Origin of Fluoride and Arsenic in the Main Ethiopian Rift Waters
Abstract
1. Introduction
- (1)
- Lake and hot and cold spring waters (preliminarily studied by Belete et al. [26]);
- (2)
- Bulk rocks and constituent minerals of MER aquifer matrices;
- (3)
- Leachates obtained from water extraction tests with the MER rhyolitic volcanic rocks and fluvio-lacustrine sediments, performed to simulate the water–rock interaction processes in the area.
2. Geological Setting
3. Materials and Methods
3.1. Sampling
3.2. Analytical Methods
4. Results
4.1. Geochemistry of the MER Natural Water
4.2. Geochemistry of the MER Natural Waters
5. Discussion
5.1. F– and As Co-Occurrence in MER Water
5.2. Geological Origin of F– and As Co-Occurrence in the MER Water
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Margat, J.; van der Gun, J. Groundwater Around the World: A Geographic Synopsis, 1st ed.; CRC Press: New York, NY, USA, 2013; p. 376. [Google Scholar]
- Bretzler, A.; Johnson, A. The geogenic contamination handbook: Addressing arsenic and fluoride in drinking water. Appl. Geochem. 2015, 63, 642–646. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organisation: Geneva, Switzerland, 2011; p. 631.
- Karagas, M.R.; Gossai, A.; Pierce, B.; Ahsan, H. Drinking water Arsenic contamination, skin lesions, and malignancies: A systematic review of the global evidence. Curr Environ. Health Rep. 2015, 2, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Nickson, R.T.; McArthur, J.M.; Ravenscrift, P.; Burgress, W.G.; Ahmed, K.M. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl. Geochem. 2000, 15, 403–413. [Google Scholar] [CrossRef]
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of drinking water by arsenic in Bangladesh: A public health emergency. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar] [PubMed]
- Hussain, I.; Arif, M.; Hussain, J. Fluoride contamination in drinking water in rural habitations of Central Rajasthan, India. Environ. Monit. Assess. 2012, 8, 5151–5158. [Google Scholar] [CrossRef]
- Amini, M.; Mueller, K.; Abbaspour, K.C.; Rosenber, T.; Afyuni, M.; Møller, K.N.; Sarr, M.; Johnson, A. Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ. Sci. Technol. 2008, 42, 3662–3668. [Google Scholar] [CrossRef]
- Missimer, T.M.; Teaf, C.M.; Beeson, W.T.; Maliva, R.G.; Woolschlager, J.; Covert, D.J. Natural background and anthropogenic arsenic enrichment in Florida soils, surface water, and groundwater: A review with a discussion on public health risk. Int. J. Environ. Res. Public Health. 2018, 15, 2278. [Google Scholar] [CrossRef]
- Alarcón-Herrera, M.T.; Bundschuh, J.; Nath, B.; Nicolli, H.B.; Gutierrez, M.; Reyes-Gomez, V.M.; Nuñez, D.; Martínez-Dominguez, I.R.; Sracek, O. Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. J. Hazard. Mater. 2013, 960–969. [Google Scholar] [CrossRef]
- Alarcón-Herrera, M.T.; Martin-Alarcon, D.A.; Gutiérrez, M.; Reynoso-Cuevas, L.; Martín-Domínguez, A.; Olmos-Márquez, M.A.; Bundschuh, J. Co-occurrence, possible origin, and health-risk assessment of arsenic and fluoride in drinking water sources in Mexico: Geographical data visualization. Sci. Total Environ. 2020, 698, 1234168. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Claesson, M.; Bundschuh, J.; Sracek, O.; Fagerberg, J.; Jacks, G.; Thir, J.M. Distribution and mobility of arsenic in the Río Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Sci. Total Environ. 2006, 358, 97–120. [Google Scholar] [CrossRef]
- Barringer, J.L.; Reilly, P.A. Arsenic in groundwater: A summary of sources and the biogeochemical and hydrogeologic factors affecting arsenic occurrence and mobility. In Current Perspectives in Contaminant Hydrology and Water Resources Sustainability; Paul, B., Ed.; InTechOpen: London, UK, 2013; pp. 82–116. [Google Scholar] [CrossRef]
- Bundschuh, J.; Maity, J.P. Geothermal arsenic: Occurrence, mobility and environmental implications. Renew. Sustain . Energy Rev. 2015, 42, 1214–1222. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gupta, S.; Coomar, P.; Fryar, A.E.; Guillot, S.; Verma, S.; Bhattacharya, P.; Bundschuh, J.; Charlet, L. Plate tectonics influence on geogenic arsenic cycling: From primary sources to global groundwater enrichment. Sci. Total Environ. 2019, 683, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Rango, T.; Bianchini, G.; Beccaluva, L.; Ayenew, T.; Colombani, N. Hydrogeochemical study in the main Ethiopian Rift: New insights to source and enrichment mechanism of fluoride. J. Environ. Geol. 2009, 58, 109–118. [Google Scholar] [CrossRef]
- Rango, T.; Bianchini, G.; Beccaluva, L.; Tassinari, R. Geochemistry and water quality assessment of central main Ethiopian Rift natural waters with emphasis on source and occurrence of fluoride and arsenic. J. Afr. Earth Sci. 2010, 57, 479–491. [Google Scholar] [CrossRef]
- Rango, T.; Petrini, R.; Stenni, B.; Bianchini, G.; Slejko, F.; Beccaluva, L.; Ayenew, T. The dynamics of central main Ethiopian Rift waters: Evidence from δD, δ18O and 87Sr/86Sr ratios. Appl. Geochem. 2010, 25, 1860–1871. [Google Scholar] [CrossRef]
- Rango, T.; Vengosh, A.; Dwyer, G.; Bianchini, G. Mobilization of arsenic and other naturally occurring contaminants in groundwater of the Main Ethiopian Rift aquifers. Water Res. 2013, 47, 5801–5818. [Google Scholar] [CrossRef]
- Beccaluva, L.; Bianchini, G.; Natali, C.; Siena, F. Continental flood basalts and mantle plumes: A case study of the northern Ethiopian plateau. J. Petrol. 2009, 50, 1377–1403. [Google Scholar] [CrossRef]
- Beccaluva, L.; Bianchini, G.; Ellam, R.M.; Natali, C.; Santato, A.; Siena, F.; Stuart, F.M. Peridotite xenoliths from Ethiopia: Inferences about mantle processes from plume to rift settings. GSA Spec. Pap. 2011, 478, 77–104. [Google Scholar] [CrossRef]
- Natali, C.; Beccaluva, L.; Bianchini, G.; Siena, F. Rhyolites associated to Ethiopian CFB: Clues for initial rifting at the Afar plume axis. Earth Planet. Sci. Lett. 2011, 312, 59–68. [Google Scholar] [CrossRef]
- Natali, C.; Beccaluva, L.; Bianchini, G.; Savo, A.; Ellam, R.M.; Siena, F.; Stuart, F.M. High-MgO lavas associated to CFB as indicators of plume-related thermochemical effects: The case of ultra-titaniferous picrite–basalt from the Northern Ethiopian–Yemeni Plateau. Gondwana Res. 2016, 34, 29–48. [Google Scholar] [CrossRef]
- Natali, C.; Beccaluva, L.; Bianchini, G.; Siena, F. The Axum-Adwa basalt-trachyte complex: A late magmatic activity at the periphery of the Afar plume. Contrib. Mineral. Petr. 2013, 166, 351–370. [Google Scholar] [CrossRef]
- Arnórsson, S. Arsenic in surface-and up to 90 °C ground waters in a basalt area, N-Iceland: Processes controlling its mobility. Appl. Geochem. 2003, 18, 1297–1312. [Google Scholar] [CrossRef]
- Belete, A.; Beccaluva, L.; Bianchini, G.; Colombani, N.; Fazzini, M.; Marchina, C.; Natali, C.; Rango, T. Water-rock intercation and lake hydrochemistry in the Main Ethiopian Rift. In Landscape and landforms of Ethiopia; Paolo, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 307–321. [Google Scholar]
- Nicolli, H.B.; Bundschuh, J.; Blanco, M.d.C.; Tujchneider, O.C.; Panarello, H.O.; Dapeña, C.; Rusansky, J.E. Arsenic and associated trace-elements in groundwater from the Chaco-Pampean plain, Argentina: Results from 100 years of research. Sci. Total Environ. 2012, 429, 36–56. [Google Scholar] [CrossRef] [PubMed]
- Armengol, S.; Ayora, C.; Manzano, M.; Bea, S.A.; Martínez, S. The role of loess weathering in the groundwater chemistry of the Chaco-Pampean Palin (Argentina). J. Hydrol. 2020, 124984. [Google Scholar] [CrossRef]
- Bundschuh, J.; Farias, B.; Martin, R.; Storniolo, A.; Bhattacharya, P.; Cortes, J.; Bonorino, G.; Albouy, R. Groundwater arsenic in the Chaco-Pampean Plain, Argentina: Case study from Robles County, Santiago del Estero Province. Appl. Geochem. 2004, 19, 231–243. [Google Scholar] [CrossRef]
- Vital, M.; Martínez, D.E.; Babay, P.; Quiroga, S.; Clément, A.; Daval, D. Control of the mobilization of arsenic and other natural pollutants in groundwater by calcium carbonate concretions in the Pampean Aquifer, southeast of the Buenos Aires province, Argentina. Sci. Total Environ. 2019, 674, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, H.B.; Bundschuh, J.; García, J.W.; Falcón, C.M.; Jean, J.S. Sources and controls for the mobility of arsenic in oxidizing groundwaters from loess-type sediments in arid/semi-arid dry climates devidence from the Chaco-Pampean plain (Argentina). Water Res. 2010, 44, 5589–5604. [Google Scholar] [CrossRef]
- Aullón, A.A.; Schulz, C.; Bundschuh, J.; Jacks, G.; Thunvik, R.; Gustafsson, J.P.; Mörth, C.M.; Sracek, O.; Ahmad, A.; Bhattacharya, P. Hydrogeochemical controls on the mobility of arsenic, fluoride and other geogenic co-contaminants in the shallow aquifers of northeastern La Pampa Province in Argentina. Sci. Total Envir. 2020, 715, 136671. [Google Scholar] [CrossRef]
- Sracek, O.; Wanke, H.; Ndakunda, N.N.; Mihaljevič, M.; Buzek, F. Geochemistry and fluoride levels of geothermal springs in Namibia. J. Geochem. Explor. 2015, 148, 96–104. [Google Scholar] [CrossRef]
- Sracek, O.; Rahobisoa, J.J.; Trubač, J.; Buzek, F.; Andriamamonjy, S.A.; Rambeloson, R.A. Geochemistry of thermal waters and arsenic enrichment at Antsirabe, Central Highlands of Madagascar. J. Hydrol. 2019, 577, 123895. [Google Scholar] [CrossRef]
- Bretzler, A.; Osenbrück, K.; Gloaguen, R.; Ruprecht, J.S.; Kebede, S.; Stadler, S. Groundwater origin and flow dynamics in active rift systems–A multi-isotope approach in the Main Ethiopian Rift. J. Hydrol. 2011, 402, 274–289. [Google Scholar] [CrossRef]
- Reimann, C.; Bjorvatn, K.; Frengstad, B.; Melaku, Z.; Tekle-Haimanot, R.; Siewers, U. Drinking water qualityin the Ethiopian section of the East African Rift Valley, part I: Data and health aspects. Sci. Total Environ. 2003, 31, 65–80. [Google Scholar] [CrossRef]
- Rango, T.; Kravchenko, J.; Atlaw, B.; McCornick, P.G.; Jeuland, M.; Merola, B.; Vengosh, A. Groundwater quality and its health impact: An assessment of dental fluorosis in rural inhabitants of the Main Ethiopian Rift. Environ. Int. 2012, 43, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Rango, T.; Jeuland, M.; Tekle-Haimanot, R.; Shankar, A.; Alemayehu, B.; Assefa, G.; Whitford, G.; Wolfe, A. Bone quality in fluoride-exposed populations: A novel application of the ultrasonic method. Bone Rep. 2020, 12, 100235. [Google Scholar] [CrossRef]
- Kravchenko, J.; Rango, T.; Akushevich, I.; Atlaw, B.; McCornick, P.G.; Merola, R.B.; Paul, C.; Weinthal, E.; Harrison, C.; Vengosh, A.; et al. The effect of non-fluoride factors on risk of dental fluorosis: Evidence from rural populations of the Main Ethiopian Rift. Sci. Total Environ. 2014, 488–489, 595–606. [Google Scholar] [CrossRef]
- Haji, M.; Wang, D.; Li, L.; Qin, D.; Guo, Y. Geochemical Evolution of Fluoride and Implication for F– Enrichment in Groundwater: Example from the Bilate River Basin of Southern Main Ethiopian Rift. Water 2018, 10, 1799. [Google Scholar] [CrossRef]
- Ayalew, D.; Jung, S.; Romer, R.L.; Kersten, F.; Pfänder, J.A.; Garbe-Schönberg, D. Petrogenesis and origin of modern Ethiopian rift basalts: Constraints from isotope and trace element geochemistry. Lithos 2016, 258–259, 1–14. [Google Scholar] [CrossRef]
- Corti, G. Continental rift evolution: From rift initiation to incipient break-up in the Main Ethiopian Rift, East Africa. Earth Sci. Rev. 2009, 96, 1–53. [Google Scholar] [CrossRef]
- Benvenuti, M.; Carnicelli, S.; Belluomini, G.; Dainelli, N.; Di Grazia, S.; Ferrari, G.A.; Iasio, C.; Sagri, M.; Ventra, D.; Atnafu, B.; et al. The Ziway–Shala lake basin (main Ethiopian rift, Ethiopia): A revision of basin evolution with special reference to the Late Quaternary. J. Afr. Earth Sci. 2002, 35, 247–269. [Google Scholar] [CrossRef]
- Kebede, S.; Travi, Y.; Asfawossen, A.; Alemayehu, T.; Ayenew, T.; Tessema, Z. Groundwater origin and flow along selected transects in Ethiopian rift volcanic aquifers. Hydrogeol. J. 2008, 16, 55–73. [Google Scholar] [CrossRef]
- Natali, C.; Bianchini, G.; Marchina, C.; Knoeller, K. Geochemistry of the Adige River water from the Eastern Alps to the Adriatic Sea (Italy): Evidences for distinct hydrological components and water-rock interactions. Environ. Sci. Pollut. Res. 2016, 23, 11677–11694. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.T.; Sanzolone, R.F. Decomposition techniques. J. Geochem. Explor. 1992, 44, 65–106. [Google Scholar] [CrossRef]
- European Union. Council Directive 98/83/EC of 3rd November 1998 on the quality of water intended for human consumption. Off. J. Eur. Community 1998, 34, L330/32–L330/54. [Google Scholar]
- United States Environmental Protection Agency. National primary drinking water regulations Long Term 1 Enhanced Surface Water Treatment Rule. Final rule. Fed. Regist. 2018, 67, 1811. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1–64. [Google Scholar]
- Macdonald, R.; Bagiński, B.; Leat, P.T.; White, J.C.; Dzierżanowski, P. Mineral stability in peralkaline silicic rocks: Information from trachytes of the Menengai volcano, Kenya. Lithos 2011, 125, 553–568. [Google Scholar] [CrossRef]
- Macdonald, R.; Bagiński, B.; Ronga, F.; Dzierżanowski, P.; Lustrino, M.; Marzoli, A.; Melluso, L. Evidence for extreme fractionation of peralkaline silicic magmas, the Boseti volcanic complex, Main Ethiopian Rift. Miner. Petrol. 2012, 104, 163–175. [Google Scholar] [CrossRef]
- Martz, A.M.; Brown, F.H. Chemistry and mineralogy of some Plio-Pleistocene tuffs from the Shugura Formation, Southwest Ethiopia. Quaternary Res. 1981, 16, 240–257. [Google Scholar] [CrossRef]
- Ayalew, D.; Barbery, P.; Marty, B.; Reisberg, L.; Yirgu, G.; Pik, R. Source, genesis and giant ignimbrite deposits associated with Ethiopian continental flood basalts. Geochim. Cosmochim. Acta 2002, 66, 1429–1448. [Google Scholar] [CrossRef]
- Ridolfi, F.; Renzulli, A.; Macdonald, R.; Upton, B. Peralkaline syenite autoliths from Kilombe volcano, Kenya Rift Valley: Evidence for subvolcanic interaction with carbonatic fluids. Lithos 2006, 91, 373–392. [Google Scholar] [CrossRef]
- Marshall, A.S.; Macdonald, R.; Rogers, N.W.; Fitton, J.G.; Tindle, A.G.; Nejbert, K.; Hinton, R.W. Fractionation of peralkaline silicic magmas: The greater Olkaria volcanic complex, Kenya Rift Valley. J. Petrol. 2009, 50, 323–359. [Google Scholar] [CrossRef]
- Petersen, E.U.; Essene, E.J.; Peacor, D.R. Fluorine end-member micas and amphiboles. Am. Mineral. 1982, 67, 538–544. [Google Scholar]
- Ryabov, V.V.; Simonov, O.N.; Snisar, S.G. Fluorine and chlorine in apatites, micas, and amphiboles of layered trap intrusions of the Siberian Platform. Russ. Geol. Geophys. 2018, 59, 363–373. [Google Scholar] [CrossRef]
- Anzolin, H.M.; Dani, N.; Remus, M.V.D.; Ribeiro, R.R.; Nunes, A.R.; Vaccari Ruppel, K.M. Apatite multi-generations in the Três Estradas Carbonatite, Southern Brazil: Physical and chemistry meaning and implications to phosphate ore quality. Braz. J. Geol. 2019, 49, e20180092. [Google Scholar] [CrossRef]
- Nicolli, H.B.; Suriano, J.; Gomez Peral, M.A.; Ferpozzi, L.H.; Baleani, O. Groundwater contamination with arsenic and other elements in an area of the Pampa, province of Cordoba, Argentina. Environ. Geol. Water Sci. 1989, 14, 3–16. [Google Scholar] [CrossRef]
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, O. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259–284. [Google Scholar] [CrossRef]
- Benson, T.R.; Coble, M.A.; Rytuba, J.J.; Mahood, G.A. Lithium enrichment in intracontinental ryolite magmas leads to Li deposits in caldera basins. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s guide to PHREEQC (version 2)—A computer program for speciation, batch-reaction, one.dimensional transport, and inverse geochemical calculations. USGS Water-Resour. Investig. Rep. 1999, 99, 312. [Google Scholar]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Fuge, R. On the behaviour of fluorine and chlorine during magmatic differentiation. Contrib. Mineral. Petrol. 1977, 61, 245–249. [Google Scholar] [CrossRef]
- Cossío, O.; Navarro-Ciurana, D.; Domènech, C.; Canals, À.; Barbieri, M.; Pittalis, D.; Soler, A.; Ghiglieri, G. F-bearing sediments and rocks in the East African Rift: Characterization and evaluation of F release capacity. Macla Rev. de la Soc. Española de Mineral. 2019, 12. [Google Scholar]
- Urann, B.M.; Le Roux, V.; Hammond, K.; Marschall, H.R.; Lee, C.T.A.; Monteleone, B.D. Fluorine and chlorine in mantle minerals and the halogen budget of the Earth’s mantle. Contrib. Mineral. Petrol. 2017, 172, 51. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhu, C.; Xue, X.; Quian, K.; Xie, X.; Wang, Y. Hydrogeochemical processes controlling the mobilization and enrichment of fluoride in groundwater of the North China Plain. Sci. Total Environ. 2020, 730, 138877. [Google Scholar] [CrossRef]
- Mukherjee, I.; Singh, U.M. Fluoride abundance and their release mechanism in groundwater along with associated human health risks in a geologically heterogeneous semi-arid region of east India. Microchem. J. 2020, 152, 104304. [Google Scholar] [CrossRef]
- Casentini, B.; Pettine, M.; Millero, F.J. Release of Arsenic from volcanic rocks through interactions with inorganic anions and organic ligands. Aquat. Geochem. 2010, 16, 373–393. [Google Scholar] [CrossRef]
- Mazziotti-Tagliani, S.; Angelone, M.; Armiento, G.; Pacifico, R. Arsenic and fluorine in the Etnean volcanics from Biancavilla, Sicily, Italy: Environmental implications. Environ. Earth Sci. 2012, 66, 561–572. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Zeng, H.; Wang, D.; Liu, J.; Liu, H.; Qian, M.; Xu, L. Dissolution and solubility of the arsenate–phosphate hydroxylapatite solid solution [Ca5(PxAs1-xO4)3(OH)] at 25 °C. Environ. Chem. 2011, 8, 133–145. [Google Scholar] [CrossRef]
- Liu, W.; Mei, Y.; Etschmann, B.; Brugger, J.; Pearce, M.; Ryan, C.G.; Borg, S.; Wykes, J.; Kappen, P.; Paterson, D.; et al. Arsenic in hydrothermal apatite: Oxidation state, mechanism of uptake, and comparison between experiments and nature. Geochim. Cosmochim. Acta 2017, 196, 144–159. [Google Scholar] [CrossRef]
- Puzio, B.; Manecki, M.; Kwasniak-Kominek, M. Transition from endothermic to exothermic dissolution of Hydroxyapatite Ca5(PO4)3OH–Johnbaumite Ca5(AsO4)3OH solid solution series at temperatures ranging from 5 to 65 °C. Minerals 2018, 8, 281. [Google Scholar] [CrossRef]
- Moore, P.B. Welshite, Ca2Mg4Fe3+Sb5+O2[Si4Be2O18], a new member of the aenigmatite group. Mineral. Mag. 1978, 42, 129–132. [Google Scholar] [CrossRef]
- Grew, E.S.; Barbier, J.; Britten, J.; Halenius, U.; Shearer, C.K. The crystal chemistry of welshite, a non-centrosymmetric (P1) aenigmatite-sapphirine-surinamite group mineral. Am. Mineral. 2007, 92, 80–90. [Google Scholar] [CrossRef]
- Liu, C.H.; Chuang, Y.H.; Chen, T.Y.; Yuan Tian, Y.; Li, H.; Wang, M.K.; Zhang, W. Mechanism of Arsenic Adsorption on Magnetite Nanoparticles from Water: Thermodynamic and Spectroscopic Studies. Environ. Sci. Technol. 2015, 49, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P.; Bellieni, G.; Dudek, A.; Efremova, S.; Keller, J.; et al. Igneous Rocks: A Classification and Glossary of Terms, Recommendations of the International Union of Geological Sciences Subcommission of the Systematics of Igneous Rocks; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Macdonald, R. Nomenclature and petrochemistry of the peralkaline oversaturated extrusive rocks. Bull. Volc. 1974, 54, 78–83. [Google Scholar] [CrossRef]
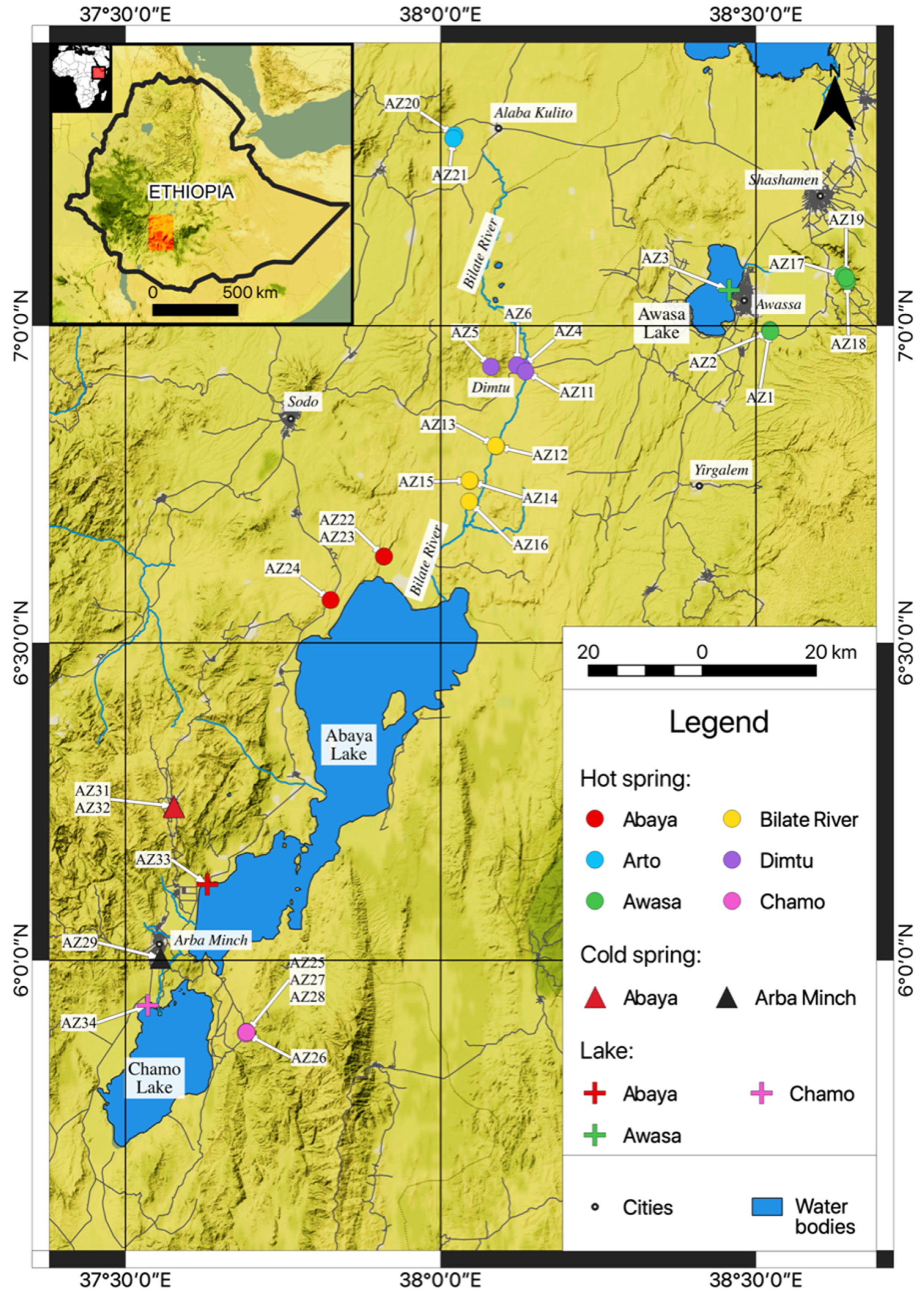




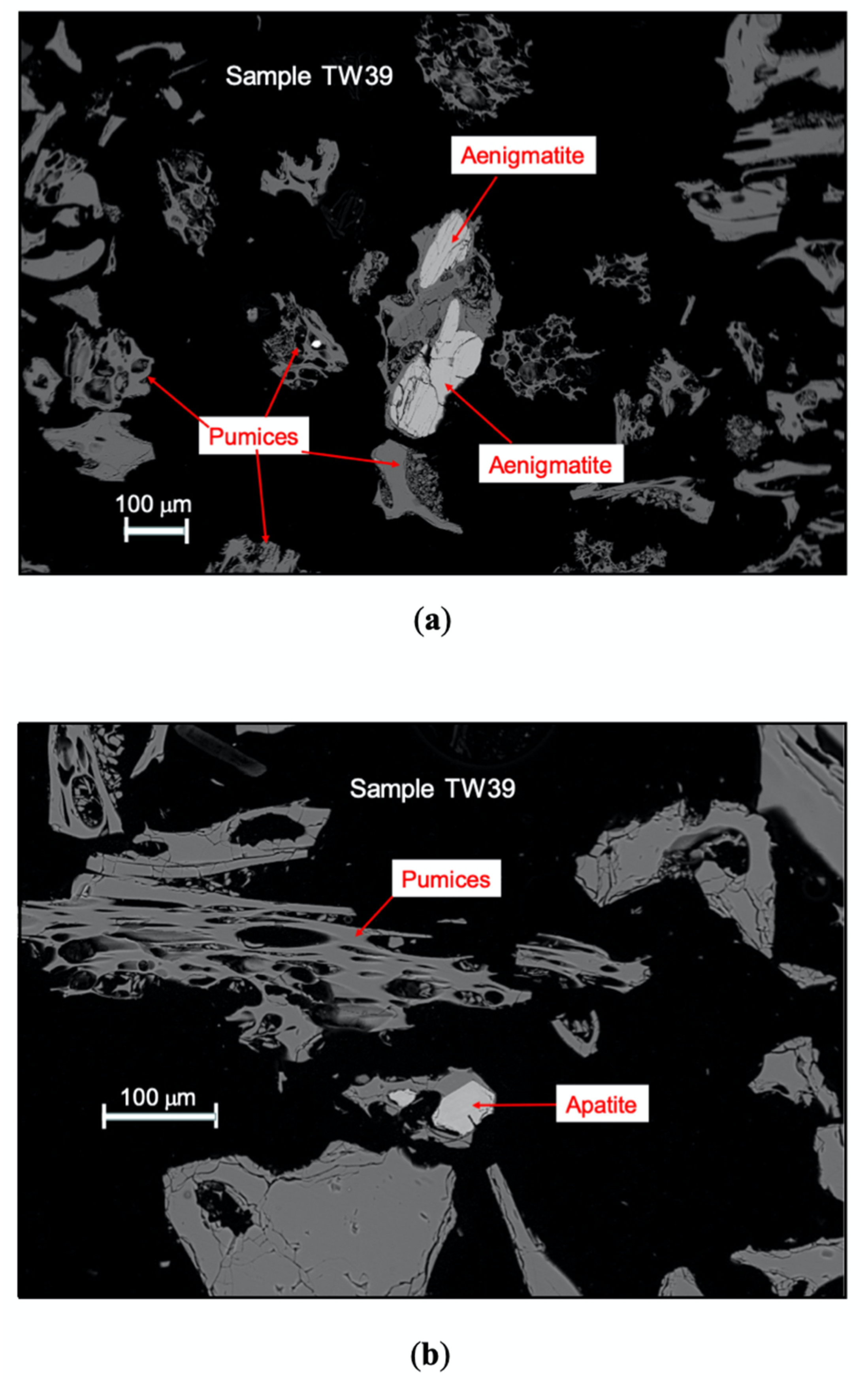


| Sample | Latitude | Longitude | Elevation (m) | pH | T (°C) | EC (µS/cm) | TDS (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| Awasa Lake and surroundings | ||||||||
| AZ1 | 06°59′36″ N | 38°31′19″ E | 1706 | 6.2 | 61 | 1200 | 671 | Hot spring |
| AZ2 | 06°59′27″ N | 38°31′22″ E | 1707 | 6.9 | >80 | 2330 | 1131 | Hot spring |
| AZ3 | 07°03′22″ N | 38°27′27″ E | 1687 | 9.9 | 25 | 810 | 574 | Lake |
| AZ17 | 07°04′46″ N | 38°38′17″ E | 1914 | 7.4 | 66 | 1160 | 573 | Hot spring |
| AZ18 | 07°04′14″ N | 38°38′40″ E | 1912 | 7.9 | 59 | 1120 | 611 | Hot spring |
| AZ19 | 07°04′32″ N | 38°38′33″ E | 1936 | 7.7 | 59 | 1020 | 692 | Hot spring |
| Dimtu town | ||||||||
| AZ4 | 06°56′05″ N | 38°07′46″ E | 1489 | 7.2 | 37 | 1030 | 692 | Hot spring |
| AZ5 | 06°56′07″ N | 38°04′46″ E | 1491 | 6.9 | 39 | 950 | 666 | Hot spring |
| AZ6 | 06°56′16″ N | 38°07′14″ E | 1491 | 6.8 | 39 | 960 | 656 | Hot spring |
| AZ11 | 06°55′41″ N | 38°08′04″ E | 1490 | 8.3 | 57 | 1150 | 745 | Hot spring |
| Bilate River and surroundings | ||||||||
| AZ12 | 06°48′35″ N | 38°05′17″ E | 1346 | 7.8 | 45 | 1340 | 852 | Hot spring |
| AZ13 | 06°48′39″ N | 38°05′13″ E | 1346 | 7.5 | 56 | 1480 | 913 | Hot spring |
| AZ14 | 06°45′18″ N | 38°02′47″ E | 1248 | 7.6 | 67 | 5530 | 3667 | Hot spring |
| AZ15 | 06°45′22″ N | 38°02′44″ E | 1248 | 7.8 | 63 | 5500 | 3736 | Hot spring |
| AZ16 | 06°43′22″ N | 38°02′42″ E | 1243 | 7.9 | > 80 | 6200 | 3722 | Hot spring |
| Abaya Lake and surroundings | ||||||||
| AZ22 | 06°38′10″ N | 37°54′34″ E | 1197 | 6.6 | 65 | 2700 | 1750 | Hot spring |
| AZ23 | 06°38′10″ N | 37°54′35″ E | 1199 | 7.3 | 61 | 2560 | 1903 | Hot spring |
| AZ24 | 06°34′03″ N | 37°49′30″ E | 1259 | 6.4 | 36 | 420 | 280 | Hot spring |
| AZ31 | 06°14′35″ N | 37°34′35″ E | 2682 | 6.0 | 17 | 60 | 67 | Cold spring |
| AZ32 | 06°14′29″ N | 37°34′37″ E | 2681 | 5.3 | 18 | 10 | 26 | Cold spring |
| AZ33 | 06°07′10″ N | 37°37′46″ E | 1192 | 9.1 | 26 | 970 | 799 | Lake |
| Chamo Lake and surroundings | ||||||||
| AZ25 | 05°53′14″ N | 37°41′29″ E | 1116 | 7.9 | 40 | 2270 | 1770 | Hot spring |
| AZ26 | 05°53′04″ N | 37°41′26″ E | 1136 | 8.0 | 46 | 2400 | 1734 | Hot spring |
| AZ27 | 05°53′11″ N | 37°41′30″ E | 1126 | 7.5 | 45 | 2450 | 1779 | Hot spring |
| AZ28 | 05°53′11″ N | 37°41′30″ E | 1125 | 7.7 | 56 | 2600 | 1757 | Hot spring |
| AZ34 | 05°55′44″ N | 37°32′07″ E | 1121 | 9.3 | 29 | 1640 | 1225 | Lake |
| Arto (near Alba Kulito) | ||||||||
| AZ20 | 07°18′02″ N | 38°01′19″ E | 1797 | 9.3 | 74 | 1930 | 909 | Hot spring |
| AZ21 | 07°17′43″ N | 38°01′11″ E | 1777 | 8.9 | 70 | 1800 | 1009 | Hot spring |
| Arba Minch | ||||||||
| AZ29 | 06°00′13″ N | 37°33′20″ E | 1199 | 7.3 | 24 | 300 | 222 | Cold spring |
| Sample | Major Ions (mg/L) | Trace Elements (µg/L) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F– | Cl– | NO3– | SO42– | HCO3– | Ca2+ | Mg2+ | Na+ | K+ | Li | Rb | Sr | Ba | As | Mo | Cd | Sb | Hg | |
| WHO * | 1.5 | n.g. | 50 | n.g. | n.m. | n.g. | n.g. | n.g. | n.g. | n.g. | n.m. | n.m. | 700 | 10 | n.g. | 3 | 20 | 6 |
| EU ** | 1.5 | 0.25 | 50 | 250 | n.m. | n.m. | n.m. | 200 | n.m. | n.m. | n.m. | n.m. | n.m. | 10 | n.m. | 5 | 5 | n.m. |
| EPA *** | 4 | n.m. | 10 | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.g. | 2000 | 10 | n.g. | 50 | 6 | 2 |
| Awasa Lake and surroundings | ||||||||||||||||||
| AZ1 | 36.3 | 444 | 12.3 | 5.64 | 203 | 15.0 | 142 | 75 | 183 | 13 | 37 | 18 | 4 | <6 | <2 | |||
| AZ2 | 14.6 | 73.2 | 118 | 600 | 9.44 | 3.80 | 380 | 25.3 | 277 | 130 | 304 | 22 | 74 | 20 | 5 | 7 | 3 | |
| AZ3 | 9.56 | 31.1 | 0.52 | 1.55 | 372 | 8.74 | 6.38 | 167 | 25.2 | 79 | 33 | 100 | 7 | 3 | 12 | 4 | <6 | <2 |
| AZ17 | 1.58 | 24.7 | 0.22 | 0.25 | 393 | 5.05 | 3.92 | 169 | 36.1 | 36 | 51 | 96 | 58 | 5 | 14 | 5 | 0 | <2 |
| AZ18 | 1.76 | 27.0 | 0.12 | 0.25 | 429 | 5.34 | 3.93 | 178 | 38.0 | 39 | 50 | 99 | 56 | 7 | 11 | 4 | <6 | <2 |
| AZ19 | 1.54 | 24.9 | 0.14 | 0.27 | 426 | 5.35 | 4.45 | 188 | 41.6 | 41 | 54 | 109 | 68 | <4 | 12 | 4 | <6 | <2 |
| Dimtu town | ||||||||||||||||||
| AZ4 | 15.9 | 20.2 | 4.08 | 26.5 | 390 | 4.66 | 3.52 | 211 | 16.4 | 64 | 30 | 47 | <5 | 11 | 29 | <4 | <6 | <2 |
| AZ5 | 16.9 | 17.4 | 3.66 | 25.0 | 387 | 8.38 | 3.17 | 190 | 14.9 | 63 | 34 | 44 | <5 | 10 | 32 | 5 | <6 | <2 |
| AZ6 | 17.0 | 17.7 | 2.91 | 25.2 | 372 | 6.53 | 3.50 | 197 | 15.1 | 66 | 34 | 43 | <5 | 5 | 28 | <4 | <6 | <2 |
| AZ11 | 26.5 | 36.5 | 0.33 | 15.5 | 420 | 1.52 | 0.53 | 230 | 14.6 | 98 | 19 | 17 | <5 | 17 | 36 | <4 | <6 | 2 |
| Bilate River and surroundings | ||||||||||||||||||
| AZ12 | 18.4 | 51.0 | 0.30 | 13.3 | 462 | 5.49 | 3.77 | 282 | 15.9 | 99 | 52 | 63 | 20 | 13 | 28 | <4 | <6 | 2 |
| AZ13 | 21.0 | 52.4 | 0.35 | 13.7 | 516 | 3.11 | 2.14 | 291 | 13.9 | 131 | 56 | 59 | 45 | 23 | 27 | <4 | <6 | <2 |
| AZ14 | 45.2 | 147 | 0.63 | 73.1 | 2181 | 0.46 | 0.60 | 1158 | 60.7 | 1069 | 481 | 283 | 26 | 970 | 13 | 4 | 76 | 27 |
| AZ15 | 43.5 | 162 | 75.2 | 2241 | 0.16 | 0.69 | 1146 | 67.6 | 1024 | 501 | 126 | 14 | 940 | 17 | 6 | 80 | 29 | |
| AZ16 | 44.9 | 144 | 1.09 | 72.9 | 2193 | 3.34 | 0.76 | 1195 | 67.2 | 1085 | 522 | 289 | 43 | 1019 | 22 | 8 | 75 | 30 |
| Abaya Lake | ||||||||||||||||||
| AZ22 | 18.1 | 65.5 | 14.9 | 1347 | 6.15 | 5.70 | 505 | 26.9 | 390 | 94 | 108 | 14 | 64 | 17 | <4 | <6 | <2 | |
| AZ23 | 18.3 | 70.4 | 0.19 | 15.9 | 1542 | 5.18 | 6.06 | 531 | 28.2 | 408 | 94 | 111 | 9 | 54 | 18 | <4 | <6 | <2 |
| AZ24 | 2.26 | 4.43 | 0.98 | 0.97 | 189 | 11.3 | 9.04 | 52.5 | 9.70 | 20 | 18 | 102 | <5 | <4 | <10 | <4 | <6 | <2 |
| AZ31 | 0.61 | 8.56 | 15.45 | 3.24 | 600 | 4.96 | 1.90 | 8.07 | 9.21 | 2 | 6 | 54 | 53 | <4 | <10 | <4 | <6 | <2 |
| AZ32 | 0.13 | 1.39 | 2.64 | 0.91 | 0.20 | 2.99 | 1.85 | 2.32 | 2.26 | 1 | <2 | 42 | 20 | <4 | <10 | <4 | <6 | <2 |
| AZ33 | 8.97 | 76.1 | 0.33 | 21.3 | 6.89 | 12.7 | 3.58 | 243 | 13.4 | 2 | 4 | 130 | 11 | <4 | 28 | <4 | <6 | <2 |
| Chamo Lake and surroundings | ||||||||||||||||||
| AZ25 | 10.9 | 116 | 954 | 225 | 21.1 | 5.97 | 430 | 22.5 | 494 | 154 | 956 | 4 | <4 | 15 | 0 | <6 | <2 | |
| AZ26 | 11.1 | 115 | 938 | 210 | 14.3 | 6.22 | 419 | 20.2 | 478 | 157 | 961 | <5 | <4 | 26 | 4 | <6 | <2 | |
| AZ27 | 11.4 | 118 | 981 | 195 | 25.4 | 6.43 | 450 | 22.4 | 502 | 167 | 1002 | <5 | 8 | 26 | 5 | <6 | <2 | |
| AZ28 | 10.9 | 115 | 0.15 | 930 | 195 | 25.4 | 6.67 | 441 | 33.3 | 491 | 172 | 966 | 11 | 5 | 19 | 4 | <6 | <2 |
| AZ34 | 9.33 | 156 | 0.13 | 16.6 | 600 | 8.30 | 9.71 | 411 | 15.0 | 1 | 3 | 133 | 21 | <4 | 29 | 6 | <6 | <2 |
| Arto (near Alba Kulito) | ||||||||||||||||||
| AZ20 | 33.4 | 37.3 | 0.10 | 57.5 | 585 | 0.00 | 0.50 | 309 | 16.7 | 177 | 81 | 44 | <5 | 41 | 26 | 6 | 2 | 2 |
| AZ21 | 27.8 | 37.1 | 0.16 | 57.4 | 669 | 0.69 | 0.64 | 295 | 15.5 | 175 | 79 | 104 | <5 | 31 | 23 | 2 | 3 | 3 |
| Arba Minch | ||||||||||||||||||
| AZ29 | 0.37 | 3.74 | 6.39 | 2.05 | 138 | 33.7 | 14.1 | 16.8 | 9.02 | 1 | 12 | 221 | <5 | 5 | <10 | 6 | 1 | <2 |
| Sample | Ted4 | TW31 | TW39 | TW22 | Ted38 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyroclastic Deposit | Pyroclastic Deposit | Pyroclastic Deposit | Fluvio-Lacustrine Sediments | Fluvio-Lacustrine Sediments | |||||||||||
| 15 days | 30 days | 90 days | 15 days | 30 days | 90 days | 15 days | 30 days | 90 days | 15 days | 30 days | 90 days | 15 days | 30 days | 90 days | |
| pH | 8.38 | 8.33 | 8.02 | 8.67 | 8.52 | 8.45 | 8.26 | 7.76 | 8.10 | 8.64 | 7.80 | 8.33 | 8.06 | 8.10 | n.a. |
| EC (µS/cm) | 460 | 401 | 437 | 143 | 170 | 209 | 199 | 219 | 191 | 1210 | 1280 | 1350 | 416 | 455 | n.a. |
| F– (mg/L) | 7.73 | 7.48 | 8.93 | 3.16 | 3.66 | 3.66 | 1.62 | 1.82 | 2.35 | 8.00 | 8.01 | 8.52 | 8.73 | 7.64 | n.a. |
| Cl– | 25.5 | 24.5 | 26.7 | 1.67 | 7.75 | 17.7 | 10.0 | 23.3 | 11.9 | 70.6 | 70.6 | 160 | 17.1 | 1 | n.a. |
| NO3– | 35.4 | 35.2 | 35.6 | n.d. | 0.08 | 0.05 | 4.78 | 6.44 | 19.9 | 0.45 | 0.46 | 0.94 | 15.0 | 16.1 | n.d. |
| SO42– | 33.0 | 32.3 | 34.2 | 0.85 | 2.80 | 0.69 | 4.21 | 4.25 | 4.65 | 300 | 300 | 310 | 12.1 | 12.4 | n.a. |
| * HCO3– | 96 | 72 | 27 | 65 | 71 | 96 | 72 | 63 | 55 | 120 | 154 | 46 | 139 | 176 | n.a |
| Ca2+ | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | 2.80 | 4.79 | 5.93 | 4.35 | 7.18 | 9.64 | 10.8 | 7.61 | 9.51 | 4.10 |
| Mg2+ | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | 0.50 | <0.4 | 1.26 | 1.65 | 2.69 | 1.54 | 2.12 | 3.80 |
| Na+ | 84.3 | 64.9 | 74.3 | 27.0 | 34.1 | 44.9 | 29.8 | 34.0 | 31.5 | 217 | 225 | 243 | 61.9 | 68.9 | 108 |
| K+ | 11.0 | 16.7 | 8.71 | 2.92 | 4.01 | 6.00 | 7.47 | 8.02 | 6.32 | 28.3 | 30.6 | 36.7 | 20.6 | 24.1 | 26.9 |
| Li (µg/L) | 7 | 7 | 16 | 8 | 13 | 22 | 6 | 6 | 9 | 152 | 159 | 218 | 52 | 63 | 70 |
| Rb | 18 | 13 | 20 | 3 | 3 | 6 | 11 | 12 | 8 | 18 | 20 | 25 | 12 | 14 | 12 |
| Sr | <2 | <2 | <2 | <2 | <2 | 4 | 12 | 17 | 8 | 61 | 73 | 97 | 83 | 123 | 156 |
| Ba | <5 | <5 | 5 | 6 | <5 | 8 | <5 | 10 | 5 | 17 | 14 | 15 | 6 | 16 | 5 |
| As | <4 | 5 | 7 | <4 | 4 | 4 | <4 | <4 | 5 | 155 | 170 | 187 | 7 | 7 | <4 |
| Mo | <1 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 416 | 419 | 468 | <10 | <10 | <10 |
| Cd | <4 | <4 | <4 | 4 | <4 | <4 | <4 | <4 | <4 | 4 | <4 | <4 | <4 | <4 | <4 |
| Sb | <6 | <6 | 6 | <6 | <6 | <6 | <6 | 7 | <6 | 12 | 9 | 7 | <6 | 14 | <6 |
| Hg | <2 | <2 | <2 | <2 | 3 | 4 | <2 | <2 | <2 | 17 | <2 | 16 | <2 | <2 | <2 |
| Sample | Ted4 | TW31 | TW39 | TW22 | Ted38 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyroclastic Deposit | Pyroclastic Deposit | Pyroclastic Deposit | Fluvio-Lacustrine Sediments | Fluvio-Lacustrine Sediments | |||||||||||
| 15 days | 30 days | 90 days | 15 days | 30 days | 90 days | 15 days | 30 days | 90 days | 15 days | 30 days | 90 days | 15 days | 30 days | 90 days | |
| pH | 8.35 | 8.55 | 8.30 | 8.21 | 8.00 | 8.18 | 7.70 | 7.76 | 7.68 | 8.15 | 7.86 | 8.30 | 8.00 | 8.10 | 7.81 |
| EC (µS/cm) | 588 | 483 | 700 | 302 | 323 | 306 | 312 | 308 | 278 | 1300 | 1200 | 1350 | 540 | 565 | 533 |
| F– (mg/L) | 6.42 | 6.79 | 7.79 | 2.94 | 3.22 | 3.53 | 1.77 | 1.98 | 2.41 | 7.80 | 7.76 | 8.24 | 5.97 | 6.68 | 8.26 |
| Cl– | 32.3 | 33.5 | 36.2 | 7.33 | 17.1 | 8.10 | 17.4 | 17.2 | 18.5 | 7.97 | 80.3 | 78.5 | 23.8 | 24.7 | 23.6 |
| NO3– | 34.2 | 31.1 | 37.6 | 0.26 | n.d. | 0.2 | 4.60 | 5.76 | 20.4 | n.d. | n.d. | 1.21 | 15.1 | 18.0 | 16.7 |
| SO42– | 34.0 | 35.1 | 38.0 | 2.46 | 1.96 | 2.30 | 6.06 | 6.24 | 6.30 | 290 | 310 | 300 | 14.1 | 14.8 | 13.7 |
| * HCO3– | 48 | 63 | 93 | 144 | 161 | 166 | 139 | 127 | 113 | 146 | 201 | 201 | 216 | 242 | 233 |
| Ca2+ | <0.3 | <0.3 | <0.3 | 1.19 | 4.51 | 4.12 | 11.9 | 10.6 | 11.7 | 9.10 | 11.3 | 12.5 | 14.3 | 14.1 | 15.9 |
| Mg2+ | <0.4 | <0.4 | <0.4 | <0.4 | 0.66 | <0.4 | 2.69 | 2.01 | 1.56 | 2.29 | 2.86 | 3.03 | 3.40 | 4.65 | 4.52 |
| Na+ | 70.4 | 59.2 | 86.3 | 56.7 | 64.5 | 62.5 | 45.1 | 43.8 | 44.6 | 221 | 246 | 238 | 77.3 | 85.6 | 81.4 |
| K+ | 17.9 | 13.0 | 21.9 | 8.68 | 9.72 | 9.11 | 13.5 | 11.7 | 12.1 | 35.8 | 34.5 | 38.9 | 28.8 | 31.2 | 30.3 |
| Li (µg/L) | 11 | 14 | 21 | 15 | 17 | 15 | 8 | 7 | 6 | 191 | 191 | 217 | 70 | 95 | 81 |
| Rb | 24 | 26 | 32 | 9 | 9 | 9 | 20 | 18 | 16 | 23 | 22 | 27 | 17 | 20 | 18 |
| Sr | 2 | 3 | 4 | 5 | 10 | 7 | 47 | 40 | 29 | 90 | 98 | 107 | 178 | 193 | 210 |
| Ba | <5 | <5 | <5 | 54 | <5 | 5 | <5 | <5 | <5 | 11 | 20 | 13 | 10 | 6 | 8 |
| As | <4 | <4 | 62 | <4 | <4 | <4 | 4 | 6 | 9 | 145 | 144 | 188 | 8 | 8 | 6 |
| Mo | <10 | <10 | 11 | <10 | <10 | <10 | <10 | <10 | <10 | 454 | 432 | 477 | <10 | 10 | <10 |
| Cd | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 |
| Sb | <6 | <6 | <6 | <6 | <6 | <6 | <6 | <6 | <6 | <6 | 10 | <6 | <6 | <6 | <6 |
| Hg | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchini, G.; Brombin, V.; Marchina, C.; Natali, C.; Godebo, T.R.; Rasini, A.; Salani, G.M. Origin of Fluoride and Arsenic in the Main Ethiopian Rift Waters. Minerals 2020, 10, 453. https://doi.org/10.3390/min10050453
Bianchini G, Brombin V, Marchina C, Natali C, Godebo TR, Rasini A, Salani GM. Origin of Fluoride and Arsenic in the Main Ethiopian Rift Waters. Minerals. 2020; 10(5):453. https://doi.org/10.3390/min10050453
Chicago/Turabian StyleBianchini, Gianluca, Valentina Brombin, Chiara Marchina, Claudio Natali, Tewodros Rango Godebo, Alessandro Rasini, and Gian Marco Salani. 2020. "Origin of Fluoride and Arsenic in the Main Ethiopian Rift Waters" Minerals 10, no. 5: 453. https://doi.org/10.3390/min10050453
APA StyleBianchini, G., Brombin, V., Marchina, C., Natali, C., Godebo, T. R., Rasini, A., & Salani, G. M. (2020). Origin of Fluoride and Arsenic in the Main Ethiopian Rift Waters. Minerals, 10(5), 453. https://doi.org/10.3390/min10050453









