Mineralogy and Fluid Regime of Formation of the REE-Late-Stage Hydrothermal Mineralization of Petyayan-Vara Carbonatites (Vuoriyarvi, Kola Region, NW Russia)
Abstract
:1. Introduction
- Ancylite-dominant magnesiocarbonatites with ancylite–baryte–strontianite–calcite–quartz (±late Ca–Fe–Mg carbonates) ore assemblage.
- Breccias of magnesiocarbonatites with a quartz–bastnäsite matrix (±late Ca–Fe–Mg carbonates).
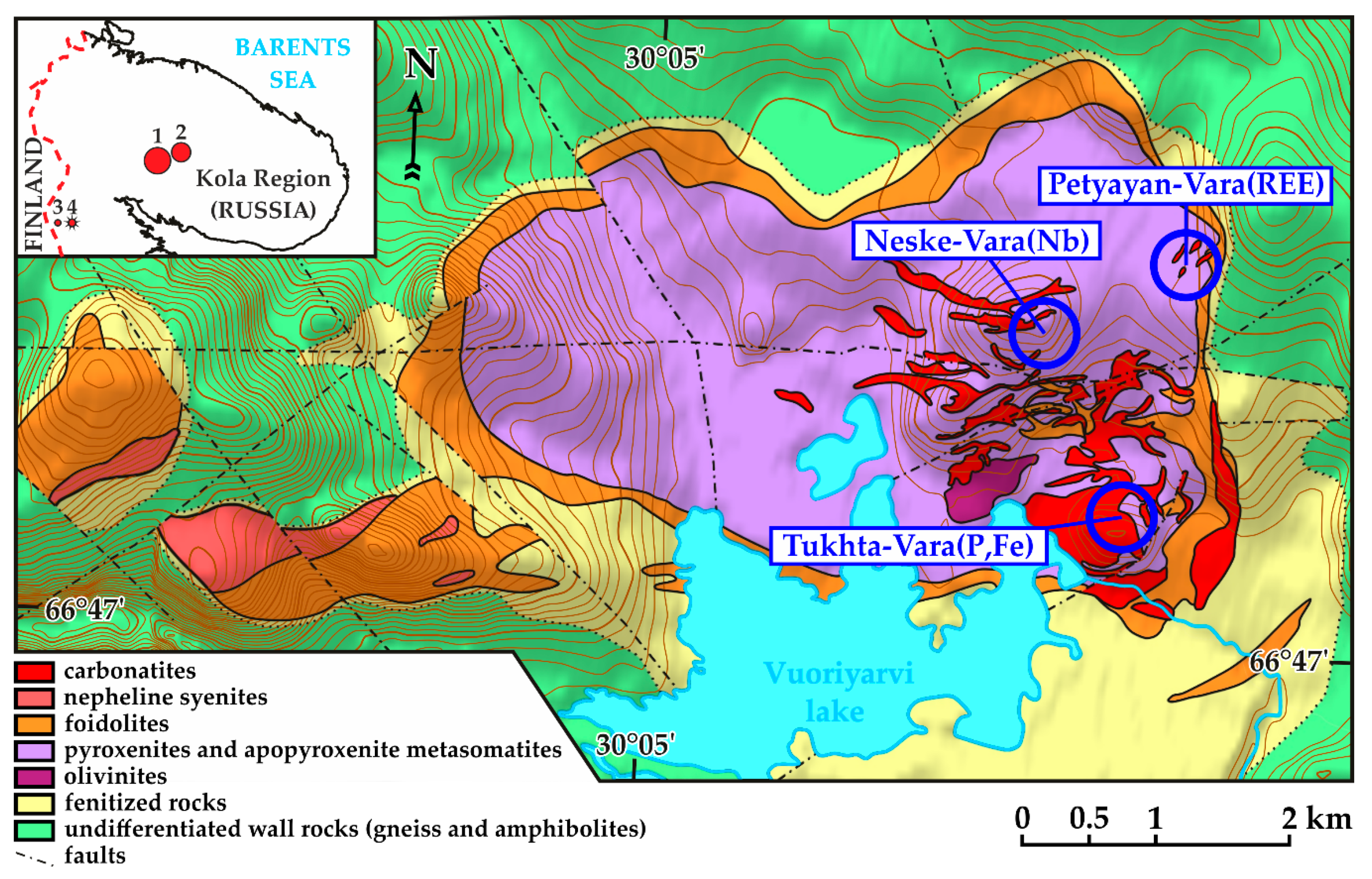
2. Geological Setting
- Magnesiocarbonatites, consisting of dolomite and ancylite–strontianite–baryte pseudomorphs after burbankite. These carbonatites are primarily magmatic. They were altered to varying degrees during several metasomatic events.
- High-Ti carbonatites composed of dolomite, microcline, Ti oxides (±phlogopite, pyrochlore, and pyrite). Locally high-Ti carbonatites are enriched in apatite (+zircon; ±aegirine and albite). These rocks were formed as a result of the introduction of a carbonatitic fluid (or fluid-saturated melt) rich in K, Al, Si, Fe, and Ti at the final stage of magmatic activity.
- Baryte-dominant (dolomite + baryte) and ancylite-dominant (ancylite–baryte–strontianite–calcite–quartz ± late Ca–Fe–Mg carbonates; “ancylite ores”) magnesiocarbonatites. Alteration of primary magnesiocarbonatites is caused by a magmatic S-rich fluid with high concentrations of Ba, Sr, and REE.
- Late giant-grained carbothermal calciocarbonatites localized near the ancylite-dominant rocks.
- Breccias of magnesiocarbonatites with a quartz–bastnäsite matrix (“bastnäsite ores”). These are the product of the final stage of the formation of Petyayan-Vara carbonatites during which the ancylite was dissolved, and REEs were deposited in bastnäsite (predominantly hydroxylated) in cracks and crush zones. Strontianite-dominant rocks, in which Sr was released from ancylite, are complementary to bastnäsite ores. Also, monazite, thorite, iron oxides and hydroxides, late Ca–Fe–Mg carbonates and calcite were crystallized at the late stage of carbonatite formation.
3. Sampling Procedure and Analytical Methods
4. Results
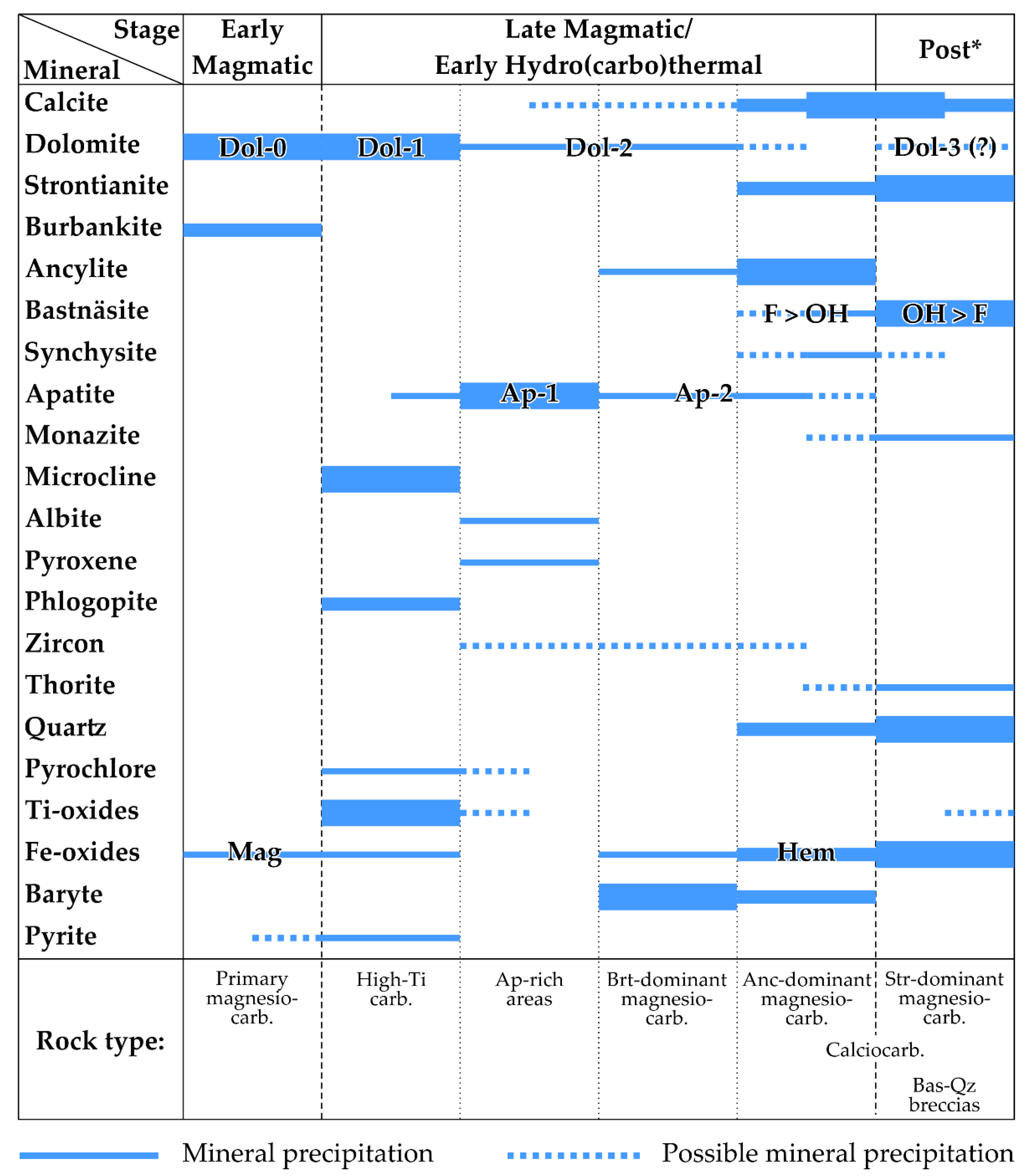
4.1. Mineralogy of REE–Carbonatites
4.2. Fluid Inclusion Study
5. Discussion
6. Conclusions
- The Petyayan-Vara magnesiocarbonatites underwent intense hydrothermal-metasomatic processes. The Ba–Sr and REE ore-related late-stage hydrothermal mineralization is represented by the ancylite–baryte–strontianite–celestine–calcite–quartz (±late Ca–Fe–Mg carbonates), with monazite-(Ce)–bastnäsite-(Ce)–thorite, and hydroxylbastnäsite-(Ce) mineral assemblages.
- The ore-forming late-stage hydrothermal fluids wee oxidized and elemental compositions evolved, which resulted in auto-metasomatic alteration and recrystallization. The fluid regime was changed from highly concentrated (30–50 wt.%) sulphate–carbonate at 275–300 °C to medium–low concentrated (<15 wt.% of NaCl-equ.) hydrocarbonate–chloride at 150–250 °C.
- The REE-late-stage hydrothermal mineralization of Petyayan-Vara magnesiocarbonatites is most likely a product of hydrothermal-metasomatic remobilization of the REE mineral phases by the high activity of the sulphate–carbonate–hydrocarbonate–chloride hydrothermal ore-forming fluids.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P.; Bellieni, G.; Dudek, A.; Efremova, S.; Keller, J.; et al. Igneous Rocks, 2nd ed.; Le Maitre, R.W., Streckeisen, A., Zanettin, B., Le Bas, M.J., Bonin, B., Bateman, P., Eds.; Cambridge University Press: Cambridge, UK, 2002; ISBN 9780511535581. [Google Scholar]
- Weng, Z.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A detailed assessment of global rare earth element resources: Opportunities and challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Wall, F. Rare earth elements: Minerals, mines, magnets (and more). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Wall, F. Rare earth elements. In Critical Metals Handbook; John Wiley & Sons: Oxford, UK, 2013; pp. 312–339. ISBN 9780470671719. [Google Scholar]
- Goodenough, K.M.; Wall, F.; Merriman, D. The rare earth elements: Demand, global resources, and challenges for resourcing future generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Kalashnikov, A.O.; Konopleva, N.G.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Rare earth deposits of the Murmansk region, Russia—A review. Econ. Geol. 2016, 111, 1529–1559. [Google Scholar] [CrossRef]
- Kukharenko, A.A.; Orlova, M.P.; Bulakh, A.G.; Bagdasarov, E.A.; Rimskaya-Korsakova, O.M.; Nefedov, Y.I.; II’inskii, G.A.; Sergeev, A.S.; Abakumova, N.B. The Caledonian Complex of Ultrabasic and Alkaline Rocks and Carbonatites of the Kola Peninsula and Northern Karelia; Nedra: Moscow, Russia, 1965. (In Russian) [Google Scholar]
- Gerasimovsky, V.I.; Volkov, V.P.; Kogarko, L.M.; Polyakov, A.I. Kola peninsula. In The Alkaline Rocks; Sørensen, H., Ed.; John Wiley & Sons: New York, NY, USA, 1974; pp. 206–220. ISBN 9780471813835. [Google Scholar]
- Kramm, U.; Kogarko, L.N.; Kononova, V.A.; Vartiainen, H. The Kola Alkaline Province of the CIS and Finland: Precise Rb-Sr ages define 380–360 Ma age range for all magmatism. Lithos 1993, 30, 33–44. [Google Scholar] [CrossRef]
- Arzamastsev, A.A.; Glaznev, V.N.; Arzamastseva, L.V.; Bea, F.; Montero, P. Kola alkaline province in the Paleozoic: Evaluation of primary mantle magma composition and magma generation conditions. Russ. J. Earth Sci. 2001, 3, 1–32. [Google Scholar] [CrossRef]
- Bulakh, A.G.; Ivanikov, V.V.; Orlova, M.P. Overview of carbonatite-phoscorite complexes of the Kola Alkaline Province. In Phoscorites and Carbonatites from Mantle to Mine; Wall, F., Zaitsev, A.N., Eds.; Mineralogical Society of Great Britain and Ireland: London, UK, 2004; pp. 1–43. ISBN 9780903056229. [Google Scholar]
- Downes, H.; Balaganskaya, E.; Beard, A.; Liferovich, R.; Demaiffe, D. Petrogenetic processes in the ultramafic, alkaline and carbonatitic magmatism in the Kola Alkaline Province: A review. Lithos 2005, 85, 48–75. [Google Scholar] [CrossRef] [Green Version]
- Arzamastsev, A.A.; Mitrofanov, F.P. Paleozoic plume-lithospheric processes in northeastern Fennoscandia: Evaluation of the composition of the parental mantle melts and magma generation conditions. Petrology 2009, 17, 300–313. [Google Scholar] [CrossRef]
- Rukhlov, A.S.; Bell, K. Geochronology of carbonatites from the Canadian and Baltic Shields, and the Canadian Cordillera: Clues to mantle evolution. Mineral. Petrol. 2010, 98, 11–54. [Google Scholar] [CrossRef]
- Afanasyev, B.V. Mineral Resources of the Alkaline–Ultramafic Massifs of the Kola Peninsula; Roza Vetrov: St. Petersburg, Russia, 2011. (In Russian) [Google Scholar]
- Kozlov, E.; Fomina, E.; Sidorov, M.; Shilovskikh, V.; Bocharov, V.; Chernyavsky, A.; Huber, M. The Petyayan-Vara Carbonatite-Hosted Rare Earth Deposit (Vuoriyarvi, NW Russia): Mineralogy and Geochemistry. Minerals 2020, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhao, J.; Chen, J.; Keisuke, N.; Hirochika, S.; Shen, K.; Men, L.; Chen, L. Ore-forming mechanism for the Xiaoxinancha Au-rich Cu deposit in Yanbian, Jilin Province, China: Evidence from noble gas isotope geochemistry of fluid inclusions in minerals. Sci. China Ser. D Earth Sci. 2008, 51, 216–228. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y. Mechanisms of element precipitation in carbonatite-related rare-earth element deposits: Evidence from fluid inclusions in the Maoniuping deposit, Sichuan Province, southwestern China. Ore Geol. Rev. 2019, 107, 218–238. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Y. Occurrence and geochemistry of bastnäsite in carbonatite-related REE deposits, Mianning–Dechang REE belt, Sichuan Province, SW China. Ore Geol. Rev. 2019, 107, 266–282. [Google Scholar] [CrossRef]
- Cangelosi, D.; Smith, M.; Banks, D.; Yardley, B. The role of sulfate-rich fluids in heavy rare earth enrichment at the Dashigou carbonatite deposit, Huanglongpu, China. Mineral. Mag. 2020, 84, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chakhmouradian, A.R.; Hou, Z.; Song, W.; Kynický, J. Development of REE mineralization in the giant Maoniuping deposit (Sichuan, China): Insights from mineralogy, fluid inclusions, and trace-element geochemistry. Miner. Depos. 2019, 54, 701–718. [Google Scholar] [CrossRef]
- Samson, I.M.; Wood, S.A.; Finucane, K. fluid inclusion characteristics and genesis of the fluorite-parisite Mineralization in the Snowbird Deposit, Montana. Econ. Geol. 2004, 99, 1727–1744. [Google Scholar] [CrossRef]
- Shu, X.; Liu, Y. Fluid inclusion constraints on the hydrothermal evolution of the Dalucao Carbonatite-related REE deposit, Sichuan Province, China. Ore Geol. Rev. 2019, 107, 41–57. [Google Scholar] [CrossRef]
- Nikolenko, A.M.; Redina, A.A.; Doroshkevich, A.G.; Prokopyev, I.R.; Ragozin, A.L.; Vladykin, N.V. The origin of magnetite-apatite rocks of Mushgai-Khudag Complex, South Mongolia: Mineral chemistry and studies of melt and fluid inclusions. Lithos 2018, 320–321, 567–582. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, Z.; Yin, S.; Dominy, S.C.; Xu, J.; Tian, S.; Xu, W. Continuous carbonatitic melt–fluid evolution of a REE mineralization system: Evidence from inclusions in the Maoniuping REE Deposit, Western Sichuan, China. Ore Geol. Rev. 2009, 36, 90–105. [Google Scholar] [CrossRef]
- Prokopyev, I.R.; Doroshkevich, A.G.; Ponomarchuk, A.V.; Sergeev, S.A. Mineralogy, age and genesis of apatite-dolomite ores at the Seligdar apatite deposit (Central Aldan, Russia). Ore Geol. Rev. 2017, 81, 296–308. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Z. A synthesis of mineralization styles with an integrated genetic model of carbonatite-syenite-hosted REE deposits in the Cenozoic Mianning-Dechang REE metallogenic belt, the eastern Tibetan Plateau, southwestern China. J. Asian Earth Sci. 2017, 137, 35–79. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Brady, A.E.; Wall, F.; Gunn, G.; Dawes, W. REE minerals at the Songwe Hill carbonatite, Malawi: HREE-enrichment in late-stage apatite. Ore Geol. Rev. 2017, 81, 23–41. [Google Scholar] [CrossRef] [Green Version]
- Prokopyev, I.R.; Doroshkevich, A.G.; Redina, A.A.; Obukhov, A.V. Magnetite-apatite-dolomitic rocks of Ust-Chulman (Aldan shield, Russia): Seligdar-type carbonatites? Mineral. Petrol. 2018, 112, 257–266. [Google Scholar] [CrossRef]
- Kozlov, E.; Fomina, E.; Sidorov, M.; Shilovskikh, V. Ti-Nb mineralization of late carbonatites and role of fluids in its formation: Petyayan-vara rare-earth carbonatites (Vuoriyarvi Massif, Russia). Geosciences 2018, 8, 281. [Google Scholar] [CrossRef] [Green Version]
- Roedder, E. Fluid Inclusions. Reviews in Mineralogy; Ribbe, P.H., Ed.; Mineralogical Society of America: Blacksburg, VA, USA, 1984; Volume 12, ISBN 978-0-939950-16-4. [Google Scholar]
- Kotel’nikova, Z.A.; Kotel’nikov, A.R. Method of synthetic fluid inclusions in quartz in experimental study of the water-sodium sulfate system. Geol. Ore Depos. 2009, 51, 68–73. [Google Scholar] [CrossRef]
- Koster van Groos, A.F. High-pressure DTA study of the upper three-phase region in the system Na2CO3-H2O. Am. Mineral. 1990, 75, 667–675. [Google Scholar]
- Borisenko, A.S. Cryometric technique applied to studies of the saline composition of solution in gaseous fluid inclusions in minerals. Geol. i Geofiz. AN SSSR SO 1977, 8, 16–27. (In Russian) [Google Scholar]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal mobilisation of the rare earth elements-a tale of ‘ceria’ and ‘yttria’. Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- Tropper, P.; Manning, C.E.; Harlov, D.E. Solubility of CePO4 monazite and YPO4 xenotime in H2O and H2O–NaCl at 800 °C and 1GPa: Implications for REE and Y transport during high-grade metamorphism. Chem. Geol. 2011, 282, 58–66. [Google Scholar] [CrossRef]
- Tropper, P.; Manning, C.E.; Harlov, D.E. Experimental determination of CePO4 and YPO4 solubilities in H2O-NaF at 800 °C and 1 GPa: Implications for rare earth element transport in high-grade metamorphic fluids. Geofluids 2013, 13, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Doroshkevich, A.G.; Ripp, G.S.; Moore, K.R. Genesis of the Khaluta alkaline-basic Ba-Sr carbonatite complex (West Transbaikala, Russia). Mineral. Petrol. 2010, 98, 245–268. [Google Scholar] [CrossRef]
- Duraiswami, R.; Shaikh, T. Fluid-rock interaction in the Kangankunde Carbonatite Complex, Malawi: SEM based evidence for late stage pervasive hydrothermal mineralisation. Open Geosci. 2014, 6, 476–491. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.F.; Collins, A.K.; Palin, J.M.; Spratt, J. Mineralogical evolution and REE mobility during crystallisation of ancylite-bearing ferrocarbonatite, Haast River, New Zealand. Lithos 2015, 216–217, 324–337. [Google Scholar] [CrossRef]
- Nadeau, O.; Cayer, A.; Pelletier, M.; Stevenson, R.; Jébrak, M. The Paleoproterozoic Montviel carbonatite-hosted REE–Nb deposit, Abitibi, Canada: Geology, mineralogy, geochemistry and genesis. Ore Geol. Rev. 2015, 67, 314–335. [Google Scholar] [CrossRef]
- Andersen, A.K.; Clark, J.G.; Larson, P.B.; Neill, O.K. Mineral chemistry and petrogenesis of a HFSE(+HREE) occurrence, peripheral to carbonatites of the Bear Lodge alkaline complex, Wyoming. Am. Mineral. 2016, 101, 1604–1623. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Styles, M.T.; Appleton, J.D.; Gunn, G.; Wall, F. Evidence for dissolution-reprecipitation of apatite and preferential LREE mobility in carbonatite-derived late-stage hydrothermal processes. Am. Mineral. 2016, 101, 596–611. [Google Scholar] [CrossRef] [Green Version]
- Prokopyev, I.R.; Borisenko, A.S.; Borovikov, A.A.; Pavlova, G.G. Origin of REE-rich ferrocarbonatites in southern Siberia (Russia): Implications based on melt and fluid inclusions. Mineral. Petrol. 2016, 110, 845–859. [Google Scholar] [CrossRef]
- Prokopyev, I.R.; Doroshkevich, A.G.; Sergeev, S.A.; Ernst, R.E.; Ponomarev, J.D.; Redina, A.A.; Chebotarev, D.A.; Nikolenko, A.M.; Dultsev, V.F.; Moroz, T.N.; et al. Petrography, mineralogy and SIMS U-Pb geochronology of 1.9–1.8 Ga carbonatites and associated alkaline rocks of the Central-Aldan magnesiocarbonatite province (South Yakutia, Russia). Mineral. Petrol. 2019, 113, 329–352. [Google Scholar] [CrossRef]
- Andersen, A.K.; Larson, P.B.; Cosca, M.A. C–O stable isotope geochemistry and 40Ar/39Ar geochronology of the Bear Lodge carbonatite stockwork, Wyoming, USA. Lithos 2019, 324–325, 640–660. [Google Scholar] [CrossRef]
- Dalsin, M.L.; Groat, L.A.; Creighton, S.; Evans, R.J. The mineralogy and geochemistry of the Wicheeda Carbonatite Complex, British Columbia, Canada. Ore Geol. Rev. 2015, 64, 523–542. [Google Scholar] [CrossRef]
- Trofanenko, J.; Williams-Jones, A.E.; Simandl, G.J.; Migdisov, A.A. The nature and origin of the REE mineralization in the Wicheeda Carbonatite, British Columbia, Canada. Econ. Geol. 2016, 111, 199–223. [Google Scholar] [CrossRef] [Green Version]
- Zaitsev, A.N.; Wall, F.; Le Bas, M.J. REE-Sr-Ba minerals from the Khibina carbonatites, Kola Peninsula, Russia: Their mineralogy, paragenesis and evolution. Mineral. Mag. 1998, 62, 225–250. [Google Scholar] [CrossRef]
- Ngwenya, B.T. Hydrothermal rare earth mineralisation in carbonatites of the Tundulu complex, Malawi: Processes at the fluid/rock interface. Geochim. Cosmochim. Acta 1994, 58, 2061–2072. [Google Scholar] [CrossRef]
- Ruberti, E.; Enrich, G.E.R.; Gomes, C.B.; Comin-Chiaramonti, P. Hydrothermal REE fluorocarbonate mineralization at Barra do Itapirapuã, a multiple stockwork carbonatite, southern Brazil. Can. Mineral. 2008, 46, 901–914. [Google Scholar] [CrossRef]
- Rankin, A.H. Carbonatite-associated rare metal deposits: Composition and evolution of ore-forming fluids—The fluid inclusion evidence. In Rare-Element Geochemistry and Mineral Deposits. GAC Short Course Notes 17; Linnen, R.L., Samson, I.M., Eds.; Geological Association of Canada: St. John’s, NL, Canada, 2005; pp. 299–314. ISBN 978-1-897095-08-9. [Google Scholar]
- Borisenko, A.S.; Borovikov, A.A.; Vasyukova, E.A.; Pavlova, G.G.; Ragozin, A.L.; Prokop’ev, I.R.; Vladykin, N.V. Oxidized magmatogene fluids: Metal-bearing capacity and role in ore formation. Russ. Geol. Geophys. 2011, 52, 144–164. [Google Scholar] [CrossRef]




| Mineral | CaO | MgO | FeOt | MnO | SrO | BaO | SO3 | Total |
|---|---|---|---|---|---|---|---|---|
| Dolomite | 29.45 | 16.72 | 5.41 | 2.11 | b.d.l. | - | - | 53.65 |
| 28.73 | 17.38 | 4.63 | 1.14 | b.d.l. | - | - | 51.88 | |
| 30.21 | 17.31 | 4.31 | 0.19 | b.d.l. | - | - | 52.02 | |
| Calcite | 55.91 | 0.95 | b.d.l. | b.d.l. | b.d.l. | - | - | 56.86 |
| 54.26 | 1.69 | 0.24 | b.d.l. | b.d.l. | - | - | 56.19 | |
| 54.05 | 1.13 | b.d.l. | b.d.l. | 0.28 | - | - | 55.46 | |
| Ankerite | 22.85 | 2.04 | 34.21 | 0.19 | b.d.l. | - | - | 59.29 |
| 20.85 | 0.73 | 33.61 | 0.39 | 1.81 | - | - | 57.35 | |
| Siderite | 2.55 | 0.86 | 57.59 | 0.27 | b.d.l. | - | - | 61.27 |
| 0.73 | 1.08 | 60.52 | 0.44 | b.d.l. | - | - | 62.77 | |
| Strontianite | 1.04 | - | - | - | 68.62 | - | - | 69.64 |
| 2.53 | - | - | - | 66.63 | - | - | 69.16 | |
| 0.62 | - | - | - | 69.04 | - | - | 69.66 | |
| Celestite | - | - | - | - | 52.48 | 3.98 | 42.88 | 99.34 |
| - | - | - | - | 47.02 | 8.97 | 43.08 | 99.07 | |
| Baryte | - | - | - | - | b.d.l. | 65.12 | 33.92 | 99.02 |
| - | - | - | - | 1.16 | 63.78 | 34.33 | 99.24 |
| Mineral | CaO | SrO | La2O3 | Ce2O3 | Pr2O3 | Nd2O3 | Sm2O3 | ThO2 | P2O5 | SO3 | F | –O=F2 | OH | Total | La/Ce | La/Nd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ancylite-(Ce) | ||||||||||||||||
| 0.54 | 16.59 | 18.46 | 25.33 | 1.95 | 5.69 | 0.97 | b.d.l. | - | - | b.d.l. | - | - | 69.53 | 0.73 | 3.24 | |
| 0.44 | 18.15 | 20.42 | 25.76 | 2.06 | 4.61 | b.d.l. | b.d.l. | - | - | b.d.l. | - | - | 71.44 | 0.79 | 4.43 | |
| 0.91 | 15.83 | 20.09 | 25.38 | 1.65 | 4.86 | b.d.l. | b.d.l. | - | - | b.d.l. | - | - | 68.72 | 0.79 | 4.13 | |
| Bastnäsite-(Ce) | ||||||||||||||||
| 0.87 | b.d.l. | 20.31 | 35.47 | 3.04 | 9.16 | b.d.l. | b.d.l. | - | - | 6.02 | 2.54 | - | 74.86 | 0.57 | 2.22 | |
| 1.09 | b.d.l. | 21.17 | 35.07 | 2.95 | 9.39 | b.d.l. | b.d.l. | - | - | 5.96 | 2.51 | - | 75.63 | 0.6 | 2.25 | |
| 0.76 | b.d.l. | 20.79 | 36.33 | 3.04 | 9.18 | b.d.l. | b.d.l. | - | - | 5.36 | 2.26 | - | 75.46 | 0.57 | 2.26 | |
| Formulae based on the anions for the mineral | ||||||||||||||||
| 0.036 | 0.294 | 0.509 | 0.043 | 0.128 | 0.996 | 0.004 | ||||||||||
| 0.045 | 0.302 | 0.496 | 0.041 | 0.129 | 0.972 | 0.028 | ||||||||||
| 0.031 | 0.296 | 0.513 | 0.043 | 0.126 | 0.872 | 0.127 | ||||||||||
| Hydroxylbastnäsite-(Ce) | ||||||||||||||||
| 0.32 | b.d.l. | 20.35 | 36.61 | 2.61 | 8.82 | b.d.l. | b.d.l. | - | - | b.d.l. | - | - | 68.71 | 0.56 | 2.31 | |
| 0.31 | b.d.l. | 20.86 | 36.74 | 2.75 | 8.83 | b.d.l. | b.d.l. | - | - | b.d.l. | - | - | 69.49 | 0.57 | 2.36 | |
| 0.29 | b.d.l. | 20.85 | 36.63 | 2.81 | 8.89 | b.d.l. | b.d.l. | - | - | b.d.l. | - | - | 69.46 | 0.57 | 2.35 | |
| Formulae based on the anions for the mineral | ||||||||||||||||
| 0.013 | 0.297 | 0.531 | 0.037 | 0.125 | - | |||||||||||
| 0.013 | 0.301 | 0.527 | 0.039 | 0.123 | - | |||||||||||
| 0.012 | 0.301 | 0.526 | 0.040 | 0.124 | - | |||||||||||
| Monazite-(Ce) | ||||||||||||||||
| 3.86 | 4.91 | 16.12 | 29.85 | 2.36 | 9.01 | 1.28 | 1.77 | 25.35 | 4.72 | - | - | - | 99.22 | 0.54 | 1.79 | |
| 3.48 | 5.04 | 16.34 | 30.03 | 2.21 | 9.27 | b.d.l. | b.d.l. | 27.39 | 5.77 | - | - | - | 99.53 | 0.54 | 1.76 | |
| 4.41 | 4.08 | 16.27 | 29.81 | 2.42 | 9.51 | b.d.l. | b.d.l. | 28.41 | 4.12 | - | - | - | 99.02 | 0.55 | 1.71 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokopyev, I.; Kozlov, E.; Fomina, E.; Doroshkevich, A.; Dyomkin, M. Mineralogy and Fluid Regime of Formation of the REE-Late-Stage Hydrothermal Mineralization of Petyayan-Vara Carbonatites (Vuoriyarvi, Kola Region, NW Russia). Minerals 2020, 10, 405. https://doi.org/10.3390/min10050405
Prokopyev I, Kozlov E, Fomina E, Doroshkevich A, Dyomkin M. Mineralogy and Fluid Regime of Formation of the REE-Late-Stage Hydrothermal Mineralization of Petyayan-Vara Carbonatites (Vuoriyarvi, Kola Region, NW Russia). Minerals. 2020; 10(5):405. https://doi.org/10.3390/min10050405
Chicago/Turabian StyleProkopyev, Ilya, Evgeniy Kozlov, Ekaterina Fomina, Anna Doroshkevich, and Maxim Dyomkin. 2020. "Mineralogy and Fluid Regime of Formation of the REE-Late-Stage Hydrothermal Mineralization of Petyayan-Vara Carbonatites (Vuoriyarvi, Kola Region, NW Russia)" Minerals 10, no. 5: 405. https://doi.org/10.3390/min10050405







