Petrogenesis of Ultramafic Lamprophyres from the Terina Complex (Chadobets Upland, Russia): Mineralogy and Melt Inclusion Composition
Abstract
1. Introduction
2. Geological Setting
3. Sampling Procedure and Analytical Methods
4. Results of Investigations
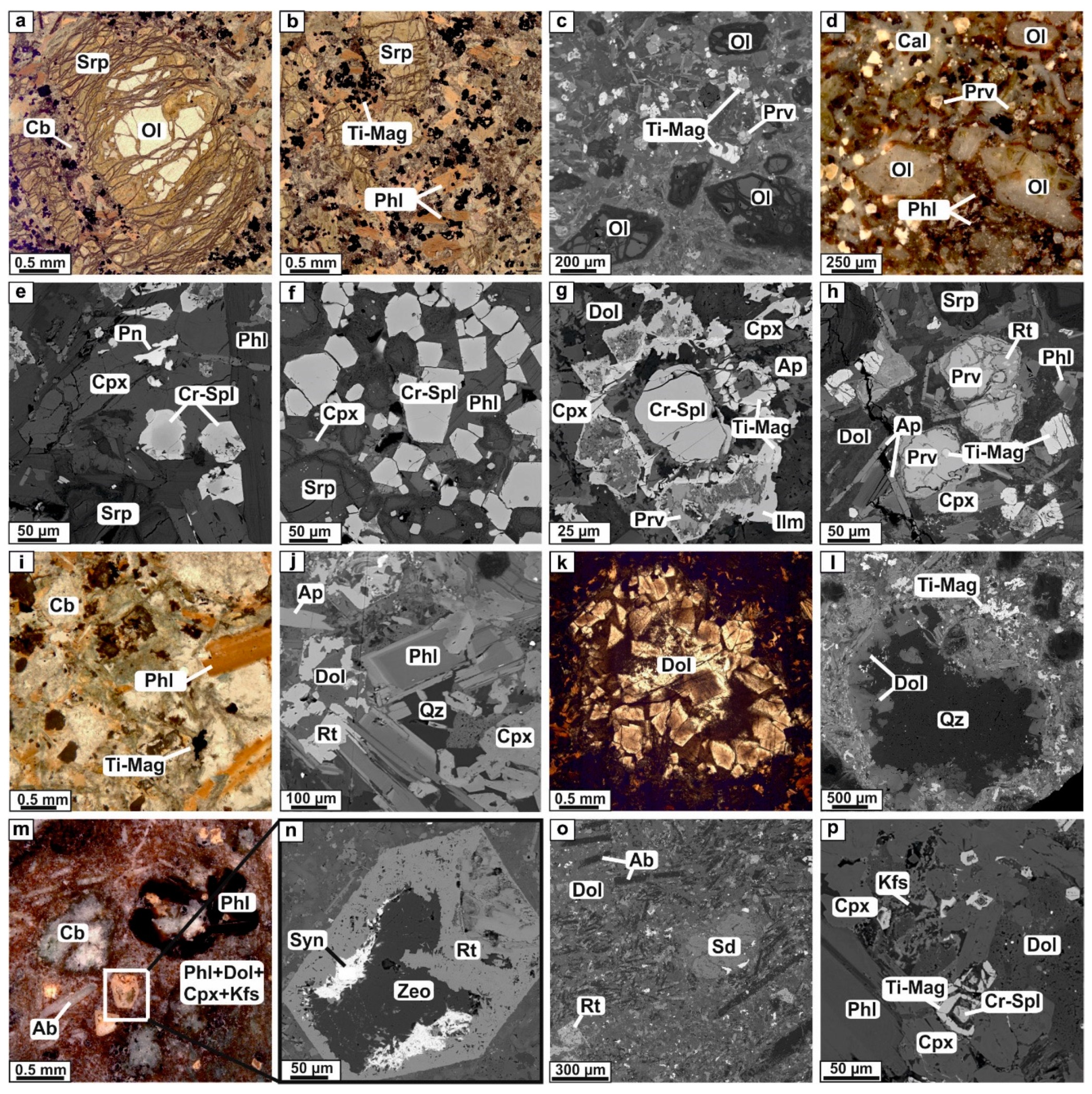
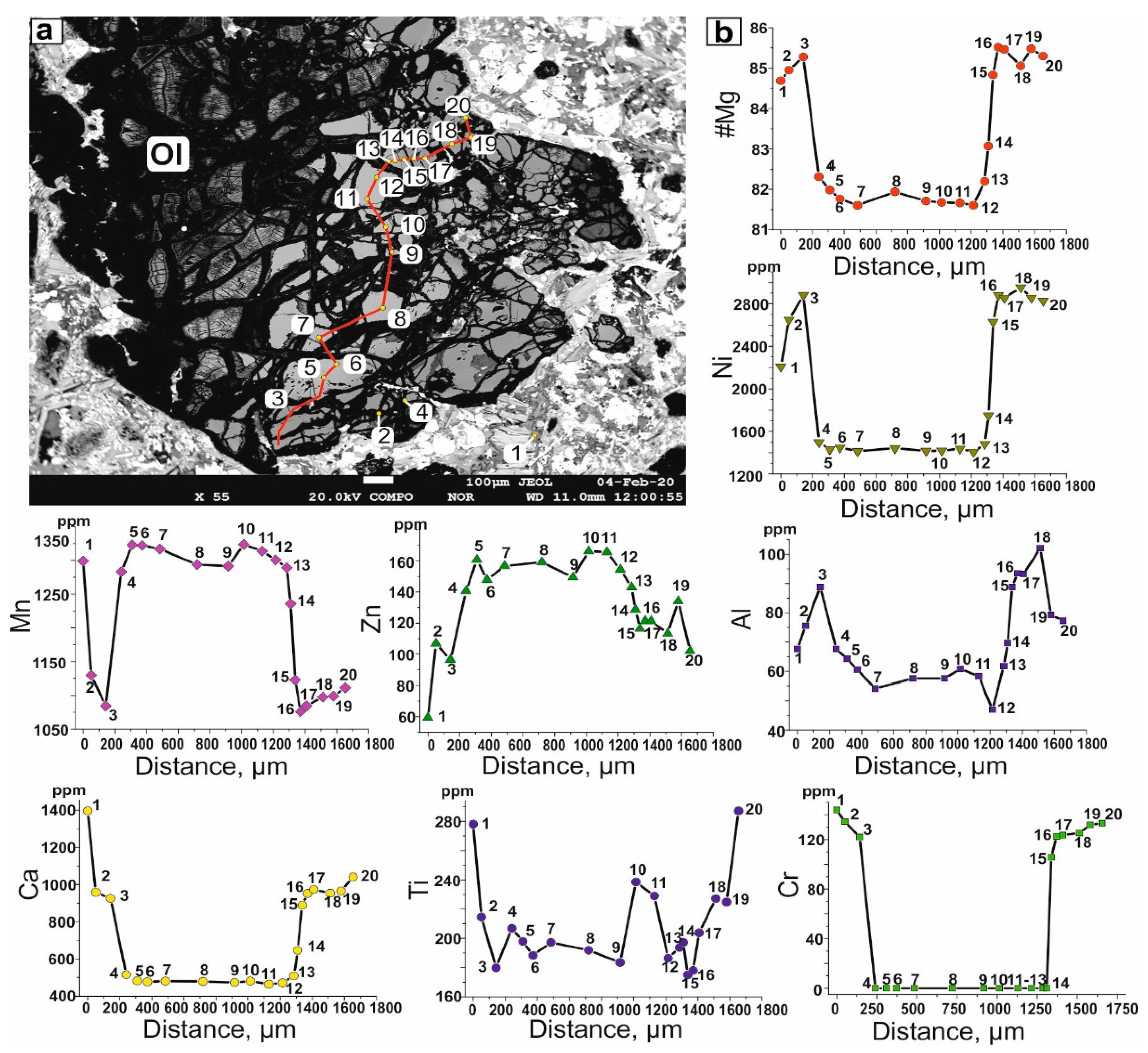
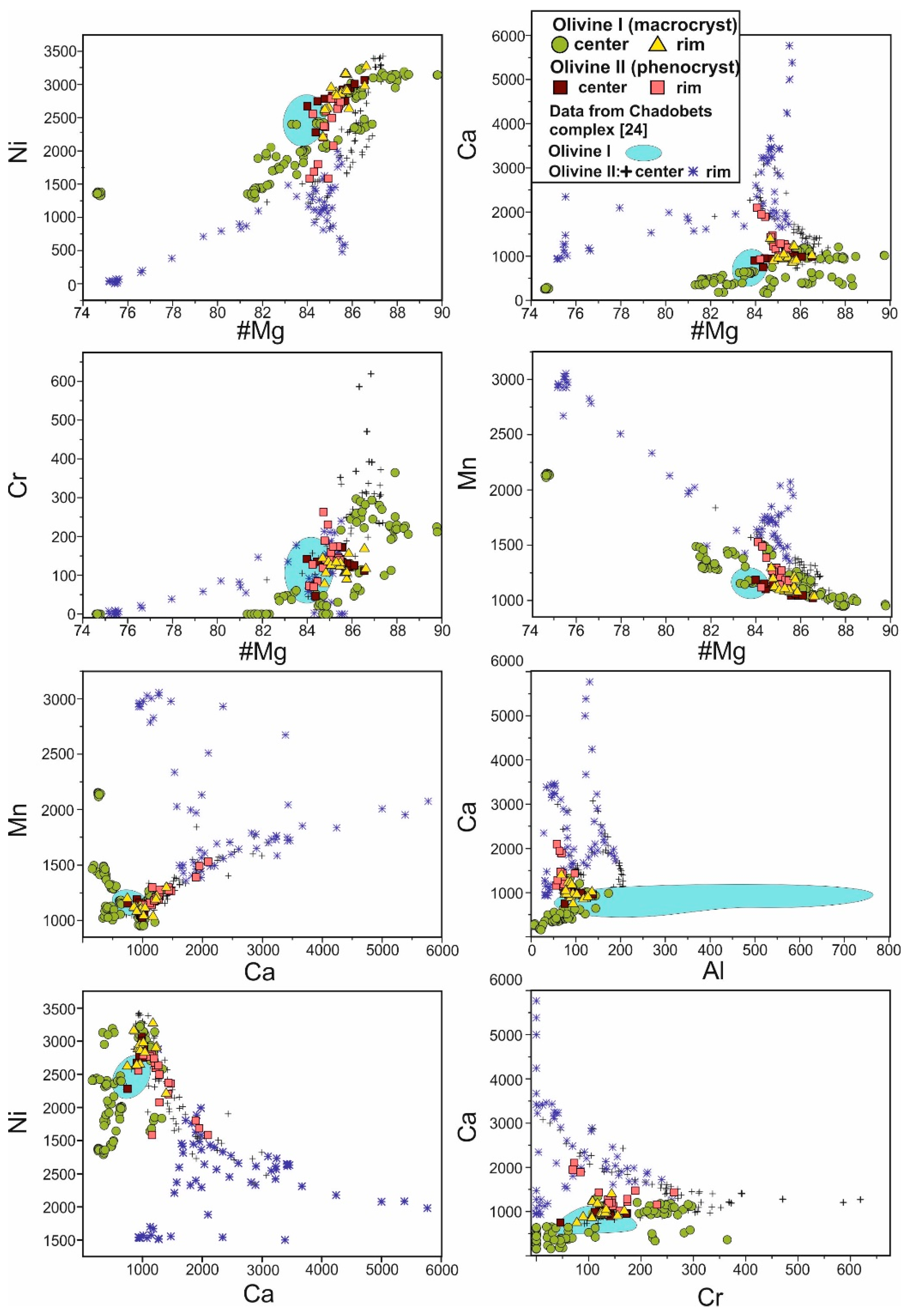
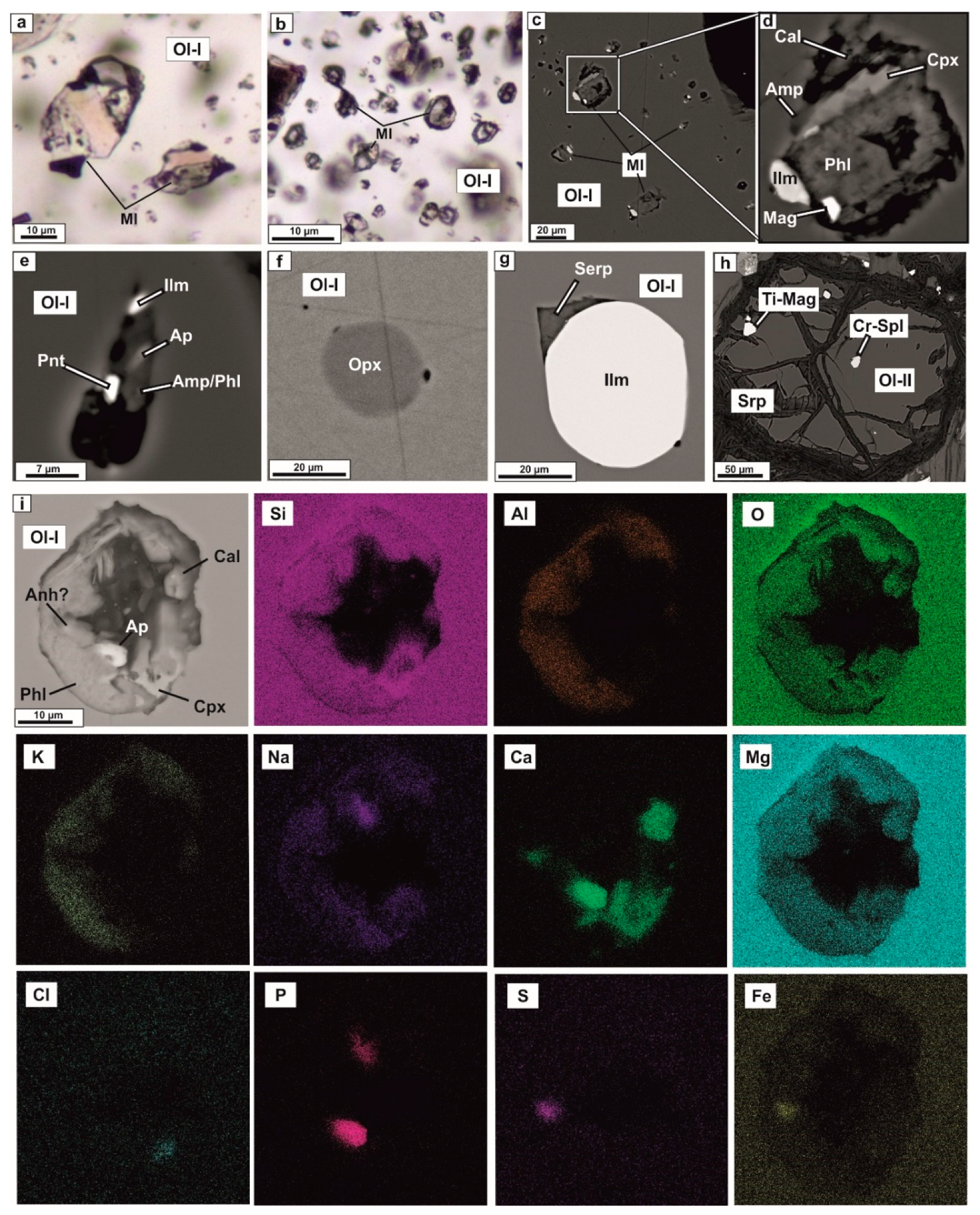
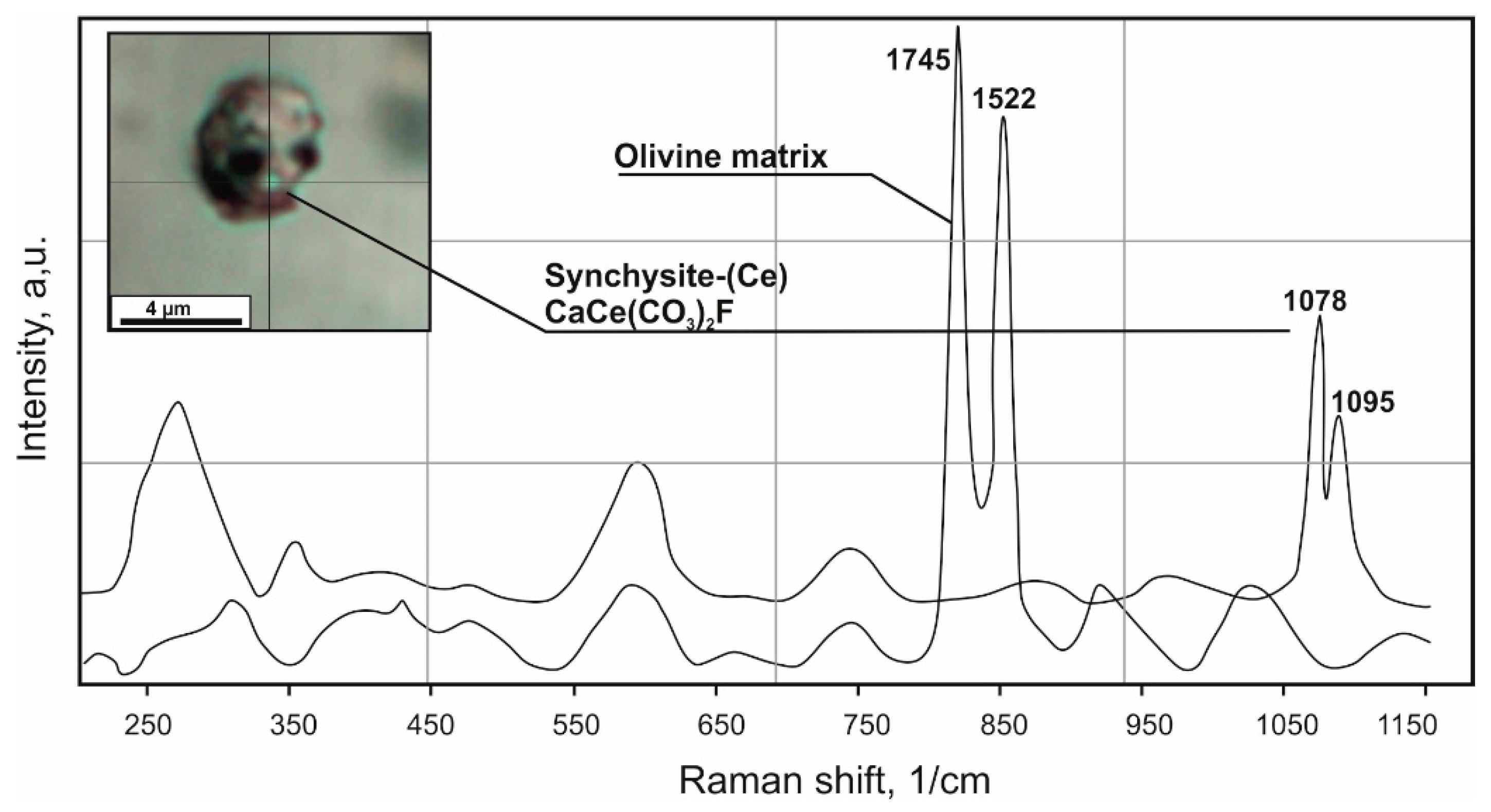
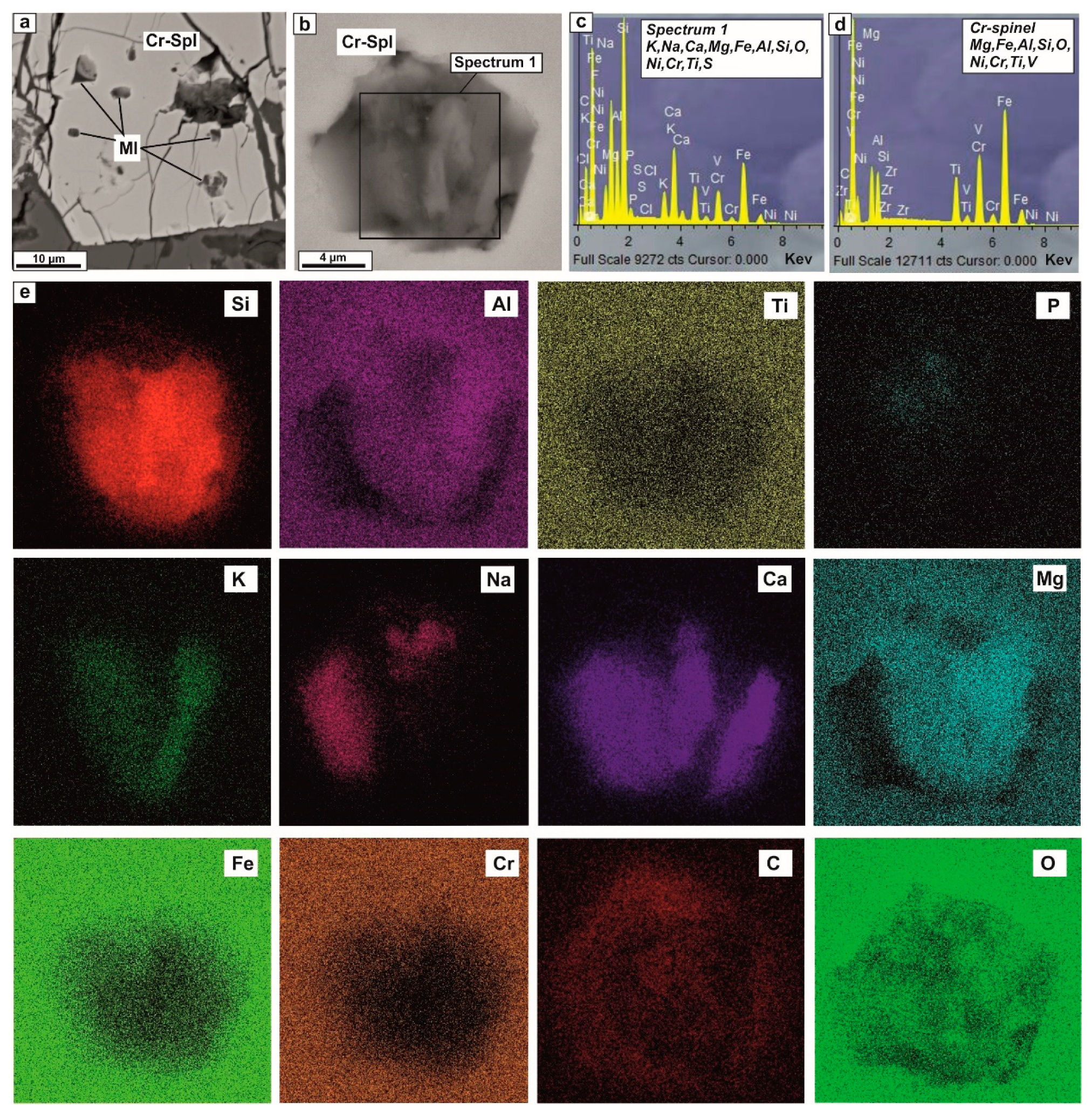
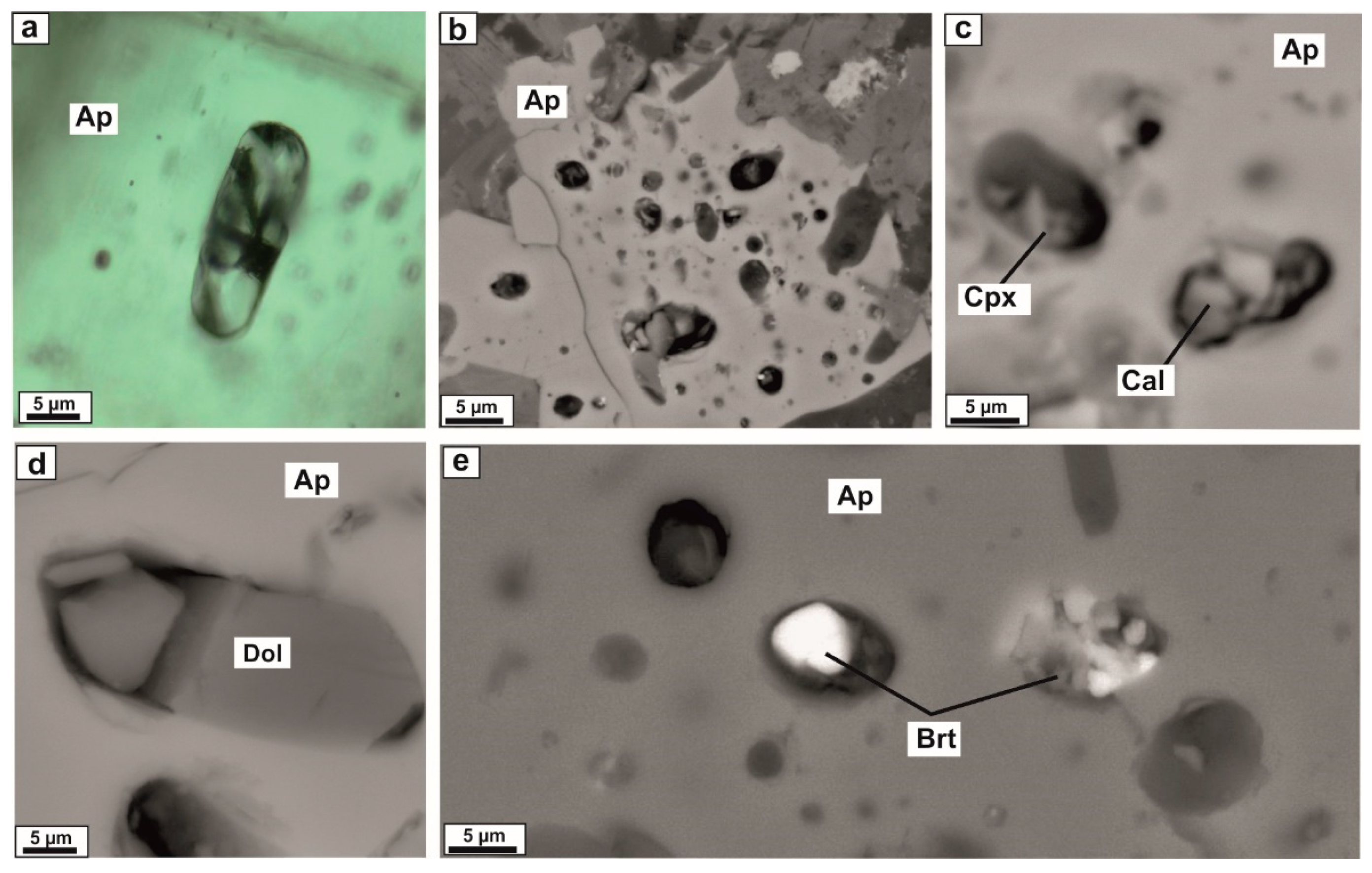
4.1. Petrography and Mineralogy of the UML Rocks
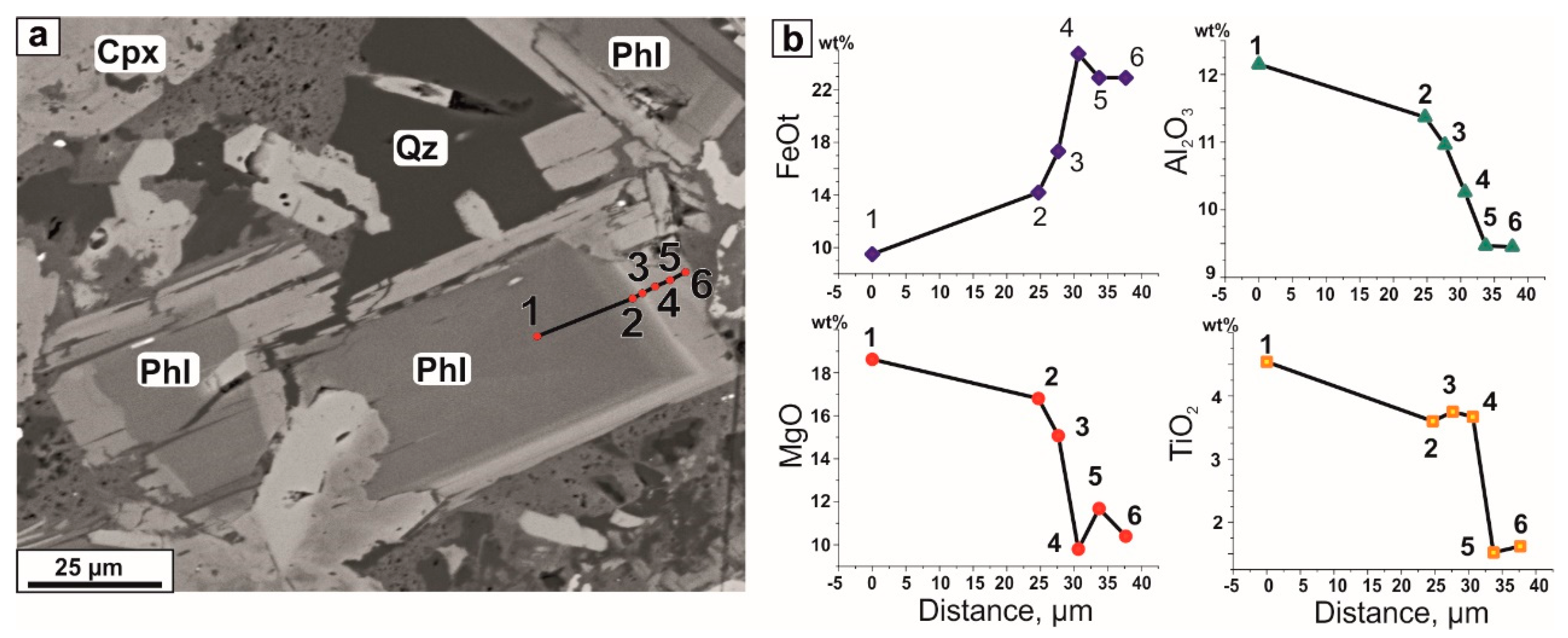
4.2. Melt and Mineral Inclusion Investigations
5. Discussion
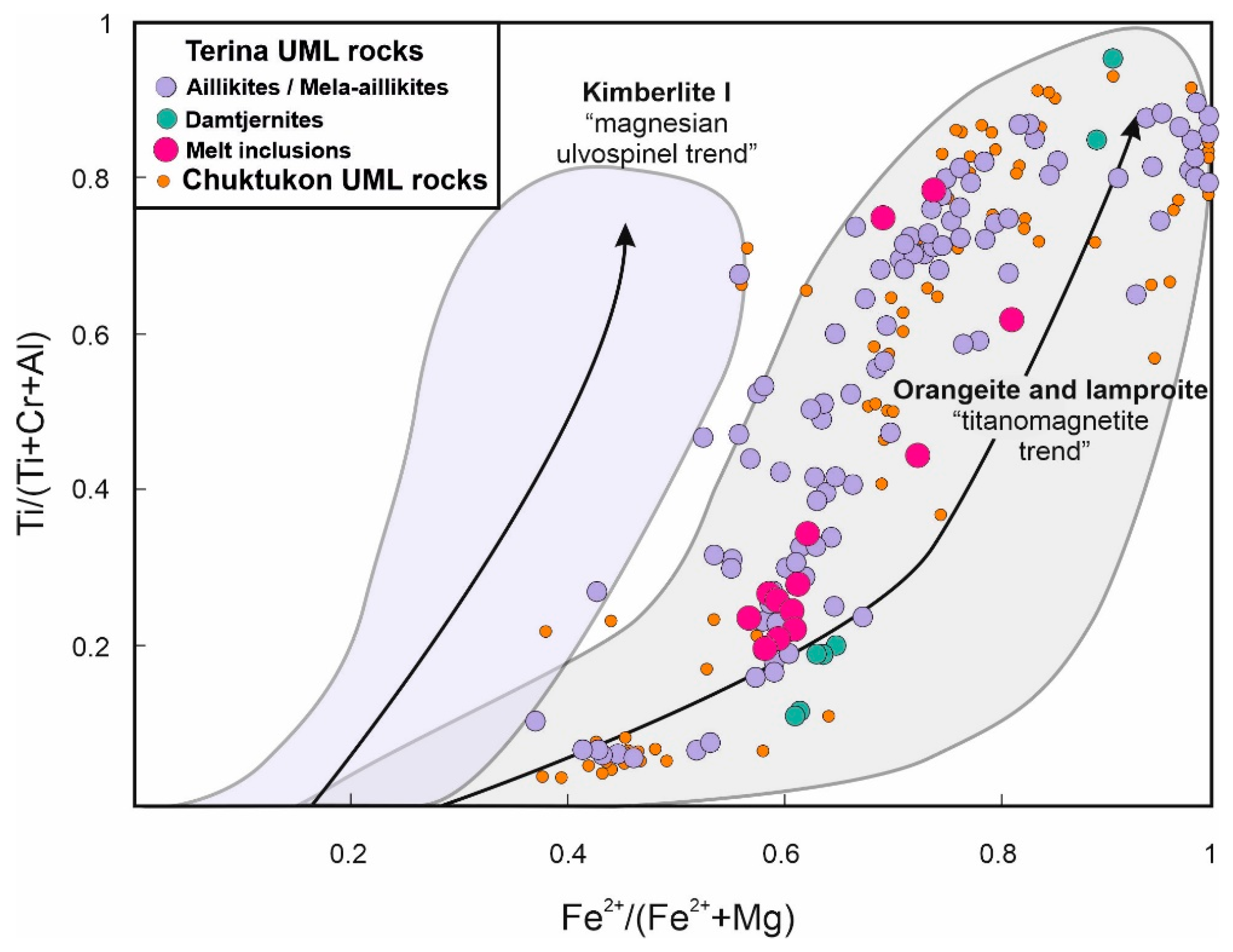
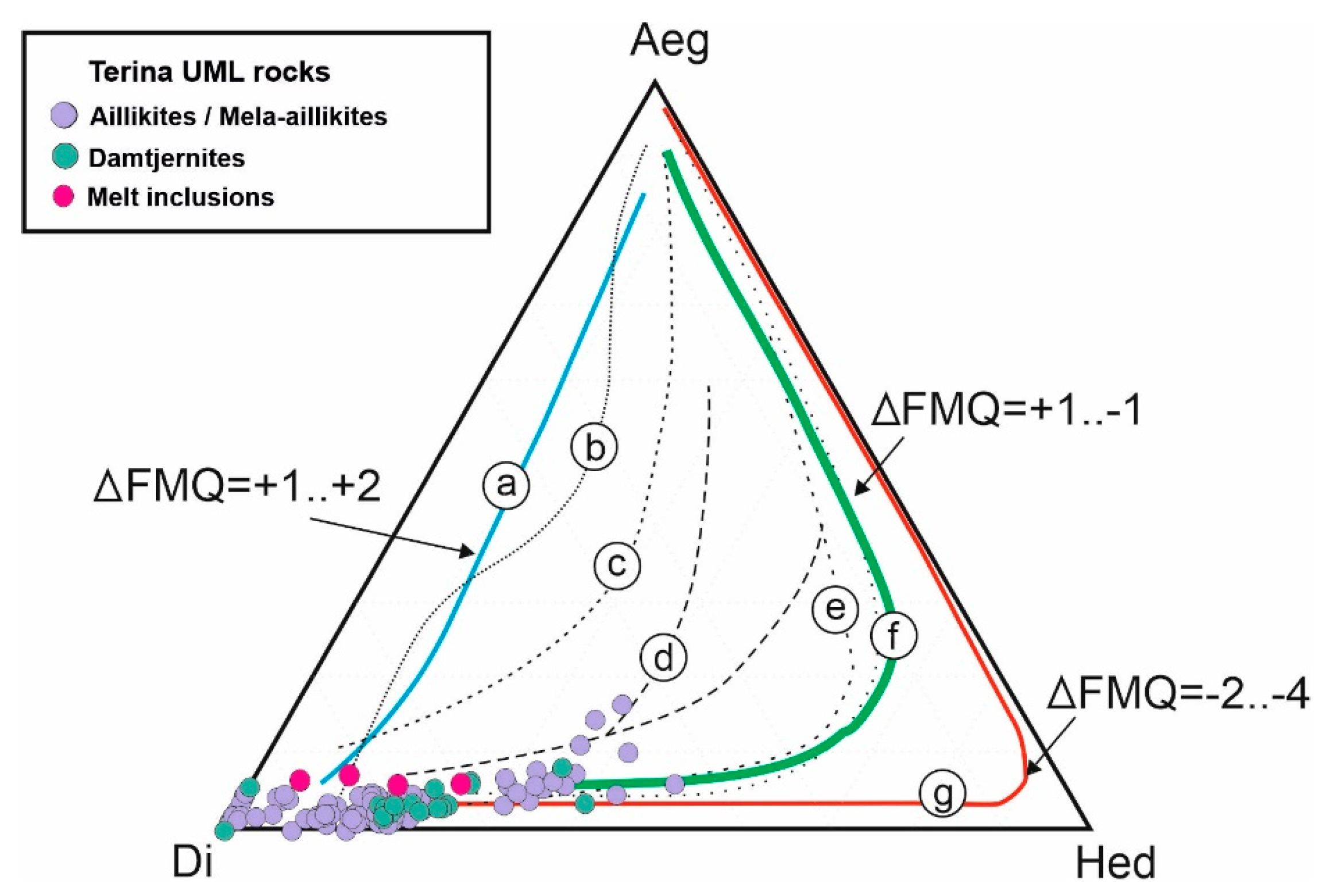
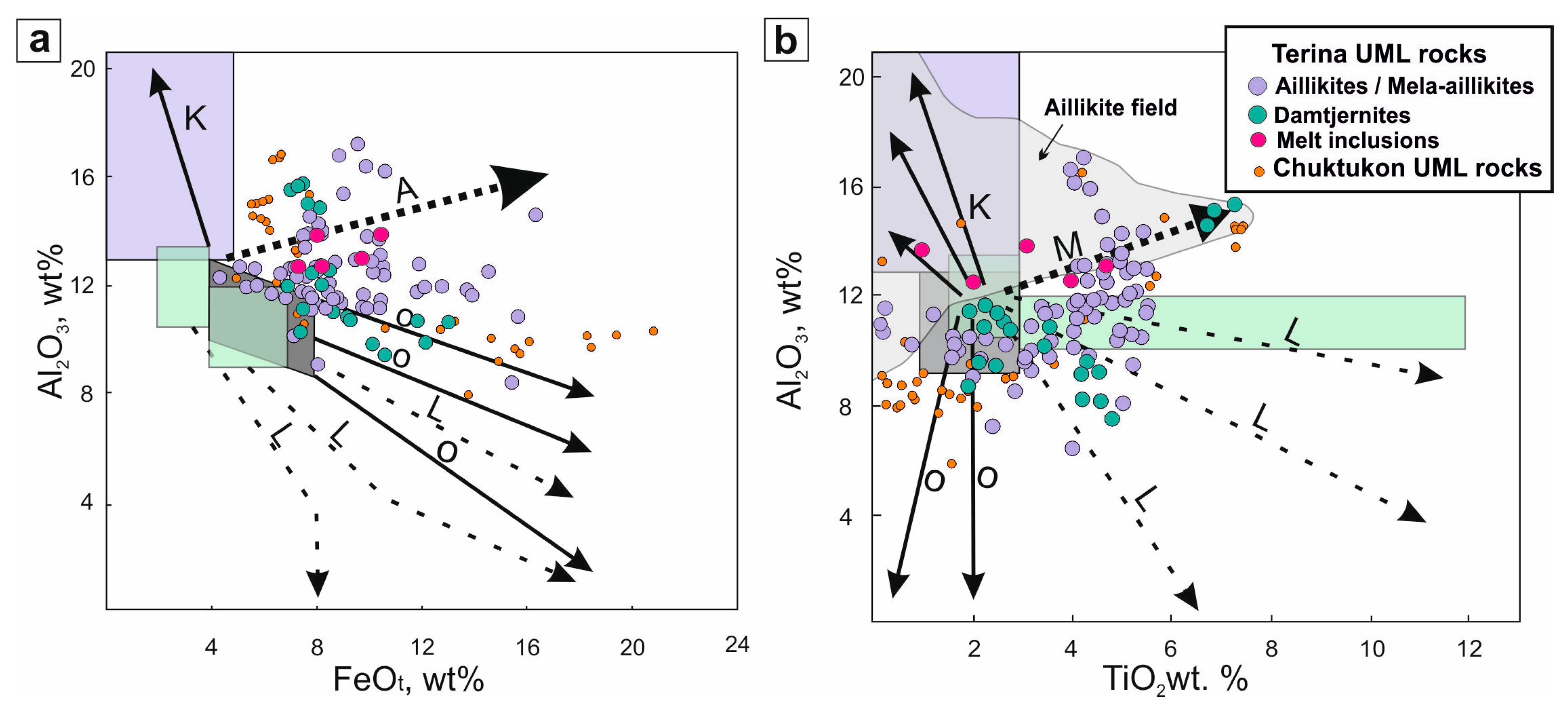
5.1. Genesis of the UMLs of the Chadobets Complex
5.2. Comparison of Inclusion Data with Well-Known in the World
5.3. PTX Estimations for the Chadobets UMLs
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kranck, E.H. The rock-ground of the coast of Labrador and the connection between the pre-Cambrian of Greenland and North America. Bull. Comm. Geol. Finl. 1939, 22, 65–86. [Google Scholar]
- Rock, N.M.S. The nature and origin of ultramafic lamprophyres: Alnöites and allied rocks. J. Petrol. 1986, 27, 155–196. [Google Scholar] [CrossRef]
- Le Maitre, R.W.A. Classification of Igneous Rocks and Glossary of Terms: Recommendations of the International Union of Geological Sciences, Subcommission on the Systematics of Igneous Rocks; Blackwell: Oxford, UK, 1989; p. 193. [Google Scholar]
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P.; Bellieni, G.; Dudek, A.; Efremova, S.; Keller, J.; et al. Igneous Rocks: A Classification and Glossary of Terms. Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks, 2nd ed.; Le Maitre, R.W., Ed.; Cambridge University Press: Cambridge, UK, 2002; ISBN 9780521619486. [Google Scholar]
- Tappe, S.; Foley, S.F.; Jenner, G.A.; Kjarsgaard, B.A. Integrating ultramafic lamprophyres into the IUGS classification of igneous rocks: Rationale and implications. J. Petrol. 2005, 46, 1893–1900. [Google Scholar] [CrossRef]
- Tappe, S.; Foley, S.F.; Jenner, G.A.; Heaman, L.M.; Kjarsgaard, B.A.; Romer, R.L.; Stracke, A.; Joyce, N.; Hoefs, J. Genesis of ultramafic lamprophyres and carbonatites at Aillik Bay, labrador: A consequence of incipient lithospheric thinning beneath the North Atlantic Craton. J. Petrol. 2006, 47, 1261–1315. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Chebotarev, D.A.; Sharygin, V.V.; Prokopyev, I.R.; Nikolenko, A.M. Petrology of alkaline silicate rocks and carbonatites of the Chuktukon massif, Chadobets upland, Russia: Sources, evolution and relation to the Triassic Siberian LIP. Lithos 2019, 332–333, 245–260. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Doroshkevich, A.G. Multiphase inclusions in zircons from Chuktukon carbonatite massif, Chadobets upland, Russia. In Proceedings of the 34th International Conference “Magmatism of the Earth and Related Strategic Metal Deposits”, Miass, Russia, 4–9 August 2017; pp. 244–247. [Google Scholar]
- Nosova, A.A.; Sazonova, L.V.; Kargin, A.V.; Smirnova, M.D.; Lapin, A.V.; Shcherbakov, V.D. Olivine in ultramafic lamprophyres: Chemistry, crystallisation, and melt sources of Siberian Pre- and post-trap aillikites. Contrib. Mineral. Petrol. 2018, 173, 55. [Google Scholar] [CrossRef]
- Nosova, A.A.; Kargin, A.V.; Sazonova, L.V.; Dubinina, E.O.; Chugaev, A.V.; Lebedeva, N.M.; Yudin, D.S.; Larionova, Y.O.; Abersteiner, A.; Gareev, B.I.; et al. Sr-Nd-Pb isotopic systematic and geochronology of ultramafic alkaline magmatism of the southwestern margin of the Siberian Craton: Metasomatism of the sub-continental lithospheric mantle related to subduction and plume events. Lithos 2020. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Sharygin, V.V.; Seryotkin, Y.V.; Karmanov, N.S.; Belogub, E.V.; Moroz, T.N.; Nigmatulina, E.N.; Eliseev, A.P.; Vedenyapin, V.N.; Kupriyanov, I.N. Rippite, IMA 2016-025. CNMNC Newsletter No. 32, August 2016, page 919. Mineral. Mag. 2016, 80, 915–922. [Google Scholar] [CrossRef]
- Chebotarev, D.A.; Doroshkevich, A.G.; Sharygin, V.V.; Yudin, D.S.; Ponomarchuk, A.V.; Sergeev, S.A. Geochronology of the Chuktukon carbonatite massif, Chadobets uplift (Krasnoyarsk Territory). Russ. Geol. Geophys. 2017, 58, 1222–1231. [Google Scholar] [CrossRef]
- Basu, A.R.; Poreda, R.J.; Renne, P.R.; Telchmann, F.; Vasiliev, Y.R.; Sobolev, N.V.; Turrin, B.D. High-3He plume origin and temporal–spatial evolution of the Siberian flood basalts. Science 1995, 269, 825–882. [Google Scholar] [CrossRef]
- Dalrymple, G.B.; Czamanske, G.K.; Fedorenko, V.A.; Simonov, O.N.; Lanphere, M.A.; Likhachev, A.P. A reconnaissance 40Ar/39Ar geochronologic study of ore-bearing and related rocks, Siberian Russia. Geochim. Cosmochim. Acta 1995, 59, 2071–2083. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Zartman, R.E. New data on the age of the Guli intrusion and implications for the relationships between alkaline magmatism in the Maymecha–Kotuy province and the Siberian Superplume: U–Th–Pb isotopic systematics. Geochem. Int. 2011, 49, 439–448. [Google Scholar] [CrossRef]
- Malich, K.N.; Khiller, V.V.; Badanina, I.Y.; Belousova, E.A. Results of dating of thorianite and baddeleyite from carbonatites of the Guli massif, Russia. Dokl. Earth Sci. 2015, 464, 1029–1032. [Google Scholar] [CrossRef]
- Ghobadi, M.; Gerdes, A.; Kogarko, L.; Hoefer, H.; Brey, G. In situ LA-ICPMS Isotopic and Geochronological Studies on Carbonatites and Phoscorites from the Guli Massif, Maymecha-Kotuy, Polar Siberia. Geochem. Int. 2018, 56, 766–783. [Google Scholar] [CrossRef]
- Vrublevskii, V.V.; Voitenko, N.N.; Romanov, A.P.; Polyakov, G.V.; Izokh, A.E.; Gertner, I.F.; Krupchatnikov, V.I. Magma sources of Triassic lamproites of Gornyi Altai and Taimyr: Sr and Nd isotope evidence for plume-lithosphere interaction. Dokl. Earth. Sci. 2005, 405, 1365–1367. [Google Scholar]
- Carlson, R.W.; Czamanske, G.; Fedorenko, V.; Ilupin, I. A comparison of Siberian meimechites and kimberlites: Implications for the source of high-Mg alkalic magmas and flood basalts. Geochem. Geophys. Geosyst. 2006, 7. [Google Scholar] [CrossRef]
- Sun, J.; Liu, C.; Tappe, S.; Kostrovitsky, S.I.; Wu, F.-Y.; Yakovlev, D.; Yang, Y.-H.; Yang, J.-H. Repeated kimberlite magmatism beneath Yakutia and its relationship to Siberian flood volcanism: Insights from in situ U-Pb and Sr–Nd perovskite isotope analysis. Earth Planet. Sci. Lett. 2014, 404, 283–295. [Google Scholar] [CrossRef]
- Ivanov, A.V.; He, H.; Yan, L.; Ryabov, V.V.; Shevko, A.Y.; Palesskii, S.V.; Nikolaeva, I.V. Siberian Traps large igneous province: Evidence for two food basalt pulses around the Permo–Triassic boundary and in the Middle Triassic, and contemporaneous granitic magmatism. Earth Sci. Rev. 2013, 122, 58–76. [Google Scholar] [CrossRef]
- Letnikova, E.F.; Izokh, A.E.; Nikolenko, E.I.; Pokhilenko, N.P.; Shelestov, V.O.; Hilen, G.; Lobanov, S.S. Late Triassic high-potassium trachitic volcanism of the northeast of the Siberian platform: Evidence in the sedimentary record. Dokl. Earth. Sci. 2014, 459, 1344–1347. [Google Scholar] [CrossRef]
- Ernst, R.E.; Davies, D.R.; Jowitt, S.M.; Campbell, I.H. When do mantle plumes destroy diamonds? Earth Planet. Sci. Lett. 2018, 502, 244–252. [Google Scholar] [CrossRef]
- Pernet-Fisher, J.F.; Howarth, G.H.; Pearson, D.G.; Woodland, S.; Barry, P.H.; Pokhilenko, N.P.; Agashev, A.M.; Taylor, L.A. Plume impingement on the Siberian SCLM: Evidence from Re-Os isotope systematics. Lithos 2015, 218–219, 141–154. [Google Scholar] [CrossRef]
- Kirichenko, T.; Zuev, K.; Perfilova, O.Y.; Sosnovskaya, O.; Smokotina, I.; Markovich, L.A.; Borodin, M.E. State Geological Map of Russian Federation; Scale 1:1000000 (Third Generation). Ser. Angaro-Eniseysk. Sheet O-47 Bratsk. Explanatory Note; VSEGEI: St. Petersburg, Russia, 2012; pp. 163–179. (In Russian) [Google Scholar]
- Dashkevich, N.N. Regional prediction of kimberlite magmatism in the southwestern Siberian Platform. In Geologiya i Poleznye Iskopaemye Krasnoyarskogo Kraya (Geology and Mineral Resources of Krasnoyarsk District); 1999; pp. 1–42. (In Russian) [Google Scholar]
- Staroseltsev, V.S. Identifying paleorifts as promising tectonic elements for active oil and gas generation. Russ. Geol. Geophys. 2009, 50, 358–364. [Google Scholar] [CrossRef]
- Lapin, A.V.; Lisitsin, D.V. On the mineralogical typomorphism of alkaline ultrabasic migmatites of the Chadobets ulift. Otechestvennaya Geologiya 2004, 4, 83–93. (In Russian) [Google Scholar]
- Lapin, A.V. Structure, formation conditions and ore-bearing of the main types of REE in the carvonatite weathering crusts. Otechestvennaya Geologiya 1997, 11, 15–22. (In Russian) [Google Scholar]
- Slukin, A.D. Bauxite deposits with unusually high concentrations of REE, Nb, Ti, and Th, Chadobets uplift, Siberian platform. Int. Geol. Rev. 1994, 36, 179–193. [Google Scholar] [CrossRef]
- Lomayev, V.G.; Serdyuk, S.S. The Chuktukon Nb-TR deposit—The priority object for modernization of the Russian rare-earth industry. J. Sib. Fed. Univ. 2011, 4, 132–154. (In Russian) [Google Scholar]
- Kargin, A.V.; Nosova, A.A.; Chugaev, A.V.; Sazonova, L.V.; Dokuchaev, A.Y.; Smirnova, M.D.; Postnikov, A.V.; Postnikova, O.V.; Popova, L.P.; Poshibaev, V.V. Devonian ultramafic lamprophyre in the Irkineeva–Chadobets trough in the southwest of the Siberian platform: Age, composition, and implications for diamond potential prediction. Geol. Ore Dep. 2016, 58, 383–403. [Google Scholar] [CrossRef]
- Mitchell, R.H. Kimberlite, Orangeites and Related Rocks; Plenum Press: New York, NY, USA, 1995. [Google Scholar]
- Mann, U.; Marks, M.A.W.; Markl, G. Influence of oxygen fugacity on mineral compositions in peralkaline melts: The Katzenbuckel volcano, Southwest Germany. Lithos 2006, 91, 262–285. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Vladykin, N.V. Compositional variation of pyroxene and mica from the little Murun ultrapotassic complex, Aldan Shield, Russia. Mineral. Mag. 1996, 60, 907–925. [Google Scholar] [CrossRef]
- Korobeinikov, A.N.; Laajoki, K. Petrological aspects of the evolution of clinopyroxene composition in the intrusive rocks of the Lovozero alkaline massif. Geochem. Int. 1994, 31, 69–76. [Google Scholar]
- Vuorinen, J.H.; Hafi Lenius, U.; Whitehouse, M.J.; Mansfeld, J.; Skelton, A.D.L. Compositional variations (major and trace elements) of clinopyroxene andTi-andradite from pyroxenite, ijolite and nepheline syenite, Alnö Island, Sweden. Lithos 2005, 81, 55–77. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Platt, R.G. Mineralogy and petrology of nepheline syenites from the Coldwell alkaline complex, Ontario, Canada. J. Petrol. 1982, 23, 186–214. [Google Scholar] [CrossRef]
- Coulson, I.M. Evolution of the North Qộroq centre nepheline syenites, South Greenland: Alkali-mafic silicates and the role of metasomatism. Mineral. Mag. 2003, 67, 873–892. [Google Scholar] [CrossRef]
- Marks, M.; Markl, G. Fractionation and assimilation processes in the alkaline augite syenite unit of the Ilimaussaq Intrusion, South Greenland, as deduced from phase equilibria. J. Petrol. 2001, 42, 1947–1969. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Ryabchikov, I.D.; Kuzmin, D.V. High-Ba mica in olivinites of the Guli massif (Maymecha–Kotuy province, Siberia). Russ. Geol. Geophys. 2012, 53, 1209–1215. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Kurat, G.; Ntaflos, T. Henrymeyerite in the metasomatized upper mantle of eastern Antarctica. Can. Mineral. 2007, 45, 497–501. [Google Scholar] [CrossRef]
- Harlov, D.E.; Förster, H.-J. Fluid-induced nucleation of (Y+REE)-phosphate minerals within apatite: Nature and experiment. Part II. Fluorapatite. Am. Mineral. 2004, 88, 1209–1229. [Google Scholar] [CrossRef]
- Bühn, B.; Wall, F.; Le Bas, M. Rare-earth element systematics of carbonatitic fluorapatites, and their significance for carbonatite magma evolution. Contrib. Mineral. Petrol. 2001, 141, 572–591. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Zaitsev, A.N.; Couëslan, C.; Xu, C.; Kynický, J.; Mumin, A.H.; Yang, P. Apatite in carbonatitic rocks: Compositional variation, zoning, element partitioning and petrogenetic significance. Lithos 2017, 274–275, 188–213. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Styles, M.T.; Appleton, J.D.; Gunn, G.; Wall, F. Evidence for dissolution-reprecipitation of apatite and preferential LREE mobility in carbonatite-derived late-stage hydrothermal processes. Am. Mineral. 2016, 101, 596–611. [Google Scholar] [CrossRef]
- Prokopyev, I.R.; Doroshkevich, A.G.; Ponomarchuk, A.V.; Sergeev, S.A. Mineralogy, age and genesis of apatite-dolomite ores at the Seligdar apatite deposit (Central Aldan, Russia). Ore Geol. Rev. 2017, 81, 296–308. [Google Scholar] [CrossRef]
- Prokopyev, I.R.; Doroshkevich, A.G.; Sergeev, S.A.; Ernst, R.E.; Ponomarev, J.D.; Redina, A.A.; Chebotarev, D.A.; Nikolenko, A.M.; Dultsev, V.F.; Moroz, T.N.; et al. Petrography, mineralogy and SIMS U-Pb geochronology of 1.9–1.8 Ga carbonatites and associated alkaline rocks of the Central-Aldan magnesiocarbonatite province (South Yakutia, Russia). Mineral. Petrol. 2019, 113, 329–352. [Google Scholar] [CrossRef]
- Kozlov, E.; Fomina, E.; Sidorov, M.; Shilovskikh, V.; Chernyavsky, A.; Huber, M. The Petyayan-Vara Carbonatite-Hosted Rare Earth Deposit (Vuoriyarvi, NW Russia): Mineralogy and Geochemistry. Minerals 2020, 10, 73. [Google Scholar] [CrossRef]
- Giuliani, A.; Foley, S.F. The Geochemical Complexity of Kimberlite Rocks and their Olivine Populations: A Comment on Cordier et al. (Journal of Petrology 2015, 56, 1775–1796). J. Petrol. 2016, 57, 921–926. [Google Scholar] [CrossRef]
- Ammannati, E.; Jacob, D.E.; Avanzinelli, R.; Foley, S.F.; Conticelli, S. Low Ni olivine in silica-undersaturated ultrapotassic igneous rocks as evidence for carbonate metasomatism in the mantle. Earth Planet. Sci. Lett. 2016, 444, 64–74. [Google Scholar] [CrossRef]
- Arndt, N.T.; Guitreau, M.; Boullier, A.-M.; Roex, A.L.; Tommasi, A.; Cordier, P.; Sobolev, A. Olivine, and the Origin of Kimberlite. J. Petrol. 2010, 51, 573–602. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Sobolev, A.V.; Golovin, A.V.; Demouchy, S.; Faure, K.; Sharygin, V.V.; Kuzmin, D.V. Olivine in the Udachnaya-East Kimberlite (Yakutia, Russia): Types, Compositions and Origins. J. Petrol. 2008, 49, 823–839. [Google Scholar] [CrossRef]
- Brett, R.C.; Russell, J.K.; Moss, S. Origin of olivine in kimberlite: Phenocryst or impostor? Lithos 2009, 112, 201–212. [Google Scholar] [CrossRef]
- Veter, M.; Foley, S.F.; Mertz-Kraus, R.; Groschopf, N. Trace elements in olivine of ultramafic lamprophyres controlled by phlogopite-rich mineral assemblages in the mantle source. Lithos 2017, 292–293, 81–95. [Google Scholar] [CrossRef]
- Roedder, E. Fluid Inclusions. In Reviews in Mineralogy; Ribbe, P.H., Ed.; Mineralogical Society of America: Blacksburg, VA, USA, 1984; Volume 12, ISBN 978-0-939950-16-4. [Google Scholar]
- Roedder, E. Origin and significance of magmatic inclusions. Bull. Minéral. 1979, 102, 487–510. [Google Scholar] [CrossRef]
- Chebotarev, D.A.; Doroshkevich, A.G.; Klemd, R.; Karmanov, N.S. Evolution of Nb mineralization in the Chuktukon carbonatite massif, Chadobets upland (Krasnoyarsk Territory, Russia). Period. Mineral. 2017, 86, 99–118. [Google Scholar] [CrossRef]
- Downes, H.; Balaganskaya, E.; Beard, A.D.; Liferovich, R.; Demaiffe, D. Petrogenetic processes in the ultramafic, alkaline and carbonatitic magmatism in the Kola Alkaline Province: A review. Lithos 2005, 85, 48–75. [Google Scholar] [CrossRef]
- Agashev, A.M.; Pokhilenko, N.P.; Takazawa, E.; McDonald, J.A.; Vavilov, M.A.; Watanabe, T.; Sobolev, N.V. Primary melting sequence of a deep (N250 km) lithospheric mantle as recorded in the geochemistry of kimberlite–carbonatite assemblages, Snap Lake dyke system, Canada. Chem. Geol. 2008, 255, 317–328. [Google Scholar] [CrossRef]
- Golovin, A.V.; Sharygin, V.V.; Pokhilenko, N.P.; Mal’kovets, V.G.; Kolesov, B.A.; Sobolev, N.V. Secondary melt inclusions in olivine from unaltered kimberlites of the Udachnaya-East pipe, Yakutia. Dokl. Earth Sci. 2003, 388, 93–96. [Google Scholar]
- Golovin, A.V.; Sharygin, V.V.; Pokhilenko, N.P. Melt inclusions in olivine phenocrysts in unaltered kimberlites from the Udachnaya-East pipe, Yakutia: Some aspects of kimberlite magma evolution during late crystallization stages. Petrology 2007, 15, 168–183. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Golovin, A.V.; Pokhilenko, N.P.; Kamenetsky, V.S. Djerfisherite in the Udachnaya-East pipe kimberlites (Sakha Yakutia, Russia): Paragenesis, composition and origin. Eur. J. Mineral. 2007, 19, 51–63. [Google Scholar] [CrossRef]
- Kamenetsky, M.B.; Sobolev, A.V.; Kamenetsky, V.S.; Maas, R.; Danyushevsky, L.V.; Thomas, R.; Pokhilenko, N.V.; Sobolev, N.V. Kimberlite melts rich in alkali chlorides and carbonates: A potent metasomatic agent in the mantle. Geology 2004, 32, 845–848. [Google Scholar] [CrossRef]
- Abersteiner, A.; Kamenetsky, V.S.; Goemann, K.; Kjarsgaard, B.A.; Rodemann, T.; Kamenetsky, M.; Ehrig, K. A genetic story of olivine crystallisation in the Mark kimberlite (Canada) revealed by zoning and melt inclusions. Lithos 2020, 358–359, 105405. [Google Scholar] [CrossRef]
- Giuliani, A.; Phillips, D.; Woodhead, J.; Kamenetsky, V.S.; Fiorentini, M.L.; Maas, R.; Soltys, A.; Armstrong, R.A. Did diamond-bearing orangeites originate from MARID-veined peridotites in the lithospheric mantle? Nat. Commun. 2015, 6, 6837. [Google Scholar] [CrossRef]
- Guzmics, T.; Kodolányi, J.; Kovács, I.; Szabó, C.; Bali, E.; Ntaflos, T. Primary carbonatite melt inclusions in apatite and in K-feldspar of clinopyroxene-rich mantle xenoliths hosted in lamprophyre dikes (Hungary). Mineral. Petrol. 2008, 94, 225. [Google Scholar] [CrossRef]
- Panina, L.I.; Rokosova, E.Y.; Isakova, A.T.; Tolstov, A.V. Lamprophyres of the Tomtor Massif: A result of mixing between potassic and sodic alkaline mafic magmas. Petrology 2016, 24, 608–625. [Google Scholar] [CrossRef]
- Panina, L.I.; Stoppa, F.; Usol’tseva, L.M. Genesis of melilitite rocks of Pian di Celle volcano, umbrian kamafugite province, Italy: Evidence from melt inclusions in minerals. Petrology 2003, 11, 365–382. [Google Scholar]
- Bussweiler, Y.; Brey, G.P.; Pearson, D.G.; Stachel, T.; Stern, R.A.; Hardman, M.F.; Kjarsgaard, B.A.; Jackson, S.E. The aluminum-in-olivine thermometer for mantle peridotites—Experimental versus empirical calibration and potential applications. Lithos 2017, 272–273, 301–314. [Google Scholar] [CrossRef]
- Lapin, A.V.; Pyatenko, I.K. Chadobets complex of ultrabasic alkaline rocks and carbonatites: New data about composition and condition of formation. Dokl. Earth Sci. 1992, 6, 88–101. (In Russian) [Google Scholar]
- Nosova, A.A.; Sazonova, L.V. Ocelli in the early Triassic Chadobets ultramafic lamprophyre (SW Siberian craton): Evidence of late-stage evolution of the lamprophyre fluid—Melt system. In Proceedings of the XXXIV International Conference “Magmatism of the Earth and Related Strategic Metal Deposits”, Miass, Russia, 4–9 August 2017; pp. 168–171. [Google Scholar]
- Skinner, E.M.W. Kimberlites and Related Rocks: Their Composition, Occurrence, Origin and Emplacement Proceedings of the 4th International Kimberlite Conference; Glover, J.E., Harris, P.G., Eds.; Geological Society of Australia: Sydney, Australia, 1989; pp. 528–544. [Google Scholar]
- Smith, C.B. Pb, Sr and Nd isotopic evidence for sources of southern African Cretaceous kimberlites. Nature 1983, 304, 51–54. [Google Scholar] [CrossRef]
- Downes, P.J.; Wartho, J.-A.; Griffin, B.J. Magmatic Evolution and Ascent History of the Aries Micaceous Kimberlite, Central Kimberley Basin, Western Australia: Evidence from Zoned Phlogopite Phenocrysts, and UV Laser 40Ar/39Ar Analysis of Phlogopite-Biotite. J. Petrol. 2006, 47, 1751–1783. [Google Scholar] [CrossRef]
- Mitchell, R.H. Potassic magmas derived from metasomatized lithospheric mantle: Nomenclature and relevance to exploration for diamond-bearing rocks. J. Geol. Soc. India 2006, 67, 317–327. [Google Scholar]
- Becker, M.; Le Roex, A.P. Geochemistry of South African on- and off-craton, Group I and Group II kimberlites: Petrogenesis and source region evolution. J. Petrol. 2006, 47, 673–703. [Google Scholar] [CrossRef]
- De Hoog, J.C.M.M.; Gall, L.; Cornell, D.H. Trace-element geochemistry of mantle olivine and application to mantle petrogenesis and geothermobarometry. Chem. Geol. 2010, 270, 196–215. [Google Scholar] [CrossRef]
- Smirnova, M.; Sazonova, L.; Nosova, A.; Kargin, A.; Scherbacov, V. Phenocrysts and megacrysts of olivines from ultramafic lamprophyres ofthe Chadobets and Il’bokich uplifts, Southwestern Siberia. Geophys. Res. Abstr. EGU 2017, 19, 335. [Google Scholar]
- Howarth, G.H.; Barry, P.H.; Pernet-Fisher, J.F.; Baziotis, I.P.; Pokhilenko, N.P.; Pokhilenko, L.N.; Bodnar, R.J.; Taylor, L.A.; Agashev, A.M. Superplume metasomatism: Evidence from Siberian mantle xenoliths. Lithos 2014, 184–187, 209–224. [Google Scholar] [CrossRef]
- Ballhaus, C.B.; Berry, R.F.; Green, D.H. High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen barometer—Implications for redox conditions in the upper mantle. Contrib. Mineral. Petrol. 1991, 107, 27–40. [Google Scholar] [CrossRef]
- Ludington, S. The biotite-apatite geothermometer revisited. Am. Mineral. 1978, 63, 551–553. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokopyev, I.; Starikova, A.; Doroshkevich, A.; Nugumanova, Y.; Potapov, V. Petrogenesis of Ultramafic Lamprophyres from the Terina Complex (Chadobets Upland, Russia): Mineralogy and Melt Inclusion Composition. Minerals 2020, 10, 419. https://doi.org/10.3390/min10050419
Prokopyev I, Starikova A, Doroshkevich A, Nugumanova Y, Potapov V. Petrogenesis of Ultramafic Lamprophyres from the Terina Complex (Chadobets Upland, Russia): Mineralogy and Melt Inclusion Composition. Minerals. 2020; 10(5):419. https://doi.org/10.3390/min10050419
Chicago/Turabian StyleProkopyev, Ilya, Anastasiya Starikova, Anna Doroshkevich, Yazgul Nugumanova, and Vladislav Potapov. 2020. "Petrogenesis of Ultramafic Lamprophyres from the Terina Complex (Chadobets Upland, Russia): Mineralogy and Melt Inclusion Composition" Minerals 10, no. 5: 419. https://doi.org/10.3390/min10050419
APA StyleProkopyev, I., Starikova, A., Doroshkevich, A., Nugumanova, Y., & Potapov, V. (2020). Petrogenesis of Ultramafic Lamprophyres from the Terina Complex (Chadobets Upland, Russia): Mineralogy and Melt Inclusion Composition. Minerals, 10(5), 419. https://doi.org/10.3390/min10050419





