Jianite: Massive Dunite Solely Made of Virtually Pure Forsterite from Ji’an County, Jilin Province, Northeast China
Abstract
:1. Introduction
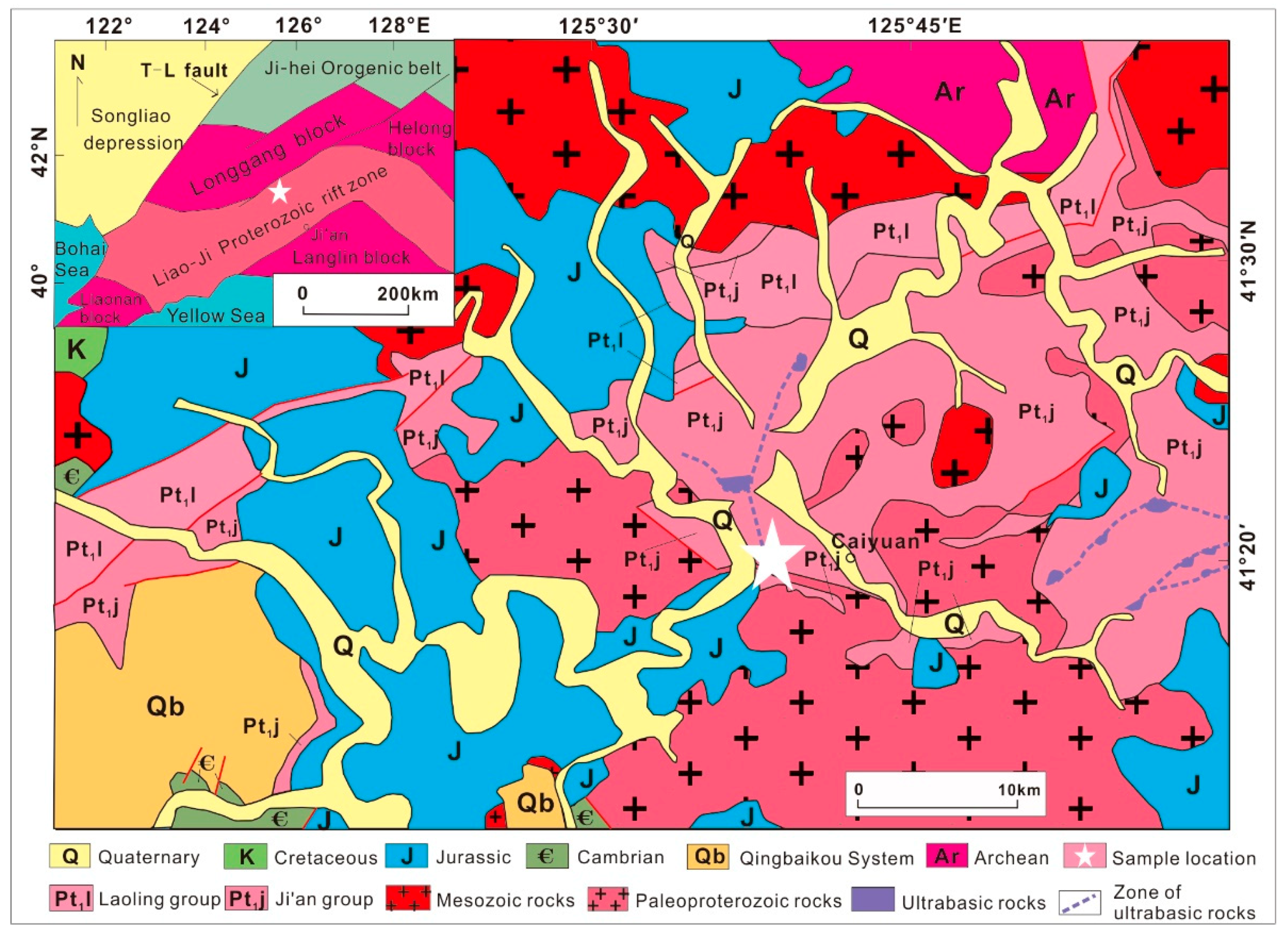
2. Sampling Location and Analytical Methods
3. Results
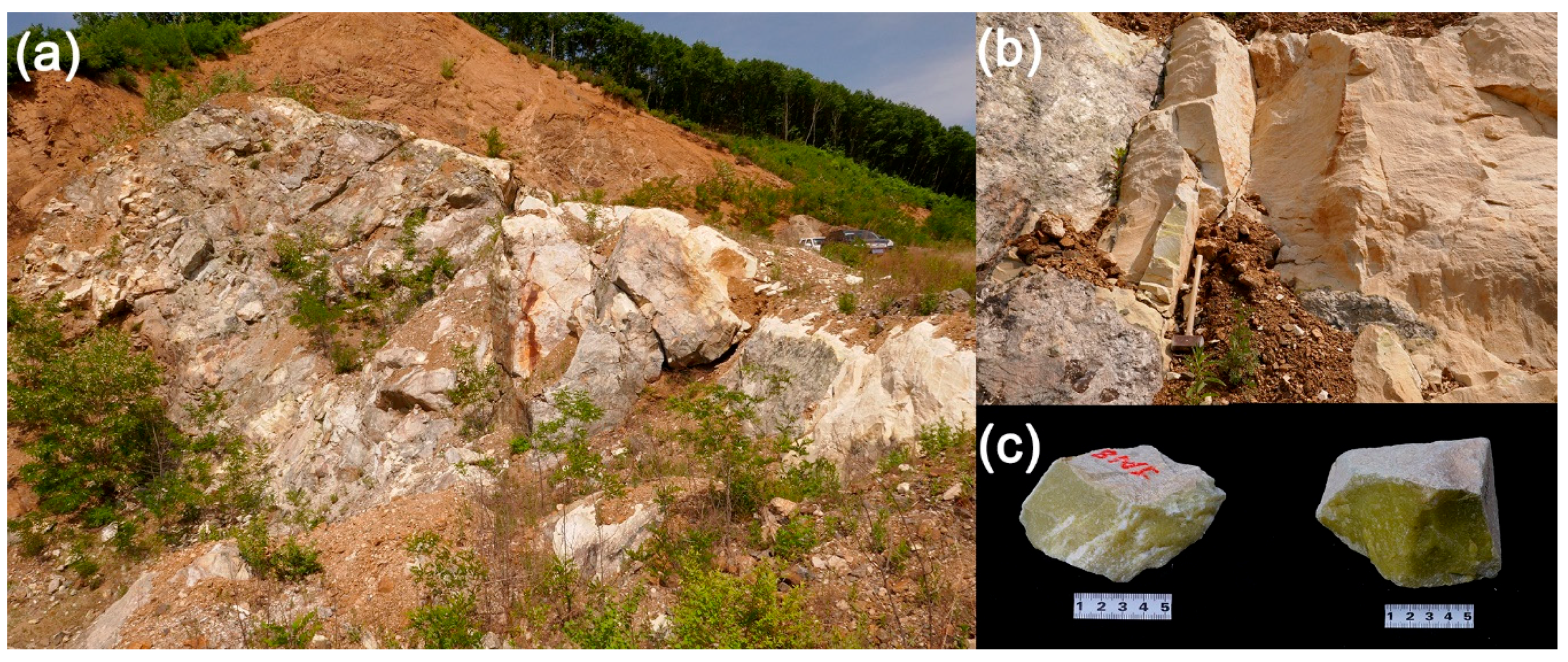
3.1. Petrography
3.2. Phase Identification
3.3. Chemistry of Minerals
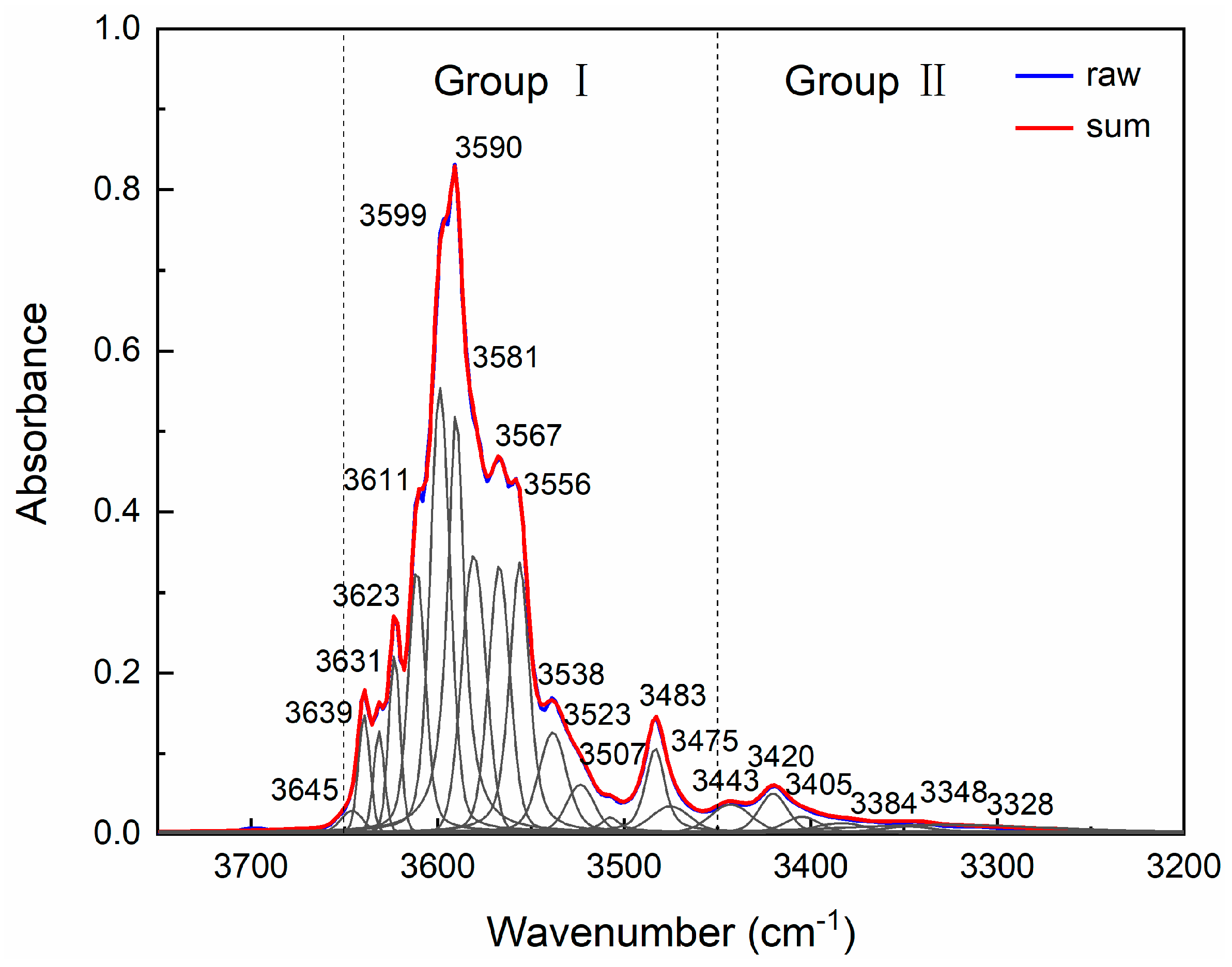
3.4. Water in Olivine
4. Discussion: Jianite and Its Petrogenesis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walter, M.J. Melting residues of fertile peridotite and the origin of cratonic lithosphere. Spec. Publ. Geochem. Soc. 1999, 6, 225–239. [Google Scholar]
- Bernstein, S.; Kelemen, P.B.; Hanghøj, K. Consistent olivine Mg# in cratonic mantle reflects Archean mantle melting to the exhaustion of orthopyroxene. Geology 2007, 35, 459–462. [Google Scholar]
- Boyd, F.R.; Nixon, P.H. Ultramafic nodules from the Kimberley pipes, South Africa. Geochim. Cosmochim. Acta 1978, 42, 1367–1382. [Google Scholar] [CrossRef]
- Griffin, W.L.; O’Reilly, S.Y.; Ryan, C.G. The composition and origin of sub-continental lithospheric mantle. Spec. Publ. Geochem. Soc. 1999, 6, 13–45. [Google Scholar]
- Fan, W.M.; Zhang, H.F.; Baker, J.; Jarvis, K.E.; Mason, P.R.D.; Menzies, M.A. On and off the north China craton: Where is the Archaean keel? J. Petrol. 2000, 41, 933–950. [Google Scholar] [CrossRef]
- Zheng, J.; O’Reilly, S.Y.; Griffin, W.L.; Lu, F.; Zhang, M.; Pearson, N.J. Relic refractory mantle beneath the eastern North China block: Significance for lithosphere evolution. Lithos 2001, 57, 43–66. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S.; Ling, W.; Liu, Y.; McDonough, W.F. Petrology and geochemistry of spinel peridotite xenoliths from Hannuoba and Qixia, North China craton. Lithos 2004, 77, 609–637. [Google Scholar] [CrossRef]
- Jaques, A.L.; Green, D.H. Anhydrous melting of peridotite at 0–15 Kb pressure and the genesis of tholeiitic basalts. Contrib. Mineral. Petrol. 1980, 73, 287–310. [Google Scholar] [CrossRef]
- Falloon, T.J.; Green, D.H.; Hatton, C.J.; Harris, K.L. Anhydrous partial melting of a fertile and depleted peridotite from 2 to 30 kb and application to basalt petrogenesis. J. Petrol. 1988, 29, 1257–1282. [Google Scholar] [CrossRef]
- Barnes, S.J.; Roeder, P.L. The range of spinel compositions in terrestrial mafic and ultramafic rocks. J. Petrol. 2001, 42, 2279–2302. [Google Scholar] [CrossRef]
- Liu, X.; O’Neill, H.S.C. The effect of Cr2O3 on the partial melting of spinel lherzolite in the system CaO-MgO-Al2O3-SiO2-Cr2O3 at 1.1 GPa. J. Petrol. 2004, 45, 2261–2286. [Google Scholar] [CrossRef]
- Green, D.H.; Ringwood, A.E. The genesis of basaltic magmas. Contrib. Mineral. Petrol. 1967, 15, 103–190. [Google Scholar] [CrossRef]
- Roeder, P.L.; Emslie, R.F. Olivine-liquid equilibrium. Contrib. Mineral. Petrol. 1970, 29, 275–289. [Google Scholar] [CrossRef]
- Plechov, P.Y.; Shcherbakov, V.D.; Nekrylov, N.A. Extremely magnesian olivine in igneous rocks. Russ. Geol. Geophys. 2018, 59, 1702–1717. [Google Scholar] [CrossRef]
- Sui, J.; Fan, Q.; Liu, J. Discovery and significance of high-purity forsterite (Fo~98.5) and its dissolution structure in Longgang volcano, Jilin Province. In Proceedings of the 11th Annual Conference of the Chinese Society for Mineralogy, Petrology and Geochemistry, Beijing, China, 27–30 August 2007; p. 37. [Google Scholar]
- Blondes, M.S.; Brandon, M.T.; Reiners, P.W.; Page, F.Z.; Kita, N.T. Generation of forsteritic olivine (Fo99.8) by subsolidus oxidation in basaltic flows. J. Petrol. 2012, 53, 971–984. [Google Scholar] [CrossRef] [Green Version]
- Bai, W.; Fan, Q.; Zhan, Z.; Yan, B.; Yang, J. Crystal structure of forsterite from podiform chromitite in Luobusa ophiolite of Tibet and its implications. Acta Petrol. Mineral. 2001, 20, 1–10. [Google Scholar]
- Owens, B.E. High-temperature contact metamorphism of calc-silicate xenoliths in the Kiglapait Intrusion, Labrador. Am. Mineral. 2000, 85, 1595–1605. [Google Scholar] [CrossRef]
- Wenzel, T.; Baumgartner, L.P.; Konnikov, E.G.; Brugmann, G.E.; Kislov, E.V. Partial melting and assimilation of dolomitic xenoliths by mafic magma: The Ioko-Dovyren intrusion (North Baikal region, Russia). J. Petrol. 2002, 43, 2049–2074. [Google Scholar] [CrossRef]
- Ferry, J.M.; Ushikubo, T.; Valley, J.W. Formation of forsterite by silicification of dolomite during contact metamorphism. J. Petrol. 2011, 52, 1619–1640. [Google Scholar] [CrossRef] [Green Version]
- Arai, S.; Ishimaru, S.; Mizukami, T. Methane and propane micro-inclusions in olivine in titanoclinohumite-bearing dunites from the Sanbagawa high-P metamorphic belt, Japan: Hydrocarbon activity in a subduction zone and Ti mobility. Earth Planet. Sci. Lett. 2012, 353–354, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, F.; Shi, G.-H.; Wu, F.-Y.; Chen, X.; Jin, Q.-Z.; Su, B.; Guo, S.; Sein, K.; Nyunt, T.T. Magnesium isotope composition of subduction zone fluids as constrained by jadeitites from Myanmar. J. Geophys. Res. 2018, 123, 7566–7585. [Google Scholar] [CrossRef]
- Majumdar, A.S.; Hovelmann, J.; Vollmer, C.; Berndt, J.; Mondal, S.K.; Putnis, A. Formation of Mg-rich olivine pseudomorphs in serpentinized dunite from the Mesoarchean Nuasahi Massif, eastern India: Insights into the evolution of fluid composition at mineral-fluid interface. J. Petrol. 2016, 57, 3–26. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Chen, D.; Liang, Y.; Zou, C.; Zhang, Q.; Bai, L. Geochronology of Ji’an group in Tonghua area, southern Jilin province. J. Earth Sci. 2014, 39, 1487–1499. [Google Scholar]
- Zhou, X.; Di, X.; Lu, X.; Kong, F. Discovery and significance of the ophiolite in Ji’an rock group, southern of Jilin Province. Jilin Geol. 2018, 37, 1–6. [Google Scholar]
- Zhang, W.; Liu, F.; Cai, J.; Liu, C.; Liu, J.; Liu, P.; Liu, L.; Wang, F.; Yang, H. Geochemistry, zircon U-Pb dating and tectonic implications of the Palaeoproterozoic Ji’an and Laoling groups, northeastern Jiao-Liao-Ji Belt, North China Craton. Precambrian Res. 2018, 314, 264–287. [Google Scholar] [CrossRef]
- Meng, E.; Wang, C.-Y.; Li, Z.; Li, Y.-G.; Yang, H.; Cai, J.; Ji, L.; Jin, M.-Q. Palaeoproterozoic metasedimentary rocks of the Ji’an group and their significance for the tectonic evolution of the northern segment of the Jiao–Liao–Ji Belt, North China Craton. Geol. Mag. 2017, 155, 149–173. [Google Scholar] [CrossRef]
- Lu, X. Paleoproterozoic Tectonic Magmatic Event in Tonghua Area. Ph.D. Dissertation, Jilin University, Changchun, China, 15 June 2004. [Google Scholar]
- Liu, L.; Liu, X.; Bao, X.; He, Q.; Yan, W.; Ma, Y.; He, M.; Tao, R.; Zou, R. Si-disordering in MgAl2O4-spinel under high P-T conditions, with implications for Si-Mg disorder in MgAl2O4-ringwoodite. Minerals 2018, 8, 210. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, L.; Wei, C.; Slabunov, A.I.; Bader, T. Quartz and orthopyroxene exsolution lamellae in clinopyroxene and the metamorphic P-T path of Belomorian eclogites. J. Metamorph. Geol. 2017, 36, 1–22. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Y.; Yan, W.; Liu, X. Trace element partitioning between MgAl2O4-spinel and carbonatitic silicate melt from 3 to 6 GPa, with emphasis on the role of cation order-disorder. Solid Earth Sci. 2019, 4, 43–65. [Google Scholar] [CrossRef]
- Li, X.-W.; Mo, X.-X.; Yu, X.-H.; Ding, Y.; Huang, X.-F.; Wei, P.; He, W.-Y. Petrology and geochemistry of the early mesozoic pyroxene andesites in the Maixiu Area, west Qinling, China: Products of subduction or syn-collision? Lithos 2013, 172–173, 158–174. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of reference values for NIST SRM 610-617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Jackson, S.E. The Application of Nd: YAG Lasers in LA-ICP-MS. In Principles and Applications of Laser Ablation-Mass Spectrometry in the Earth Sciences, 2nd ed.; Sylvester, P., Ed.; Mineralogical Association of Canada: Quebec City, QC, Canada, 2001; Volume 29, pp. 29–45. [Google Scholar]
- Liu, X.; Ma, Y.; He, Q.; He, M. Some IR features of SiO4, and OH in coesite, and its amorphization and dehydration at ambient pressure. J. Asian Earth Sci. 2017, 148, 315–323. [Google Scholar] [CrossRef]
- He, M.; Yan, W.; Chang, Y.; Liu, K.; Liu, X. Fundamental infrared absorption features of α-quartz: An unpolarized single-crystal absorption infrared spectroscopic study. Vib. Spectrosc. 2019, 101, 52–63. [Google Scholar] [CrossRef]
- Liu, X.; O’Neill, H.S.C.; Berry, A.J. The effects of small amounts of H2O, CO2 and Na2O on the partial melting of spinel lherzolite in the system CaO–MgO–Al2O3–SiO2 ± H2O ± CO2 ± Na2O at 1.1 GPa. J. Petrol. 2006, 47, 409–434. [Google Scholar] [CrossRef] [Green Version]
- Bowen, N.L.; Tuttle, O.F. The system MgO-SiO2-H2O. Geol. Soc. Am. Bull. 1949, 60, 439–460. [Google Scholar] [CrossRef]
- Evans, B.W. The serpentinite multisystem revisited: Chrysotile is metastable. Inter. Geol. Rev. 2004, 46, 479–506. [Google Scholar] [CrossRef]
- Evans, B.W. Lizardite versus antigorite serpentinite: Magnetite, hydrogen, and life (?). Geology 2010, 38, 879–882. [Google Scholar] [CrossRef]
- Huang, R.; Lin, C.-T.; Sun, W.; Ding, X.; Zhan, W. The production of iron oxide during peridotite serpentinization: Influence of pyroxene. Geosci. Front. 2017, 8, 1311–1321. [Google Scholar] [CrossRef]
- Mohanan, K.; Sharma, S.K.; Bishop, F.C. A Raman spectral study of forsterite-monticellite solid solutions. Am. Mineral. 1993, 78, 42–48. [Google Scholar]
- Ishibashi, H.; Arakawa, M.; Yamamoto, J.; Kagi, H. Precise determination of Mg/Fe ratios applicable to terrestrial olivine samples using Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 331–337. [Google Scholar] [CrossRef]
- Auzende, A.L.; Daniel, L.; Reynard, B.; Lemaire, C.; Guyot, F. High-pressure behaviour of serpentine minerals: A Raman spectroscopic study. Phys. Chem. Mineral. 2004, 31, 267–277. [Google Scholar] [CrossRef]
- Groppo, C.; Rinaudo, C.; Cairo, S.; Gastaldi, D.; Compagnoni, R. Micro-Raman spectroscopy for a quick and reliable identification of serpentine minerals from ultramafics. Eur. J. Mineral. 2006, 18, 319–329. [Google Scholar] [CrossRef]
- Reynard, B.; Wunder, B. High-pressure behavior of synthetic antigorite in the MgO-SiO2-H2O system from Raman spectroscopy. Am. Mineral. 2006, 91, 459–462. [Google Scholar] [CrossRef]
- Rinaudo, C.; Gastaldi, D.; Belluso, E. Characterization of chrysotile, antigorite and lizardite by FT-Raman spectroscopy. Can. Mineral. 2003, 41, 883–890. [Google Scholar] [CrossRef]
- Prencipe, M.; Noel, Y.; Bruno, M.; Dovesi, R. The vibrational spectrum of lizardite-1 T [Mg3Si2O5(OH)4] at the Г point: A contribution from an ab initio periodic B3LPY calculation. Am. Mineral. 2009, 94, 986–994. [Google Scholar] [CrossRef]
- Schwartz, S.; Guillot, S.; Reynard, B.; Lafay, R.; Debret, B.; Nicollet, C.; Lanari, P.; Line Auzende, A. Pressure-temperature estimates of the lizardite/antigorite transition in high pressure serpentinites. Lithos 2013, 178, 197–210. [Google Scholar] [CrossRef] [Green Version]
- Dawson, P.; Hadfield, C.D.; Wilkinson, G.R. The polarized infrared and Raman spectra of Mg(OH)2 and Ca(OH)2. J. Phys. Chem. Solids. 1973, 34, 1217–1225. [Google Scholar] [CrossRef]
- Duffy, T.S.; Meade, C.; Fei, Y.; Mao, H.-K.; Hemley, R.J. High-pressure phase transition in brucite, Mg(OH)2. Am. Mineral. 1995, 80, 222–230. [Google Scholar] [CrossRef]
- Mével, C. Serpentinization of abyssal peridotites at mid-ocean ridges. C. R. Geosci. 2017, 335, 825–852. [Google Scholar] [CrossRef]
- Witt-Eickschen, G.; O’Neill, H.S.C. The effect of temperature on the equilibrium distribution of trace elements between clinopyroxene, orthopyroxene, olivine and spinel in upper mantle peridotite. Chem. Geol. 2005, 221, 65–101. [Google Scholar] [CrossRef]
- De Hoog, J.C.M.; Gall, L.; Cornell, D.H. Trace-element geochemistry of mantle olivine and application to mantle petrogenesis and geothermobarometry. Chem. Geol. 2010, 270, 196–215. [Google Scholar] [CrossRef] [Green Version]
- Tollan, P.M.E.; O’Neill, H.S.C.; Hermann, J.; Benedictus, A.; Arculus, R.J. Frozen melt–rock reaction in a peridotite xenolith from sub-arc mantle recorded by diffusion of trace elements and water in olivine. Earth Planet. Sci. Lett. 2015, 422, 169–181. [Google Scholar] [CrossRef]
- Straub, S.M.; LaGatta, A.B.; Lillian Martin-Del Pozzo, A.; Langmuir, C.H. Evidence from high-Ni olivines for a hybirdized peridotite/pyroxenite source for orogenic andesites from the central Mexican Volcanic Belt. Geochem. Geophys. Geosys. 2008, 9, Q03007. [Google Scholar] [CrossRef]
- Su, B.; Chen, Y.; Mao, Q.; Zhang, D.; Jia, L.-H.; Guo, S. Minor elements in olivine inspect the petrogenesis of orogenic peridotites. Lithos 2019, 344–345, 207–216. [Google Scholar] [CrossRef]
- Foley, S.F.; Prelevic, D.; Rehfeldt, T.; Jacob, D.E. Minor and trace elements in olivines as probes into early igneous and mantle melting processes. Earth Planet. Sci. Lett. 2013, 363, 181–191. [Google Scholar] [CrossRef]
- Shea, J.J.; Foley, S.F. Evidence for carbonatite-influenced source assemblage for intraplate basalts from the Buckland Volcanic Province, Queensland, Australia. Minerals 2019, 9, 546. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.R.; Rossman, G.R.; Maldener, J.; Endisch, D.; Rauch, F. Hydroxide in olivine: A quantitative determination of the absolute amount and calibration of the IR spectrum. J. Geophys. Res. 2003, 108, 2105. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Jiang, H.; Kovács, I.; Xia, Q.-K.; Yang, X. Quantitative analysis of H-species in anisotropic minerals by unpolarized infrared spectroscopy: An experimental evaluation. Am. Mineral. 2018, 103, 1761–1769. [Google Scholar] [CrossRef]
- Bai, Q.; Kohlstedt, D.L. Effects of chemical environment on the solubility and incorporation mechanism for hydrogen in olivine. Phys. Chem. Mineral. 1993, 19, 460–471. [Google Scholar] [CrossRef]
- Matveev, S.; O’Neill, H.S.C.; Ballhaus, C.; Taylor, W.R.; Green, D.H. Effect of silica activity on OH-IR spectra of olivine: Implications for low-a SiO2 mantle metasomatism. J. Petrol. 2001, 42, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, C.; Kohn, S.C.; Brooker, R.A. The effect of silica activity on the incorporation mechanisms of water in synthetic forsterite: A polarised infrared spectroscopic study. Contrib. Mineral. Petrol. 2004, 147, 48–57. [Google Scholar]
- Matsyuk, S.S.; Langer, K. Hydroxyl in olivines from mantle xenoliths in kimberlites of the Siberian platform. Contrib. Mineral. Petrol. 2004, 147, 413–437. [Google Scholar] [CrossRef]
- Huang, R.; Sun, W.; Ding, X.; Wang, Y.; Zhan, W. Experimental investigation of iron mobility during serpentinization. Acta Petrol. Sin. 2015, 31, 883–890. [Google Scholar]
- Cai, J.; Liu, F.; Liu, P.; Wang, F. Metamorphic P-T evolution and tectonic implications of pelitic granulites in the Ji’an area, northeastern Jiao-Liao-Ji Belt, North China Craton. J. Asian Earth Sci. 2020, 191, 104197. [Google Scholar] [CrossRef]
- Finkelstein, G.J.; Dera, P.K.; Jahn, S.; Oganov, A.R.; Holl, C.M.; Meng, Y.; Duffy, T.S. Phase transitions and equation of state of forsterite to 90 GPa from single-crystal X-ray diffraction and molecular modeling. Am. Mineral. 2014, 99, 35–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, Y.; Liu, X. A metastable Fo-III wedge in cold slabs subducted to the lower part of the mantle transition zone: A hypothesis based on first-principles simulations. Minerals 2019, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.; Jacobsen, S.D.; Jiang, F.; Smyth, J.R.; Holl, C.M.; Frost, D.J.; Duffy, T.S. Velocity crossover between hydrous and anhydrous forsterite at high pressures. Earth Planet. Sci. Lett. 2010, 293, 250–258. [Google Scholar] [CrossRef]









| Rim(10) a | Mantle(10) | Core(5) | |
|---|---|---|---|
| SiO2 | 40.90(158)b | 41.29(55) | 41.32(47) |
| TiO2 | 0.02(2) | 0.02(2) | 0.01(1) |
| Al2O3 | 0.00(0) | 0.01(1) | 0.01(1) |
| Cr2O3 | 0.02(2) | 0.00(1) | 0.02(2) |
| FeO | 0.35(3) | 0.36(3) | 0.37(1) |
| MnO | 0.04(2) | 0.03(2) | 0.02(2) |
| NiO | 0.02(2) | 0.01(2) | 0.01(2) |
| MgO | 57.79(144) | 57.74(56) | 57.66(40) |
| CaO | 0.01(1) | 0.01(1) | 0.01(1) |
| Na2O | 0.02(2) | 0.01(1) | 0.00(0) |
| K2O | 0.00(1) | 0.01(1) | 0.00(0) |
| Total | 99.16(117) | 99.48(79) | 99.44(72) |
| Si | 0.97(3) | 0.98(1) | 0.98(1) |
| Ti | 0.00(0) | 0.00(0) | 0.00(0) |
| Al | 0.00(0) | 0.00(0) | 0.00(0) |
| Cr | 0.00(0) | 0.00(0) | 0.00(0) |
| Fe | 0.01(0) | 0.01(0) | 0.01(0) |
| Mn | 0.00(0) | 0.00(0) | 0.00(0) |
| Ni | 0.00(0) | 0.00(0) | 0.00(0) |
| Mg | 2.05(6) | 2.04(2) | 2.04(1) |
| Ca | 0.00(0) | 0.00(0) | 0.00(0) |
| Na | 0.00(0) | 0.00(0) | 0.00(0) |
| K | 0.00(0) | 0.00(0) | 0.00(0) |
| Total | 3.03(3) | 3.02(1) | 3.02(1) |
| Mg#c | 99.67(3) | 99.65(3) | 99.64(1) |
| Rim(10) a | Core(9) | |
|---|---|---|
| Li | 1.87(48)b | 1.97(38) |
| B | 1763(23) | 1759(33) |
| Na | 0.92(28) | 1.06(73) |
| Al | 38.0(73) | 38.1(64) |
| P | 121(13) | 120(11) |
| Ca | 23.7(75) | 20.7(50) |
| Sc | 1.20(4) | 1.20(4) |
| Ti | 7.22(188) | 7.59(216) |
| V | 0.57(6) | 0.57(6) |
| Cr | 0.92(8) | 0.71(8) |
| Mn | 198(5) | 199(5) |
| Co | 0.58(3) | 0.58(2) |
| Ni | 0.91(6) | 0.91(3) |
| Cu | b.d.l.c | b.d.l. |
| Zn | 44.0(9) | 44.0(11) |
| Ga | 0.28(2) | 0.27(2) |
| Sr | b.d.l. | b.d.l. |
| Y | 0.068(12) | 0.063(12) |
| Zr | 0.87(12) | 1.04(35) |
| Nb | 0.094(24) | 0.103(32) |
| Ce | b.d.l. | b.d.l. |
| Antigorite(13) a | Brucite(8) | |
|---|---|---|
| SiO2 | 40.48(317) b | 1.21(101) |
| TiO2 | 0.02(3) | 0.01(1) |
| Al2O3 | 0.11(14) | 0.07(8) |
| Cr2O3 | 0.01(1) | 0.01(2) |
| FeO | 0.48(20) | 0.39(8) |
| MnO | 0.02(3) | 0.07(3) |
| NiO | 0.01(2) | 0.02(2) |
| MgO | 43.30(292) | 80.98(464) |
| CaO | 0.02(1) | 0.03(2) |
| Na2O | 0.02(2) | 0.03(4) |
| K2O | 0.02(2) | 0.02(2) |
| Total | 84.50(147) | 82.83(459) c |
| Si | 3.87(24) | 0.01(1) |
| Ti | 0.00(0) | 0.00(0) |
| Al | 0.01(2) | 0.00(0) |
| Cr | 0.00(0) | 0.00(0) |
| Fe | 0.04(2) | 0.00(0) |
| Mn | 0.00(0) | 0.00(0) |
| Ni | 0.00(0) | 0.00(0) |
| Mg | 6.19(49) | 0.98(2) |
| Ca | 0.00(0) | 0.00(0) |
| Na | 0.00(0) | 0.00(0) |
| K | 0.00(0) | 0.00(0) |
| Total | 10.12(24) | 0.99(1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; He, M.; Yan, W.; Yang, M.; Liu, X. Jianite: Massive Dunite Solely Made of Virtually Pure Forsterite from Ji’an County, Jilin Province, Northeast China. Minerals 2020, 10, 220. https://doi.org/10.3390/min10030220
Wang Y, He M, Yan W, Yang M, Liu X. Jianite: Massive Dunite Solely Made of Virtually Pure Forsterite from Ji’an County, Jilin Province, Northeast China. Minerals. 2020; 10(3):220. https://doi.org/10.3390/min10030220
Chicago/Turabian StyleWang, Yuwei, Mingyue He, Wei Yan, Mei Yang, and Xi Liu. 2020. "Jianite: Massive Dunite Solely Made of Virtually Pure Forsterite from Ji’an County, Jilin Province, Northeast China" Minerals 10, no. 3: 220. https://doi.org/10.3390/min10030220





