Phase Transformation and Dissolution Behavior of Pyrite in the Roasting-Sulfuric Acid Leaching Process of Vanadium-Bearing Stone Coal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Roasting Treatment
2.3. Acid Leaching Experiments
2.4. X-Ray Diffraction (XRD) Analysis
2.5. Microscopic Morphology Analysis
3. Results
3.1. The Correlation Between the Dissolution of V and Fe in the Blank Roasting-Sulfuric Acid Leaching Process
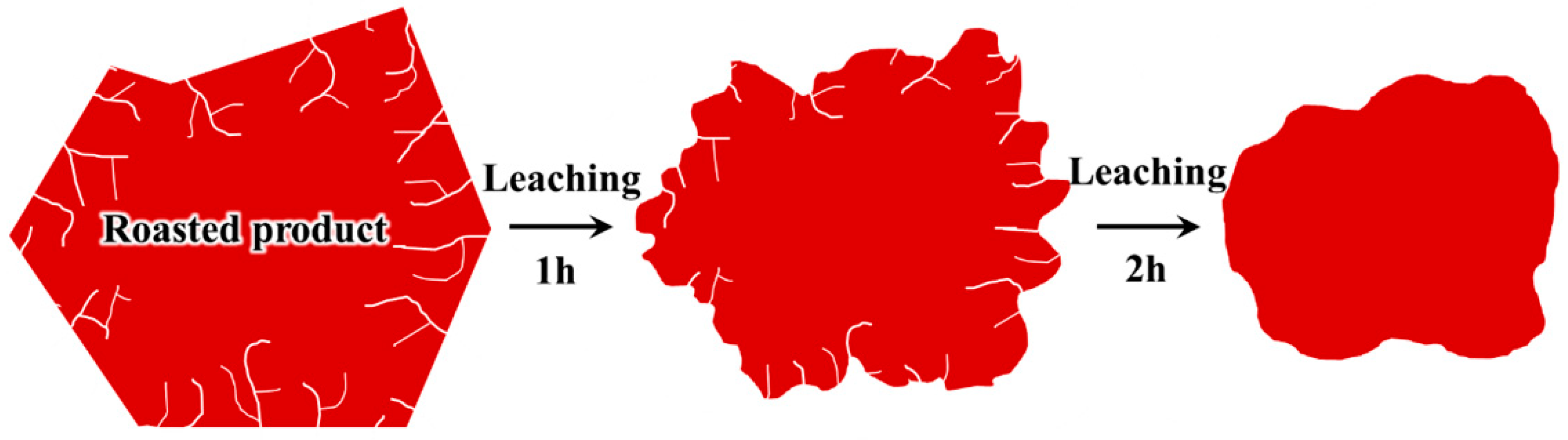
3.2. The Dissolution Behavior of Pyrite and Its Roasted Product
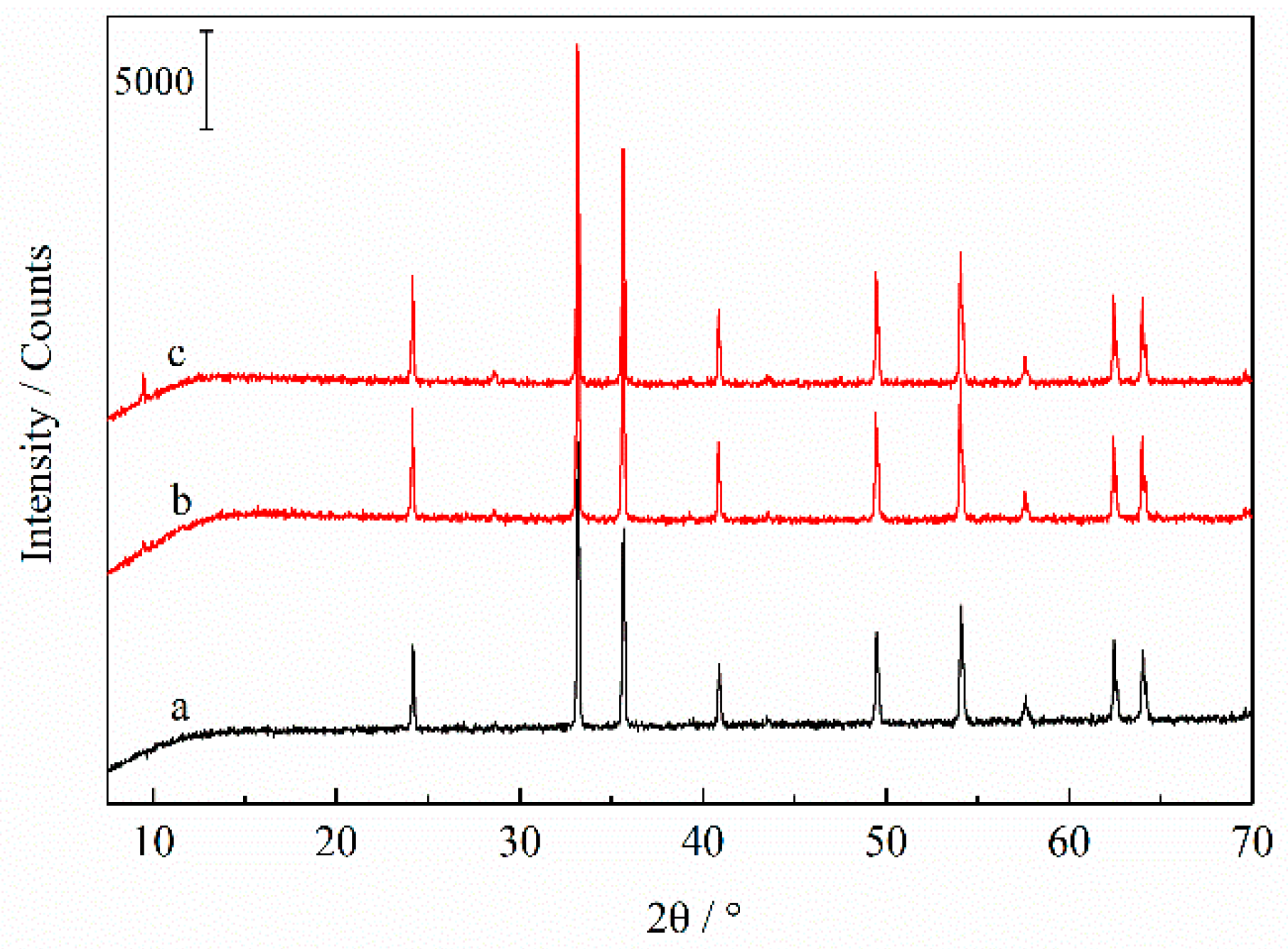
3.3. The Phase and Structure Transformations of Pyrite after Roasting and Acid Leaching Treatment
3.4. Implications for the Extraction of Vanadium from Stone Coal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Bao, S.; Liu, T.; Chen, T.; Huang, J. The technology of extracting vanadium from stone coal in china: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Zhang, Q. Recovery of vanadium and carbon from low-grade stone coal by flotation. Trans. Nonferrous Met. Soc. China 2015, 25, 3767–3773. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, G.; Feng, Q.; Yiping, L.U.; Leming, O.U.; Huang, S. Acid leaching of vanadium from roasted residue of stone coal. Trans. Nonferrous Met. Soc. China 2010, 20, S107–S111. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Luo, W.; Ou, L. Study on leaching vanadium from roasted residue of stone coal. Min. Met. Explor. 2008, 25, 181–184. [Google Scholar] [CrossRef]
- Chen, B.; Bao, S.; Zhang, Y.; Li, S. A high-efficiency and sustainable leaching process of vanadium from shale in sulfuric acid systems enhanced by ultrasound. Sep. Purif. Technol. 2020, 240, 116624. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Deng, Z.; Li, M.; Li, C.; Fan, G. Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by d2ehpa/tbp. Hydrometallurgy 2011, 105, 359–363. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Fan, G.; Li, C.; Deng, Z.; Li, X. Extraction of vanadium from black shale using pressure acid leaching. Hydrometallurgy 2009, 98, 308–313. [Google Scholar] [CrossRef]
- Ye, P.; Wang, X.; Wang, M.; Fan, Y.; Xiang, X. Recovery of vanadium from stone coal acid leaching solution by coprecipitation, alkaline roasting and water leaching. Hydrometallurgy 2012, 117, 108–115. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, L.; Yi, H.; Zhang, Y.; Song, S.; Bao, S. Vanadium transitions during roasting-leaching process of vanadium extraction from stone coal. Minerals 2018, 8, 63. [Google Scholar] [CrossRef]
- Li, X.; Deng, Z.; Wei, C.; Li, C.; Li, M.; Fan, G.; Huang, H. Solvent extraction of vanadium from a stone coal acidic leach solution using d2ehpa/tbp: Continuous testing. Hydrometallurgy 2015, 154, 40–46. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. Solvent extraction of vanadium(v) from sulfate solutions using lix 63 and pc 88a. J. Ind. Eng. Chem. 2015, 31, 118–123. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Zheng, Q. Highly selective separation of vanadium over iron from stone coal by oxalic acid leaching. J. Ind. Eng. Chem. 2017, 45, 241–247. [Google Scholar] [CrossRef]
- Lopezarce, P.; Garciaguinea, J.; Garrido, F. Chemistry and phase evolution during roasting of toxic thallium-bearing pyrite. Chemosphere 2017, 181, 447–460. [Google Scholar] [CrossRef]
- Jorgensen, F.R.A.; Moyle, F.J. Phases formed during the thermal analysis of pyrite in air. J. Therm. Anal. Calorim. 1982, 25, 473–485. [Google Scholar] [CrossRef]
- Dunn, J.G.; De, G.C.; O’Connor, B.H. The effect of experimental variables on the mechanism of the oxidation of pyrite: Part 2. Oxidation of particles of size 90–125 μm. Thermochim. Acta 1989, 155, 135–149. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, P.; Cheng, H.; Liu, Q. Thermal phase transition of pyrite from coal. J. Therm. Anal. Calorim. 2018, 134, 2391–2396. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.; Xu, B.; Yang, Y.; Jiang, T. A thermodynamic analysis on the roasting of pyrite. Minerals 2019, 9, 220. [Google Scholar] [CrossRef]
- Hu, G.; Damjohansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Srinivasachar, S.; Helble, J.J.; Boni, A.A. Mineral behavior during coal combustion 1. Pyrite transformations. Prog. Energy Combust. Sci. 1990, 16, 281–292. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, L.; Liu, H.; Qian, P.; Ye, S.; Chen, Y. Acid leaching pretreatment on two-stage roasting pyrite cinder for gold extraction and co-precipitation of arsenic with iron. Hydrometallurgy 2018, 179, 192–197. [Google Scholar] [CrossRef]
- Lee, S.O.; Tran, T.; Park, Y.Y.; Kim, S.J.; Kim, M.J. Study on the kinetics of iron oxide leaching by oxalic acid. Int. J. Miner. Process. 2006, 80, 144–152. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Liu, T.; Cai, Z.; Sun, K. Pre-concentration of vanadium from stone coal by gravity using fine mineral spiral. Minerals 2016, 6, 82. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Liu, T.; Huang, J.; Xue, N. Vanadium extraction from black shale: Enhanced leaching due to fluoride addition. Hydrometallurgy 2019, 187, 141–148. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, L.; Li, Q.; Wang, X.; Xiang, X. Leaching of vanadium from stone coal with sulfuric acid. Rare Met. 2009, 28, 1–4. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Ou, L.; Lu, Y. An environmentally-friendly technology of vanadium extraction from stone coal. Miner. Eng. 2007, 20, 1184–1186. [Google Scholar] [CrossRef]
- Dunn, J.G.; De, G.C.; O’Connor, B.H. The effect of experimental variables on the mechanism of the oxidation of pyrite: Part 1. Oxidation of particles less than 45 μm in size. Thermochim. Acta 1989, 145, 115–130. [Google Scholar] [CrossRef]

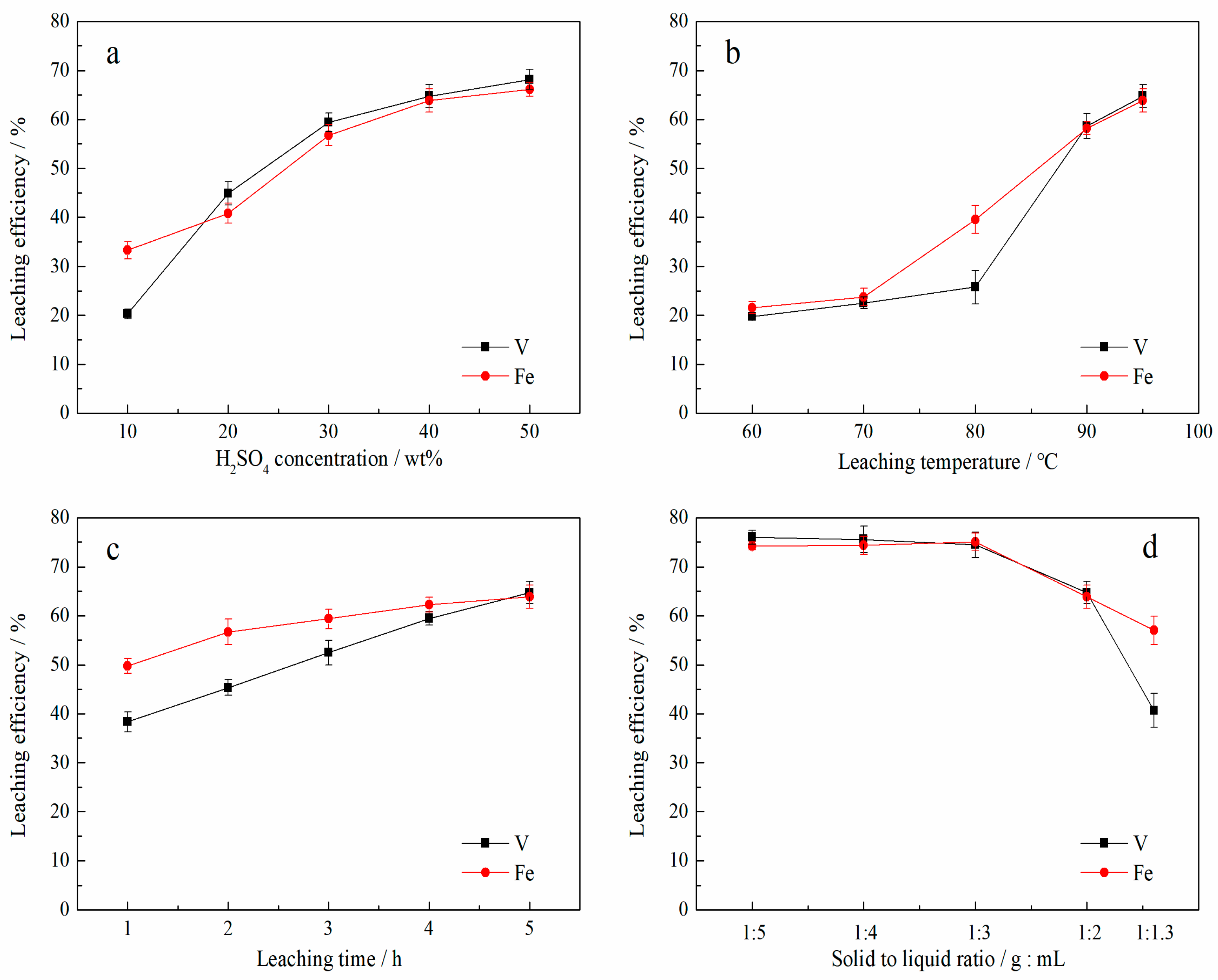
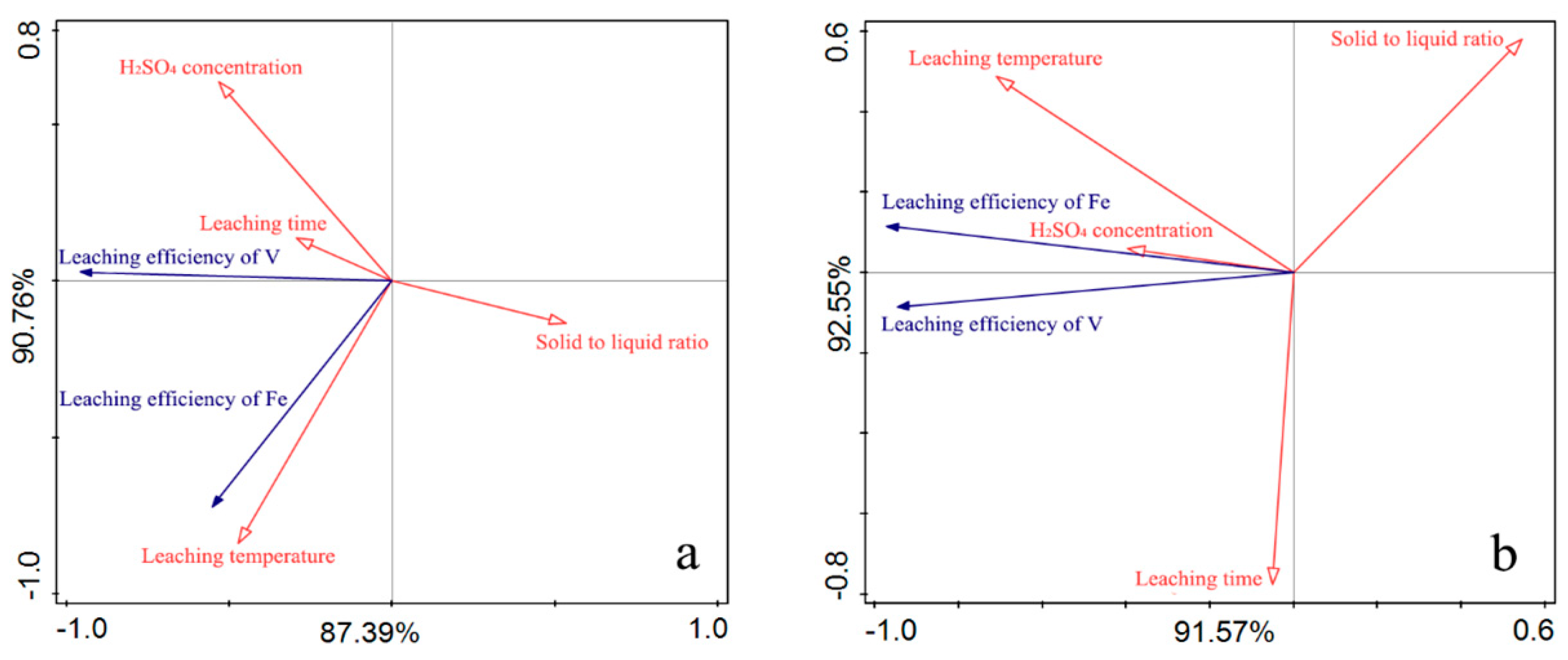


| Groups | H2SO4 Concentration (wt.%) | Leaching Temperature (°C) | Leaching Time (h) | Solid to Liquid Ratio (g:mL) |
|---|---|---|---|---|
| a | variable | 90 | 4 | 1:2 |
| b | 40 | variable | 4 | 1:2 |
| c | 40 | 90 | variable | 1:2 |
| d | 40 | 90 | 4 | variable |
| Groups | H2SO4 Concentration (wt.%) | Leaching Temperature (°C) | Leaching Time (h) | Solid to Liquid Ratio (g:mL) |
|---|---|---|---|---|
| a | variable | 90 | 4 | 1:40 |
| b | 40 | variable | 4 | 1:40 |
| c | 40 | 90 | variable | 1:40 |
| d | 40 | 90 | 4 | variable |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Y.; Liu, T.; Yuan, Y. Phase Transformation and Dissolution Behavior of Pyrite in the Roasting-Sulfuric Acid Leaching Process of Vanadium-Bearing Stone Coal. Minerals 2020, 10, 526. https://doi.org/10.3390/min10060526
Wang X, Zhang Y, Liu T, Yuan Y. Phase Transformation and Dissolution Behavior of Pyrite in the Roasting-Sulfuric Acid Leaching Process of Vanadium-Bearing Stone Coal. Minerals. 2020; 10(6):526. https://doi.org/10.3390/min10060526
Chicago/Turabian StyleWang, Xingjie, Yimin Zhang, Tao Liu, and Yizhong Yuan. 2020. "Phase Transformation and Dissolution Behavior of Pyrite in the Roasting-Sulfuric Acid Leaching Process of Vanadium-Bearing Stone Coal" Minerals 10, no. 6: 526. https://doi.org/10.3390/min10060526
APA StyleWang, X., Zhang, Y., Liu, T., & Yuan, Y. (2020). Phase Transformation and Dissolution Behavior of Pyrite in the Roasting-Sulfuric Acid Leaching Process of Vanadium-Bearing Stone Coal. Minerals, 10(6), 526. https://doi.org/10.3390/min10060526





