 | 2956.00 | 7866.00 | 1122.00 | N/A | 115.00 | 451.00 | 1717.00 | 3226.00 | 1082.00 | |
| Max | 3128.00 | 10,000.00 | 9233.00 | 9290.00 | 2628.00 | N/A | 1273.00 | 812.00 | 7086.00 | 7644.00 | 1558.00 | |
| Co (19.00) * | Mean | 10.78 | 14.62 | 11.76 | 75.50 | 11.58 | 39.40 | 9.57 | 31.10 | 41.20 | 51.70 | 8.90 |
| SD | 1.01 | 2.69 | 1.36 | 37.90 | 2.29 | N/A | 6.81 | 21.07 | 12.38 | 58.41 | 3.54 | |
| Min | 9.30 | 10.50 | 10.80 | 48.70 | 9.00 | N/A | 2.80 | 16.20 | 26.90 | 10.40 | 6.40 | |
| Max | 11.60 | 18.00 | 14.10 | 102.30 | 13.70 | N/A | 20.60 | 46.00 | 48.40 | 93.00 | 11.40 | |
| Ni (68.00) * | Mean | 24.05 | 153.74 | 55.08 | 93.80 | 29.75 | 117.60 | 30.52 | 45.60 | 81.33 | 148.15 | 20.65 |
| SD | 5.58 | 164.56 | 5.47 | 23.19 | 1.73 | N/A | 15.16 | 22.49 | 19.58 | 176.71 | 1.48 | |
| Min | 16.40 | 38.50 | 49.30 | 77.40 | 27.50 | N/A | 13.90 | 29.70 | 59.60 | 23.20 | 19.60 | |
| Max | 29.50 | 444.80 | 61.40 | 110.20 | 31.60 | N/A | 51.90 | 61.50 | 97.60 | 273.10 | 21.70 | |
| Cu (45.00) * | Mean | 13.43 | 28.82 | 43.44 | 85.70 | 20.05 | 93.20 | 31.17 | 16.10 | 99.73 | 21.65 | 18.10 |
| SD | 2.94 | 9.21 | 3.34 | 8.06 | 2.81 | N/A | 17.79 | 3.39 | 33.76 | 11.53 | 2.83 | |
| Min | 10.10 | 16.00 | 40.60 | 80.00 | 17.60 | N/A | 11.10 | 13.70 | 61.00 | 13.50 | 16.10 | |
| Max | 16.00 | 42.00 | 49.00 | 91.40 | 24.00 | N/A | 55.30 | 18.50 | 115.30 | 29.80 | 20.10 | |
| Zn (95.00) * | Mean | 17.75 | 91.60 | 38.00 | 508.50 | 18.25 | 446.00 | 22.67 | 23.50 | 657.33 | 107.00 | 12.50 |
| SD | 3.77 | 73.38 | 14.71 | 321.73 | 2.22 | N/A | 10.58 | 12.02 | 488.82 | 134.35 | 0.71 | |
| Min | 15.00 | 25.00 | 25.00 | 281.00 | 16.00 | N/A | 10.00 | 15.00 | 153.00 | 12.00 | 12.00 | |
| Max | 23.00 | 216.00 | 54.00 | 736.00 | 21.00 | N/A | 36.00 | 32.00 | 1129.00 | 202.00 | 13.00 | |
| Cd (0.30) * | Mean | 0.10 | 0.20 | 0.10 | 0.55 | 0.10 | 0.70 | 0.10 | 0.10 | 0.50 | 0.30 | 0.10 |
| SD | 0.00 | 0.17 | 0.00 | 0.21 | 0.00 | N/A | 0.00 | 0.00 | 0.27 | 0.28 | 0.00 | |
| Min | 0.10 | 0.10 | 0.10 | 0.40 | 0.10 | N/A | 0.10 | 0.10 | 0.30 | 0.10 | 0.10 | |
| Max | 0.10 | 0.50 | 0.10 | 0.70 | 0.10 | N/A | 0.10 | 0.10 | 0.80 | 0.50 | 0.10 | |
| Hg (0.40) * | Mean | 0.03 | 0.14 | 0.04 | 0.23 | 0.03 | 1.36 | 0.04 | 0.02 | 0.25 | 0.04 | 0.02 |
| SD | 0.01 | 0.20 | 0.01 | 0.01 | 0.01 | N/A | 0.01 | 0.01 | 0.09 | 0.01 | 0.00 | |
| Min | 0.02 | 0.03 | 0.03 | 0.22 | 0.02 | N/A | 0.03 | 0.01 | 0.15 | 0.03 | 0.02 | |
| Max | 0.04 | 0.50 | 0.04 | 0.24 | 0.04 | N/A | 0.06 | 0.02 | 0.26 | 0.04 | 0.02 | |
| Pb (20.00) * | Mean | 5.20 | 13.66 | 8.14 | 16.50 | 6.00 | 79.60 | 7.57 | 9.35 | 71.67 | 9.70 | 7.55 |
| SD | 0.82 | 3.61 | 2.09 | 2.69 | 2.02 | N/A | 3.36 | 4.31 | 21.17 | 3.82 | 2.33 | |
| Min | 4.60 | 7.50 | 6.30 | 14.60 | 3.60 | N/A | 3.60 | 6.30 | 50.00 | 7.00 | 5.90 | |
| Max | 6.40 | 16.40 | 10.60 | 18.40 | 8.20 | N/A | 13.20 | 12.40 | 92.30 | 12.40 | 9.20 | |
| As (13.00) * | Mean | 5.83 | 6.30 | 7.04 | 15.45 | 4.13 | 15.90 | 8.48 | 3.95 | 130.07 | 5.00 | 3.65 |
| SD | 0.78 | 1.66 | 0.55 | 1.910 | 0.72 | N/A | 2.79 | 1.49 | 46.47 | 1.98 | 0.49 | |
| Min | 5.20 | 4.10 | 6.40 | 14.10 | 3.20 | N/A | 4.20 | 2.90 | 79.20 | 3.60 | 3.30 | |
| Max | 6.80 | 8.60 | 7.60 | 16.80 | 4.70 | N/A | 11.60 | 5.00 | 170.30 | 6.40 | 4.00 | |
| V (130.00) * | Mean | 36.00 | 51.40 | 89.80 | 64.50 | 50.50 | 59.00 | 55.33 | 58.50 | 57.00 | 45.50 | 57.50 |
| SD | 9.70 | 7.02 | 5.07 | 7.78 | 9.54 | N/A | 23.11 | 17.68 | 9.64 | 2.12 | 6.36 | |
| Min | 25.00 | 39.00 | 85.00 | 59.00 | 40.00 | N/A | 24.00 | 46.00 | 46.00 | 44.00 | 53.00 | |
| Max | 46.00 | 56.00 | 97.00 | 70.00 | 63.00 | N/A | 82.00 | 71.00 | 64.00 | 47.00 | 62.00 | |
| Fe (47,200.00) * | Mean | 29,925.00 | 49,020.00 | 44,800.00 | 34,900.00 | 28,425.00 | 42,700.00 | 53,016.67 | 26,650.00 | 38,666.67 | 27,250.00 | 27,100.00 |
| SD | 6935.60 | 21,154.72 | 3430.74 | 989.95 | 4045.06 | N/A | 33,853.53 | 7990.31 | 3955.17 | 1909.19 | 3394.11 | |
| Min | 21,400.00 | 23,800.00 | 41,500.00 | 38,700.00 | 28,200.00 | N/A | 16,700.00 | 21,000.00 | 35,500.00 | 25,900.00 | 24,700.00 | |
| Max | 36,900.00 | 82,300.00 | 48,500.00 | 40,100.00 | 34,000.00 | N/A | 92,200.00 | 32,300.00 | 43,100.00 | 28,600.00 | 29,500.00 | |
| Study Sites | ||||||||
|---|---|---|---|---|---|---|---|---|
| PHE | Krugersdorp Game Reserve | Smallholdings | Rand Uranium Mine | Mintails Mogale Gold | Range of MAC in Agricultural Soils a | Average Composition of Living Matter b | Comments | |
| As | Mean | 7.56 | 4.33 | 3.95 | 130.07 | 15–20 | 0.31 | Toxic; readily absorbed by plants from groundwater and soil |
| SD | 3.54 | 1.41 | 1.49 | 46.47 | ||||
| Min | 3.20 | 3.30 | 2.90 | 79.20 | ||||
| Max | 16.80 | 6.40 | 5.00 | 170.30 | ||||
| N | 23 | 4 | 2 | 2 | ||||
| Co | Mean | 17.37 | 30.30 | 31.10 | 41.20 | 20–50 | 0.2 | Microorganisms (Rhizobium sp.); essential to animals |
| SD | 19.45 | 41.86 | 21.07 | 12.38 | ||||
| Min | 2.80 | 6.40 | 16.20 | 26.90 | ||||
| Max | 102.30 | 93.00 | 46.00 | 48.40 | ||||
| N | 23 | 4 | 2 | 2 | ||||
| Cu | Mean | 35.07 | 19.88 | 16.10 | 99.73 | 60–150 | 2 | Vital to health of all living organisms. Necessary for haemoglobin and melanin formation; component of several blood proteins and enzyme systems |
| SD | 23.21 | 7.15 | 3.39 | 33.76 | ||||
| Min | 10.10 | 13.50 | 13.70 | 61.00 | ||||
| Max | 93.20 | 29.80 | 18.50 | 115.30 | ||||
| N | 23 | 4 | 2 | 2 | ||||
| Hg | Mean | 0.12 | 0.03 | 0.02 | 0.25 | 0.5–5 | 0.00 | Toxic. Fish can contain unusual amounts |
| SD | 0.27 | 0.01 | 0.01 | 0.09 | ||||
| Min | 0.02 | 0.02 | 0.01 | 0.15 | ||||
| Max | 1.36 | 0.04 | 0.02 | 0.26 | ||||
| N | 23 | 4 | 2 | 2 | ||||
| Pb | Mean | 11.55 | 8.63 | 9.35 | 71.67 | 20–300 | 0.51 | Present in plants and animals; easily absorbed and accumulates in different plant parts |
| SD | 14.26 | 2.87 | 4.31 | 21.17 | ||||
| Min | 3.60 | 5.90 | 6.30 | 50.00 | ||||
| Max | 79.60 | 12.40 | 12.40 | 92.30 | ||||
| N | 23 | 4 | 2 | 2 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapi, M.; Jordaan, M.A.; Nadasan, D.S.; Davies, T.C.; Chirenje, E.; Dube, M.; Lekoa, M.R. Analysis of the Distribution of Some Potentially Harmful Elements (PHEs) in the Krugersdorp Game Reserve, Gauteng, South Africa. Minerals 2020, 10, 151. https://doi.org/10.3390/min10020151
Shapi M, Jordaan MA, Nadasan DS, Davies TC, Chirenje E, Dube M, Lekoa MR. Analysis of the Distribution of Some Potentially Harmful Elements (PHEs) in the Krugersdorp Game Reserve, Gauteng, South Africa. Minerals. 2020; 10(2):151. https://doi.org/10.3390/min10020151
Chicago/Turabian StyleShapi, Michael, Maryam Amra Jordaan, Devandren Subramoney Nadasan, Theophilus C. Davies, Emmanuel Chirenje, Mpumelelo Dube, and Mammusa R. Lekoa. 2020. "Analysis of the Distribution of Some Potentially Harmful Elements (PHEs) in the Krugersdorp Game Reserve, Gauteng, South Africa" Minerals 10, no. 2: 151. https://doi.org/10.3390/min10020151
APA StyleShapi, M., Jordaan, M. A., Nadasan, D. S., Davies, T. C., Chirenje, E., Dube, M., & Lekoa, M. R. (2020). Analysis of the Distribution of Some Potentially Harmful Elements (PHEs) in the Krugersdorp Game Reserve, Gauteng, South Africa. Minerals, 10(2), 151. https://doi.org/10.3390/min10020151





