Mineralogy of Silicate-Natrophosphate Immiscible Inclusion in Elga IIE Iron Meteorite †
Abstract
1. Introduction
2. History of Finding and Brief Review on Studies for the Elga Meteorite
3. Analytical Methods
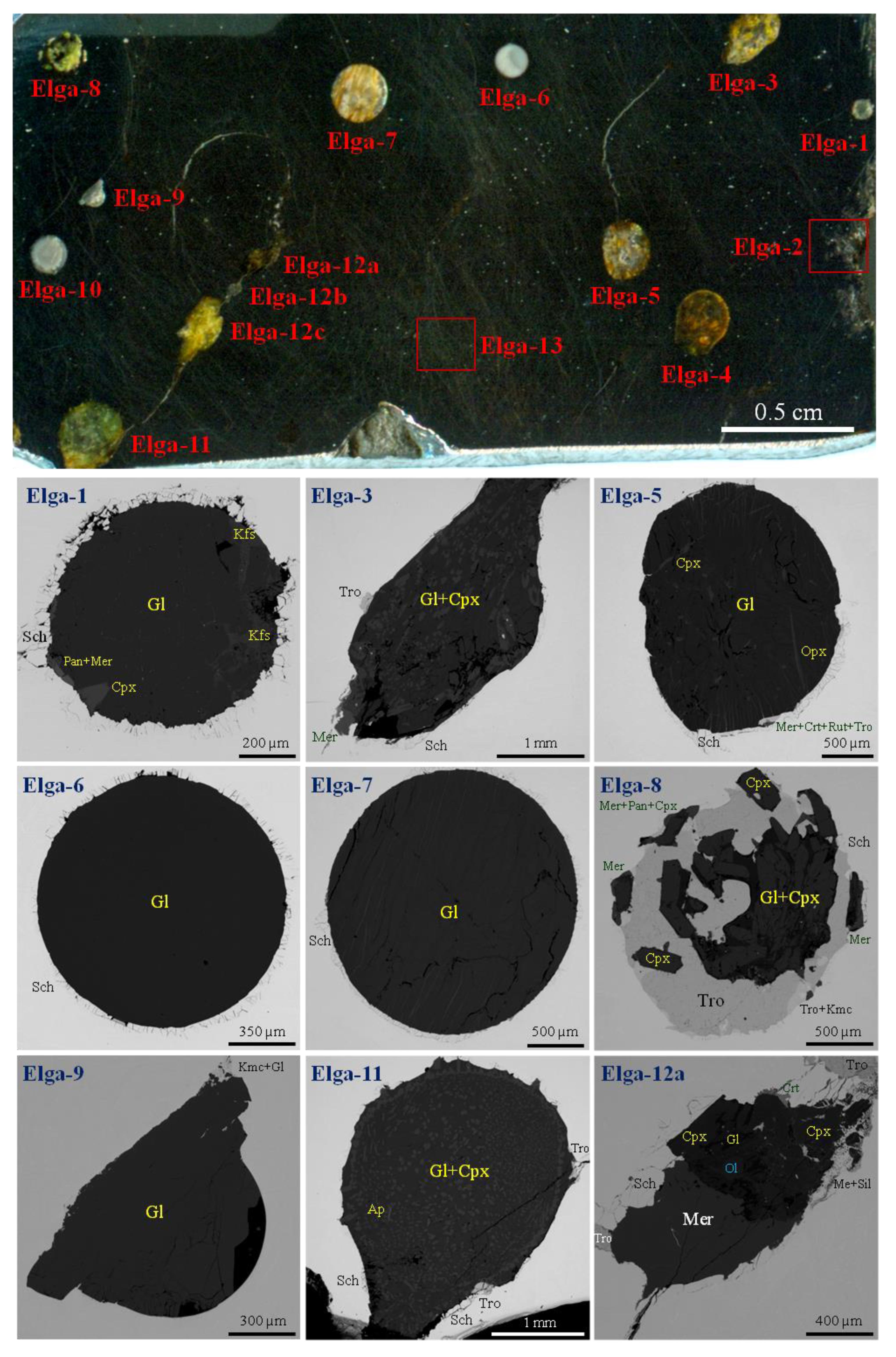
4. Description of Studied Cut-off from the Elga Meteorite
5. Inclusion with Silicate-Natrophosphate Immisciblity (Elga-4)
5.1. General Description of the Immiscible Inclusion
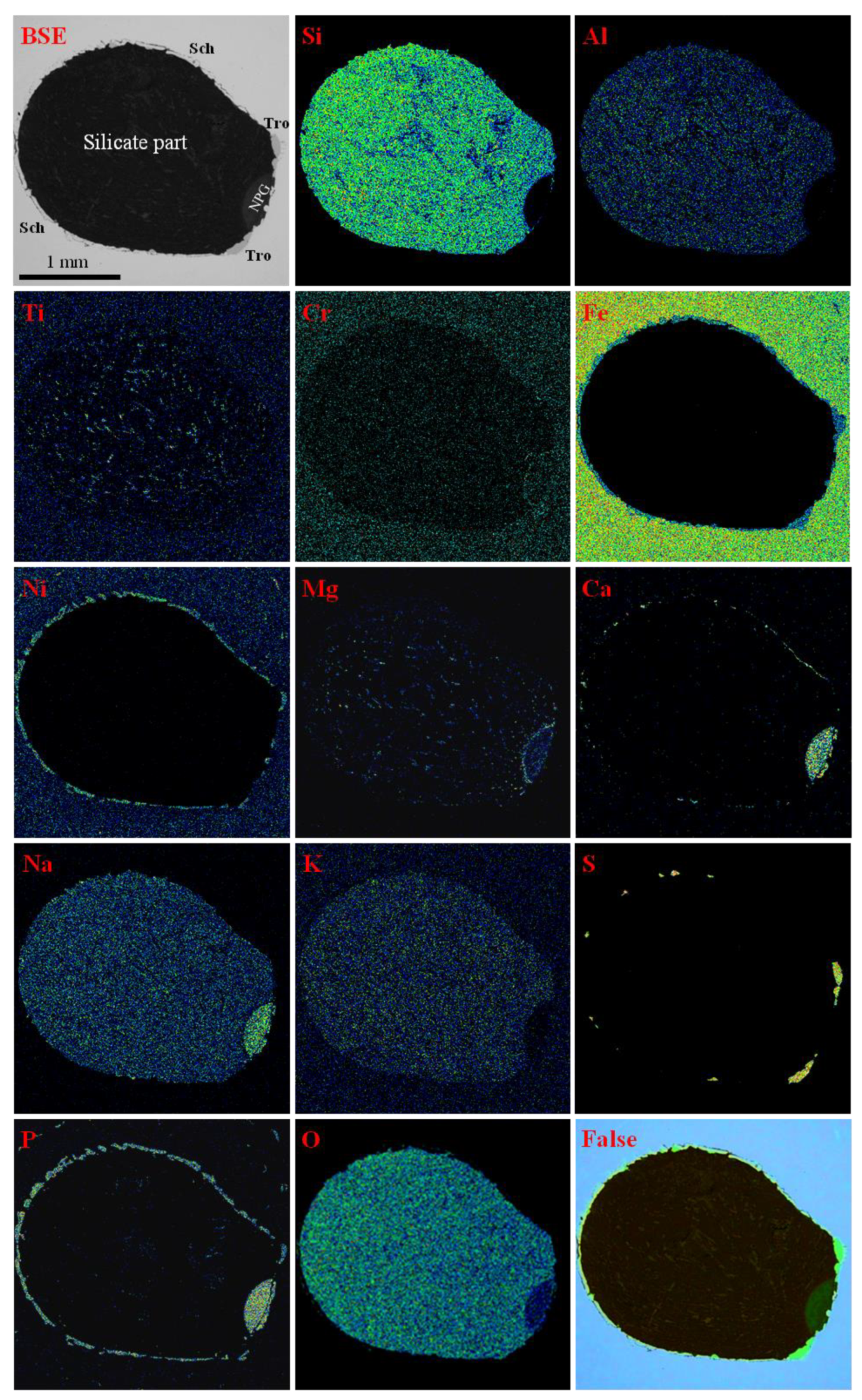
5.2. Mineralogy of Silicate Part
5.3. Mineralogy of Natrophosphate Part
5.4. Chemistry of Minerals in the Elga-4 Immiscible Inclusion
5.4.1. Feldspars, Quartz and Siliceous Glass
5.4.2. Mineral of the Obertiite Subgroup (Amphibole Supergroup)
5.4.3. Minerals of the Aenigmatite-Subgroup (Sapphirine Supergroup)
5.4.4. Orthopyroxene
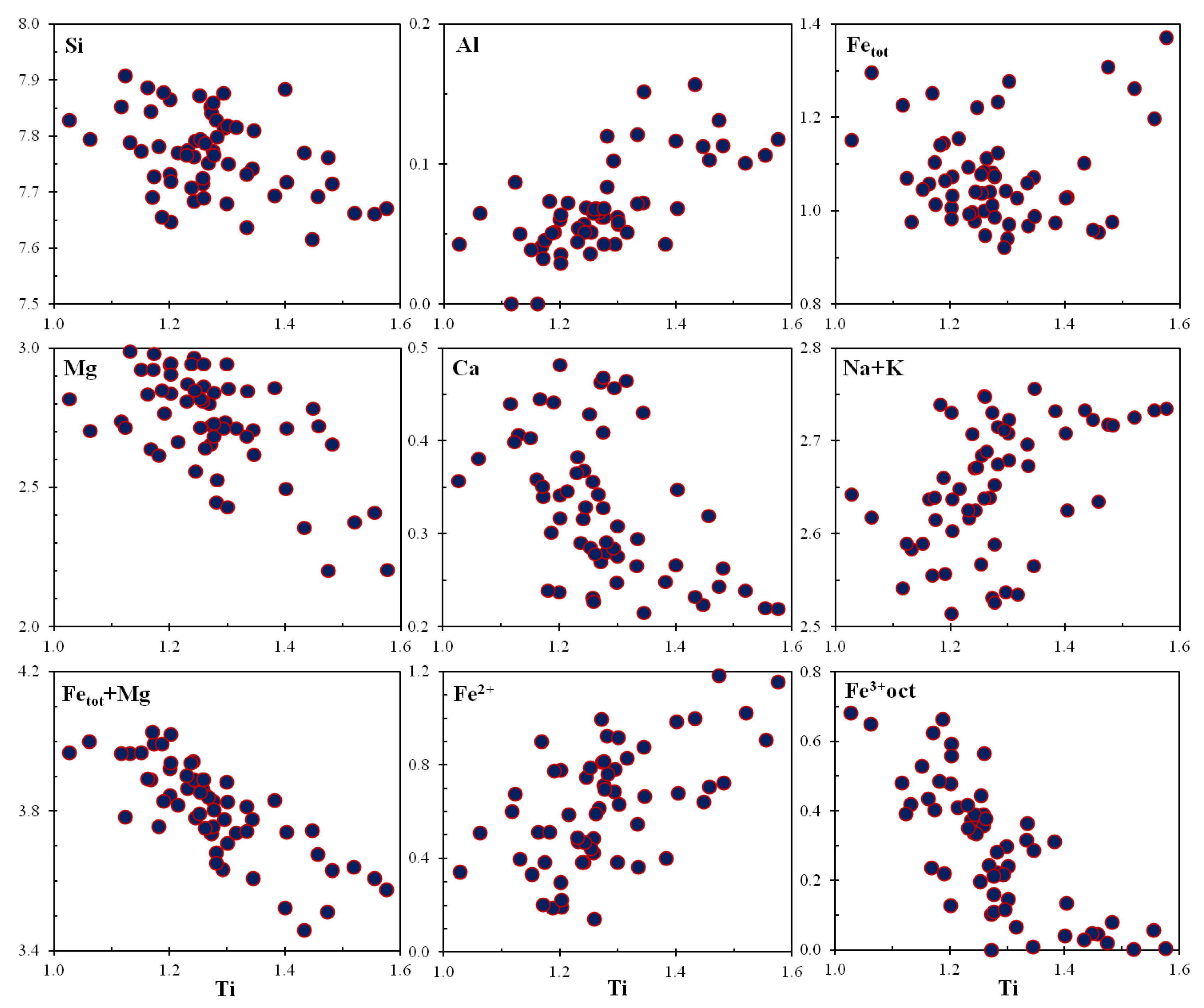
5.4.5. Na-Ca-Cr-Ti-Mg-Clinopyroxenes
5.4.6. Na-Ca-Mg-Fe-Orthophosphates
5.4.7. Other Minerals
6. Raman Spectroscopy for Minerals of the Elga-4 Inclusion
7. Discussion and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plyashkevich, L.N. Some data regarding the composition and structure of the iron meteorite Elga. Meteoritika 1962, 22, 51–60. (In Russian) [Google Scholar]
- Vronskii, V.I. On the find of the Elga iron meteorite. Meteoritika 1962, 22, 47–50. (In Russian) [Google Scholar]
- D’yakonova, M.I.; Kharitonova, V.Y. Composition of Ni-rich iron from some iron and iron-stone meteorites of different types. Meteoritika 1963, 23, 42–43. (In Russian) [Google Scholar]
- Wasson, J.T. Ni, Ga, Ge and Ir in the metal of iron meteorites with silicate inclusions. Geochim. Cosmochim. Acta 1970, 34, 957–964. [Google Scholar] [CrossRef]
- Kvasha, L.G.; Lavrent’ev, Y.G.; Sobolev, N.V. Silicate inclusions and evidence of impact metamorphism in the Elga octahedrite. Meteoritika 1974, 33, 143–147. (In Russian) [Google Scholar]
- Kashkarov, L.L.; Korotkova, N.N.; Lavrukhina, A.V. Relict radiation by cosmic rays low-energy heavy nuclei of matter of iron meteorite. Dokl. Acad. Nauk SSSR 1975, 221, 198–200. (In Russian) [Google Scholar]
- D’yakonova, M.I.; Kharitonova, V.Y.; Anvel, A.A. Chemical Composition of Meteorites; Nauka: Moscow, Russia, 1979. (In Russian) [Google Scholar]
- Osadchii, E.G.; Baryshnikova, G.V.; Novikov, G.V. The Elga meteorite: Silicate inclusions and shock metamorphism. Proc. Lunar Planet. Sci. 1981, 12, 1049–1068. [Google Scholar]
- Sharygin, I.S. Mineralogy of the Elga meteorite. In Thesis for the Bachelor of Science; Department of Geology and Geophysics, Novosibirsk State University: Novosibirsk, Russia, 2005; p. 32. (In Russian) [Google Scholar]
- Teplyakova, S.N.; Khisina, N.R.; Artemov, V.V.; Vasil’ev, A.L. Nanomineralogy of dendritic inclusions in the Elga iron meteorite. Zap. Ross. Mineral. O-va 2012, 141, 42–52. (In Russian) [Google Scholar]
- Nazarov, M.A.; Teplyakova, S.N.; Brandstaetter, F.; Ntaflos, T. Fluorine-bearing merrillite from a silicate inclusion of the Elga (IIE) iron meteorite. Lunar Planet. Sci. Conf. 2013, 44, 1342. [Google Scholar]
- Sharygin, V.V. Phosphate minerals in silicate inclusions of the Elga meteorite. In 5th Conference “Meteorites, Asteroids, Comets”; PH Fort Dialog-Iset’: Ekaterinburg, Russia, 2017; pp. 153–157. (In Russian) [Google Scholar]
- Litasov, K.D.; Podgornykh, N.M. Raman spectroscopy of various phosphate minerals and occurrence of tuite in the Elga IIE iron meteorite. J. Raman Spectrosc. 2017, 48, 1518–1527. [Google Scholar] [CrossRef]
- Khisina, N.R.; Teplyakova, S.N.; Wirth, R.; Senin, V.G.; Averin, A.A.; Shiryaev, A.A. Carbon-bearing phases in shock-induced melt zones of the Elga meteorite. Geochem. Inter. 2017, 55, 317–329. [Google Scholar] [CrossRef]
- Teplyakova, S.N.; Lorenz, C.A.; Ivanova, M.A.; Kononkova, N.N.; Anosova, M.O.; Ryazantsev, K.M.; Kostitsyn, Y.A. Mineralogy of silicate inclusions in the Elga IIE iron meteorite. Geochem. Inter. 2018, 56, 1–23. [Google Scholar] [CrossRef]
- Senin, V.G.; Zinovieva, N.G.; Pankrushina, E.A.; Averin, A.A.; Khisina, N.R. Identification of minerals in shock melted regions in meteorite Elga. Exp. Geosci. 2018, 24, 47–49. [Google Scholar]
- Sharygin, V.V. Mineralogy of inclusion with silicate-natrophosphate immiscibility, meteorite Elga (IIE). Meteorit. Planet. Sci. 2018, 53, 6013. [Google Scholar]
- Teplyakova, S.N.; Lorenz, C.A. Metal crystallization in IIE irons and their possible meteorite analogues. Geochem. Inter. 2019, 57, 893–902. [Google Scholar] [CrossRef]
- Khisina, N.R.; Wirth, R.; Abdrakhimov, A.M. Liquid immiscibility in regions of localized shock-induced melting in the Elga meteorite. Geochem. Inter. 2019, 57, 903–911. [Google Scholar] [CrossRef]
- Litasov, K.D.; Teplyakova, S.N.; Shatskiy, A.; Kuper, K.E. Fe-Ni-P-S melt pockets in Elga IIE iron meteorite: Evidence for the origin at high-pressures up to 20 GPa. Minerals 2019, 9, 616. [Google Scholar] [CrossRef]
- Wasson, J.T.; Wang, J. A nonmagmatic origin of group-IIE iron meteorites. Geochim. Cosmochim. Acta 1986, 50, 725–732. [Google Scholar] [CrossRef]
- Wasson, J.T. Formation of non-magmatic iron-meteorite group IIE. Geochim. Cosmochim. Acta 2017, 53, 396–416. [Google Scholar] [CrossRef]
- Pouchou, I.L.; Pichoir, F. “PaP” (phi-rho-z) procedure for improved quantitative microanalysis. In Microbeam Analysis; Armstrong, I.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Rubin, A.E.; Ma, C. Meteoritic minerals and their origins. Chem. Erde 2017, 77, 325–385. [Google Scholar] [CrossRef]
- Floran, R.; Prinz, M.; Hlava, P.; Keil, K.; Nehru, C.; Hinthorne, J. The Chassigny meteorite: A cumulate dunite with hydrous amphibole-bearing melt inclusions. Geochim. Cosmochim. Acta 1978, 42, 1213–1229. [Google Scholar] [CrossRef]
- Treiman, A.H. Amphibole and hercynite spinel in Shergotty and Zagami: Magmatic water, depth of crystallization, and metasomatism. Meteoritics 1985, 20, 229–243. [Google Scholar] [CrossRef]
- Johnson, M.C.; Rutherford, M.J.; Hess, P.C. Chassigny petrogenesis: Melt compositions, intensive parameters and water contents of Martian (?) magmas. Geochim. Cosmochim. Acta 1991, 55, 349–366. [Google Scholar] [CrossRef]
- Ikeda, Y. Petrology of magmatic silicate inclusions in the Allan Hills 77005 lherzolitic shergottite. Meteorit. Planet. Sci. 1998, 33, 803–812. [Google Scholar] [CrossRef]
- Monkawa, A.; Mikouchi, T.; Koizumi, E.; Sugiyama, K.; Miyamoto, M. Determination of the Fe oxidation state of the Chassigny kaersutite: A microXANES spectroscopic study. Meteorit. Planet. Sci. 2006, 41, 1321–1329. [Google Scholar] [CrossRef]
- Hagen, E. A Comparative Study of Kaersutite in the Egersund Dikes and SNC Meteorites. Master’s Thesis, Ohio State University, Columbus, OH, USA, 2017. [Google Scholar]
- Ivanov, A.V.; Kononkova, N.N.; Zolensky, M.E.; Migdisova, L.F.; Stroganov, I.A. The Kaidun meteorite: A large albite crystal-fragment of an alkaline rock. Lunar Planet. Sci. 2001, 32, 1080. [Google Scholar]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Oberti, R.; Cannillo, E.; Toscani, G. How to name amphiboles after the IMA2012 report: Rules of thumb and a new PC program for monoclinic amphiboles. Period. Mineral. 2012, 81, 257–267. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Oberti, R.; Zanetti, A.; Czamanske, G.K. The role of Ti in hydrogen-deficient amphiboles: Sodic-calcic and sodic amphiboles from Coyote Peak, California. Can. Mineral. 1998, 36, 1253–1265. [Google Scholar]
- Hawthorne, F.C.; Cooper, M.A.; Grice, J.D.; Ottolini, L. A new anhydrous amphibole from the Eifel region, Germany: Description and crystal structure of obertiite, NaNa (Mg3,Fe3+,Ti4+)Si8O22O2. Am. Mineral. 2000, 85, 236–241. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Ball, N.A.; Czamanske, G.K. Ferro-obertiite, NaNa2(Fe2+3Fe3+Ti)Si8O22O2, new mineral of the amphibole group from Coyote Peak, Humboldt County, California. Can. Mineral. 2010, 48, 301–306. [Google Scholar] [CrossRef]
- Oberti, R.; Boiocchi, M.; Hawthorne, F.C.; Ball, N.A.; Blass, G. Ferri-obertiite from the Rothenberg quarry, Eifel volcanic complex (Germany): Mineral data and crystal-chemistry of a new amphibole end-member. Mineral. Mag. 2017, 81, 641–651. [Google Scholar] [CrossRef]
- Kullerud, K.; Zozulya, D.; Erambert, M.; Ravna, E.J.K. Solid solution between potassic alkali amphiboles from the silica-rich Kvaløya lamproite, West Troms Basement Complex, northern Norway. Eur. J. Mineral. 2014, 25, 935–945. [Google Scholar] [CrossRef]
- Oberti, R.; Ungaretti, L.; Cannillo, E.; Hawthrone, F. The behavior of Ti in amphiboles: I. Four- and six-coordinated Ti in richterite. Eur. J. Mineral. 1992, 4, 425–439. [Google Scholar] [CrossRef]
- Kunzmann, T. The aenigmatite-rhönite mineral group. Eur. J. Mineral. 1999, 11, 743–756. [Google Scholar] [CrossRef]
- Grew, E.S.; Hålenius, U.; Pasero, M.; Barbier, J. Recommended nomenclature for the sapphirine and surinamite groups (sapphirine supergroup). Mineral. Mag. 2008, 72, 839–876. [Google Scholar] [CrossRef]
- Brearley, A.J.; Jones, R.H. Chondritic Meteorites. In Planetary Materials; Papike, J.J., Ed.; Mineralogical Society of America: Washington, DC, USA, 1998; Volume 3, p. 398. [Google Scholar]
- Zolensky, M.; Ivanov, A. The Kaidun microbreccia meteorite: A harvest from the inner and outer asteroid belt. Geochemistry 2003, 63, 185–246. [Google Scholar] [CrossRef]
- Sokol, A.K.; Bischoff, A.; Marhas, K.K.; Mezger, K.; Zinner, E. Late accretion and lithification of chondritic parent bodies: Mg isotope studies on fragments from primitive chondrites and chondritic breccias. Meteorit. Planet. Sci. 2007, 42, 1291–1308. [Google Scholar] [CrossRef]
- Price, R.C.; Johnson, R.W.; Gray, C.M.; Frey, F.A. Geochemistry of phonolites and trachytes from the summit region of Mt. Kenya. Contrib. Mineral. Petrol. 1985, 89, 394–409. [Google Scholar] [CrossRef]
- Starikova, A.E.; Sklyarov, E.V.; Kanakin, S.V. Mg-aenigmatite from the Tazheran massif (East Siberia, Russia). In Proceedings of the Goldschmidt 2013 Conference Abstracts, Florence, Italy, 25–30 August 2013; p. 2253. [Google Scholar]
- Morimoto, N.; Fabries, J.; Ferguson, A.K.; Ginzburg, I.V.; Ross, M.; Seibert, F.A.; Zussman, J.; Aoki, K.; Gottardi, G. Nomenclature of pyroxenes. Mineral. Mag. 1988, 52, 535–550. [Google Scholar] [CrossRef]
- Frondel, C.; Klein, C., Jr. Ureyite, NaCr2Si2O6, a new meteoritic pyroxene. Science 1965, 149, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Greshake, A.; Bischoff, A. Chromium-bearing phases in Orgueil (CI): Discovery of magnesiochromite (MgCr2O4), ureyite (NaCrSi2O6), and chromium oxide (Cr2O3). Lunar Planet. Sci. 1996, 27, 461. [Google Scholar]
- Sobolev, V.S.; Sobolev, N.V.; Lavrentiev, U.G. Chrome-rich clinopyroxenes from the kimberlities of Yakutia. Neues Jahrb. für Mineral. 1975, 123, 213–218. [Google Scholar]
- Mével, C.; Kiénast, J.-R. Jadeite-kosmochlor solid solution and chromian sodic amphiboles in jadeitites and associated rocks from Taw maw (Burma). Bull. Minéral. 1986, 109, 617–633. [Google Scholar] [CrossRef]
- Secco, L.; Martignago, F.; Dal Negro, A.; Reznitskii, L.Z.; Sklyarov, E.V. Crystal chemistry of Cr3+-V3+-rich clinopyroxenes. Am. Mineral. 2002, 87, 709–714. [Google Scholar] [CrossRef]
- Reznitsky, L.Z.; Sklyarov, E.V.; Galuskin, E.V. Complete isomorphic join diopside–kosmochlor CaMgSi2O6 – NaCrSi2O6 in metamorphic rocks of the Sludyanka complex (southern Baikal region). Rus. Geol. Geophys. 2011, 52, 40–51. [Google Scholar] [CrossRef]
- Franz, L.; Sun, T.T.; Hänni, H.A.; de Capitani, C.; Thanasuthipitak, T.; Atichat, W. A comparative study of jadeite, omphacite and kosmochlor jades from Myanmar, and suggestions for a practical nomenclature. J. Gemmol. 2014, 34, 210–229. [Google Scholar] [CrossRef]
- Krátký, O.; Rapprich, V.; Racek, M.; Míková, J.; Magna, T. On the chemical composition and possible origin of Na–Cr-rich clinopyroxene in silicocarbonatites from Samalpatti, Tamil Nadu, South India. Minerals 2018, 8, 355. [Google Scholar] [CrossRef]
- Karwowski, Ł.; Helios, K.; Kryza, R.; Muszyński, A.; Drożdżewski, P. Raman spectra of selected mineral phases of the Morasko iron meteorite. J. Raman Spectrosc. 2013, 44, 1181–1186. [Google Scholar] [CrossRef]
- Karwowski, Ł.; Kusz, J.; Muszyński, A.; Kryza, R.; Sitarz, M.; Galuskin, E.V. Moraskoite, Na2Mg(PO4)F, a new mineral from the Morasko IAB-MG iron meteorite (Poland). Mineral. Mag. 2015, 79, 387–398. [Google Scholar] [CrossRef]
- Karwowski, Ł.; Kryza, R.; Muszyński, A.; Kusz, J.; Helios, K.; Drożdżewski, P.; Galuskin, E.V. Czochralskiite, Na4Ca3Mg(PO4)4, a second new mineral from the Morasko IAB-MG iron meteorite (Poland). Eur. J. Mineral. 2016, 28, 890–899. [Google Scholar] [CrossRef]
- Fuchs, L.H.; Olsen, E.; Henderson, E.P. On the occurrence of brianite and panethite, two new phosphate minerals from the Dayton meteorite. Geochim. Cosmochim. Acta 1967, 31, 1711–1719. [Google Scholar] [CrossRef]
- Sturman, B.D.; Mandarino, J.A.; Corlett, M.I. Marićite, a sodium iron phosphate, from the Big Fish River area, Yukon Territory, Canada. Can. Mineral. 1977, 15, 396–398. [Google Scholar]
- Kracher, A.; Kurat, G.; Buchwald, V.F. Cape York: The extraordinary mineralogy of an ordinary iron meteorite and its implications for the genesis of IIIAB irons. Geochem. J. 1977, 11, 207–217. [Google Scholar] [CrossRef][Green Version]
- Lauretta, D.S.; Buseck, P.R.; Zega, T.J. Opaque minerals in the matrix of the Bishunpur (LL3.1) meteorite: Constraints on the chondrule formation environment. Geochim. Cosmochim. Acta 2001, 65, 1337–1353. [Google Scholar] [CrossRef]
- Barth, M.I.F.; Harries, D.; Langenhorst, F.; Hoppe, P. Sulfide-oxide assemblages in Acfer 094—Clues to nebular metal-gas interactions. Meteorit. Planet. Sci. 2018, 53, 187–203. [Google Scholar] [CrossRef]
- Fornero, E.; Allegrina, M.; Rinaudo, C.; Mazziotti-Tagliani, S.; Gianfagna, A. Micro-Raman spectroscopy applied on oriented crystals of fluoro-edenite amphibole. Period. Mineral. 2008, 77, 5–14. [Google Scholar] [CrossRef]
- Apopei, A.I.; Buzgar, N. The Raman study of amphiboles. An. Ştiinţifice Univ. Al. I. Cuza Iaşi Geol. 2010, 56, 57. [Google Scholar]
- Leissner, L. Crystal chemistry of amphiboles studied by Raman spectroscopy. Master’s Thesis, Mineralogisch-Petrographisches Institut, Universität Hamburg, Hamburg, Germany, 2014; p. 53. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. de Gruyter GmbH: Berlin, Germany, 2016; pp. 1–29. [Google Scholar] [CrossRef]
- Buchwald, V. Handbook of Iron Meteorites; University of California Press: Berkeley, CA, USA, 1975; p. 1418. [Google Scholar]
- Bogard, D.D.; Garrison, D.H.; McCoy, T.J. Chronology and petrology of silicates from IIE iron meteorites: Evidence of complex parent body evolution. Geochim. Cosmochim. Acta 2000, 64, 2133–2154. [Google Scholar] [CrossRef]
- Ruzicka, A.; Hutson, M.; Floss, C. Petrology of silicate inclusions in the Sombrerete ungrouped iron meteorite: Implications for the origins of IIE-type silicate-bearing irons. Meteorit. Planet. Sci. 2006, 41, 1797–1831. [Google Scholar] [CrossRef]
- Ruzicka, A. Silicate-bearing iron meteorites and their implications for the evolution of asteroidal parent bodies. Chem. Erde 2014, 74, 3–48. [Google Scholar] [CrossRef]
- Beckett, J.R.; Live, D.; Tsay, F.D.; Grossman, L.; Stolper, E. Ti3+ in meteoritic and synthetic hibonite. Geochim. Cosmochim. Acta 1988, 52, 1479–1495. [Google Scholar] [CrossRef]
- Bonaccorsi, E.; Merlino, S.; Pasero, M. Rhönite: Structure and microstructural features, crystal chemistry and polysomatic relationships. Eur. J. Mineral. 1990, 2, 203–218. [Google Scholar] [CrossRef]
- Ma, C.; Rossman, G.R. Tistarite, Ti2O3, a new refractory mineral from the Allende meteorite. Am. Mineral. 2009, 94, 841–844. [Google Scholar] [CrossRef]
- Ma, C.; Rossman, G.R. Grossmanite, CaTi3+AlSiO6, a new pyroxene from the Allende meteorite. Am. Mineral. 2009, 94, 1491–1494. [Google Scholar] [CrossRef]
- Ma, C.; Tschauner, O.; Beckett, J.R.; Rossman, G.R.; Liu, W. Panguite, (Ti4+,Sc,Al,Mg,Zr,Ca)1.8O3, a new ultra-refractory titania mineral from the Allende meteorite: Synchrotron micro-diffraction and EBSD. Am. Mineral. 2012, 97, 1219–1225. [Google Scholar] [CrossRef]
- Ma, C.; Krot, A.N. Hutcheonite, Ca3Ti2(SiAl2)O12, a new garnet mineral from the Allende meteorite: An alteration phase in a Ca-Al-rich inclusion. Am. Mineral. 2014, 99, 667–670. [Google Scholar] [CrossRef]
- Ma, C. Paqueite, IMA 2013-053. CNMNC Newsletter No. 17, October 2013, page 3002. Mineral. Mag. 2013, 77, 2997–3005. [Google Scholar]
- Ma, C.; Yoshizaki, T.; Nakamura, T.; Muto, J. Rubinite, IMA 2016-110. CNMNC Newsletter No. 36, April 2017, page 408. Mineral. Mag. 2017, 81, 403–409. [Google Scholar]
- Ma, C. Kaitianite, IMA 2017-078. CNMNC Newsletter No. 42, April 2018, page 450. Mineral. Mag. 2018, 82, 445–451. [Google Scholar]













| Mineral | Formula | Mineral | Formula |
|---|---|---|---|
| Graphite | sp2-C | Rutile | TiO2 |
| Iron (kamacite) | α-(Fe,Ni) | Chromite | FeCr2O4 |
| Taenite | γ-(Fe,Ni) | Magnetite | FeFe2O4 |
| Tetrataenite | FeNi | Trevorite | NiFe2O4 |
| Schreibersite | (Fe,Ni)3P | Ilmenite | FeTiO3 |
| “Ferro-melliniite” | (Fe,Ni)4P | Armalcolite | (Mg,Fe)Ti2O5 |
| Troilite | FeS | Quartz | SiO2 |
| Millerite | NiS | Forsterite | (Mg,Fe)2SiO4 |
| Pentlandite | (Fe,Ni)9S8 | Enstatite | (Mg,Fe)2Si2O6 |
| Fluorapatite | Ca5(PO4)3F | Diopside | Ca(Mg,Fe)Si2O6 |
| Merrillite | Ca9NaMg(PO4)7 | Kosmochlor | NaCrSi2O6 |
| Tuite | Ca3(PO4)2 | “Na-Ti-clinopyroxene” | Na(Mg,Fe)0.5Ti0.5Si2O6 |
| Panethite | (Na,Ca,K)2(Mg,Fe)2(PO4)2 | Ferri-obertiite | NaNa2Mg3Fe3+Ti[Si8O22]O2 |
| Czochralskiite | Na4Ca3Mg(PO4)4 | “Titan-obertiite” | NaNa2Mg2Fe2+0.5Ti1.5[Si8O22]O2 |
| Brianite | Na2CaMg(PO4)2 | Aenigmatite | Na2(Fe2+,Mg)5TiSi6O18O2 |
| Marićite | NaFe(PO4) | "Magnesio-aenigmatite" | Na2(Mg,Fe2+)5TiSi6O18O2 |
| Chladniite | Na2Ca(Mg,Fe)7(PO4)6 | Albite | NaAlSi3O8 |
| “Na-Fe-Mg-phosphate” | Na2(Fe,Mn)(Mg,Ca)(PO4)2 | K-feldspar | KAlSi3O8 |
| "Na-Fe-H2O-phosphate" | Na-rich Fe2+3(PO4)2∙8H2O | Chlorite (chamosite ?) | Fe5Al[AlSi3O10](OH)8 |
| Siderite | (Fe,Ni)(CO3) | Serpentine | Fe3Si2O5(OH)4 |
| Goethite | α-FeOOH | Siliceous glass | >70 wt.% SiO2 |
| Hematite | Fe2O3 | Ca-Mg-Fe silicate glass | 40–45 wt.% SiO2 |
| Mineral | Kamacite | Schreibersite | Troilite |
|---|---|---|---|
| N = 5 | N = 6 | N = 3 | |
| P | n.d. | 15.20 | n.d. |
| S | n.d. | n.d. | 36.64 |
| Fe | 93.08 | 60.10 | 63.51 |
| Co | 0.45 | 0.13 | n.d. |
| Ni | 6.44 | 24.69 | n.d. |
| Sum | 99.97 | 100.11 | 100.15 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| Phase | Albite | K-feldspar | Quartz | Glass | ||||
| Position | El4-2 | El4-5 | El4-3 | El4-2 | El4-2 | El4-2 | El4 | El4 |
| Area | N = 2 | N = 1 | N = 1 | N = 4 | N = 4 | N = 2 | N = 8 | N = 6 |
| SiO2 | 69.80 | 68.85 | 69.43 | 69.59 | 67.04 | 66.69 | 99.44 | 72.18 |
| Al2O3 | 16.50 | 16.03 | 16.19 | 16.18 | 15.75 | 15.41 | n.d. | 14.40 |
| FeO | 0.87 | 1.78 | 0.91 | 1.27 | 1.56 | 2.41 | 0.47 | 2.05 |
| MnO | 0.04 | n.d. | n.d. | n.d. | n.d. | 0.04 | n.d. | n.d. |
| MgO | 0.68 | 0.29 | 0.85 | 0.80 | 0.81 | 0.83 | n.d. | 0.36 |
| CaO | 0.01 | n.d. | n.d. | n.d. | n.d. | 0.18 | n.d. | n.d. |
| Na2O | 10.53 | 11.26 | 9.09 | 8.66 | 5.67 | 2.95 | n.d. | 7.55 |
| K2O | 1.29 | 1.73 | 3.87 | 3.30 | 8.52 | 11.50 | n.d. | 3.09 |
| Sum | 99.73 | 99.94 | 100.34 | 99.81 | 99.34 | 100.01 | 99.91 | 99.62 |
| Albite | 92.46 | 90.82 | 78.12 | 79.96 | 50.27 | 27.81 | ||
| Orthoclase | 7.47 | 9.18 | 21.88 | 20.04 | 49.73 | 71.27 | ||
| Anorthite | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.92 | ||
| Quartz | 100.00 | |||||||
| Position | Elga-4 | El4-3 | El4-4 | El4-5 | El4-6 | El4-7 | El4-12 | El4-13 | El4-r | El4-K | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | N = 57 | sd | max | min | N = 8 | N = 5 | N = 7 | N = 4 | N = 10 | N = 2 | N = 3 | N = 5 | N = 6 |
| SiO2 | 53.62 | 0.50 | 54.63 | 52.16 | 53.75 | 53.76 | 53.92 | 52.48 | 53.54 | 53.18 | 53.51 | 53.59 | 54.03 |
| TiO2 | 11.72 | 1.03 | 14.25 | 9.46 | 11.02 | 11.76 | 11.68 | 13.80 | 11.53 | 12.97 | 11.62 | 11.55 | 11.06 |
| Cr2O3 | 0.26 | 0.28 | 1.34 | 0.02 | 0.12 | 0.20 | 0.15 | 0.29 | 0.37 | 0.18 | 0.52 | 0.22 | 0.18 |
| V2O3 | 0.01 | 0.02 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.04 | 0.00 | 0.06 | 0.00 | 0.00 |
| Al2O3 | 0.40 | 0.19 | 0.91 | 0.00 | 0.30 | 0.37 | 0.31 | 0.66 | 0.31 | 0.69 | 0.41 | 0.36 | 0.32 |
| Fe2O3 | 3.46 | 2.09 | 8.86 | 0.38 | 5.22 | 4.03 | 1.70 | 1.88 | 4.65 | 3.79 | 3.25 | 4.42 | 2.62 |
| FeO | 5.75 | 2.07 | 9.64 | 1.15 | 4.32 | 4.66 | 7.15 | 8.85 | 4.66 | 4.64 | 7.27 | 4.20 | 6.72 |
| MnO | 0.45 | 0.08 | 0.65 | 0.26 | 0.46 | 0.42 | 0.39 | 0.48 | 0.51 | 0.55 | 0.45 | 0.47 | 0.38 |
| MgO | 12.64 | 0.93 | 13.95 | 10.05 | 13.19 | 13.23 | 12.59 | 10.53 | 13.04 | 12.79 | 11.39 | 13.32 | 12.52 |
| CaO | 2.12 | 0.50 | 3.09 | 1.39 | 2.18 | 2.41 | 2.85 | 1.47 | 1.88 | 1.80 | 1.89 | 1.76 | 2.57 |
| Na2O | 8.98 | 0.28 | 9.40 | 8.21 | 9.01 | 9.03 | 8.67 | 9.18 | 9.09 | 9.23 | 9.15 | 9.14 | 8.47 |
| K2O | 0.72 | 0.18 | 1.21 | 0.40 | 0.67 | 0.47 | 0.61 | 0.61 | 0.75 | 0.61 | 0.61 | 0.83 | 1.09 |
| F | 0.28 | 0.13 | 0.50 | 0.09 | 0.31 | 0.18 | 0.19 | 0.14 | 0.43 | 0.11 | 0.09 | 0.39 | 0.27 |
| Sum | 100.41 | 100.56 | 100.52 | 100.25 | 100.41 | 100.80 | 100.51 | 100.23 | 100.23 | 100.25 | |||
| O-F2 | 0.12 | 0.13 | 0.07 | 0.09 | 0.06 | 0.18 | 0.04 | 0.04 | 0.16 | 0.11 | |||
| Sum | 100.29 | 100.43 | 100.44 | 100.16 | 100.36 | 100.62 | 100.47 | 100.19 | 100.07 | 100.13 | |||
| Formula based on 13 cations and 24 anions (O + F) | |||||||||||||
| Si | 7.766 | 7.757 | 7.747 | 7.827 | 7.680 | 7.720 | 7.670 | 7.810 | 7.744 | 7.852 | |||
| Al | 0.068 | 0.052 | 0.063 | 0.054 | 0.114 | 0.053 | 0.117 | 0.070 | 0.061 | 0.055 | |||
| Fe3+ | 0.166 | 0.192 | 0.190 | 0.119 | 0.206 | 0.228 | 0.214 | 0.120 | 0.195 | 0.093 | |||
| Sum T | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | |||
| Ti | 1.277 | 1.196 | 1.275 | 1.276 | 1.519 | 1.250 | 1.407 | 1.276 | 1.255 | 1.209 | |||
| Cr+V | 0.031 | 0.014 | 0.023 | 0.017 | 0.039 | 0.047 | 0.020 | 0.067 | 0.025 | 0.020 | |||
| Fe3+ | 0.212 | 0.375 | 0.247 | 0.066 | 0.001 | 0.276 | 0.198 | 0.237 | 0.285 | 0.194 | |||
| Fe2+ | 0.697 | 0.522 | 0.562 | 0.869 | 1.083 | 0.562 | 0.559 | 0.887 | 0.508 | 0.816 | |||
| Mn | 0.055 | 0.056 | 0.051 | 0.048 | 0.059 | 0.062 | 0.067 | 0.056 | 0.058 | 0.047 | |||
| Mg | 2.729 | 2.837 | 2.843 | 2.735 | 2.298 | 2.802 | 2.749 | 2.477 | 2.870 | 2.712 | |||
| Sum C | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | |||
| Ca | 0.328 | 0.337 | 0.372 | 0.444 | 0.230 | 0.290 | 0.278 | 0.295 | 0.272 | 0.400 | |||
| Na | 1.672 | 1.663 | 1.628 | 1.556 | 1.770 | 1.710 | 1.722 | 1.705 | 1.728 | 1.600 | |||
| Sum B | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | |||
| Na | 0.848 | 0.858 | 0.895 | 0.884 | 0.835 | 0.833 | 0.858 | 0.884 | 0.832 | 0.787 | |||
| K | 0.133 | 0.123 | 0.086 | 0.112 | 0.114 | 0.138 | 0.113 | 0.113 | 0.152 | 0.203 | |||
| Sum A | 0.981 | 0.982 | 0.981 | 0.996 | 0.949 | 0.970 | 0.971 | 0.997 | 0.984 | 0.989 | |||
| F | 0.129 | 0.142 | 0.081 | 0.098 | 0.062 | 0.196 | 0.048 | 0.042 | 0.179 | 0.125 | |||
| O | 1.871 | 1.858 | 1.919 | 1.913 | 1.938 | 1.804 | 1.952 | 1.958 | 1.821 | 1.875 | |||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Mean | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | El4-13 | El4-6 | El4-N | |||||||||
| SiO2 | 46.39 | 45.95 | 46.27 | 46.44 | 46.18 | 46.18 | 46.60 | 46.24 | 44.71 | 44.65 | 46.29 | 45.99 |
| TiO2 | 10.20 | 10.33 | 10.29 | 10.36 | 10.29 | 10.10 | 10.09 | 10.14 | 10.47 | 10.59 | 10.20 | 10.28 |
| Cr2O3 | 0.21 | 0.20 | 0.44 | 0.34 | 0.33 | 0.30 | 0.34 | 0.23 | 0.06 | 0.18 | 0.16 | 0.25 |
| Al2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.45 | 0.45 | n.d. | 0.08 |
| Fe2O3 | 2.56 | 2.65 | 2.64 | 0.91 | 2.77 | 3.81 | 1.27 | 1.92 | 3.68 | 1.89 | 2.17 | 2.39 |
| FeO | 17.53 | 17.74 | 17.68 | 19.30 | 18.08 | 16.01 | 18.77 | 18.78 | 20.12 | 22.59 | 18.38 | 18.63 |
| MnO | 0.50 | 0.62 | 0.53 | 0.67 | 0.70 | 0.54 | 0.57 | 0.45 | 0.67 | 0.80 | 0.57 | 0.60 |
| MgO | 14.59 | 14.20 | 14.58 | 14.18 | 13.91 | 15.06 | 14.53 | 14.43 | 12.01 | 11.34 | 14.60 | 13.95 |
| CaO | 0.02 | n.d. | 0.03 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.01 |
| Na2O | 8.47 | 8.44 | 8.43 | 8.24 | 8.53 | 8.55 | 8.24 | 8.21 | 8.42 | 8.10 | 8.24 | 8.35 |
| Sum | 100.46 | 100.13 | 100.89 | 100.44 | 100.79 | 100.55 | 100.41 | 100.40 | 100.60 | 100.59 | 100.60 | 100.54 |
| Formula based on 14 cations and 20 oxygens | ||||||||||||
| Si | 5.935 | 5.915 | 5.906 | 5.964 | 5.918 | 5.891 | 5.973 | 5.938 | 5.820 | 5.848 | 5.929 | 5.913 |
| Al | 0.070 | 0.069 | 0.012 | |||||||||
| Fe3+ | 0.065 | 0.085 | 0.094 | 0.036 | 0.082 | 0.109 | 0.027 | 0.062 | 0.111 | 0.082 | 0.071 | 0.075 |
| Sum | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 |
| Ti | 0.981 | 1.000 | 0.988 | 1.001 | 0.992 | 0.969 | 0.973 | 0.979 | 1.025 | 1.043 | 0.982 | 0.994 |
| Cr | 0.021 | 0.020 | 0.044 | 0.035 | 0.033 | 0.030 | 0.034 | 0.023 | 0.006 | 0.019 | 0.016 | 0.026 |
| Fe3+ | 0.182 | 0.171 | 0.160 | 0.052 | 0.185 | 0.256 | 0.095 | 0.124 | 0.250 | 0.104 | 0.138 | 0.156 |
| Fe2+ | 1.875 | 1.909 | 1.888 | 2.073 | 1.937 | 1.708 | 2.012 | 2.017 | 2.190 | 2.474 | 1.968 | 2.003 |
| Mn | 0.054 | 0.068 | 0.057 | 0.073 | 0.076 | 0.058 | 0.062 | 0.049 | 0.074 | 0.089 | 0.062 | 0.066 |
| Mg | 2.783 | 2.725 | 2.774 | 2.715 | 2.657 | 2.864 | 2.776 | 2.763 | 2.331 | 2.214 | 2.788 | 2.673 |
| Na | 0.103 | 0.106 | 0.089 | 0.052 | 0.119 | 0.115 | 0.048 | 0.044 | 0.125 | 0.057 | 0.046 | 0.082 |
| Sum | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 |
| Ca | 0.002 | 0.004 | 0.001 | |||||||||
| Na | 1.998 | 2.000 | 1.996 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 1.999 |
| Sum | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| End-members (mol.%) | ||||||||||||
| Mg-Aen | 55.66 | 54.50 | 55.49 | 54.30 | 53.15 | 57.28 | 55.53 | 55.25 | 46.61 | 44.28 | 55.75 | 53.46 |
| Aen | 37.51 | 38.19 | 37.75 | 41.47 | 38.75 | 34.16 | 40.23 | 40.35 | 43.80 | 49.49 | 39.37 | 40.07 |
| Others | 6.83 | 7.31 | 6.76 | 4.23 | 8.10 | 8.56 | 4.24 | 4.40 | 9.59 | 6.23 | 4.88 | 6.47 |
| 1 | 2 | 3 | 4 | 5 c | 5 r | ||
|---|---|---|---|---|---|---|---|
| Position | SP | CONT | SP | SP | CONT | CONT | |
| Area | Elga-4 | Elga-4 | Elga-4 | El4-5-2 | El4-5-3 | El4-5-3 | |
| N = 3 | N = 19 | sd | N = 3 | N = 1 | N = 1 | N = 1 | |
| SiO2 | 55.69 | 56.12 | 0.43 | 53.94 | 55.41 | 56.56 | 54.37 |
| TiO2 | 0.41 | 0.28 | 0.06 | n.d. | 0.27 | 0.28 | n.d. |
| Cr2O3 | 0.54 | 0.34 | 0.08 | n.d. | 0.34 | 0.32 | n.d. |
| Al2O3 | n.d. | 0.03 | 0.05 | 0.11 | 0.20 | n.d. | 0.11 |
| Fe2O3 | 0.82 | 0.98 | 0.81 | 1.31 | 0.27 | 0.53 | 0.95 |
| FeO | 11.39 | 10.16 | 0.99 | 18.62 | 14.14 | 9.81 | 18.24 |
| MnO | 0.90 | 0.75 | 0.09 | 1.66 | 0.65 | 0.75 | 1.54 |
| MgO | 29.42 | 30.97 | 0.74 | 23.84 | 27.90 | 31.41 | 24.41 |
| CaO | 0.70 | 0.43 | 0.08 | 0.34 | 0.50 | 0.43 | 0.29 |
| Na2O | 0.29 | 0.15 | 0.06 | 0.28 | 0.29 | 0.17 | 0.29 |
| Sum | 100.17 | 100.22 | 100.10 | 99.97 | 100.26 | 100.20 | |
| Formula based on 4 cations and 6 oxygens | |||||||
| Si | 1.980 | 1.979 | 1.989 | 1.990 | 1.987 | 1.995 | |
| Al | 0.001 | 0.005 | 0.008 | 0.005 | |||
| Fe3+ | 0.020 | 0.020 | 0.006 | 0.001 | 0.013 | 0.000 | |
| Sum | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | |
| Ti | 0.011 | 0.008 | 0.007 | 0.007 | |||
| Cr | 0.015 | 0.010 | 0.010 | 0.009 | |||
| Fe3+ | 0.002 | 0.006 | 0.031 | 0.006 | 0.001 | 0.026 | |
| Fe2+ | 0.339 | 0.300 | 0.574 | 0.425 | 0.288 | 0.560 | |
| Mn | 0.027 | 0.022 | 0.052 | 0.020 | 0.022 | 0.048 | |
| Mg | 1.559 | 1.628 | 1.310 | 1.493 | 1.644 | 1.335 | |
| Ca | 0.027 | 0.016 | 0.014 | 0.019 | 0.016 | 0.011 | |
| Na | 0.020 | 0.010 | 0.020 | 0.020 | 0.012 | 0.021 | |
| Sum | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | |
| End-members (mol.%) | |||||||
| En | 78.20 | 81.30 | 65.31 | 75.25 | 82.35 | 66.72 | |
| Fs | 19.46 | 17.39 | 33.03 | 22.76 | 16.26 | 31.68 | |
| Wo | 2.34 | 1.32 | 1.66 | 1.99 | 1.39 | 1.60 | |
| Position | SP | SP | SP | SP | SP | SP | SP | CONT | CONT | CONT | NFG | NFG | NFG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | El4-5-1 | El4-5-1 | El4-5-1 | El4-5-2 | El4-5-2 | El4-5-2 | El4-5-2 | El4-5-3 | El4-5-3 | El4-1 | El4-6x | El4-6x | El4-6x |
| N = 3 | N = 4 | N = 4 | N = 2 | N = 2 | N = 2 | N = 2 | N = 1 | N = 1 | N = 1 | N = 2 | N = 2 | N = 2 | |
| SiO2 | 54.11 | 53.28 | 53.22 | 53.71 | 53.36 | 54.03 | 53.26 | 53.45 | 53.50 | 53.05 | 53.17 | 53.45 | 53.19 |
| TiO2 | 4.28 | 3.34 | 3.74 | 5.28 | 4.89 | 2.29 | 4.07 | 3.15 | 2.92 | 4.55 | 12.05 | 10.61 | 9.38 |
| V2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.11 | 0.08 | 0.08 |
| Cr2O3 | 10.89 | 16.92 | 14.94 | 17.58 | 17.25 | 10.06 | 15.41 | 9.30 | 7.54 | 7.33 | 10.87 | 12.22 | 11.04 |
| Al2O3 | 0.13 | 0.45 | 0.46 | 0.25 | 0.34 | 0.41 | 0.53 | 0.25 | 0.42 | 0.19 | 0.70 | 0.39 | 1.03 |
| Fe2O3 | 0.39 | 2.30 | 1.91 | 0.28 | 0.19 | 2.53 | 2.27 | 6.62 | 5.33 | 4.65 | 0.79 | 0.73 | 3.00 |
| FeO | 1.65 | 0.94 | 3.11 | 1.64 | 2.23 | 0.03 | 0.99 | 0.05 | 3.24 | 0.00 | 1.41 | 2.60 | 0.33 |
| MnO | 0.19 | 0.23 | 0.30 | 0.20 | 0.21 | 0.22 | 0.27 | 0.23 | 0.44 | 0.27 | 0.20 | 0.17 | 0.21 |
| MgO | 9.51 | 5.64 | 6.34 | 5.32 | 5.16 | 10.48 | 6.24 | 9.24 | 9.45 | 10.33 | 4.44 | 4.27 | 4.78 |
| CaO | 11.57 | 6.78 | 6.71 | 5.07 | 5.62 | 13.41 | 7.10 | 11.15 | 10.26 | 12.39 | 3.08 | 2.90 | 4.54 |
| Na2O | 7.53 | 10.09 | 9.42 | 11.03 | 10.65 | 6.59 | 9.89 | 7.70 | 7.10 | 7.11 | 13.14 | 12.80 | 12.33 |
| Sum | 100.25 | 99.96 | 100.15 | 100.35 | 99.88 | 100.06 | 100.03 | 101.14 | 100.20 | 99.87 | 99.94 | 100.20 | 99.90 |
| Formula based on 4 cations and 6 oxygens | |||||||||||||
| Si | 1.984 | 1.980 | 1.979 | 1.982 | 1.983 | 1.981 | 1.973 | 1.955 | 1.980 | 1.953 | 1.950 | 1.966 | 1.954 |
| Al | 0.005 | 0.020 | 0.020 | 0.011 | 0.015 | 0.018 | 0.023 | 0.011 | 0.018 | 0.008 | 0.030 | 0.017 | 0.045 |
| Fe3+ | 0.011 | 0.001 | 0.001 | 0.007 | 0.002 | 0.001 | 0.004 | 0.034 | 0.002 | 0.039 | 0.020 | 0.017 | 0.001 |
| Sum | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| Ti | 0.118 | 0.093 | 0.105 | 0.146 | 0.137 | 0.063 | 0.113 | 0.087 | 0.081 | 0.126 | 0.332 | 0.293 | 0.259 |
| Cr+V | 0.316 | 0.497 | 0.439 | 0.513 | 0.507 | 0.292 | 0.451 | 0.269 | 0.221 | 0.213 | 0.318 | 0.357 | 0.323 |
| Fe3+ | 0.000 | 0.064 | 0.052 | 0.001 | 0.004 | 0.069 | 0.059 | 0.148 | 0.147 | 0.090 | 0.002 | 0.003 | 0.082 |
| Fe2+ | 0.051 | 0.029 | 0.097 | 0.051 | 0.069 | 0.001 | 0.031 | 0.002 | 0.100 | 0.000 | 0.043 | 0.080 | 0.010 |
| Mn | 0.006 | 0.007 | 0.010 | 0.006 | 0.007 | 0.007 | 0.008 | 0.007 | 0.014 | 0.008 | 0.006 | 0.005 | 0.007 |
| Mg | 0.519 | 0.312 | 0.351 | 0.293 | 0.286 | 0.573 | 0.344 | 0.504 | 0.521 | 0.567 | 0.243 | 0.234 | 0.262 |
| Sum | 1.009 | 1.003 | 1.053 | 1.010 | 1.009 | 1.004 | 1.008 | 1.017 | 1.084 | 1.004 | 0.945 | 0.953 | 0.943 |
| Ca | 0.455 | 0.270 | 0.267 | 0.201 | 0.224 | 0.527 | 0.282 | 0.437 | 0.407 | 0.489 | 0.121 | 0.114 | 0.179 |
| Na | 0.536 | 0.727 | 0.679 | 0.789 | 0.767 | 0.469 | 0.710 | 0.546 | 0.509 | 0.507 | 0.934 | 0.913 | 0.878 |
| Sum | 0.991 | 0.997 | 0.947 | 0.990 | 0.991 | 0.996 | 0.992 | 0.983 | 0.916 | 0.996 | 1.055 | 1.027 | 1.057 |
| End-members (mol.%) | |||||||||||||
| Kosmochlor | 31.57 | 49.71 | 43.92 | 51.30 | 50.69 | 29.18 | 45.13 | 26.90 | 22.06 | 21.33 | 31.81 | 35.74 | 32.30 |
| Aegirine | 0.01 | 6.37 | 5.20 | 0.11 | 0.38 | 6.90 | 5.94 | 14.85 | 14.66 | 8.96 | 0.19 | 0.29 | 8.23 |
| “Na-Ti-cpx” | 21.99 | 16.63 | 18.81 | 27.53 | 25.65 | 10.78 | 19.97 | 12.88 | 14.23 | 20.46 | 61.45 | 55.25 | 47.30 |
| Diopside | 40.78 | 23.63 | 21.45 | 15.38 | 15.69 | 52.38 | 25.04 | 44.49 | 37.64 | 48.43 | 1.60 | 0.19 | 10.50 |
| Hedenbergite | 5.65 | 3.66 | 10.62 | 5.68 | 7.58 | 0.77 | 3.92 | 0.88 | 11.41 | 3.92 | 4.95 | 8.52 | 1.67 |
| Mineral | Czochralskiite | Brianite | Marićite | NFP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 32 | sd | min | max | N = 23 | sd | min | max | N = 16 | sd | min | max | N = 4 | |
| SiO2 | 0.13 | 0.14 | 0.00 | 0.75 | 0.09 | 0.09 | 0.00 | 0.44 | 0.13 | 0.19 | 0.00 | 0.67 | 0.54 |
| P2O5 | 45.16 | 0.35 | 44.38 | 45.86 | 46.68 | 46.68 | 45.62 | 47.12 | 41.88 | 0.50 | 41.10 | 42.46 | 31.30 |
| FeO | 3.31 | 0.70 | 2.27 | 5.07 | 3.39 | 3.39 | 2.95 | 4.23 | 26.74 | 0.42 | 25.80 | 27.74 | 34.58 |
| MnO | 0.64 | 0.21 | 0.41 | 1.48 | 0.48 | 0.48 | 0.26 | 0.67 | 5.81 | 0.28 | 5.34 | 6.17 | 0.59 |
| MgO | 4.88 | 0.34 | 4.22 | 5.64 | 11.03 | 11.03 | 10.40 | 11.89 | 5.65 | 0.32 | 5.28 | 6.24 | 3.77 |
| CaO | 26.03 | 0.59 | 25.00 | 27.06 | 17.76 | 17.76 | 17.40 | 18.85 | 0.77 | 0.25 | 0.52 | 1.46 | 2.26 |
| SrO | 0.10 | 0.05 | 0.00 | 0.18 | 0.34 | 0.34 | 0.10 | 0.53 | 0.09 | 0.06 | 0.00 | 0.17 | |
| Na2O | 19.85 | 0.25 | 19.50 | 20.45 | 20.27 | 20.27 | 19.87 | 20.60 | 18.90 | 0.29 | 18.60 | 19.60 | 2.22 |
| Sum | 100.10 | 100.04 | 99.98 | 76.37 | |||||||||
| Formula | |||||||||||||
| based on | 16 oxy | 8 oxy | 4 oxy | ||||||||||
| Si | 0.014 | 0.004 | 0.004 | ||||||||||
| P | 3.981 | 2.007 | 0.986 | ||||||||||
| Fe | 0.288 | 0.144 | 0.622 | ||||||||||
| Mn | 0.056 | 0.021 | 0.137 | ||||||||||
| Mg | 0.758 | 0.835 | 0.234 | ||||||||||
| Ca | 2.907 | 0.967 | 0.023 | ||||||||||
| Sr | 0.006 | 0.010 | 0.001 | ||||||||||
| Na | 4.008 | 1.996 | 1.019 | ||||||||||
| Sum cations | 12.018 | 5.983 | 3.027 | ||||||||||
| Phase | Ferri- Obertiite | “Ti3+- Obertiite” | “Fe2+-Ti4+- Obertiite” | Kosmo- Chlor | “Ti3+-kosmo- Chlor” | “Ti3+-Na- Cpx” | “Mg-Ti4+-Na- Cpx” |
|---|---|---|---|---|---|---|---|
| Formula | NaNa2 Mg3Fe3+Ti4+ (Si8O22)O2 | NaNa2 Mg3Ti3+Ti4+ (Si8O22)O2 | NaNa2 Mg3Fe2+0.5Ti4+1.5 (Si8O22)O2 | Na Cr Si2O6 | Na Cr0.5Ti3+0.5 Si2O6 | Na Ti3+ Si2O6 | Na Mg0.5Ti4+0.5 Si2O6 |
| SiO2 | 56.27 | 58.03 | 56.53 | 52.90 | 55.60 | 58.59 | 56.88 |
| TiO2 tot | 9.35 | 16.88 | 14.09 | 0.00 | 13.87 | 29.23 | 18.91 |
| Cr2O3 | 0.00 | 0.00 | 0.00 | 33.46 | 17.58 | 0.00 | 0.00 |
| FeO tot | 8.39 | 0.00 | 4.22 | 0.00 | 0.00 | 0.00 | 0.00 |
| MgO | 14.15 | 14.60 | 14.22 | 0.00 | 0.00 | 0.00 | 9.54 |
| Na2O | 10.88 | 11.22 | 10.93 | 13.64 | 14.34 | 15.11 | 14.67 |
| Sum | 99.04 | 100.72 | 100.00 | 100.00 | 101.39 | 102.93 | 100.00 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharygin, V.V. Mineralogy of Silicate-Natrophosphate Immiscible Inclusion in Elga IIE Iron Meteorite. Minerals 2020, 10, 437. https://doi.org/10.3390/min10050437
Sharygin VV. Mineralogy of Silicate-Natrophosphate Immiscible Inclusion in Elga IIE Iron Meteorite. Minerals. 2020; 10(5):437. https://doi.org/10.3390/min10050437
Chicago/Turabian StyleSharygin, Victor V. 2020. "Mineralogy of Silicate-Natrophosphate Immiscible Inclusion in Elga IIE Iron Meteorite" Minerals 10, no. 5: 437. https://doi.org/10.3390/min10050437
APA StyleSharygin, V. V. (2020). Mineralogy of Silicate-Natrophosphate Immiscible Inclusion in Elga IIE Iron Meteorite. Minerals, 10(5), 437. https://doi.org/10.3390/min10050437




