In Situ Geochemical Analysis of Organics in Growth Lines of Antarctic Scallop Shells: Implications for Sclerochronology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
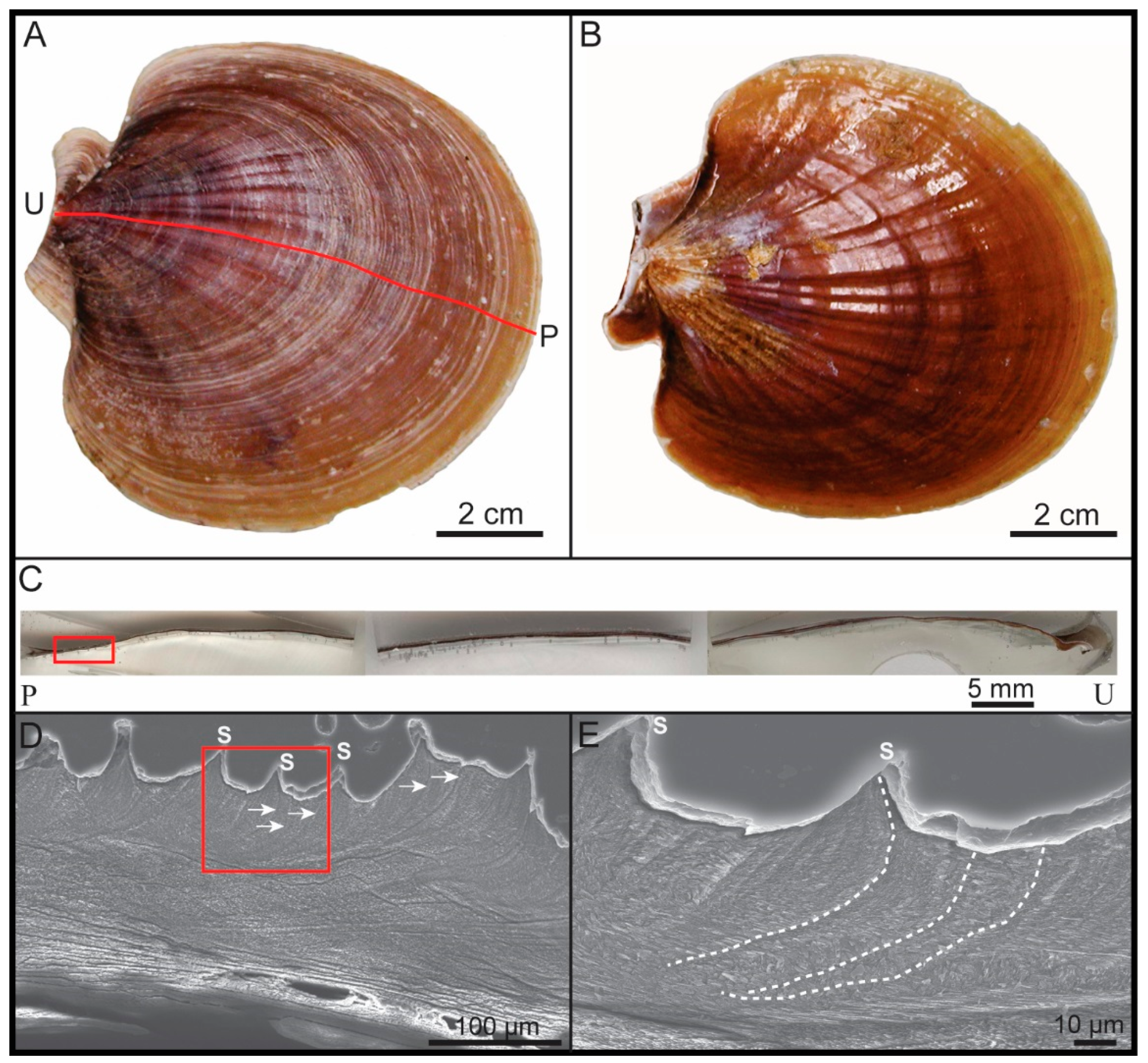
2.2.1. General Sample Preparation and Selection of Regions of Interest
2.2.2. Scanning Electron Microscopy (SEM) Imaging
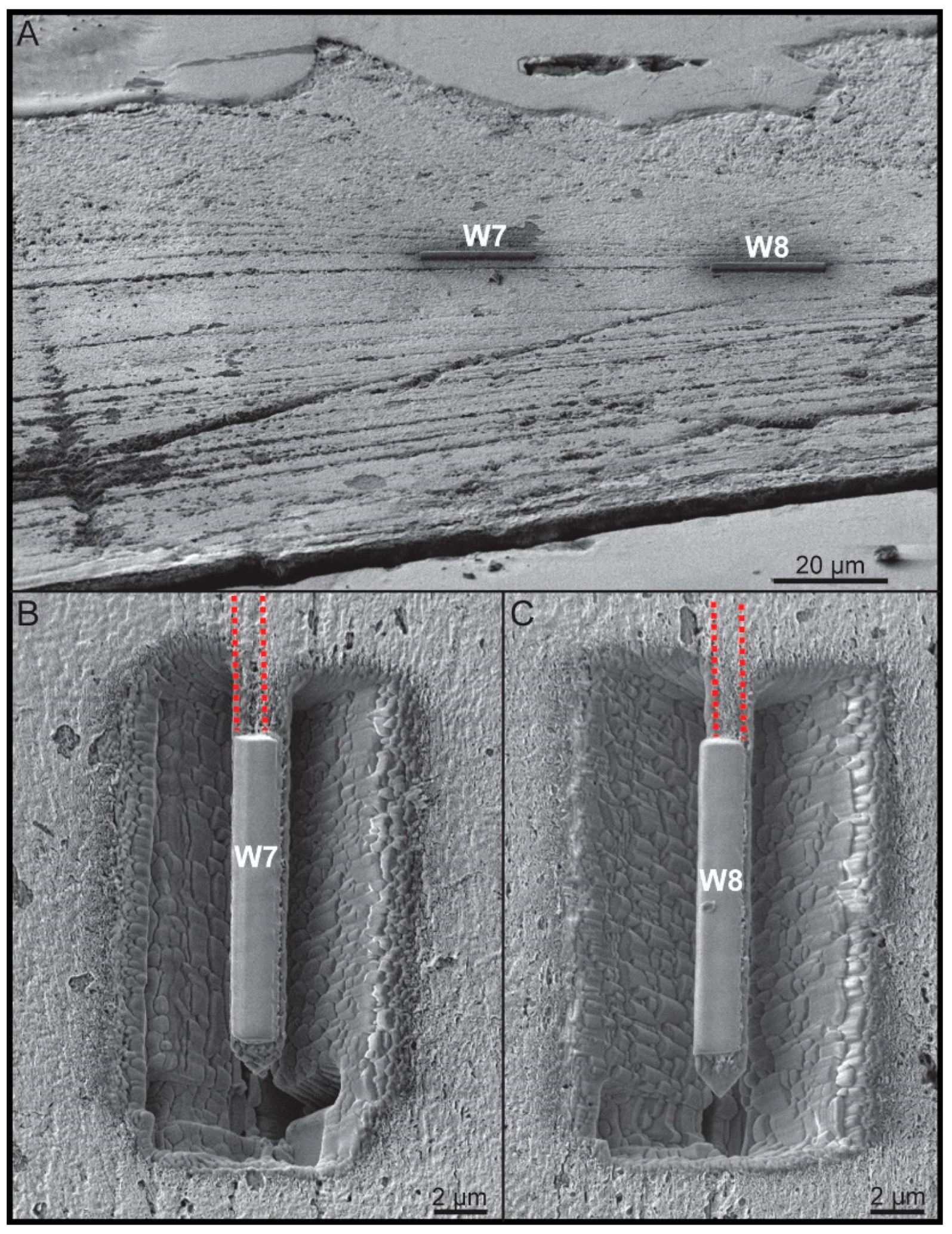
2.2.3. Focused Ion Beam (FIB) Work
2.2.4. Local Electrode Atom Probe (LEAP) Work and Data Analysis
3. Results
4. Discussion
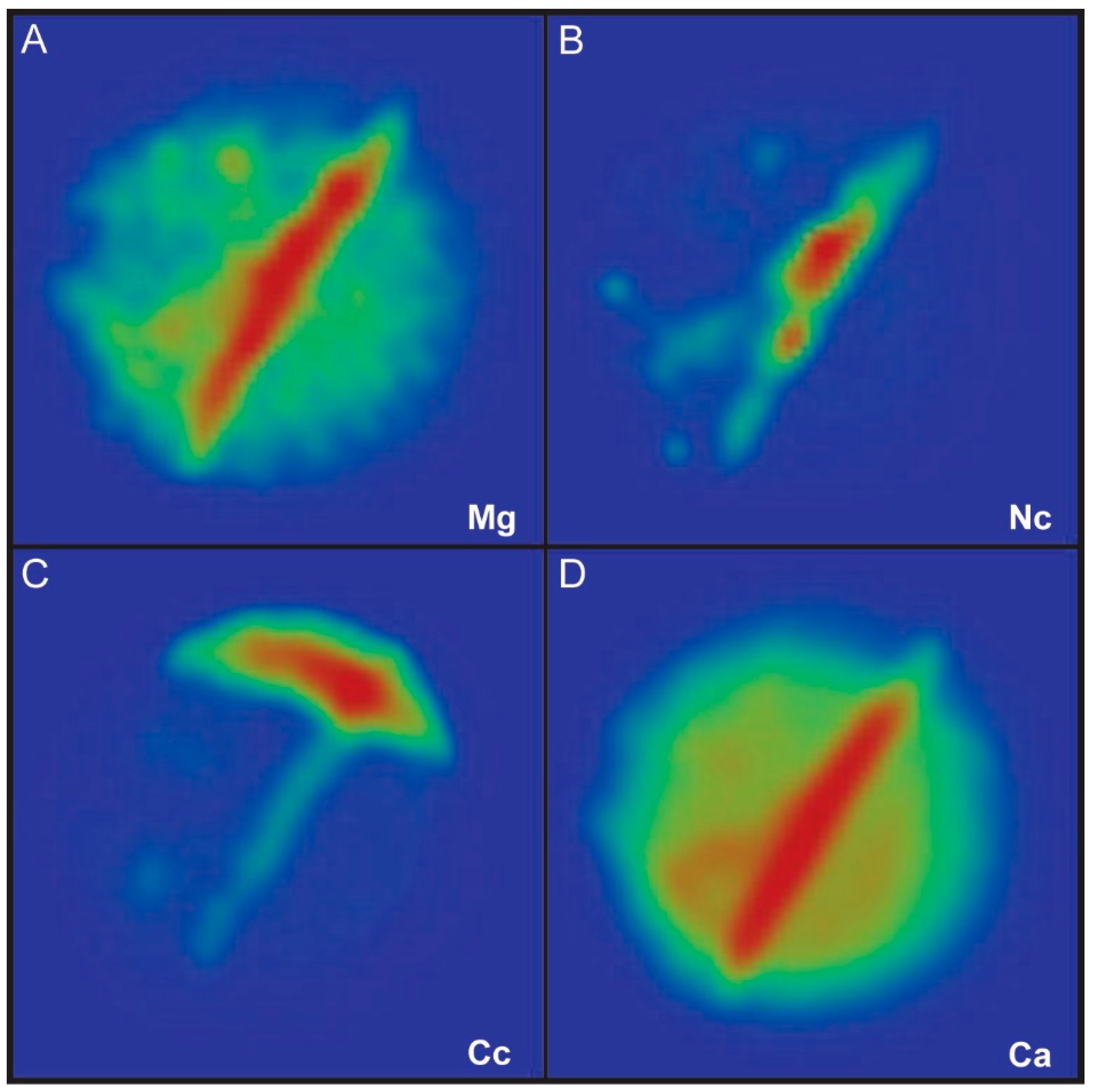
4.1. Chemical Composition of Growth Lines
4.2. APT Geochemical Data: Implications for Sclerochronology in the Context of Diagenesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cuif, J.-P.; Dauphin, Y.; Sorauf, J.E. Biominerals and Fossils through Time, 1st ed.; Cambridge University Press: Cambridge, UK, 2010; p. 503. [Google Scholar]
- Pérez-Huerta, A.; Coronado, I.; Hegna, T. Understanding biomineralization in the fossil record. Earth-Sci. Rev. 2018, 179, 95–122. [Google Scholar]
- Schöne, B.R.; Surge, D. Bivalve sclerochronology and geochemistry. Part N, Bivalvia, revised, Chapter 14. In Treatise Online; Seldon, P., Hardesty, J., Carter, J.G., Eds.; University of Kansas Press: Lawrence, KS, USA, 2012; Volume 46, pp. 1–24. [Google Scholar]
- Steinhardt, J.; Butler, P.G.; Carroll, M.L.; Hartley, J. The application of long-lived bivalve sclerochronology in environmental baseline monitoring. Front. Mar. Sci. 2016, 3, 176. [Google Scholar] [CrossRef] [Green Version]
- Schöne, B.R.; Zhang, Z.; Jacob, D.; Gillikin, D.P.; Tutken, T.; Garbe-Schönberg, D.; McConnaughey, T.; Soldati, A. Effect of organic matrices on the determination of the trace element chemistry (Mg, Sr, Mg/Ca, Sr/Ca) of aragonitic bivalve shells (Artica islandica) – Comparison of ICP-OES and LA-ICP-MS data. Geochem. J. 2010, 44, 23–37. [Google Scholar]
- Hancock, L.G.; Walker, S.E.; Pérez-Huerta, A.; Bowser, S.S. Population dynamics and parasite load of a foraminifer and its Antarctic scallop host with their carbonate biomass contributions. PLoS ONE 2015, 10, e0132534. [Google Scholar] [CrossRef] [PubMed]
- Lartaud, F.; Chauvaud, L.; Richard, J.; Toulot, A.; Bollinger, C.; Testud, L.; Paulet, Y.-M. Experimental growth pattern calibration of Antarctic scallop shells (Adamussium colbecki, Smith 1902) to provide a biogenic archive of high-resolution records of environmental and climate changes. J. Exper. Mar. Biol. Ecol. 2010, 393, 158–167. [Google Scholar] [CrossRef]
- Trevisiol, A.; Bergamasco, A.; Montagna, P.; Sprovieri, M.; Taviani, M. Antarctic seawater temperature evaluation based on stable isotope measurement on Adamussium colbecki shells: Kinetic vs. isotopic equilibrium. J. Mar. Syst. 2013, 126, 43–55. [Google Scholar] [CrossRef]
- Pérez-Huerta, A.; Laiginhas, F. Preliminary data on the nanoscale characterization of the inter-crystalline organic matrix of a calcium carbonate biomineral. Minerals 2018, 8, 223. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Huerta, A.; Laiginhas, F.; Reinhard, D.A.; Prosa, T.J.; Martens, R.L. Atom probe tomography (APT) of carbonate minerals. Micron 2016, 80, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Huerta, A.; Suzuki, M.; Cappelli, C.; Laiginhas, F.; Kintsu, H. Atom probe tomography (APT) characterization of organics occluded in single calcite crystals: Implications for biomineralization studies. J. Carbon Res. 2019, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Karney, G.B.; Butler, P.G.; Scourse, J.D.; Richardson, C.A.; Lau, K.H.; Czernuszka, J.T.; Grovernor, C.R.M. Identification of growth increments in the shell of the bivalve mollusk Artica islandica using backscattered electron imaging. J. Microsc. 2011, 241, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dauphin, Y.; Cuif, J.-P.; Doucet, J.; Salomé, M.; Susini, J.; Williams, C.T. In situ mapping of growth lines in the calcitic prismatic layers of mollusc shells using X-ray absorption near-edge structure (XANES) spectroscopy at the sulphur K-edge. Mar. Biol. 2003, 142, 299–304. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G. Molluscan shell proteins. C. R. Palevol 2004, 3, 469–492. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan shell proteins: Primary structure, origin, and evolution. Curr. Top. Develop. Biol. 2008, 80, 209–276. [Google Scholar]
- Arias, J.L.; Fernández, M.S. Polysaccharides and proteoglycans in calcium carbonate-based biomineralization. Chem. Rev. 2008, 108, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, N.; Takahashi, K.; Onoda, A.; Okamura, T.; Yamamoto, H. Inorganic-organic calcium carbonate composite of synthetic polymer ligands with an intramolecular NH…O hydrogen bond. Top. Curr. Chem. 2007, 271, 155–193. [Google Scholar]
- Wang, D.; Wallace, A.F.; DeYoreo, J.J.; Dove, P.M. Carboxylated molecules regulate Mg content of ACC during calcification. Proc. Natl. Acad. Sci. USA 2009, 106, 21511–21516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marxen, J.C.; Becker, W. Calcium binding constituents of the organic shell matrix from the freshwater snail Biomphalaria glabrata. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 127, 235–242. [Google Scholar] [CrossRef]
- Tao, J.; Zhou, D.; Zhang, Z.; Xu, X.; Tang, R. Magnseium-aspartate-based crystallization switch inspired from shell molt of crustacean. Proc. Natl. Acad. Sci. USA 2009, 106, 22096–22101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boullion, S.; Connolly, R.M.; Gillikin, D.P. Use of stable isotopes to understand food webs and ecosystems functioning in estuaries. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D.S., Eds.; Waltham Academic Press: Waltham, MA, USA, 2011; Volume 7, pp. 143–173. [Google Scholar]
- Black, H.D.; Andrus, C.T.F.; Lambert, J.W.; Rick, T.C.; Gillikin, D.P. δ15N values in Crassostrea virginica shells provides early direct evidence for nitrogen loading to Chesapeake Bay. Sci. Rep. 2017, 7, 44241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillikin, D.P.; Lorrain, A.; Jolivet, A.; Kelemen, Z.; Chavaud, L.; Boullion, S. High-resolution nitrogen stable isotope sclerochronology of bivalve shells carbonate-bound organics. Geochim. Cosmochim. Acta 2017, 1, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Albéric, M.; Stifler, C.A.; Zou, Z.; Sun, C.-Y.; Killian, C.E.; Valencia, S.; Mawass, M.-A.; Bertinetti, L.; Gilbert, P.; Politi, Y. Growth and regrowth of adult sea urchin spines involve hydrated and anhydrous amorphous calcium carbonate precursors. J. Struct. Biol. X 2019, 1, 100004. [Google Scholar]



| Specimen/Data Set | Scallop-W7 | Scallop-W8 | |||||
|---|---|---|---|---|---|---|---|
| Reference | 4173-M13 | 4174-M19 | 4175-M20 | 4176-M21 | 4149-M3 | 4150-M4 | 4198-M10 |
| Instrument Model | LEAP 5000 XS | LEAP 5000 XS | LEAP 5000 XS | LEAP 5000 XS | LEAP 5000 XS | LEAP 5000 XS | LEAP 5000 XS |
| Instrument settings | |||||||
| Laser wavelength | 355 | 355 | 355 | 355 | 355 | 355 | 355 |
| Laser pulse energy | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Pulse frequency (kHz) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Evaporation control | Detection rate | Detection rate | Detection rate | Detection rate | Detection rate | Detection rate | Detection rate |
| Target detection rate (ions/pulse)% | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Nominal fligth path (mm) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Set point temperature (K) | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Chamber pressure (torr) | 3.00E-11 | 4.00E-11 | 4.60E-11 | 5.60E-11 | 4.90E-11 | 4.10E-11 | 5.30E-11 |
| Data summary | |||||||
| Analysis software | IVAS 3.8.4 | IVAS 3.8.5 | IVAS 3.8.6 | IVAS 3.8.7 | IVAS 3.8.8 | IVAS 3.8.9 | IVAS 3.8.10 |
| Total ions: | 12,070,475 | 5,258,176 | 9,251,680 | 7,502,224 | 16,213,824 | 12,223,734 | 4,878,030 |
| Sigle (%) | 75.5 | 74.2 | 74.5 | 75.5 | 77.8 | 74.2 | 73.4 |
| Multiple (%) | 23.4 | 24.3 | 24.5 | 23.2 | 21.2 | 24.2 | 25.5 |
| Partial (%) | 1.1 | 1.4 | 1 | 1.3 | 1 | 1.1 | 1.1 |
| Reconstructed ions: | 7,042,775 | 3,170,254 | 5,374,711 | 4,496,256 | 13,933,458 | 9,839,070 | 3,699,975 |
| Ranged (%) | 50.9 | 48 | 51.6 | 47 | 35 | 39 | 41.7 |
| Unranged (%) | 49.1 | 52 | 48.4 | 53 | 65 | 61 | 58.3 |
| Mass calib. (peaks/interp.) | Lin. Method | Lin. Method | Lin. Method | Lin. Method | Lin. Method | Lin. Method | Lin. Method |
| (M/∆M) for 40Ca++ | 261 | 149 | 388 | 282 | 435 | 208 | 294 |
| (M/∆M10)c | 122 | 77 | 172 | 112 | 196 | 90 | 109 |
| Time indipendent background (ppm/ns) | 115 | 143 | 110 | 97 | 22 | 37 | 42 |
| Recontruction | |||||||
| Final speciment state | fractured | fractured | fractured | fractured | unsure | fractured | fractured |
| Pre-/post-analysis imaging | SEM/n.a. | SEM/n.a. | SEM/n.a. | SEM/n.a. | SEM/n.a. | SEM/n.a. | SEM/n.a. |
| Radius evolution model | Shank | Shank | Shank | Shank | Shank | Shank | Shank |
| Field factor (k) | 3.5 | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 |
| Image compression factor | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| Assumed E-field (V/nm) | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Detector efficiency (%) | 80 | 80 | 80 | 80 | 80 | 80 | 80 |
| Avg. atomic volume (nm3) | 0.0435 | 0.0435 | 0.0435 | 0.0435 | 0.0435 | 0.0435 | 0.0435 |
| Vinitial; Vfinal (V) | 2500; 5800 | 1800; 3500 | 2400; 5400 | 2000; 4700 | 2200; 5300 | 3200; 3900 | 3400; 4900 |
| Peaks (Da) | Peaks (Da) | ||
|---|---|---|---|
| Mg++ | 12,12.5,13 | CO2+ | 44,45,46 |
| Mg+ | 24,25,26 | Ca2O++ | 48,49,50 |
| NH+ | 15 | CaO+ | 56,58,60 |
| O+ | 16 | CH3NO+ | 57 |
| OH+ | 17 | C3H6OH+ | 59 |
| H2O+ | 18 | CH3NS+ | 61 |
| Fe+++ | 18.6 | C4N+ | 62 |
| H3O+ | 19 | HNO3+ | 63 |
| Ca++ | 20,21,21.5,23 | unknown | 67.5 |
| Ca+ | 40,42,43 | C5H9 | 69 |
| N2+ | 28 | Ca2CO3++ | 70 |
| N2H+ | 29 | CaO2+ | 72,74,75,76 |
| N2H2+ | 30 | unknown | 80 |
| O2+, S+ | 32 | CaCO3+ | 100 |
| SH+, S+ | 33,34 |
| Reference | W7-4175-M20 | W8-4149-M3 | ||||
|---|---|---|---|---|---|---|
| Ion | Counts | Atomic % | Atomic Error % | Counts | Atomic % | Atomic Error % |
| S | 14,759 | 0.36 | 0.082 | 14,531 | 0.25 | 0.154 |
| H | 658,324 | 15.90 | 0.002 | 711,148 | 12.08 | 0.010 |
| Ca | 2,244,793 | 54.21 | 0.030 | 3,400,315 | 57.78 | 0.030 |
| C | 160,130 | 3.87 | 0.008 | 149,473 | 2.54 | 0.005 |
| N | 39,504 | 0.95 | 0.002 | 48,173 | 0.82 | 0.001 |
| O | 1,012,163 | 24.44 | 0.002 | 1,547,706 | 26.30 | 0.002 |
| Fe | 298 | 0.01 | 0.002 | - | - | - |
| Mg | 10,821 | 0.26 | 0.008 | 13,296 | 0.23 | 0.018 |
| Scallop W7 | Scallop W8 | ||||||
|---|---|---|---|---|---|---|---|
| Reference | 4173-M13 | 4174-M19 | 4175-M20 | 4176-M21 | 4149-M3 | 4150-M4 | 4198-M10 |
| Volume (nm3) | 116,376 | 56,368 | 86,300 | 70,661 | |||
| Mg | 0.37% | 0.31% | 0.44% | 0.17% | 0.36% | 0.51% | 0.25% |
| N compounds | 0.68% | 0.58% | 1.47% | 0.59% | 1.17% | 0.85% | 0.97% |
| C compounds | 0.72% | 0.33% | 0.74% | 0.46% | 0.14% | 0.27% | 6.23% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Huerta, A.; Walker, S.E.; Cappelli, C. In Situ Geochemical Analysis of Organics in Growth Lines of Antarctic Scallop Shells: Implications for Sclerochronology. Minerals 2020, 10, 529. https://doi.org/10.3390/min10060529
Pérez-Huerta A, Walker SE, Cappelli C. In Situ Geochemical Analysis of Organics in Growth Lines of Antarctic Scallop Shells: Implications for Sclerochronology. Minerals. 2020; 10(6):529. https://doi.org/10.3390/min10060529
Chicago/Turabian StylePérez-Huerta, Alberto, Sally E. Walker, and Chiara Cappelli. 2020. "In Situ Geochemical Analysis of Organics in Growth Lines of Antarctic Scallop Shells: Implications for Sclerochronology" Minerals 10, no. 6: 529. https://doi.org/10.3390/min10060529
APA StylePérez-Huerta, A., Walker, S. E., & Cappelli, C. (2020). In Situ Geochemical Analysis of Organics in Growth Lines of Antarctic Scallop Shells: Implications for Sclerochronology. Minerals, 10(6), 529. https://doi.org/10.3390/min10060529






