Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 1: Characterization of Reclamation Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Material Preparation
2.2. Physical, Hydrogeological, Chemical, and Mineralogical Characterizations
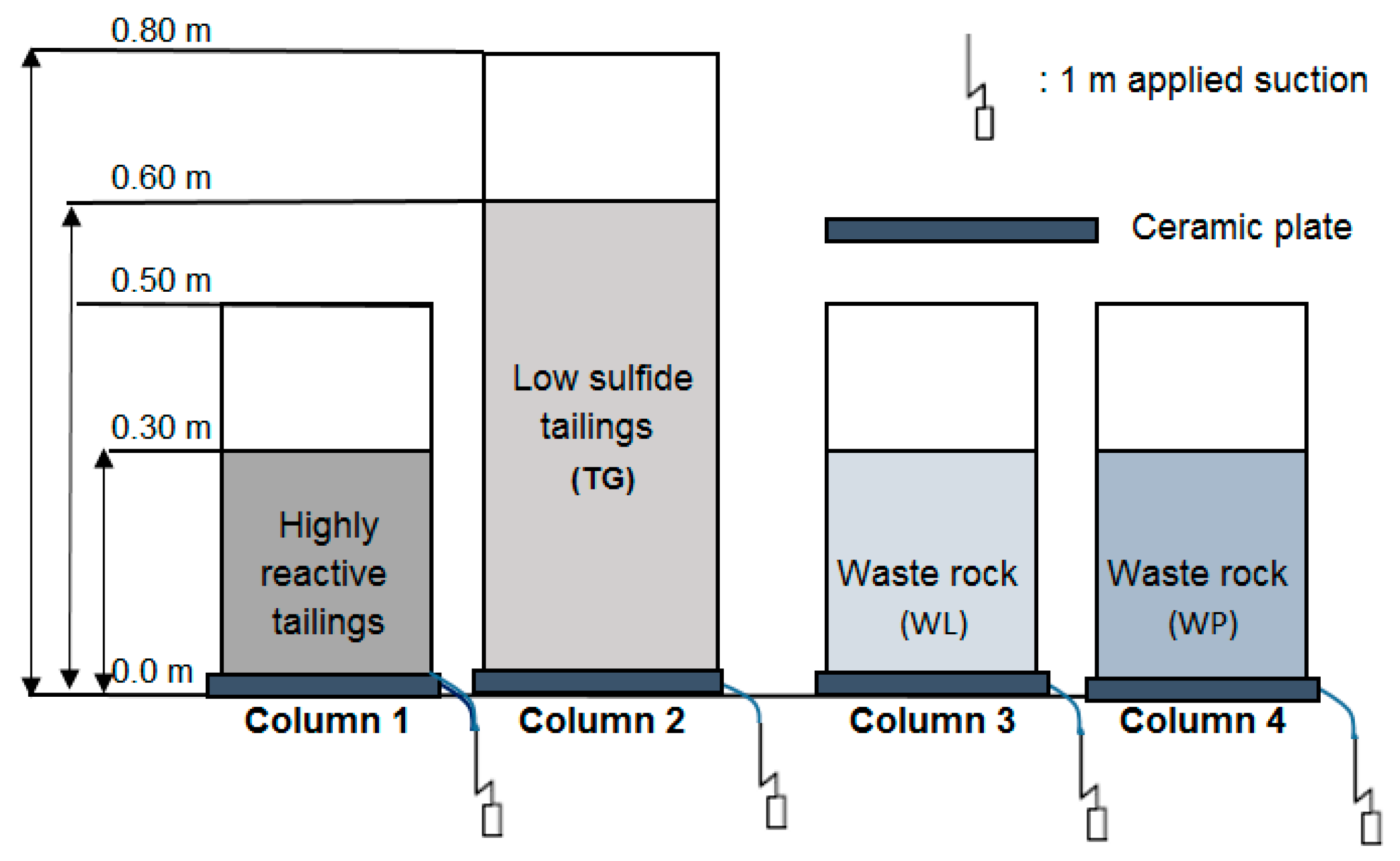
2.3. Construction of the Laboratory Column Tests and Analyses
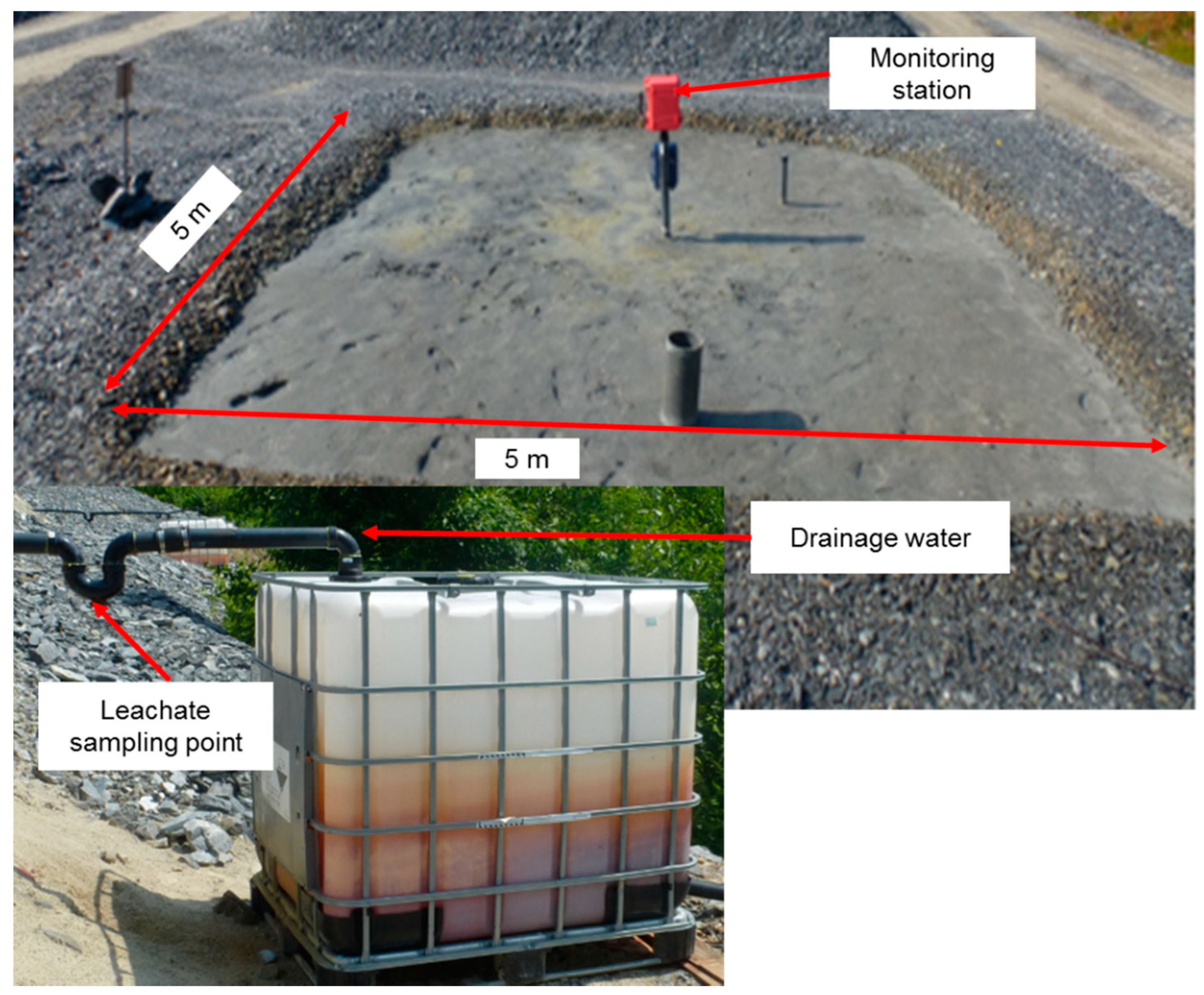
2.4. Description of the Control Field Cell and Geochemical Monitoring
3. Results and Discussion
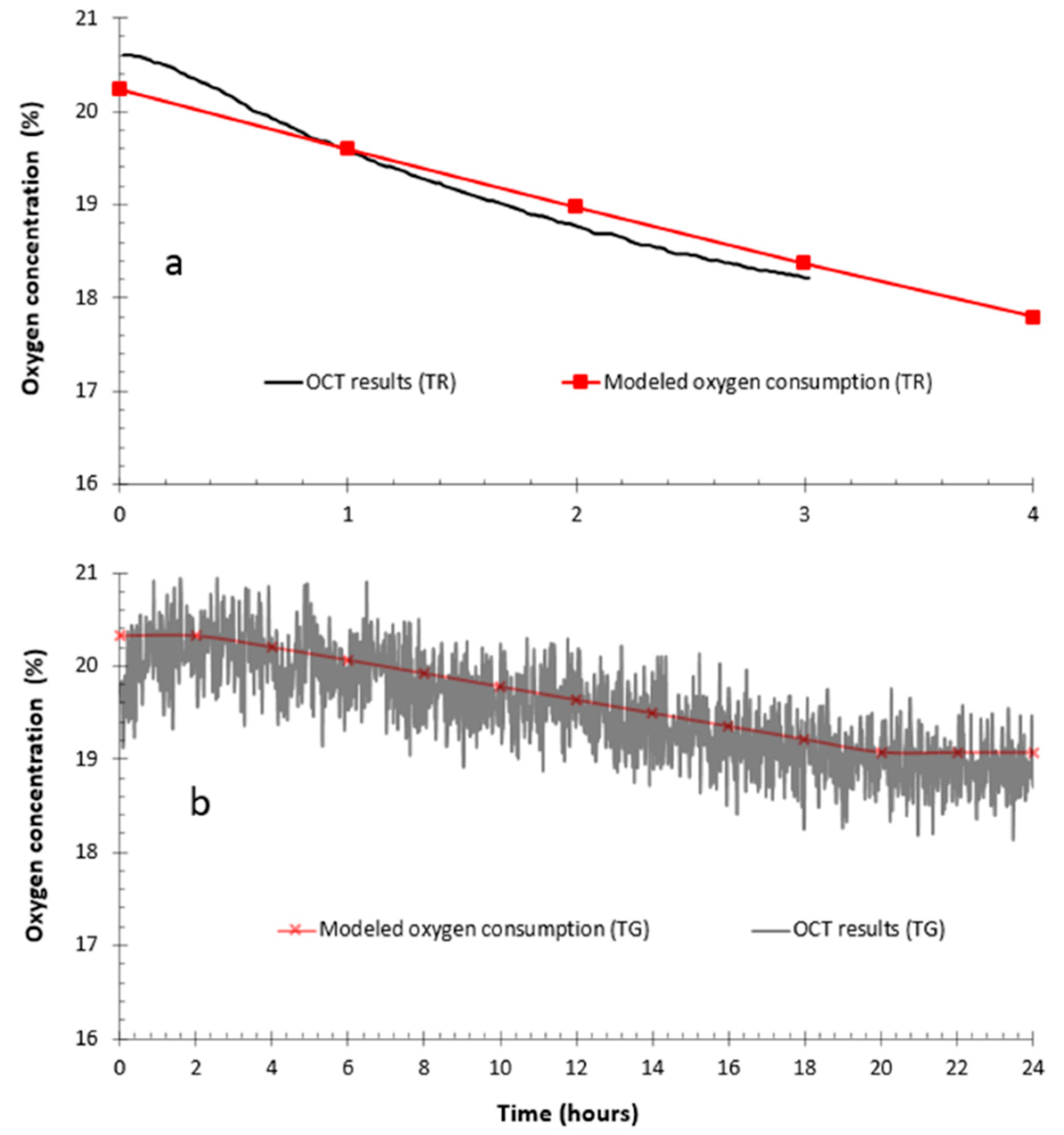
3.1. Physical, Hydrogeological, Mineralogical, and Environmental Characteristics
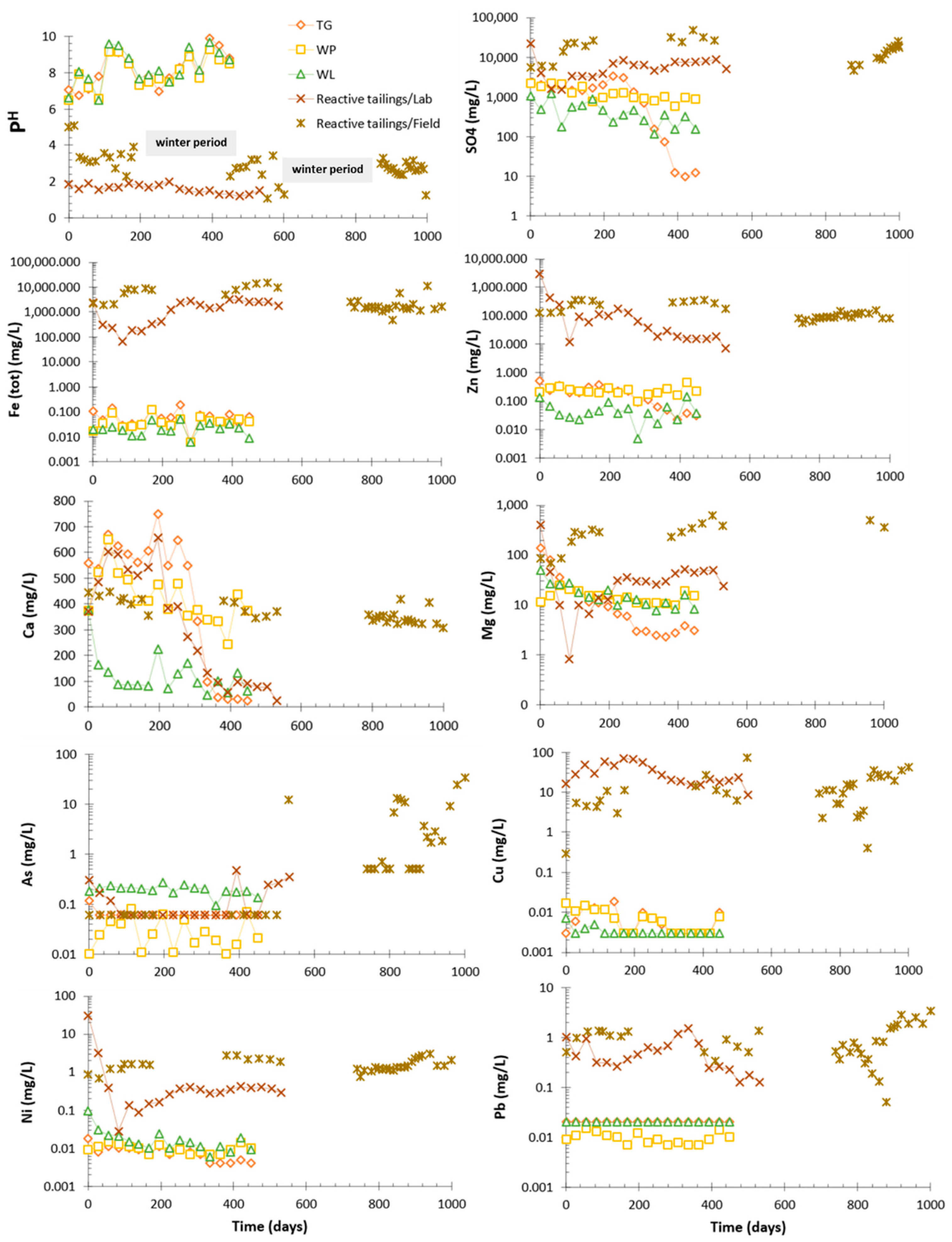
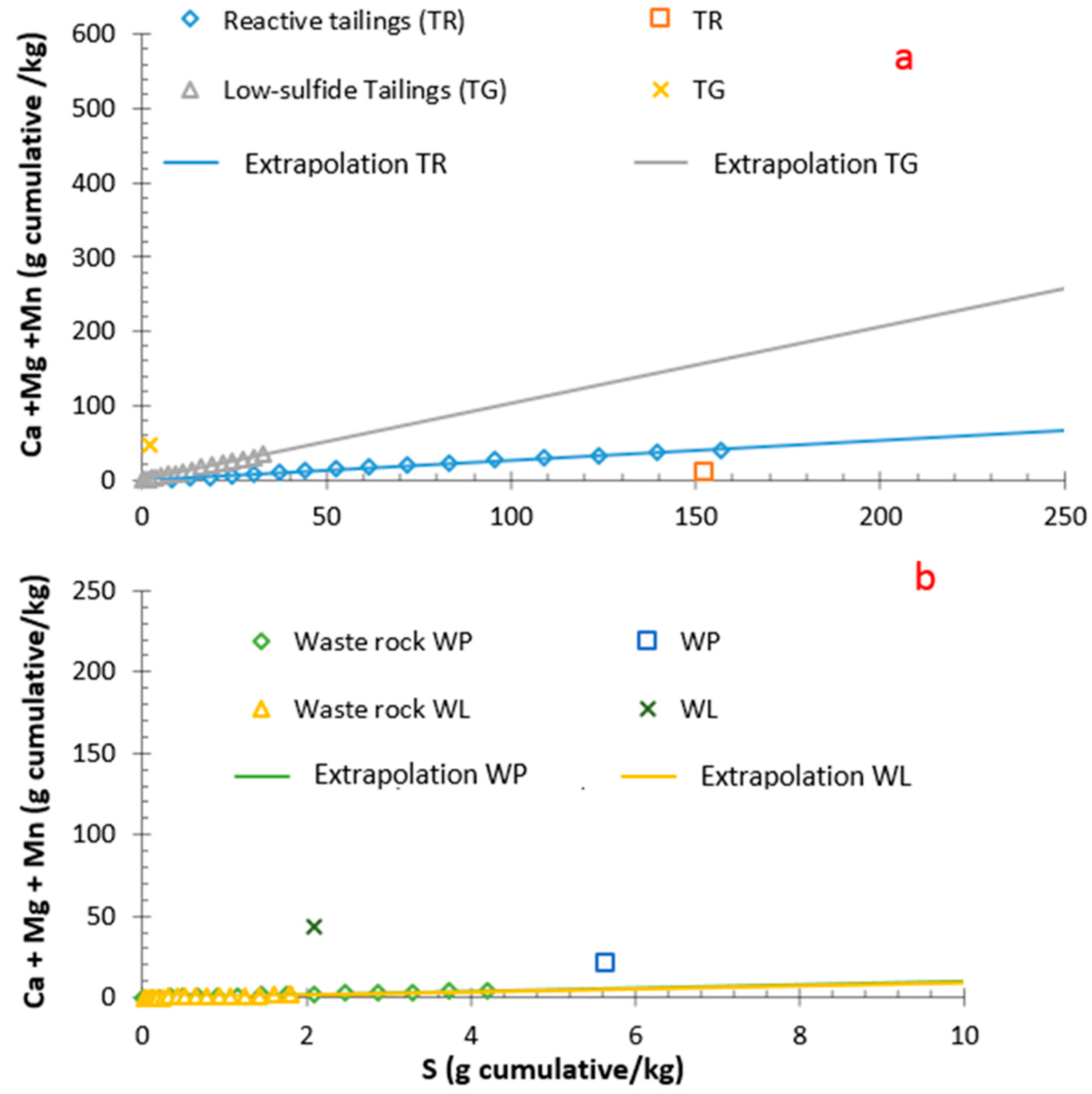
3.2. Geochemical Behavior of the Reactive Tailings and Cover Materials
3.3. Selection Criteria of Materials for a Cover with Capillary Barrier Effects
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.G. The geochemistry of acid mine drainage. In Environmental Geochemistry—Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 9, pp. 149–204. [Google Scholar]
- Rimstidt, J.D.; Vaughan, D.J. Pyrite oxidation: A stateof- the-art assessment of the reactionmechanism. Geochim. Cosmochim. Acta 2003, 67, 873–880. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistryand microbiology of mine drainage: An update. Appl. Geochem. J. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Alphane, V.; Vermeulen, P.D. Acid mine drainage and its potential impact on the water resources in the Waterberg coalfield, South Africa. S. Afr. J. Geol. 2015, 118, 55–70. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine waters. In The Environmental Geochemistry of Mineral Deposits, Part A, Processes, Techniques, & Health Issues. Reviews in Economic Geology; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 6A, pp. 133–160. [Google Scholar]
- Nicholson, R.V. Overview of Near Neutral pH Drainage and Its Mitigation: Results of a MEND Study; MEND Ontario Workshop: Sudbury, ON, Canada, 2004. [Google Scholar]
- Blowes, D.; Ptacek, C.; Jambor, J.; Weisener, C.; Paktunc, D.; Gould, W.; Johnson, D. The geochemistry of acid mine drainage. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Lindsay, M.B.J.; Moncur, M.C.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. J. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- SRK. Draft Acid Rock Drainage. Technical Guide Vol. 1, British Columbia Acid Mine Drainage Task Force Report, Prepared by Steffen, Robertson, Kirsten in Association with Norecol Environmental Consultants and Gormely Process Engineering; Ministry of Energy Mines and Petroleum Resources: Vancouver, BC, Canada, 1989.
- Aubertin, M.; Chapuis, R.P.; Aachib, M.; Bussière, B.; Ricard, J.F.; Tremblay, L. Evaluation en Laboratoire de Barrières Sèches Construites à Partir de Résidus Miniers; Rapport NEDEM/MEND Projet 2.22.2a; Ecole Polytechnique de Montréal: Ottawa, ON, Canada, 1995. [Google Scholar]
- Nicholson, R.V.; Gillham, R.W.; Cherry, J.A.; Reardon, E.J. Reduction of acid generation in mine tailings through the use of moisture-retaining layers as oxygen barriers. Can. Geotech. J. 1989, 26, 1–8. [Google Scholar] [CrossRef]
- Morel-Seytoux, H.J. L’effet de barrière capillaire à l’interface de deux couches de sol aux propriétés fort contrastées. Hydrol. Cont. 1992, 7, 117–128. [Google Scholar]
- Hotton, G.; Bussière, B.; Pabst, T.; Bresson, E.; Roy, P. Influence of climate change on the ability of cover with capillary barrier effects to control acid generation. Hydrogeol. J. 2019, 28, 763–779. [Google Scholar] [CrossRef]
- Yanful, E.K. Development of Laboratory Methodology for Evaluating the Effectiveness of Reactive Tailings Covers; Final Report (Draft); Centre de Technologie Noranda: Pointe-Claire, QC, Canada, 1991. [Google Scholar]
- Bussière, B.; Aubertin, M.; Chapuis, R.P. The behaviour of inclined covers used as oxygen barriers. Can. Geotech. J. 2003, 40, 512–535. [Google Scholar] [CrossRef]
- Aubertin, M.; Bussière, B.; Pabst, T.; James, M.; Mbonimpa, M. Review of reclamation techniques for acid generating mine wastes upon closure of disposal sites. Paper Presented at the Geo-Chicago, Sustainability, Energy and the Geoenvironment, Chicago, IL, USA, 14–18 August 2016. [Google Scholar]
- Ricard, J.F.; Aubertin, M.; Firlotte, F.W.; Knapp, R.; McMullen, J. Design and construction of a dry cover made of tailings for the closure of Les Terrains Aurifères site. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; Volume 4, pp. 1515–1530. [Google Scholar]
- Ricard, J.F.; Aubertin, M.; Garand, P. Performance d’un recouvrement multicouche au site Barrick-Bousquet de Les Terrains Aurifères. In Proceedings of the 20th Symposium on Wastewater; Delisle, C.E., Bouchard, M.A., Eds.; Collection Environnement de l’Université de Montréal: Montréal, QC, Canada, 1997; Volume 10, pp. 291–305. [Google Scholar]
- Bussière, B.; Aubertin, M.; Benzaazoua, M.; Gagnon, D. Modèle d’estimation des coûts de restauration de sites miniers générateurs de DMA. In Proceedings of the Mines Écologiques, Congrès APGGQ, Rouyn-Noranda, QC, Canada, 5 August 1999. [Google Scholar]
- Gee, G.W.; Benson, C.H.; Albright, W.H. Comment on “evaluation of evapotranspiration covers for waste containment in arid and semiarid regions in the southwestem USA”. Vadose Zone J. 2006, 5, 809–812. [Google Scholar] [CrossRef]
- Gorakhi, M.H.; Bareither, C.A. Sustainable reuse of mine tailings and waste rock as water-balance covers. Minerals 2017, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Tardif-Drolet, M.; Li, L.; Pabst, T.; Zagury, G.J.; Mermillod-Blondin, R.; Genty, T. Revue de la réglementation sur la valorisation des résidus miniers hors site au Québec. Canadian science publishing. Doss. Environ. 2019. [Google Scholar] [CrossRef]
- Aachib, M.; Aubertin, M.; Chapuis, R.P. Essais en colonne sur des couvertures avec effets de barrière capillaire. In Proceedings of the 51st Canadian Geotechnical Conference, Canadian Geotechnical Society, Edmonton, AB, Canada, 4–7 October 1998; Volume 2, pp. 837–844. [Google Scholar]
- Bussière, B.; Benzaazoua, M.; Aubertin, M.; Mbonimpa, M. A laboratory study of covers made of low sulphide tailings to prevent acid mine drainage. Environ. Geol. 2004, 45, 609–622. [Google Scholar] [CrossRef]
- Aubertin, M.; Bussière, B.; Barbera, J.M.; Chapuis, R.P.; Monzon, M.; Aachib, M. Construction and instrumentation of in situ test plots to evaluate covers built with clean tailings. In Proceedings of the 4th International Conference on Acid Rock Drainage (ICARD), Vancouver, BC, Canada, 31 May–6 June 1997; Volume 2, pp. 715–730. [Google Scholar]
- Bussière, B.; Maqsoud, A.; Aubertin, M.; Matschuk, J.; Mcmullen, J.; Julien, M. Performance of the oxygen limiting cover at the LTA site, Malartic, Québec. CIM Bull. 2006, 1, 1–11. [Google Scholar]
- Pabst, T.; Bussière, B.; Aubertin, M.; Molson, J. Comparative performance of cover systems to prevent acid mine drainage from pre-oxidized tailings: A numerical hydro geochemical assessment. J. Contam. Hydrol. 2018, 214, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Kalonji-Kabambi, A.; Bussière, B.; Demers, I. Hydrogeological Behaviour of cover with Capillary Barrier Effect made of Mining Materials. Geotech. Geol. Eng. J. 2017, 35, 1199–1220. [Google Scholar] [CrossRef]
- Maqsoud, A.; Bussière, B.; Turcotte, S.; Roy, M. Performance evaluation of covers with capillary barrier effects under deep groundwater conditions using experimental cells. In Proceedings of the Géo Ottawa Conference, Ottawa, ON, Canada, 1–4 October 2017; p. 256. [Google Scholar]
- Larochelle, C.G. Utilisation de Stériles Générateurs D’acide Comme Couche de Bris Capillaire Dans Une Couverture Avec Effet de Barrière Capillaire. Master’s Thesis, Université du Québec en Abitibi-Témiscarningue, Rouyn-Noranda, QC, Canada, 2019. [Google Scholar]
- Yanful, E.K.; Verma, A.; Straatman, A.S. Turbulence driven metal release from suspended pyrrhotite tailings. J. Geotech. Geoenviron. Eng. 2000, 126, 1157–1165. [Google Scholar] [CrossRef]
- Davé, N.K.; Paktunc, A.D. Surface reactivity of high-sulfide copper mine tailings under shallow water cover conditions. In Proceedings of the 6th ICARD, Cairns, Queensland, Australia, 14–17 July 2003; pp. 241–251. [Google Scholar]
- Awoh, A.S.; Mbonimpa, M.; Bussière, B.; Plante, B.; Hassan Bouzahzah, H. Laboratory study of highly pyritic tailings submerged beneath a water cover under various hydrodynamic conditions. Mine Water Environ. 2014, 33, 241–255. [Google Scholar] [CrossRef]
- Awoh, A.S.; Mbonimpa, M.; Bussière, B. Field study of the chemical and physical stability of highly sulphide-rich tailings stored under a shallow water cover. Mine Water Environ. 2013, 32, 42–55. [Google Scholar] [CrossRef]
- Kalonji-Kabambi, A. Étude du Comportement Hydrogéologique D’une Couverture Avec Effets de Barrière Capillaire Faite Des Matériaux Miniers à L’aide de Modélisations Physique et Numérique. Master’s Thesis, École Polytechnique de Montréal, Montréal, QC, Canada, 2014. [Google Scholar]
- Kalonji-Kabambi, A.; Demers, I.; Bussière, B. Numerical modeling of covers with capillary barrier effects made entirely of mining materials. In Proceedings of the Tailings and Mines Waste Conference, Keystone, CO, USA, 2–5 October 2016. [Google Scholar]
- Lee Black, D.; McQuay, M.Q.; Bonin, M.P. Laser-based techniques for particle-size measurement: A review of sizing methods and their industrial applications. Prog. Energy Combust. Sci. 1996, 22, 267–306. [Google Scholar] [CrossRef]
- ASTM. D422. Standard Test Method for Particle-Size Analysis of Soils; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2007. [Google Scholar]
- ASTM. D854-10. Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. In ASTM Annual CDs of Standards; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM. D5856-07. Standard Test Method for Measurement of Hydraulic Conductivity of Porous Materials Using Rigid-Wall, Compaction-Mold Permeameter; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2007. [Google Scholar]
- Peregoedova, A.; Aubertin, M.; Bussière, B. Laboratory measurement and prediction of the saturated hydraulic conductivity of mine waste rock. In Proceedings of the GeoMontréal Conference, Montréal, QC, Canada, 29 September–3 October 2013; p. 165. [Google Scholar]
- ASTM. Reapproved D3152–72. Standard Test Method for Capillary-Moisture Relationships for Fine-Textured Soils by Pressure-Membrane; 2 Annual Book of ASTM Standards (Reapproved 2000); 100 Barr Harbor Drive: West Conshohocken, PA, USA, March 1972; Volume 4. [Google Scholar]
- Van Genuchten, M.; Leij, F.J.; Yates, S.R. The RETC Code for Quantifying Hydraulic Functions of Unsaturated Soils; Environmental Protection Agency: Cincinnati, OH, USA, 1991; EPA/600/2-91/065.
- Van Genuchten, M. A Closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. 1980, 44, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Chapuis, R.; Masse, I.; Madinier, B.; Aubertin, M. A drainage column test for determining unsaturated properties of coarse materials. Geotech. Test. J. 2006, 30, 83–89. [Google Scholar]
- Hernandez, A.M. Une Étude Expérimentale Des Propriétés Hydriques Des Roches Stériles et Autres Matériaux à Grannulométrie Étalée. Master’s Thesis, École Polytechnique de Montréal, Montréal, QC, Canada, 2007. [Google Scholar]
- Peregoedova, A.; Aubertin, M.; Bussière, B. Evaluation of the water retention curve of mine waste rock using laboratory tests and predictive models. In Proceedings of the GeoRegina Conference, Regina, SK, Canada, 28 September–1 October 2014; p. 247. [Google Scholar]
- Rietveld, H.M. The Rietveld Method; Young, R.A., Ed.; Oxford University Press: Oxford, UK, 1993; Chapter 2. [Google Scholar]
- Taylor, J.C.; Hinczak, I. Rietveld Made Easy: A Practical Guide to the Understanding of the Method and Successful Phase Quantifications; University of Wollongong: Wollongong NSW, Australia, 2001. [Google Scholar]
- Bouzahzah, H. Modification et Amélioration Des Tests Statiques et Cinétiques Pour Une Prédiction Fiable du Drainage Minier Acide. Ph.D. Thesis, Université du Québec en Abitibi-Témiscamingue, Rouyn-Noranda, QC, Canada, 2013. [Google Scholar]
- Petruk, W. The MP-SEM-IPS image analysis system. In CANMET Report 87-1E (CANMET, Dept Energy, Mines, and Resources; Canada Department of Energy, Mines and Resources: Ottawa, ON, Canada, 1986. [Google Scholar]
- Miller, S.D.; Jeffery, J.J.; Wong, J.W.C. Use and misuse of the Acid-Base account for AMD prediction. Paper Presented at the 2nd International Conference, Abatement of Acidic Drainage, Montreal, QC, Canada, 16–18 September 1991; Volume 3, pp. 489–506. [Google Scholar]
- Morin, K.A.; Hutt, N.M. Environmental Geochemistry of Minesite Drainage: Practical Theory and Case Studies; MDAG Pub.: Vancouver, BC, Canada, 2001. [Google Scholar]
- Mine Environment Neutral Drainage (MEND). Report 1.20.1 Prediction Manual for Drainage Chemistry from Sulphidic Geologic Materials; CANMET-Mining and Minerals Sciences Laboratories: Smithers, BC, Canada, 2009. [Google Scholar]
- Benzaazoua, M.; Bussière, B.; Dagenais, A.M.; Archambault, M. Kinetic tests comparison and interpretation for prediction of the Joutel tailings acid generation potential. Environ. Geol. 2004, 46, 1086–1101. [Google Scholar] [CrossRef]
- Mbonimpa, M.; Aubertin, M.; Aachib, M.; Bussière, B. Diffusion and consumption of oxygen in unsaturated cover materials. Can. Geotech. J. 2003, 40, 916–932. [Google Scholar] [CrossRef]
- Gosselin, M.; Mbonimpa, M.; Aubertin, M.; Martin, V. An investigation of the effect of the degree of saturation on the oxygen reaction rate coefficient of sulphidic tailings. In Proceedings of the ICAST’07, Accra, Ghana, 10–12 December 2007. [Google Scholar]
- Ouangrawa, M.; Aubertin, M.; Molson, J.W.; Bussière, B.; Zagury, G.J. Preventing acid mine drainage with an elevated water table: Long-Term Column Experiments and parameter analysis. Water Air Soil Pollut. 2010, 213, 437–458. [Google Scholar] [CrossRef]
- Rowe, R.K.; Booker, J.R. Pollutev7 Reference Guide; GAEA Technologies Ltd.: Greater Napanee, ON, Canada, 2004. [Google Scholar]
- Demers, I.; Bussière, B.; Mbonimpa, M.; Benzaazoua, M. Oxygen diffusion and consumption in low-sulphide tailings covers. Can. Geotech. J. 2009, 46, 454–469. [Google Scholar] [CrossRef]
- Aachib, M. Étude en Laboratoire de la Performance de Barrières de Recouvrement Constituées de Rejets Miniers Pour Limiter le DMA. Ph.D. Thesis, École Polytechnique de Montréal, Montréal, QC, Canada, 1997. [Google Scholar]
- Dagenais, A.M. Techniques de Contrôle du Drainage Minier Acide Basées Sur Les Effets Capillaires. Ph.D. Thesis, École Polytechnique de Montréal, Montréal, QC, Canada, 2005. [Google Scholar]
- McCarthy, D.F. Essentials of Soil Mechanics and Foundations: Basic Geotechnics; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Bussière, B. Colloquium 2004: Hydrogeotechnical properties of hard rock tailings from metal mines and emerging geoenvironmental disposal approaches. Can. Geotech. J. 2007, 44, 1019–1052. [Google Scholar] [CrossRef]
- Sobek, A.A.; Schuller, W.A.; Freeman, J.R.; et Smith, R.M. Field and Laboratory Methods Applicable to Overburdens and Mine Soil; EPA Report No. EPA-600/2-78-054; Environmental Protection Agency: Cincinnaty, OH, USA, 1978; pp. 47–50.
- Lessard, F. Évaluation de Couverture Isolantes Avec Effets de Barrière Capillaire Faites de Résidus Désulfurés Afin de Contrôler le Drainage Minier en Conditions Nordiques. Master’s Thesis, Université du Québec en Abitibi-Témiscarningue, Rouyn-Noranda, QC, Canada, 2018. [Google Scholar]
- Nyameogo, G. Développement D’un Système de Mesure et D’un Modèle Théorique Préliminaire D’estimation du Coefficient de Diffusion de L’oxygène Dans Les Matériaux Poreux Inertes Gelés. Master’s Thesis, Université du Québec en Abitibi-Témiscamingue, Rouyn-Noranda, QC, Canada, 2017. [Google Scholar]
- Vaughan, D.J. Sulfide Mineralogy and Geochemistry. In Reviews in Mineralogy and Geochemistry; Mineralogical Society of America: Chantilly, VA, USA, 2006; Volume 61, p. 714. [Google Scholar]
- Vaughan David, J.; Corkhill Claire, L. Mineralogy of sulfides. Elements 2017, 13, 81–87. [Google Scholar] [CrossRef]
- Aubertin, M.; Chapuis, R.P. Critères de conception pour les ouvrages de retenue des résidus miniers dans la région de l’Abitibi. In Proceedings of the 1ère Conférence Canadienne de Géotechnique Environnementale, Montréal, QC, Canada, 14–16 May 1991; pp. 113–127. [Google Scholar]
- Pabst, T. Etude Expérimentale et Numérique du Comportement Hydro-Geochimique de Recouvrement Placé Sur Des Résidus Sulfureux Partiellement Oxydés. Ph.D. Thesis, École Polytechnique de Montréal, Montréal, QC, Canada, 2011. [Google Scholar]
- Mine Environment Neutral Drainage (MEND). Étude de Laboratoire Sur le Recouvrement Construits Avec Des Résidus Miners; MEND 2.22.2c; Canada Centre for Mineral and Energy Technology (CANMET): Ottawa, ON, Canada, 1999.
- Aubertin, M.; Chapuis, R.P.; Bussière, B.; Aachib, M. Propriétés des résidus miniers utilisés comme matériau de recouvrement pour limiter le DMA. In Symposium International Géologie et Confinement des Déchets Toxiques, Géoconfine’93, Arnould; Barrès, M., Côme, B., Eds.; Balkema: Rotterdam, The Netherlands, 1993; pp. 299–308. [Google Scholar]
- Bussière, B.; Aubertin, M.; Julien, M. Couvertures avec effets de barrière capillaire pour limiter le drainage minier acide, aspects théoriques et pratiques. Vecteur Environ. 2001, 34, 37–50. [Google Scholar]
- Morin, K.A.; Gerencher, E.; Jones, C.E.; Konasewich, D.E. Critical literature review of acid drainage from waste rock. In Canadian Mine Environmental Neutral Drainage (MEND) Program; Canada Centre for Mineral and Energy Technology: Ottawa, ON, Canada, 1991. [Google Scholar]
- Aubertin, M.; Bussière, B.; Bernier, L. Environnement et Gestion des Rejets Miniers; Presses Polytechnique Int.: Montréal, QC, Canada, 2002. [Google Scholar]
- Mine Environment Neutral Drainage (MEND). Étude en Laboratoire de Barrière Seches Construites à Partir de Résidus Miners; MEND 2.22.2a; Canada Centre for Mineral and Energy Technology (CANMET): Ottawa, ON, Canada, 1996.
- Bussière, B.; Potvin, R.; Dagenais, A.M.; Aubertin, M.; Maqsoud, A.; Cyr, J. Restauration du site minier Lorraine, Latulipe, Québec: Résultats de 10 ans de suivi. Déchets Revue Francophone d’écologie Industrielle 2009, 2, 54. [Google Scholar] [CrossRef] [Green Version]


| Parameters | Units | TR | TG | WP | WL |
|---|---|---|---|---|---|
| D10 | mm | 0.009 | 0.0026 | 0.160 | 0.165 |
| D50 | mm | 0.068 | 0.016 | 10 | 10 |
| D60 | mm | 0.088 | 0.022 | 17 | 15 |
| GS | - | 3.22 | 2.68 | 2.72 | 2.76 |
| ksat | cm/s | 2 × 10−4 | 5 × 10−5 | 3 × 10−2 | 2 × 10−2 |
| AEV | m of water | 2 | 2.5 | 0.002 | 0.003 |
| θs | m3/m3 | 0.45 | 0.43 | 0.35 | 0.35 |
| θr | m3/m3 | 0.01 | 0.06 | 0.00 | 0.00 |
| Mineral Phase | Reactive Tailings (TR) | Low-Sulfide Tailings (TG) | Waste Rock (WP) | Waste Rock (WL) |
|---|---|---|---|---|
| % w/w | % w/w | % w/w | % w/w | |
| Albite | 8.2% | 52% | 27% | 38% |
| Actinoline | - | 0.2% | - | 5% |
| Anhydrite | - | 0.2% | - | - |
| Calcite | - | 8.7% | - | 4% |
| Chalcopyrite | 0.9% | - | - | - |
| Chlorite | 4.4% | 13% | - | 14% |
| Dolomite | - | 1.2% | - | - |
| Gypsum | 1.4% | 0.7% | - | - |
| Muscovite | 1.8% | 2.8% | 3.4% | - |
| Pyrite | 24% | 0.3% | 1.4% | 0.5% |
| Pyrrhotite | 4.5% | - | - | - |
| Quartz | 53% | 22% | 68% | 34% |
| Rutile | 0.4% | - | - | - |
| Sphalerite | 2% | - | - | - |
| Environmental Parameters | Reactive Tailings (TR) | Low-Sulfide Tailings (TG) | Waste Rock (WP) | Waste Rock (WL) |
|---|---|---|---|---|
| Stotal | 17% | 0.13% | 0.61% | 0.21% |
| Ctotal | 0.03% | 0.85% | 0.14% | 0.26% |
| AP | 531 | 4 | 19 | 7 |
| NP | 3 | 71 | 12 | 22 |
| NNP | −528 | 67 | −7 | 15 |
| NP/AP | 0.006 | 17 | 0.63 | 3.14 |
| Kr | 3.2 × 10−4/s | 4.2 × 10−6/s | - | - |
| Physicochemical Parameters | Reactive Tailings (TR) (Laboratory Control Column) | Reactive Tailings (TR) (Field Control Cell) | Low Sulfide Tailings (TG) | Waste Rock (WP) | Waste Rock (WL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| pH | 1.2 | 2.0 | 1.6 | 1.1 | 5.1 | 2.3 | 6.7 | 9.9 | 8.2 | 6.5 | 9.3 | 8.0 | 6.5 | 9.7 | 8.3 |
| Fe (mg/L) | 65 | 3210 | 1560 | 495 | 14,700 | 4325 | 0.003 | 0.192 | 0.07 | 0.003 | 0.13 | 0.04 | 0.003 | 0.05 | 0.02 |
| Zn (mg/L) | 7 | 2940 | 230 | 57 | 368 | 163 | 0.02 | 0.5 | 0.2 | 0.10 | 0.47 | 0.24 | 0.003 | 0.14 | 0.05 |
| Sulfates (mg/L) | 1533 | 22,000 | 6300 | 4660 | 47,644 | 18,022 | 10 | 3340 | 1330 | 580 | 2250 | 1200 | 120 | 1200 | 460 |
| Ca (mg/L) | 23 | 660 | 300 | 307 | 447 | 369 | 26 | 750 | 420 | 250 | 650 | 420 | 50 | 400 | 120 |
| Mg (mg/L) | 0.8 | 400 | 50 | 69 | 621 | 298 | 3.0 | 140 | 10 | 10 | 25 | 15 | 7.0 | 50 | 20 |
| As (mg/L) | 0.06 | 0.48 | 0.14 | 0.06 | 34 | 3.9 | 0.06 | 0.1 | 0.06 | 0.06 | 0.06 | 0.06 | 0.093 | 0.27 | 0.2 |
| Cu (mg/L) | 8.53 | 72 | 32 | 0.3 | 73 | 15 | 0.002 | 0.02 | 0.007 | 0.002 | 0.02 | 0.007 | <DLM | <DLM | N.D. |
| Ni (mg/L) | 0.028 | 30 | 2.0 | 0.7 | 3.0 | 2.0 | 0.002 | 0.02 | 0.008 | 0.007 | 0.02 | 0.010 | 0.006 | 0.09 | 0.02 |
| Pb (mg/L) | 0.13 | 2.0 | 0.5 | 0.34 | 35 | 7.0 | <DLM | <DLM | N.D. | <DLM | <DLM | N.D. | <DLM | <DLM | N.D. |
| S (mg/L) | 650 | 7600 | 2100 | 0.1 | 11,900 | 5618 | 6.0 | 840 | 400 | 270 | 600 | 430 | 60 | 420 | 150 |
| EC (mS/cm) | 2.5 | 11 | 5 | 4.2 | 20 | 10 | 0.2 | 3.0 | 1.4 | 0.2 | 1.9 | 1.4 | 0.4 | 1.8 | 0.7 |
| Alkalinity(mg CaCO3/L) | <DLM | <DLM | N.D. | <DLM | <DLM | N.D. | 70 | 200 | 100 | 7.0 | 40 | 25 | 25 | 100 | 40 |
| Acidity (mg CaCO3/L) | 900 | 14,000 | 1500 | 447 | 34,020 | 15,864 | 0.0 | 14 | 7.0 | 0.0 | 28 | 9.0 | 0.0 | 28 | 6.0 |
| Redox potential (mV) | 500 | 700 | 600 | 349 | 575 | 509 | 360 | 580 | 470 | 370 | 570 | 520 | 380 | 580 | 500 |
| Criteria | Recommended Values | Values of Mining Materials from This Study | References |
|---|---|---|---|
| Moisture retaining layer (MRL) | |||
| Grain size (µm) | D90 ≤ 100 | D90 = 75 | |
| Classification | Silts of low plasticity or no plastic (ML) | Plastic silt (ML) | [10,15,64] |
| Hydraulic conductivity (cm/s) | 10−6 to 10−5 | 5 × 10−5 | [10,64,70,71] |
| Air entry values (m of water) | 1.5 to 2 and more | 2.5 | [15,72] |
| Water entry value (m of water) | >10 | 50 | [11,72,73] |
| Environmental characteristics | not potentially acid-generating | not potentially acid-generating | [24,60] |
| Capillary break layers (CBL) | |||
| Grain size (mm) | Coarse grain size | D90 = 30 | |
| Classification | Well-graded sand (SW) | well-graded sand (SW) | [17,18,28,41,47,74] |
| Hydraulic conductivity (cm/s) | 10−3 to 10−1 | 2–3 × 10−2 | [10,28,41,75,76,77] |
| Air entry value (m of water) | Low water retention capacity | 0.002 to 0.003 | [11,75,76] |
| Water entry value (m of water) | 0.5–1 | 0.15 | [11,75,76] |
| Environmental characteristics | not potentially acid-generating | not potentially acid-generating | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalonji-Kabambi, A.; Bussière, B.; Demers, I. Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 1: Characterization of Reclamation Materials. Minerals 2020, 10, 596. https://doi.org/10.3390/min10070596
Kalonji-Kabambi A, Bussière B, Demers I. Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 1: Characterization of Reclamation Materials. Minerals. 2020; 10(7):596. https://doi.org/10.3390/min10070596
Chicago/Turabian StyleKalonji-Kabambi, Alex, Bruno Bussière, and Isabelle Demers. 2020. "Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 1: Characterization of Reclamation Materials" Minerals 10, no. 7: 596. https://doi.org/10.3390/min10070596






