Adsorption and Separation of Crystal Violet, Cerium(III) and Lead(II) by Means of a Multi-Step Strategy Based on K10-Montmorillonite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pollutant/Clay Composites: Equilibrium and Kinetic Experiments
3. Results and Discussion
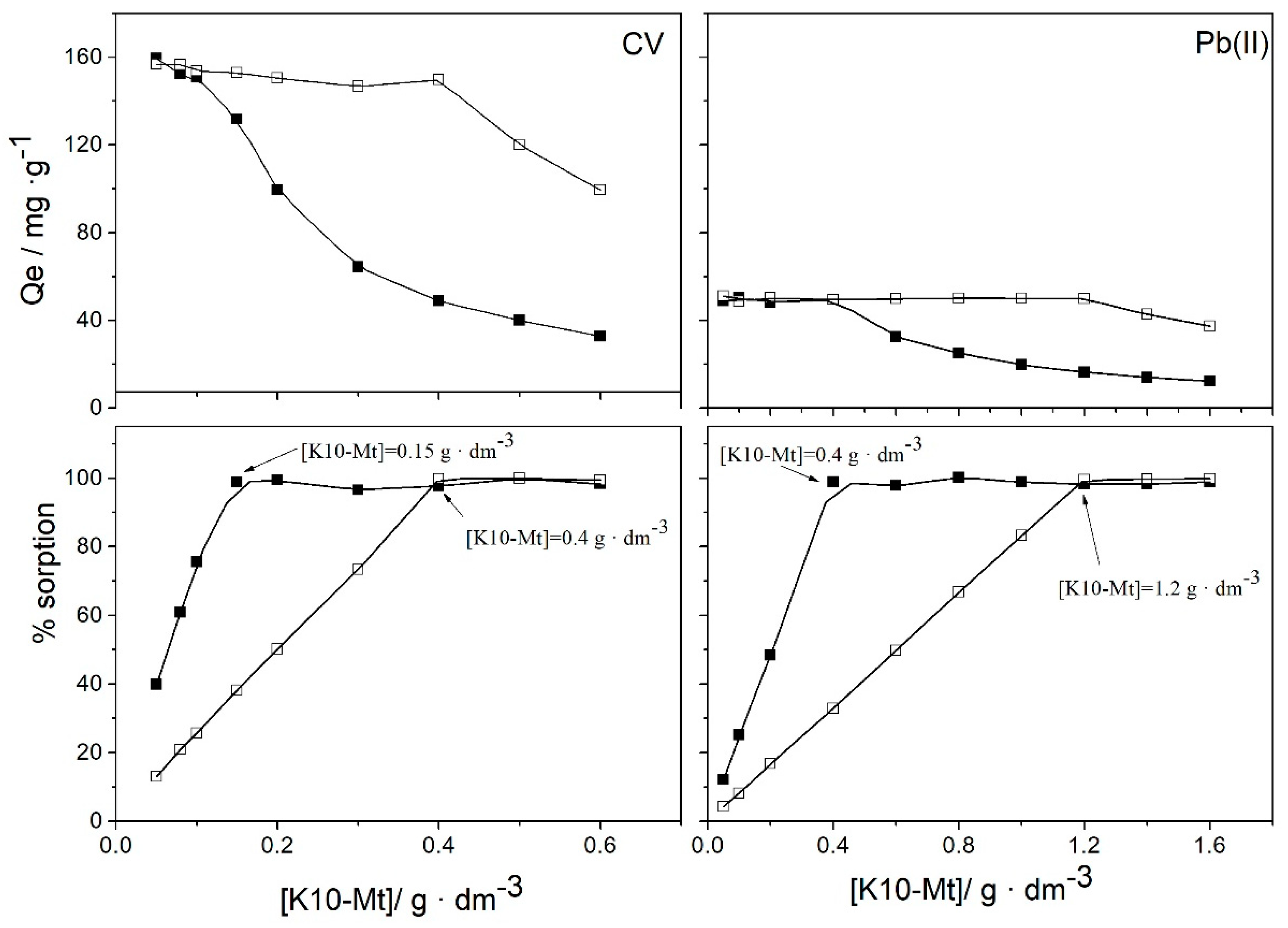
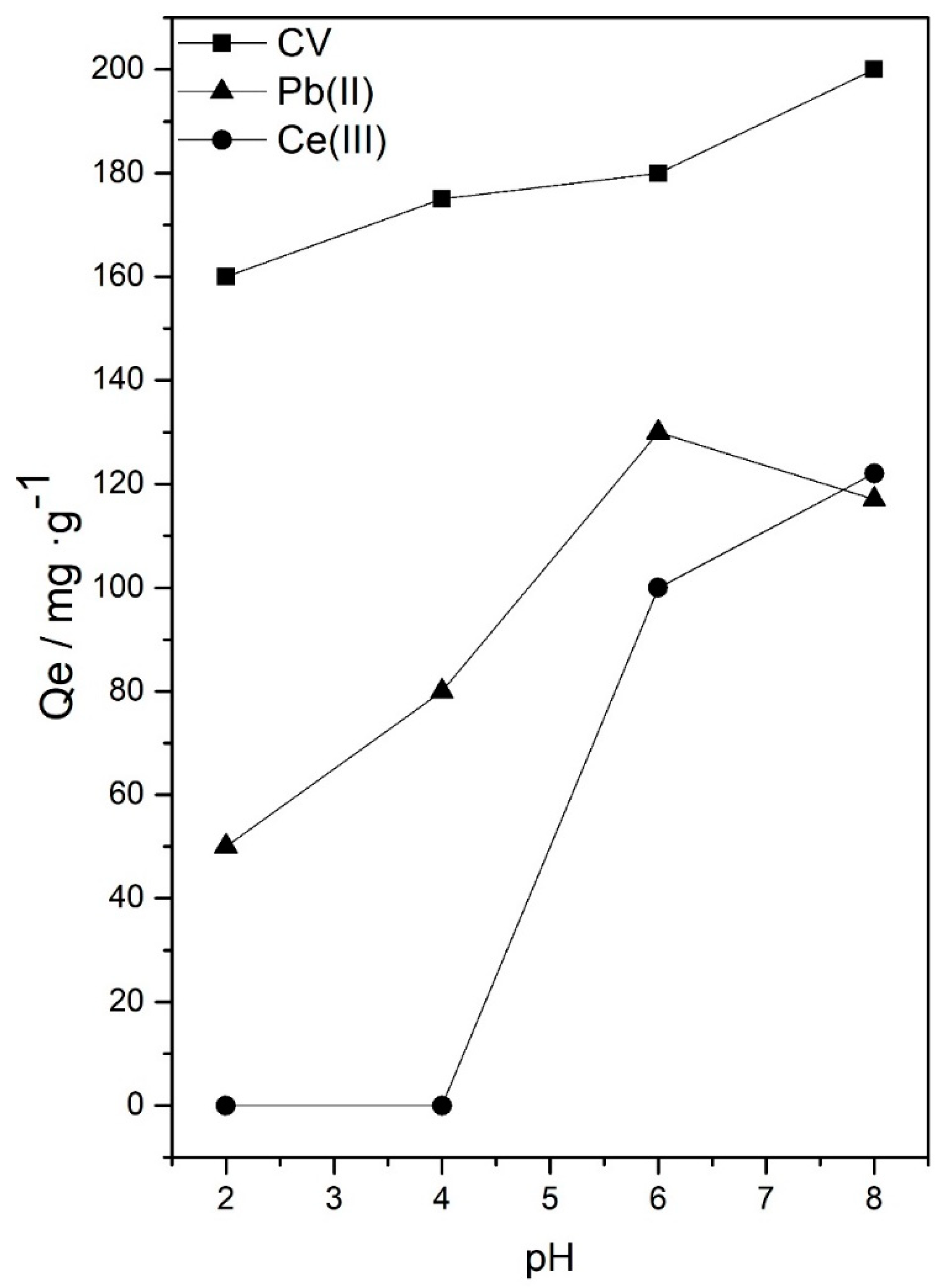
3.1. Effect of Experimental Parameters: pH Value, Initial Concentration of Adsorbate, Amount of Sorbent
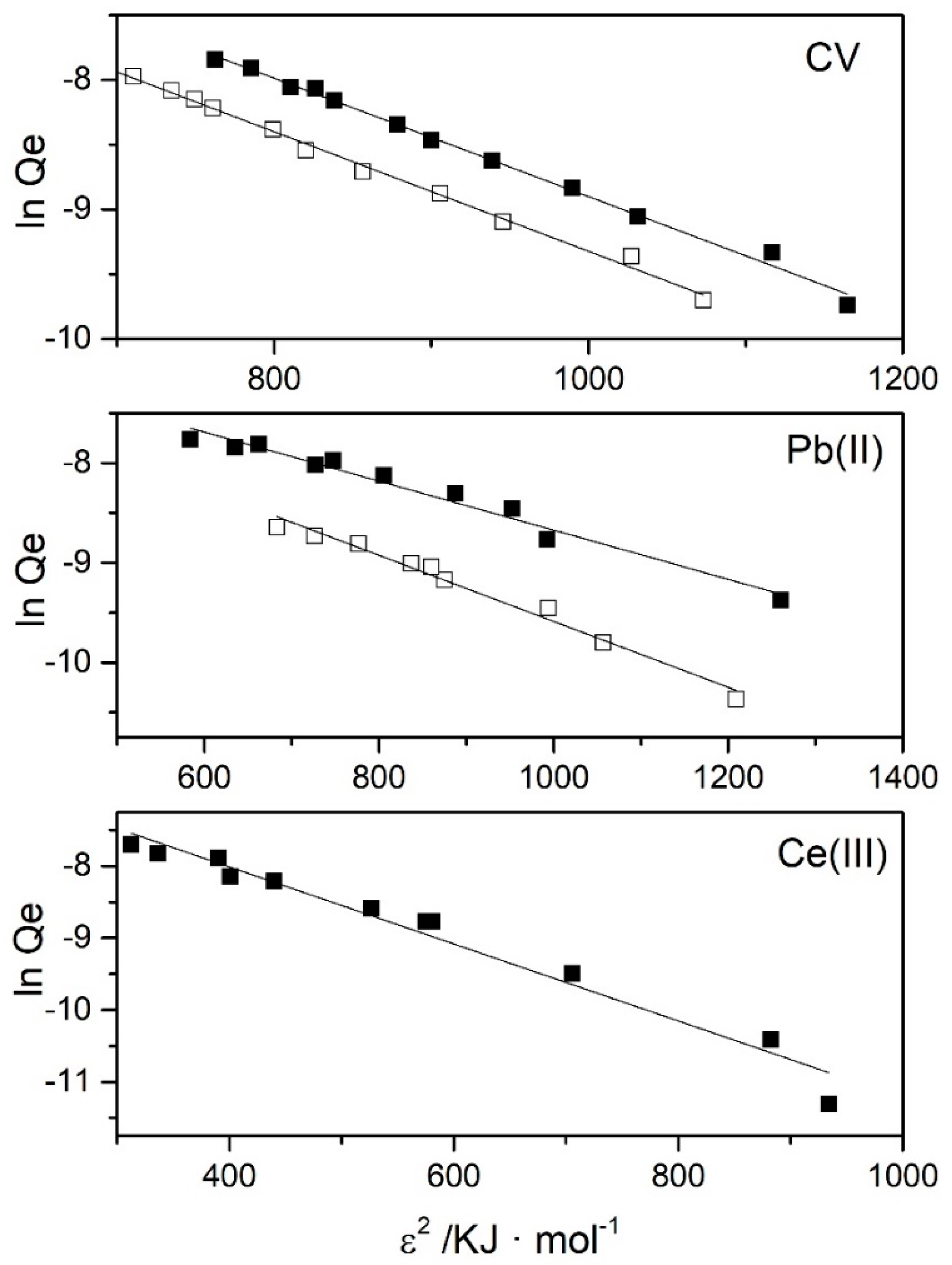
3.2. Adsorption Isotherms
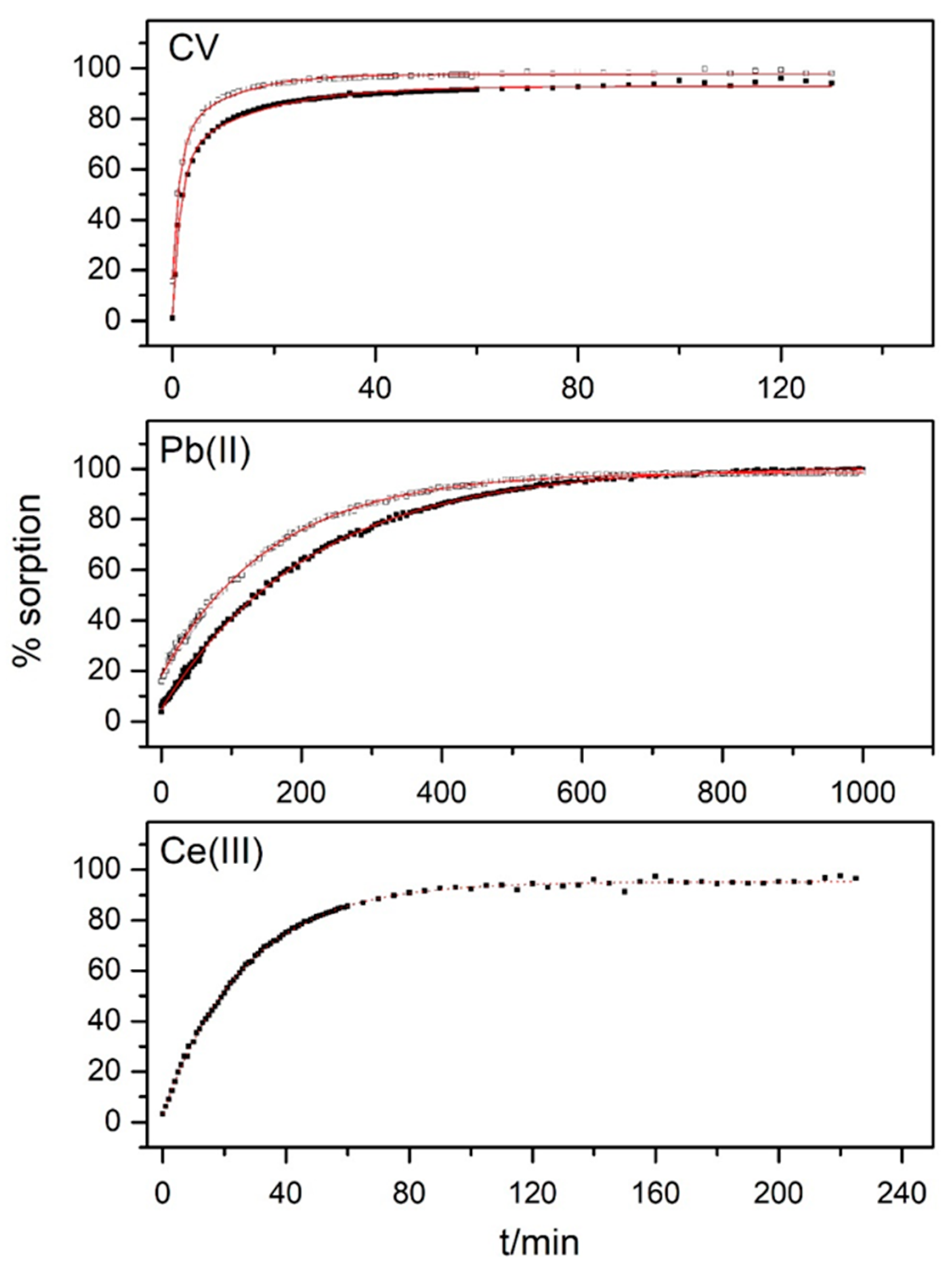
3.3. Kinetic Measurements
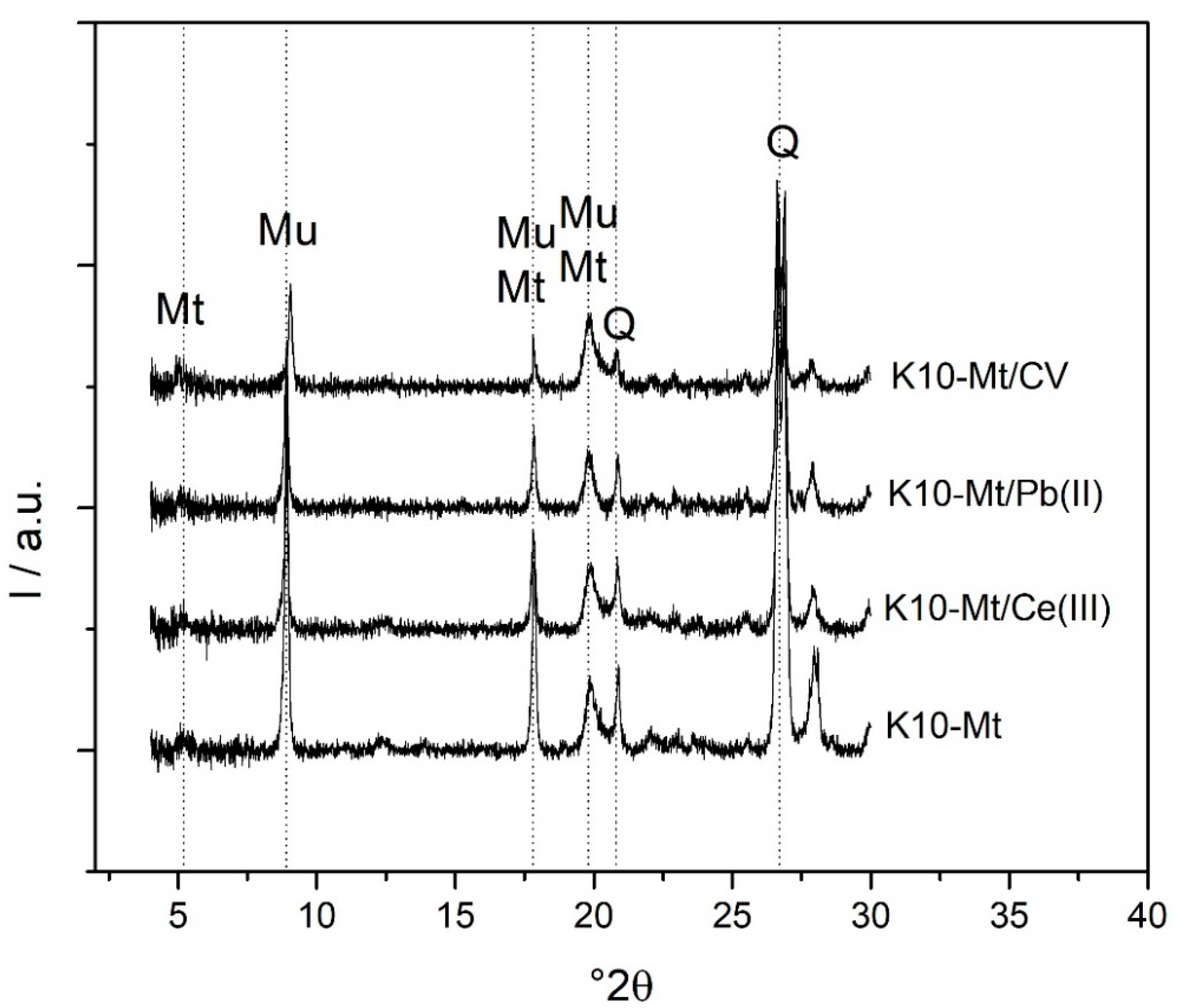
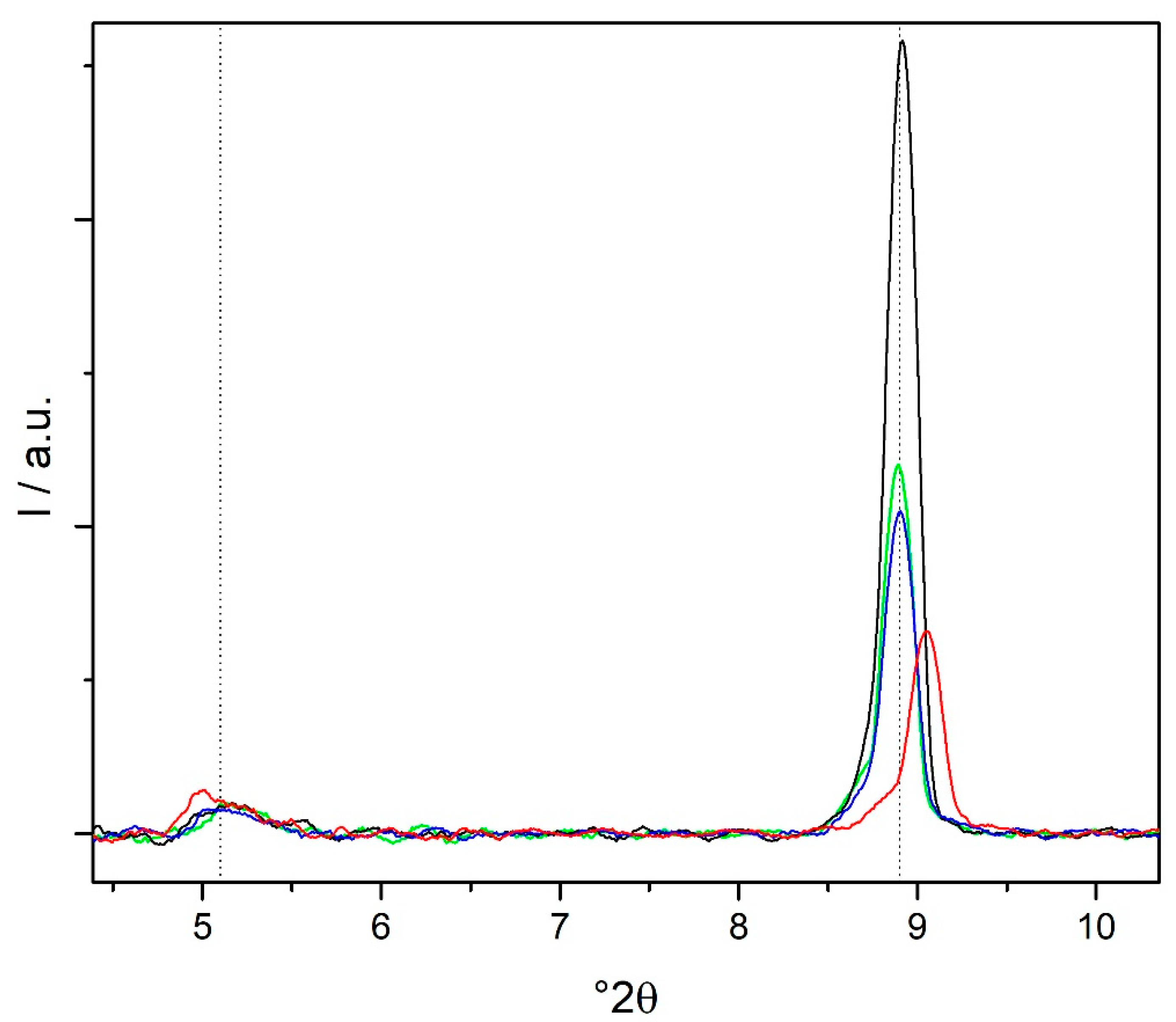
3.4. XRD Characterization
3.5. Removal and Separation of Pollutants
- First treatment of the mixture with K10-Mt at a strongly acidic pH over a short timescale (~60 min);
- Centrifugation and separation of the CV/clay composite;
- Second treatment of the supernatant with K10-Mt at a strongly acidic pH over a long timescale (>12 h);
- Centrifugation and separation of the Pb(II)/clay composite;
- Treatment of the remaining solution (containing Cerium ions) with K10-Mt at higher pH values (>6.0);
- Centrifugation and separation of the Ce(III)/clay composite.
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Bhattacharya, S.; Gupta, A.; Gupta, A.; Pandey, A. Introduction to Water Remediation: Importance and Methods. In Water Remediation; Springer Nature: Singapore, 2018; pp. 3–8. ISBN 978-981-10-7550-6. [Google Scholar]
- Camera-Roda, G.; Loddo, V.; Palmisano, L.; Parrino, F. Photocatalytic ozonation for a sustainable aquaculture: A long-term test in a seawater aquarium. Appl. Catal. B Environ. 2019, 253, 69–76. [Google Scholar] [CrossRef]
- Hao, X.; Wang, X.; Liu, R.; Li, S.; van Loosdrecht, M.C.M.; Jiang, H. Environmental impacts of resource recovery from wastewater treatment plants. Water Res. 2019, 160, 268–277. [Google Scholar] [CrossRef]
- Parrino, F.; Camera-Roda, G.; Loddo, V. Palmisano Three-Dimensional Calibration for Routine Analyses of Bromide and Nitrate Ions as Indicators of Groundwater Quality in Coastal Territories. Int. J. Environ. Res. Public. Health 2019, 16, 1419. [Google Scholar] [CrossRef] [Green Version]
- Toledano Garcia, D.; Ozer, L.Y.; Parrino, F.; Ahmed, M.; Brudecki, G.P.; Hasan, S.W.; Palmisano, G. Photocatalytic ozonation under visible light for the remediation of water effluents and its integration with an electro-membrane bioreactor. Chemosphere 2018, 209, 534–541. [Google Scholar] [CrossRef]
- Calabrese, I.; Cavallaro, G.; Lazzara, G.; Merli, M.; Sciascia, L.; Turco Liveri, M.L. Preparation and characterization of bio-organoclays using nonionic surfactant. Adsorption 2016, 22, 105–116. [Google Scholar] [CrossRef]
- Cataldo, S.; Cavallaro, G.; Gianguzza, A.; Lazzara, G.; Pettignano, A.; Piazzese, D.; Villaescusa, I. Kinetic and equilibrium study for cadmium and copper removal from aqueous solutions by sorption onto mixed alginate/pectin gel beads. J. Environ. Chem. Eng. 2013, 1, 1252–1260. [Google Scholar] [CrossRef]
- Jaspal, D.; Malviya, A. Composites for wastewater purification: A review. Chemosphere 2020, 246, 125788. [Google Scholar] [CrossRef]
- Sadraei, R.; Paganini, M.C.; Calza, P.; Magnacca, G. An Easy Synthesis for Preparing Bio-Based Hybrid Adsorbent Useful for Fast Adsorption of Polar Pollutants. Nanomaterials 2019, 9, 731. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Datta, S.; Dhanjal, D.S.; Sharma, K.; Samuel, J.; Singh, J. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total Environ. 2020, 709, 135895. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Wang, C.; Liang, Z.; Cui, F.; Zhao, Z.; Yang, C. Cr(VI) removal by micron-scale iron-carbon composite induced by ball milling: The role of activated carbon. Chem. Eng. J. 2020, 389, 122633. [Google Scholar] [CrossRef]
- Zhao, S.; Bai, Z.; Wang, B.; Tian, T.; Hu, Z. Innovative benign-to-design functionalized adsorbents from biomass for rapid azo-dyes separation. Sep. Purif. Technol. 2020, 241, 116633. [Google Scholar] [CrossRef]
- Cuevas, J.; Dirocie, N.; Yunta, F.; García Delgado, C.; González Santamaría, E.D.; Ruiz, I.A.; Fernández, R.; Eymar, E. Evaluation of the Sorption Potential of Mineral Materials Using Tetracycline as a Model Pollutant. Minerals 2019, 9, 453. [Google Scholar] [CrossRef] [Green Version]
- Kozera-Sucharda, B.; Gworek, B.; Kondzielski, I. The Simultaneous Removal of Zinc and Cadmium from Multicomponent Aqueous Solutions by Their Sorption onto Selected Natural and Synthetic Zeolites. Minerals 2020, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, R.; Bhaduri, D.; Sarkar, B.; Rusmin, R.; Hou, D.; Khanam, R.; Sarkar, S.; Kumar Biswas, J.; Vithanage, M.; Bhatnagar, A.; et al. Clay–polymer nanocomposites: Progress and challenges for use in sustainable water treatment. J. Hazard. Mater. 2020, 383, 121125. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Biopolymer-Targeted Adsorption onto Halloysite Nanotubes in Aqueous Media. Langmuir 2017, 33, 3317–3323. [Google Scholar] [CrossRef]
- Cataldo, S.; Lazzara, G.; Massaro, M.; Muratore, N.; Pettignano, A.; Riela, S. Functionalized halloysite nanotubes for enhanced removal of lead(II) ions from aqueous solutions. Appl. Clay Sci. 2018, 156, 87–95. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Sanzillo, V. Modified Halloysite Nanotubes: Nanoarchitectures for Enhancing the Capture of Oils from Vapor and Liquid Phases. ACS Appl. Mater. Interfaces 2014, 6, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Lvov, Y.; Aerov, A.; Fakhrullin, R. Clay nanotube encapsulation for functional biocomposites. Adv. Colloid Interface Sci. 2013, 207. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Wan Salleh, W.N.; Ismail, A.F.; Yusof, N.; Mohd Yusop, M.Z.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef]
- Jawed, A.; Saxena, V.; Pandey, L.M. Engineered nanomaterials and their surface functionalization for the removal of heavy metals: A review. J. Water Process Eng. 2020, 33, 101009. [Google Scholar] [CrossRef]
- Louati, I.; Elloumi-Mseddi, J.; Cheikhrouhou, W.; Hadrich, B.; Nasri, M.; Aifa, S.; Woodward, S.; Mechichi, T. Simultaneous cleanup of Reactive Black 5 and cadmium by a desert soil bacterium. Ecotoxicol. Environ. Saf. 2020, 190, 110103. [Google Scholar] [CrossRef]
- Marian, L.; Rotaru, A.; Pălărie, I.; Moanţă, A.; Popescu, M.; Morîntale, E.; Bubulică, M.; Florian, G.; Harabor, A.; Rotaru, P. Tartrazine: Physical, thermal and biophysical properties of the most widely employed synthetic yellow food-colouring azo dye. J. Therm. Anal. Calorim. 2018, 134, 209–231. [Google Scholar] [CrossRef]
- Negrea, A.; Gabor, A.; Davidescu, C.M.; Ciopec, M.; Negrea, P.; Duteanu, N.; Barbulescu, A. Rare Earth Elements Removal from Water Using Natural Polymers. Sci. Rep. 2018, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Politi, D.; Sidiras, D. Wastewater Treatment for Dyes and Heavy Metals Using Modified Pine Sawdust as Adsorbent. Procedia Eng. 2012, 42, 1969–1982. [Google Scholar] [CrossRef] [Green Version]
- Rotaru, A.; Moanţă, A.; Constantinescu, C.-D.; Dumitru, M.; Manolea, H.; Andreea Carmen, A.; Dinescu, M. Thermokinetic study of CODA azoic liquid crystal and thin films deposition by matrix-assisted pulsed laser evaporation. J. Therm. Anal. Calorim. 2017, 128, 89–105. [Google Scholar] [CrossRef]
- Rotaru, A.; Brătulescu, G.; Rotaru, P. Thermal analysis of azoic dyes: Part I. Non-isothermal decomposition kinetics of [4-(4-chlorobenzyloxy)-3-methylphenyl](p-tolyl)diazene in dynamic air atmosphere. Thermochim. Acta 2009, 489, 63–69. [Google Scholar] [CrossRef]
- Rotaru, A.; Moanţă, A.; Rotaru, P.; Segal, E. Thermal decomposition kinetics of some aromatic azomonoethers: Part III. Non-isothermal study of 4-[(4-chlorobenzyl)oxy]-4′-chloroazobenzene in dynamic air atmosphere. J. Therm. Anal. Calorim. 2009, 95, 161–166. [Google Scholar] [CrossRef]
- Rotaru, A.; Constantinescu, C.-D.; Rotaru, P.; Moanţă, A.; Dumitru, M.; Socaciu, M.; Maria, D.; Segal, E. Thermal analysis and thin films deposition by matrix assisted pulsed laser evaporation of a 4CN type azomonoether. J. Therm. Anal. Calorim. 2008, 92, 279–284. [Google Scholar] [CrossRef]
- Rotaru, A.; Dumitru, M. Thermal behaviour of CODA azoic dye liquid crystal and nanostructuring by drop cast and spin coating techniques. J. Therm. Anal. Calorim. 2017, 127, 21–32. [Google Scholar] [CrossRef]
- Varrica, D.; Tamburo, E.; Milia, N.; Vallascas, E.; Cortimiglia, V.; De Giudici, G.; Dongarrà, G.; Sanna, E.; Monna, F.; Losno, R. Metals and metalloids in hair samples of children living near the abandoned mine sites of Sulcis-Inglesiente (Sardinia, Italy). Environ. Res. 2014, 134, 366–374. [Google Scholar] [CrossRef]
- Visa, M.; Bogatu, C.; Duta, A. Simultaneous adsorption of dyes and heavy metals from multicomponent solutions using fly ash. Appl. Surf. Sci. 2010, 256, 5486–5491. [Google Scholar] [CrossRef]
- Sarma, G.K.; SenGupta, S.; Bhattacharyya, K.G. Adsorption of Monoazo Dyes (Crocein Orange G and Procion Red MX5B) from Water Using Raw and Acid-Treated Montmorillonite K10: Insight into Kinetics, Isotherm, and Thermodynamic Parameters. Water. Air. Soil Pollut. 2018, 229, 312. [Google Scholar] [CrossRef]
- Sciascia, L.; Casella, S.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Princivalle, F.; Parisi, F. Olive mill wastewaters decontamination based on organo-nano-clay composites. Thermophys. Asp. Funct. Ceram. Surf. 2019, 45, 2751–2759. [Google Scholar] [CrossRef]
- Sharma, P.; Borah, D.J.; Das, P.; Das, M.R. Cationic and anionic dye removal from aqueous solution using montmorillonite clay: Evaluation of adsorption parameters and mechanism. Desalin. Water Treat. 2016, 57, 8372–8388. [Google Scholar] [CrossRef]
- Zhang, Q.; Jing, R.; Zhao, S.; Wu, M.; Liu, X.; Shao, Y.; Lv, F.; Liu, A.; Meng, Z. Adsorption of cationic and anionic dyes on montmorillonite in single and mixed wastewater. J. Porous Mater. 2019, 26, 1861–1867. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Chi, R.; Feng, J.; Ding, Y.; Liu, Q. Preparation of Modified Montmorillonite and Its Application to Rare Earth Adsorption. Minerals 2019, 9, 747. [Google Scholar] [CrossRef] [Green Version]
- Parisi, F.; Lazzara, G.; Merli, M.; Milioto, S.; Princivalle, F.; Sciascia, L. Simultaneous Removal and Recovery of Metal Ions and Dyes from Wastewater through Montmorillonite Clay Mineral. Nanomaterials 2019, 9, 1699. [Google Scholar] [CrossRef] [Green Version]
- Rojas, J.; Suarez, D.; Moreno, A.; Silva-Agredo, J.; Torres-Palma, R. Kinetics, Isotherms and Thermodynamic Modeling of Liquid Phase Adsorption of Crystal Violet Dye onto Shrimp-Waste in Its Raw, Pyrolyzed Material and Activated Charcoals. Appl. Sci. 2019, 9, 5337. [Google Scholar] [CrossRef] [Green Version]
- Varshini, J.S.; Das, D.; Das, N. Packed bed column studies on recovery of cerium(III) from electronic wastewater using biosorbents of animal and plant origin. Indian J. Chem. Technol. 2017, 24, 294–303. [Google Scholar]
- Dubey, S.S.; Rao, B.S. Removal of cerium ions from aqueous solution by hydrous ferric oxide – A radiotracer study. J. Hazard. Mater. 2011, 186, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Duraipandian, J.; Rengasamy, T.; Vadivelu, S. Experimental and Modeling Studies for the Removal of Crystal Violet Dye from Aqueous Solutions using Eco-friendly Gracilaria corticata Seaweed Activated Carbon/Zn/Alginate Polymeric Composite Beads. J. Polym. Environ. 2017, 25, 1062–1071. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super high removal capacities of heavy metals (Pb 2+ and Cu 2+) using CNT dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yang, S.; Yu, K.; Ju, Y.; Sun, C.; Wang, L. Microwave induced catalytic degradation of crystal violet in nano-nickel dioxide suspensions. J. Hazard. Mater. 2010, 173, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Karimi, H. Effect of pH and Initial pb(II) Concentration on The Lead Removal Efficiency from Industrial Wastewater Using Ca(OH)2. Int. J. Water Wastewater Treat. 2017, 3. [Google Scholar] [CrossRef]
- Varrica, D.; Dongarrà, G.; Alaimo, M.G.; Monna, F.; Losno, R.; Sanna, E.; De Giudici, G.; Tamburo, E. Lead isotopic fingerprint in human scalp hair: The case study of Iglesias mining district (Sardinia, Italy). Sci. Total Environ. 2018, 613–614, 456–461. [Google Scholar] [CrossRef]
- Ain, Q.U.; Zhang, H.; Yaseen, M.; Rasheed, U.; Liu, K.; Subhan, S.; Tong, Z. Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb(II), Cd(II), and crystal violet from aqueous solution. J. Clean. Prod. 2020, 247, 119088. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Filipi, R.; Nesmerak, K.; Rucki, M.; Roth, Z.; Hanzlikova, I.; Tichý, M. Acute toxicity of rare earth elements and their compounds. Chem. Listy 2007, 101, 793–798. [Google Scholar]
- Masoumi, A.; Hemmati, K.; Ghaemy, M. Low-cost nanoparticles sorbent from modified rice husk and a copolymer for efficient removal of Pb(II) and crystal violet from water. Chemosphere 2016, 146, 253–262. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, Y.; Huang, L.; Li, S.; Hu, L.; Xiong, D.; Zhong, H.; He, Z. A novel composite of SiO2 decorated with nano ferrous oxalate (SDNF) for efficient and highly selective removal of Pb2+ from aqueous solutions. J. Hazard. Mater. 2020, 391, 122193. [Google Scholar] [CrossRef]
- Moja, T.N.; Bunekar, N.; Mishra, S.B.; Tsai, T.-Y.; Hwang, S.S.; Mishra, A.K. Melt processing of polypropylene-grafted-maleic anhydride/Chitosan polymer blend functionalized with montmorillonite for the removal of lead ions from aqueous solutions. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Gupt, C.B.; Bordoloi, S.; Sekharan, S.; Sarmah, A.K. Adsorption characteristics of Barmer bentonite for hazardous waste containment application. J. Hazard. Mater. 2020, 396, 122594. [Google Scholar] [CrossRef] [PubMed]
- Doi, A.; Khosravi, M.; Ejtemaei, M.; Nguyen, T.A.H.; Nguyen, A.V. Specificity and affinity of multivalent ions adsorption to kaolinite surface. Appl. Clay Sci. 2020, 190, 105557. [Google Scholar] [CrossRef]
- Imam, D.M.; Rizk, S.E.; Attallah, M.F. Adsorption studies of Ce(III) and Zr(IV) from aqueous solution using clay and humic acid—Clay materials. Part. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Chenab, K.K.; Sohrabi, B.; Jafari, A.; Ramakrishna, S. Water treatment: Functional nanomaterials and applications from adsorption to photodegradation. Mater. Today Chem. 2020, 16, 100262. [Google Scholar] [CrossRef]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Claver, C.; Fernández, E.; Margalef-Català, R.; Medina, F.; Salagre, P.; Sueiras, J.E. Studies on the Characterization of Several Iridium– and Rhodium–clay Catalysts and Their Activity in Imine Hydrogenation. J. Catal. 2001, 201, 70–79. [Google Scholar] [CrossRef]
- Dawodu, F.; Akpomie, K.; Abuh, M. Equilibrium Isotherm Studies on the Batch Sorption of Copper (II) ions from Aqueous Solution unto Nsu Clay. Int. J. Sci. Eng. Res. 2012, 3, 1–7. [Google Scholar]
- Brigatti, M.F.; Corradini, F.; Franchini, G.C.; Mazzoni, S.; Medici, L.; Poppi, L. Interaction between montmorillonite and pollutants from industrial waste-waters: Exchange of Zn2+ and Pb2+ from aqueous solutions. Appl. Clay Sci. 1995, 9, 383–395. [Google Scholar] [CrossRef]
- Oueslati, W.; Mefath, M.; ben rhaiem, H.; Ben Haj Amara, A. Cation Exchange Selectivity versus concentration of competing heavy metal cations (Pb2+,Zn2+): Case of Na-montmorillonite. Phys. Procedia 2009, 2, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Marsh, A.; Heath, A.; Patureau, P.; Evernden, M.; Walker, P. Alkali activation behaviour of un-calcined montmorillonite and illite clay minerals. Appl. Clay Sci. 2018, 166, 250–261. [Google Scholar] [CrossRef]
- Harun, F.; Almadani, E.; Radzi, S. Metal cation exchanged montmorillonite K10 (MMT K10): Surface properties and catalytic activity. J. Sci. Res. Dev. 2016, 3, 90–96. [Google Scholar]
- Zhang, S.Q.; Hou, W.G. Adsorption behavior of Pb(II) on montmorillonite. Colloids Surf. Physicochem. Eng. Asp. 2008, 320, 92–97. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, B.; Luo, J.; Ren, Y.; Zhang, Z. Efficient conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by the chromium-exchanged montmorillonite K-10 clay. Biomass Bioenergy 2014, 60, 171–177. [Google Scholar] [CrossRef]
- Gupta, S.; Bhattacharyya, K. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef]




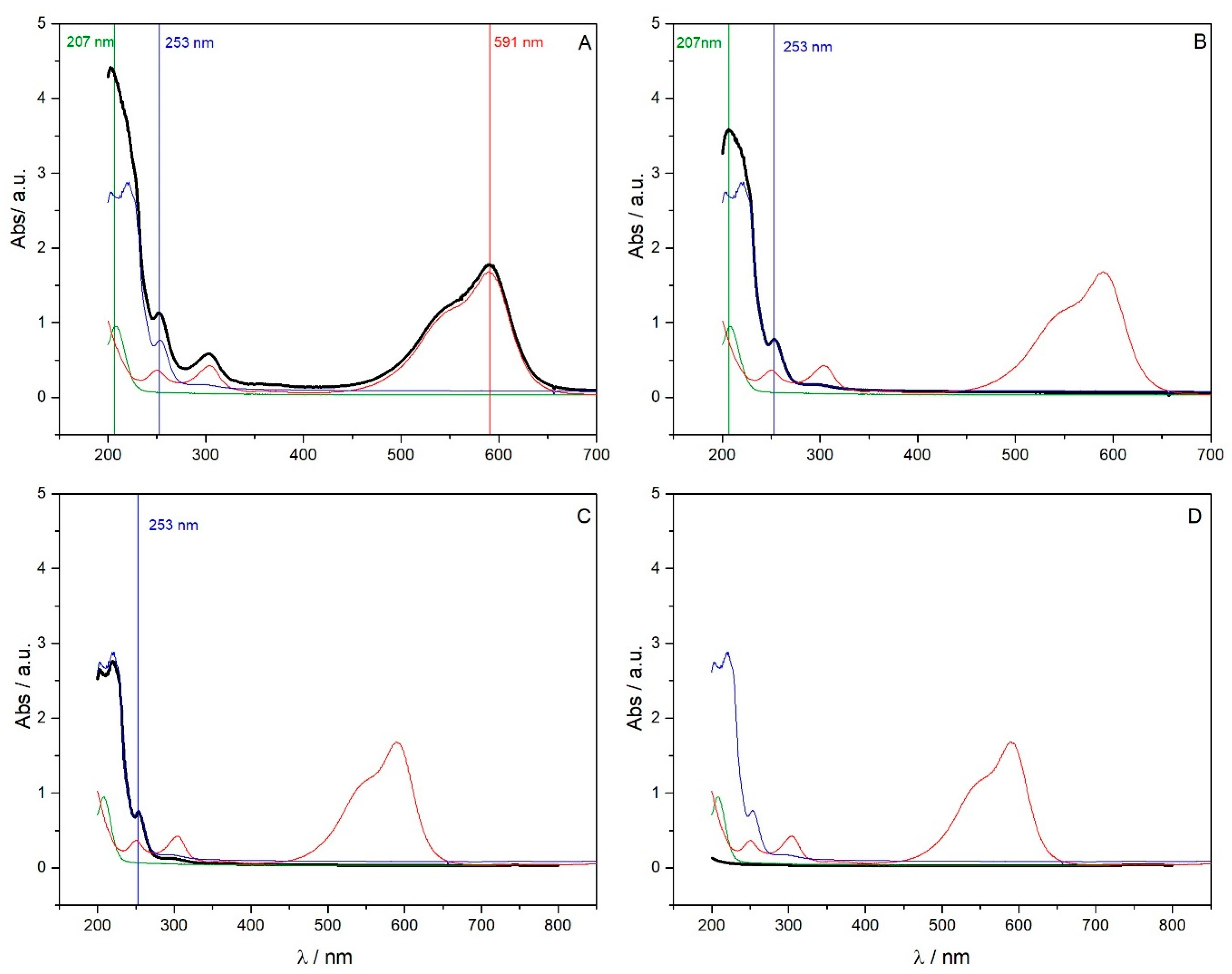
| Molar Adsorption Coefficient | pH 2.0 | pH 6.0 | ||
|---|---|---|---|---|
| ε/M−1·cm−1 | R2 | ε/M−1·cm−1 | R2 | |
| CV (λmax = 591 nm) | 89,000 ± 3000 | 0.99627 | 75,000 ± 2000 | 0.99587 |
| Pb(II) (λmax = 207 nm) | 37,000 ± 4000 | 0.99464 | 27,200 ± 800 | 0.99954 |
| Ce(III) (λmax = 253 nm) | 750 ± 30 | 0.99852 | 850 ± 40 | 0.99753 |
| Contaminants | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Sorption Parameters | pH 2.0 | pH 6.0 | Sorption Parameters | pH 2.0 | pH 6.0 | |
| CV | KL dm3·mg−1 | 0.105 ± 0.01 | 0.18 ± 0.02 | KF (mg g−1) (dm3 mg−1)1/n | 33.3 ± 0.8 | 50 ± 1 |
| qm mg·g−1 | 290 ± 20 | 300 ± 18 | n | 1.51 ± 0.03 | 1.51 ± 0.01 | |
| Pb(II) | KL dm3·mg−1 | 0.8 ± 0.1 | 0.8 ± 0,1 | KF (mg·g−1) (dm3·mg−1)1/n | 26 ± 2 | 67 ± 6 |
| qm mg·g−1 | 59 ± 3 | 141 ± 6 | n | 2.88 ± 0.05 | 3.8 ± 0.6 | |
| Ce(III) | KL dm3·mg−1 | 0.06 ± 0. 02 | KF (mg·g−1) (dm3·mg−1)1/n | 23± 6 | ||
| qm mg·g−1 | 110 ± 8 | n | 3.4 ± 0.6 | |||
| Xm/mol·g−1 | k/mol2·kJ−2 | E/kJ·mol−1 | R2 | ||
|---|---|---|---|---|---|
| Pb | pH = 2.0 | (1.88 ± 0.04)·10−3 | (3.29 ± 0.2)·10−3 | 12.3 ± 0.7 | 0.971 |
| pH = 6.0 | (2.00 ± 0.04)·10−3 | (2.5 ± 0.2)·10−3 | 14 ± 1 | 0.968 | |
| CV | pH = 2.0 | (1.08 ± 0.02)·10−2 | (4.6 ± 0.1)·10−3 | 10.4 ± 0.1 | 0.996 |
| pH = 6.0 | (1.31 ± 0.03)·10−2 | (4.6 ± 0.1)·10−3 | 10.4 ± 0.2 | 0.994 | |
| Ce(III) | pH = 6.0 | (2.82 ± 0.09)·10−3 | (5.5 ± 0.4)·10−3 | 9.5 ± 0.7 | 0.953 |
| pH 2.0 | pH 6.0 | ||
|---|---|---|---|
| CV | k1/min−1 | 0.74 ± 0.03 | 0.62 ± 0.03 |
| k2/min−1 | 0.095 ± 0.005 | 0.063 ± 0.004 | |
| Pb(II) | k/min−1 | 0.00632 ± 0.00003 | 0.00469 ± 0.00002 |
| Ce(III) | k/min−1 | 0.0376 ± 0.0003 | |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, F. Adsorption and Separation of Crystal Violet, Cerium(III) and Lead(II) by Means of a Multi-Step Strategy Based on K10-Montmorillonite. Minerals 2020, 10, 466. https://doi.org/10.3390/min10050466
Parisi F. Adsorption and Separation of Crystal Violet, Cerium(III) and Lead(II) by Means of a Multi-Step Strategy Based on K10-Montmorillonite. Minerals. 2020; 10(5):466. https://doi.org/10.3390/min10050466
Chicago/Turabian StyleParisi, Filippo. 2020. "Adsorption and Separation of Crystal Violet, Cerium(III) and Lead(II) by Means of a Multi-Step Strategy Based on K10-Montmorillonite" Minerals 10, no. 5: 466. https://doi.org/10.3390/min10050466
APA StyleParisi, F. (2020). Adsorption and Separation of Crystal Violet, Cerium(III) and Lead(II) by Means of a Multi-Step Strategy Based on K10-Montmorillonite. Minerals, 10(5), 466. https://doi.org/10.3390/min10050466





