Influence of Genetic Processes on Geochemistry of Fe-oxy-hydroxides in Supergene Zn Non-Sulfide Deposits
Abstract
:1. Introduction
2. Previous Studies on the Considered Deposits
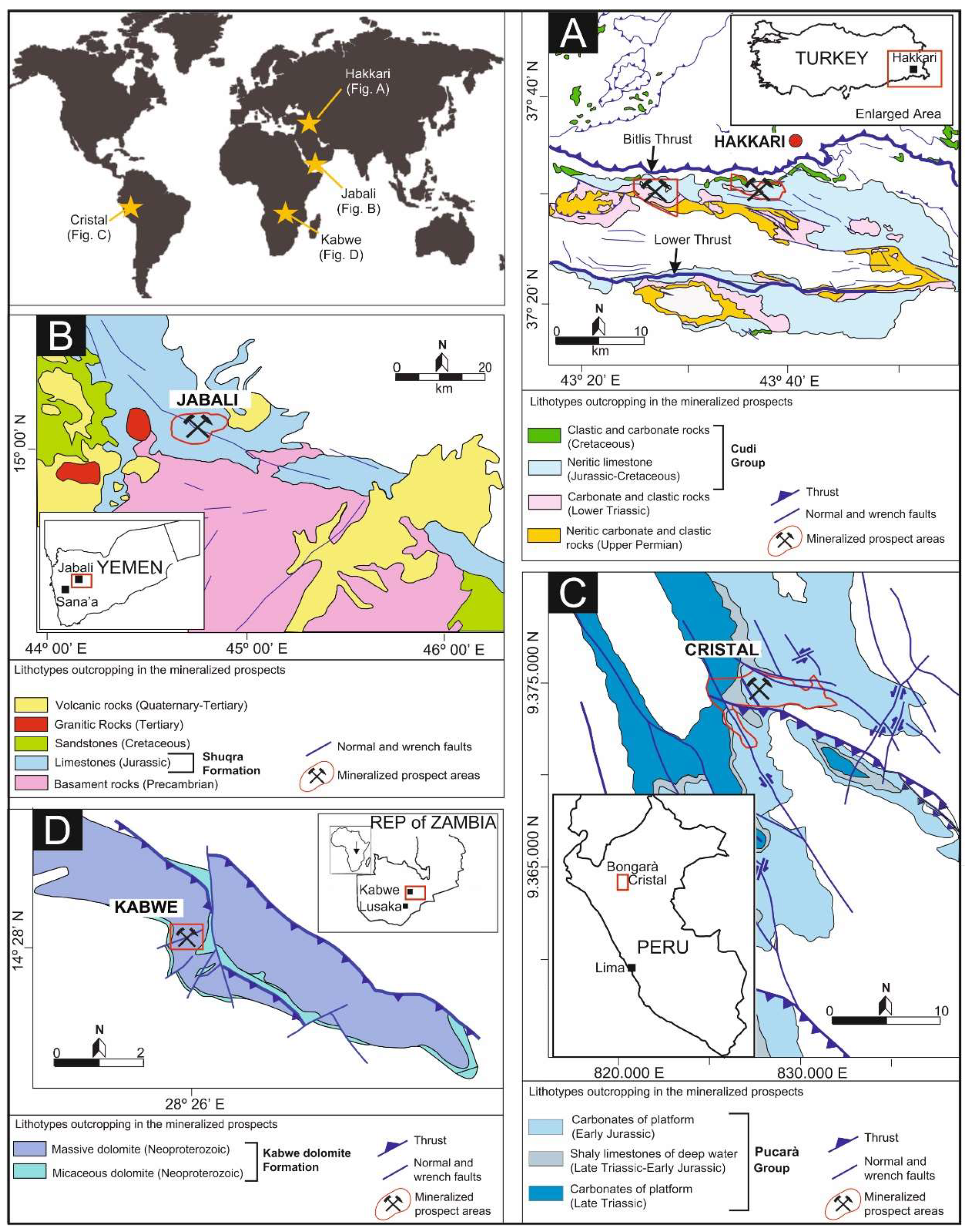
2.1. The Hakkari Prospect (Turkey)
2.2. The Jabali Deposit (Yemen)
2.3. The Cristal Prospect (Peru)
2.4. The Kabwe Deposit (Zambia)
3. Materials and Methods
4. Results
4.1. Mineralogy, Textures and LA-ICP-MS Geochemistry of the Fe-oxy-Hydroxides
4.2. Multivariate Statistical Analysis
5. Discussion
5.1. Linking the Element Deportment in Fe-oxy-Hydroxides to the Mineralization Style: Intrinsic Properties of the System Versus Environmental Conditions
- (A)
- FeO/OH, characterized by high concentrations of elements, such as Zn, Pb, Ca, Mn, Mg and Si, that are commonly found in Zn non-sulfide ores.
- (B)
- FeO/OH showing high concentrations of elements, such as Cr, V, U, Y, Ge and Ga, that occur in Zn non-sulfides only under unusual conditions (e.g., nature of the primary ore, exotic input of material, alteration history, etc.).
5.1.1. Hakkari Deposit
5.1.2. Jabali Deposit
5.1.3. Cristal Deposit
5.1.4. Kabwe Deposit
6. Conclusions
- (A)
- FeO/OH occurring in non-sulfide ores evolving by direct replacement of sulfides and/or where pyrite is abundant in the primary ore (Cristal and Hakkari, respectively), and where a negligible buffering of the solutions led to an acidity-driven ore-formation process, are significantly enriched in Zn, Si, Pb, Ga and Ge.
- (B)
- FeO/OH occurring in non-sulfide ores where the host carbonate rocks play a key role in buffering the solution (Jabali) are depleted in Zn, Pb, Si and Ge, and show relatively high contents in elements supplied to the system by the dissolution of dolomite (i.e., Mn).
- (C)
- FeO/OH occurring in deposits where the input of exotic phases from the country rocks is significant may concentrate high amounts of unconventional metals (i.e., Cr and Co at Kabwe; Y at Cristal), depending on whether optimal pH-Eh conditions occur.
- (D)
- FeO/OH occurring in the Kabwe ore body show a heterogeneous geochemical signature pointing to variable environmental conditions governing the ore formation process. Some FeO/OH show relatively high V and U concentrations, which indicate locally prevailing more basic conditions during the alteration process. Conversely, a few FeO/OH occurring in the intensively oxidized samples have high Zn and Si, commonly characteristic of an acid-driven formation process.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Large, D. The geology of nonsulfide zinc deposits—An overview. Erzmetall 2001, 54, 264–274. [Google Scholar]
- Hitzman, M.W.; Reynolds, N.A.; Sangster, D.F.; Allen, C.R.; Carman, C. Classification, genesis, and exploration guides for nonsulfide zinc deposits. Econ. Geol. 2003, 98, 685–714. [Google Scholar] [CrossRef]
- Boni, M.; Mondillo, N. The “Calamines” and the “Others”: The great family of supergene nonsulfide zinc ores. Ore Geol. Rev. 2015, 67, 208–233. [Google Scholar] [CrossRef]
- Taylor, R. Gossan and Leached Cappings, Field Assessment; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Fendorf, S.; Eick, M.J.; Grossl, P.; Sparks, D.L. Arsenate and Chromate Retention Mechanisms on Goethite. 1. Surface Structure. Environ. Sci. Technol. 1997, 31, 315–320. [Google Scholar] [CrossRef]
- Villalobos, M.; Leckie, J.O. Surface complexation modeling and FTIR study of carbonate adsorption to goethite. J. Colloid. Interface Sci. 2001, 235, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Antelo, J.; Avena, M.; Fiol, S.; Lopez, R.; Arce, F. Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite-water interface. J. Colloid. Interface Sci. 2005, 285, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; WILEY-VCH Verlag: Weinheim, Germany, 2003; p. 664. [Google Scholar]
- Granados-Correa, F.; Corral-Capulin, N.G.; Olguin, M.T.; Acosta-Leon, C.E. Comparison of the Cd(II) adsorption processes between boehmite (gamma-AlOOH) and goethite (alpha-FeOOH). Chem. Eng. J. 2011, 171, 1027–1034. [Google Scholar]
- Perelomov, L.V.; Pinskiy, D.L.; Violante, A. Effect of organic acids on the adsorption of copper, lead, and zinc by goethite. Eurasian Soil Sci. 2011, 44, 22–28. [Google Scholar] [CrossRef]
- Mikulski, S.Z.; Oszczepalski, S.; Sadłowska, K.; Chmielewski, A.; Małek, R. Trace Element Distributions in the Zn-Pb (Mississippi Valley-Type) and Cu-Ag (Kupferschiefer) Sediment-Hosted Deposits in Poland. Minerals 2020, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Blengini, G.A.; Nuss, P.; Dewulf, J.; Nita, V.; Peirò, L.T.; Vidal-Legaz, B.; Pellegrini, M. EU methodology for critical raw materials assessment: Policy needs and proposed solutions for incremental improvements. Resour. Policy 2017, 53, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Frenzel, M.; Kullik, J.; Reuter, M.A.; Gutzmer, J. Raw material ‘criticality’—Sense or nonsense? J. Phys. Appl. Phys. 2017, 50, 123002. [Google Scholar] [CrossRef]
- Bernstein, L.R. Geology and mineralogy of the Apex germanium-gallium mine, Washington County, Utah. USGS 1986, 1577, 1–9. [Google Scholar]
- Bernstein, L.R.; Waychunas, G.A. Germanium crystal chemistry in hematite and goethite from the Apex Mine, Utah, and some new data on germanium in aqueous solution and in stottite. Geochim. Cosmochim. Acta 1987, 51, 623–630. [Google Scholar] [CrossRef]
- Mondillo, N.; Boni, M.; Balassone, G.; Joachimski, M.; Mormone, A. The Jabali nonsulfide Zn–Pb–Ag deposit, western Yemen. Ore Geol. Rev. 2014, 61, 248–267. [Google Scholar] [CrossRef]
- Mondillo, N.; Boni, M.; Balassone, G.; Villa, I.M. The Yanque prospect (Peru): From polymetallic Zn-Pb mineralization to a nonsulfide deposit. Econ. Geol. 2014, 109, 1735–1762. [Google Scholar] [CrossRef] [Green Version]
- Mondillo, N.; Arfè, G.; Herrington, R.; Boni, M.; Wilkinson, C.; Mormone, A. Germanium enrichment in supergene settings: Evidence from the Cristal nonsulfide Zn prospect, Bongará district, northern Peru. Miner. Depos. 2018, 53, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Mondillo, N.; Herrington, R.; Boyce, A.J.; Wilkinson, C.; Santoro, L.; Rumsey, M. Critical elements in non-sulfide Zn deposits: A reanalysis of the Kabwe Zn-Pb ores (central Zambia). Miner. Mag. 2018, 82 (Suppl. 1), S89–S114. [Google Scholar] [CrossRef] [Green Version]
- Mondillo, N.; Accardo, M.; Boni, M.; Boyce, A.; Herrington, R.; Rumsey, M.; Wilkinson, C. New insights into the genesis of willemite (Zn2SiO4) from zinc nonsulfide deposits, through trace elements and oxygen isotope geochemistry. Ore Geol. Rev. 2020, 118, 103307. [Google Scholar] [CrossRef]
- Santoro, L.; Boni, M.; Herrington, R.; Clegg, A. The Hakkari nonsulfide Zn-Pb deposit in the context of other nonsulfide Zn–Pb deposits in the Tethyan Metallogenic Belt of Turkey. Ore Geol. Rev. 2013, 53, 244–260. [Google Scholar] [CrossRef]
- Santoro, L.; Boni, M.; Rollinson, G.K.; Mondillo, N.; Balassone, G.; Clegg, A.M. Mineralogical characterization of the Hakkari nonsulfide Zn(Pb) deposit (Turkey): The benefits of QEMSCAN®. Miner. Eng. 2014, 69, 29–39. [Google Scholar] [CrossRef]
- Santoro, L.; Rollinson, G.K.; Boni, M.; Mondillo, N. Automated Scanning Electron Microscopy (QEMSCAN@) based mineral identification and quantification of the Jabali Zn-Pb-Ag Nonsulfide deposit (Yemen). Econ. Geol. 2015, 110, 1083–1099. [Google Scholar] [CrossRef]
- Navarro-Ciurana, D.; Campos-Quispe, L.; Cardellach, E.; Catena, E.; Gómez-Gras, D.; Griera, A.; Corbella, M. Mineralogical and geochemical characterization of the Riópar non-sulfide Zn-(Fe-Pb) deposits (Prebetic Zone, SE Spain). Ore Geol. Rev. 2016, 79, 515–532. [Google Scholar] [CrossRef]
- Arfè, G.; Mondillo, N.; Boni, M.; Balassone, G.; Joachimski, M.; Mormone, A.; Di Palma, T. The karst hosted Mina Grande nonsulfide zinc deposit, Bongará district (Amazonas region, Peru). Econ. Geol. 2017, 112, 1089–1110. [Google Scholar]
- Arfè, G.; Mondillo, N.; Boni, M.; Joachimski, M.; Balassone, G.; Mormone, A.; Santoro, L.; Castro Medrano, E. The Rio Cristal Zinc prospect (Amazonas region, Northern Peru). Part II: An example of supergene zinc enrichments in tropical areas. Ore Geol. Rev. 2018, 95, 1076–1105. [Google Scholar]
- Stavinga, D.; Jamieson, H.; Paradis, S.; Falck, H. Geochemical and Mineralogical Controls on Metal(loid) Mobility in the Oxide Zone of the Prairie Creek Deposit, NWT. Geochem. Explor. Environ. A 2017, 17, 21. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Günay, Y.; Şenel, M. Turkey Geological Map, 1:500,000 Scale, Cizre Sheet, No 18; General Directorate of Mineral Research and Exploration: Ankara, Turkey, 2002. [Google Scholar]
- Yigit, O. Mineral deposits of Turkey in relation to Tethyan metallogeny: Implications for future mineral exploration. Econ. Geol. 2009, 104, 19–51. [Google Scholar] [CrossRef]
- Venter, M.; Robertson, M. Desktop, Remote Sensing and Field Validation; Internal Report; Red Crescent Resources A.Ş.: Ankara, Turkey, 2009. [Google Scholar]
- Grodner, M. The Hakkari zinc oxide project, Turkey. In Proceedings of the IAEG–ZINC Conference, Cork, Ireland, 17–19 September 2010; pp. 23–26. Available online: http://www.iaeg.org/docs/2010/Zinc2010_Abstracts.pdf (accessed on 12 April 2020).
- MSA Group Ltd. Technical Report on the Hakkari Zinc Project, NI 43–101, 3 August 2011. Available online: www.sedar.com (accessed on 12 March 2020).
- Ceyhan, N. Lead isotope geochemistry of Pb-Zn deposits from Eastern Taurides, Turkey. Unpub. Master’s Thesis, Graduate School of Natural and Applied Sciences of the Middle East Technical University, Ankara, Turkey, 2003; p. 105. [Google Scholar]
- Reynolds, N.; Large, D. Tethyan zinc–lead metallogeny in Europe, North Africa, and Asia. Econ. Geol. Spec. Publ. 2010, 15, 339–365. [Google Scholar]
- Hanilçi, N.; Öztürk, H.; Kasapci, C. Carbonate-Hosted Pb-Zn Deposits of Turkey. In Mineral Resources of Turkey; Springer: Cham, Switzerland, 2019; pp. 497–533. [Google Scholar]
- Al Ganad, I.; Lagny, P.; Lescuyer, J.L.; Rambo, C.; Touray, J.C. Jabali, a Zn-Pb-(Ag) carbonate-hosted deposit associated with Late Jurassic rifting in Yemen. Miner. Depos. 1994, 29, 44–56. [Google Scholar] [CrossRef]
- As-Saruri, M.A.; Sorkhabi, R.; Baraba, R. Sedimentary basins of Yemen: Their tectonic development and lithostratigraphic cover. Arab. J. Geosci. 2010, 3, 515–527. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusion evidence for the genesis of ores in sedimentary and volcanic rocks. In Ores in Sediments, Sedimentary and Volcanic Rocks; Wolf, K.H., Ed.; Elsevier: Amsterdam, The Netherlands, 1976; Volume 2, pp. 67–110. [Google Scholar]
- Ostendorf, J.; Henjes-Kunst, F.; Mondillo, N.; Boni, M.; Schneider, J.; Gutzmer, J. Formation of Mississippi Valley–type deposits linked to hydrocarbon generation in extensional tectonic settings: Evidence from the Jabali Zn-Pb-(Ag) deposit (Yemen). Geology 2015, 43, 1055–1058. [Google Scholar]
- Anglo Peruana. A Re–evaluation of the Geology and Mineralization of the Charlotte Bongará Zinc Project, Amazonas, Northern Peru: Technical Report; Anglo Peruana Terra S.A (former Consultora Minera Anglo Peruana S.A.): Lima, Peru, 2005. [Google Scholar]
- Workman, A.W.; Reddick, J. Technical Report on the Bongará Zinc Project, Yambrasbamba district, Amazonas Region, Northern Peru for Zinc One Resources Inc. 2019 p. 184. Available online: https://zincone.com/site/assets/files/2341/bonara_zinc_mine_technical_report.pdf (accessed on 20 April 2020).
- Mišković, A.; Spikings, R.A.; Chew, D.M.; Košler, J.; Ulianov, A.; Schaltegger, U. Tectonomagmatic evolution of Western Amazonia: Geochemical characterization and zircon U-Pb geochronologic constraints from the Peruvian Eastern Cordilleran granitoids. Geol. Soc. Am. Bull. 2009, 121, 1298–1324. [Google Scholar] [CrossRef]
- Brophy, J.A. Technical Report on the Rio Cristal Resources Corp. Bongará Zinc Project; NI 43–101; 2012; p. 104. Available online: https://www.smv.gob.pe/ConsultasP8/temp/Amended_restated_TechnicalReport_NI43101.pdf (accessed on 20 April 2020).
- Reid, C.J. Stratigraphy and Mineralization of the Bongara MVT Zinc-lead District, Northern Peru. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2001; p. 179. [Google Scholar]
- Basuki, N.I. Post-Early Cretaceous MVT Zn-Pb Mineralisation, Bongara Area, Northern Peru Fluid Characteristics and Constraints on Deposition Mechanisms. Unpublished Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2006; p. 318. [Google Scholar]
- Basuki, N.I.; Spooner, E.T.C. Fluid evolution and flow direction of MVT Zn-Pb related basinal brines, Bongará area, northern Peru: CL and fluid inclusion data [abs.]. In Proceedings of the Geological-Mineralogical Association of Canada-Society of Economic Geologists—Society for Geology Applied to Mineral Deposits Meeting, Quebec, QC, Canada, 26–28 May 2008; p. 13. [Google Scholar]
- Basuki, N.I.; Spooner, E.T.C. Post-early Cretaceous Mississippi Valley Type Zn-Pb mineralization in the Bongará Area, Northern Peru: Fluid evolution and Paleo-Flow from fluid inclusions evidence. Explor. Min. Geol. 2009, 18, 25–39. [Google Scholar] [CrossRef]
- Kamona, A.F.; Friedrich, G.H. Geology, mineralogy and stable isotope geochemistry of the Kabwe carbonate-hosted Pb–Zn deposit, Central Zambia. Ore Geol. Rev. 2007, 30, 217–243. [Google Scholar] [CrossRef]
- Kampunzu, A.B.; Cailteux, J.L.H.; Kamona, A.F.; Intiomale, M.M.; Melcher, F. Sediment hosted Zn-Pb-Cu deposits in the Central African Copperbelt. Ore Geol. Rev. 2009, 35, 263–297. [Google Scholar] [CrossRef]
- Moore, T.A. The Geology of the Chisamba Area: Explanation of Degree Sheet 1428, SW Quarter; Lusaka Govt. Priner: Lusaka, Zambia, 1964.
- Cairney, T.; Kerr, C.D. The geology of the Kabwe area: Explanation of degree sheet 1428, NW quarter. Geol. Surv. Zambia Rep. 1998, 47, 40. [Google Scholar]
- Kamona, A.F. The Carbonate-Hosted Kabwe Pb– Zn Deposit, Central Zambia. Ph.D. Thesis, Technical University of Aachen, Aachen, Germany, 1993. [Google Scholar]
- Terracciano, R. Willemite Mineralisation in Namibia and Zambia. Ph.D. Thesis, Università degli Studi di Napoli Federico II, Napoli, Italy, 2008; p. 178. [Google Scholar]
- Leach, D.L.; Sangster, D.; Kelley, K.; Large, R.; Garven, G.; Allen, C.; Gutzmer, J.; Walters, S. Sediment-hosted lead-zinc deposits: A global perspective. Econ. Geol. 2005, 100, 561–607. [Google Scholar]
- Heijlen, W.; Banks, D.A.; Muchez, P.; Stensgard, B.M.; Yardley, B.W.D. The nature of mineralizing fluids of the Kipushi Zn-Cu deposit, Katanga, Democratic Republic of Congo: Quantitative fluid inclusion analysis using Laser Ablation ICP-MS and bulk Crush-Leach methods. Econ. Geol. 2008, 103, 1459–1482. [Google Scholar] [CrossRef]
- De Putter, T.; Ruffet, G. Supergene manganese ore records 75 Myr-long Campanian to Pleistocene geodynamic evolution and weathering history of the Central African Great Lakes Region—Tectonics drives, climate assists. Gondwana Res. 2020, 83, 96–117. [Google Scholar] [CrossRef]
- Zachariáš, J.; Wilkinson, J. ExLAM 2000: Excel VBA application for processing of transient signal from laser ablation (LA-ICPMS) of fluid inclusions and solid phases. In Proceedings of the ECROFI-XIX Biennial Conference on European Current Research on Fluid Inclusions, Bern, Switzerland, 17–20 July 2007. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics with R; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Martín-Fernández, J.A.; Barceló-Vidal, C.; Pawlowsky-Glahn, V. Dealing with zeros and missing values in compositional data sets using nonparametric imputation. Math. Geol. 2003, 35, 253–278. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Miner. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Davidson, L.E.; Shaw, S.; Benning, L. The kinetics and mechanisms of schwertmannite transformation to goethite and hematite under alkaline conditions. Am. Mineral. 2008, 93, 1326–1337. [Google Scholar] [CrossRef]
- Vu, H.P.; Shaw, S.; Brinza, L.; Benning, L.G. Crystallization of hematite (alpha-Fe(2)O(3)) under alkaline condition: The effects of Pb. Cryst. Growth Des. 2010, 10, 1544–1551. [Google Scholar] [CrossRef]
- Vu, H.P.; Shaw, S.; Brinza, L.; Benning, L.G. Partitioning of Pb(II) during goethite and hematite crystallization: Implications for Pb transport in natural systems. Appl. Geochem. 2013, 39, 119–128. [Google Scholar] [CrossRef]
- Schwertmann, U.; Murad, E. Effect of pH on the formation of goethite and hematite from ferrihydrite. Clays Clay Min. 1983, 31, 277–284. [Google Scholar] [CrossRef]
- Schwertmann, U.; Friedl, J.; Stanjek, H. From Fe (III) ions to ferrihydrite and then to hematite. J. Colloid. Interface Sci. 1999, 209, 215–223. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory, 2nd ed.; Wiley-VCH Verlag: Weinheim, Germany, 2000; p. 188. [Google Scholar]
- Schwertmann, U.; Stanjek, H.; Becher, H. Long-term in vitro transformation of 2-line ferrihydrite to goethite/hematite at 4, 10, 15 and 25 °C. Clay Min. 2004, 39, 433–438. [Google Scholar] [CrossRef]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Hieronymus, B.; Kotschoubey, B.; Boulègue, J. Gallium behaviour in some contrasting lateritic profiles from Cameroon and Brazil. J. Geochem. Explor. 2001, 72, 147–163. [Google Scholar] [CrossRef]
- Lu, P.; Nuhfer, N.; Kelly, S.; Li, Q.; Konishi, H.; Elswick, E.; Zhu, C. Lead coprecipitation with iron oxyhydroxide nano-particles. Geochim. Cosmochim. Acta. 2011, 75, 4547–4561. [Google Scholar] [CrossRef] [Green Version]
- Swedlund, P.; Webster, J.; Miskelly, G. Goethite adsorption of Cu(II), Pb(II), Cd(II), and Zn(II) in the presence of sulfate: Properties of the ternary complex. Geochim. Cosmochim. Acta 2009, 73, 1548–1562. [Google Scholar] [CrossRef]
- Dash, B.; Rath, S.S. A thorough understanding of the adsorption of Ni (II), Cd (II) and Zn (II) on goethite using experiments and molecular dynamics simulation. Sep. Purif. Technol. 2020, 240, 116649. [Google Scholar] [CrossRef]
- Boni, M. Non-sulfide zinc deposits: A new-(old) type of economic mineralization. SGA News 2003, 15, 6–11. [Google Scholar]
- McPhail, D.C.; Summerhayes, E.; Jayaratne, V.; Christy, A. Hemimorphite solubility and stability of low-T zinc minerals. Geochim. Cosmochim. Acta 2006, 70, 36. [Google Scholar] [CrossRef]
- Warren, J. Dolomite: Occurrence, evolution and economically important associations. Earth Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Dublet, G.; Juillot, F.; Brest, J.; Noël, V.; Fritsch, E.; Proux, O.; Morin, G. Vertical changes of the Co and Mn speciation along a lateritic regolith developed on peridotites (New Caledonia). Geochim. Cosmochim. Acta 2017, 217, 1–15. [Google Scholar] [CrossRef]
- Liu, H.; Pourret, O.; Guo, H.; Bonhoure, J. Rare earth elements sorption to iron oxyhydroxide: Model development and application to groundwater. Appl. Geochem. 2017, 87, 158–166. [Google Scholar] [CrossRef]
- Boni, M.; Terracciano, R.; Evans, N.J.; Laukamp, C.; Schneider, J.; Bechstaädt, T. Genesis of vanadium ores in the Otavi Mountainland, Namibia. Econ. Geol. 2007, 102, 441–469. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Vanadium (V) adsorption onto goethite (α-FeOOH) at pH 1.5 to 12: A surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy. Geochim. Cosmochim. Acta 2004, 68, 1723–1733. [Google Scholar] [CrossRef]
- Sherman, D.M.; Peacock, C.L.; Hubbard, C.G. Surface complexation of U (VI) on goethite (α-FeOOH). Geochim. Cosmochim. Acta 2008, 72, 298–310. [Google Scholar] [CrossRef] [Green Version]
- Carlisle, D. Concentration of uranium and vanadium in calcretes and gypcretes. Geol. Soc. Lond. Spec. Publ. 1983, 11, 185–195. [Google Scholar] [CrossRef]
- Freyssinet, P.; Butt, C.R.M.; Morris, R.C.; Piantone, P. Ore-forming processes related to lateritic weathering. Econ. Geol. 2005, 100, 681–722. [Google Scholar]





| Deposit | Specimen ID | Description | Ref. |
|---|---|---|---|
| Jabali | JS Mon 30 | Gossan | This study |
| Hakkari | H2050 | Gossan | |
| H2058 | Gossan | ||
| Kabwe | OR5305 | Zinc-silicate ore | Mondillo et al. [19] |
| OR5309 | Zinc-silicate ore | ||
| BM.1985,MI29629 2/2 | Partly oxidized lead-zinc ore | ||
| BM.1985,MI29631 | Oxidized ore | ||
| BM1930-372 | Partly oxidized sulfide-rich ore | ||
| BM.1985,MI10900 | Hemimorphite and FeO/OH | ||
| Cristal | CR13-1 | Gossan | Mondillo et al. [18] |
| CR18-19 | Hemimorphite and FeO/OH |
| Deposit | Kabwe 3 | Hakkari | Kabwe 4 | Kabwe 2 | Jabali | Kabwe 1 | Cristal |
|---|---|---|---|---|---|---|---|
| Group | a | b | c | c | d | e | f |
| Hakkari | Jabali | Cristal | Kabwe | |
|---|---|---|---|---|
| Hypogene mineralogy | Sphalerite, pyrite, galena and barite | sphalerite, galena, pyrite/marcasite | Sphalerite, galena, pyrite | Sphalerite, galena, chalcopyrite, willemite, pyrite, Ge-sulfides |
| Supergene mineralogy | Hemimorphite, FeO/OH, Zn-carbonates, Zn-clays | Smithsonite, hydrozincite, hemimorphite, greenockite, FeO/OH | Hemimorphite, smithsonite, FeO/OH, Zn-clays | Willemite, Zn-Pb-vanadates, smithsonite, phosphates, FeO/OH |
| Geochemical signature of FeO/OH | Zn-Pb-Si-Ca-Ga | Mn-Mg | Zn-Ge-Y | Zn-Si-(Pb-Ga) and U- V-(Cr-Co) |
| Enrichment mechanism | Acidity-driven process (direct replacement of sulfides) | Wall-rock replacement process | Acidity-driven process (direct replacement of sulfides) | Local acidity-driven process, superimposed by alkaline process caused by the evolution under arid climate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, L.; Putzolu, F.; Mondillo, N.; Boni, M.; Herrington, R. Influence of Genetic Processes on Geochemistry of Fe-oxy-hydroxides in Supergene Zn Non-Sulfide Deposits. Minerals 2020, 10, 602. https://doi.org/10.3390/min10070602
Santoro L, Putzolu F, Mondillo N, Boni M, Herrington R. Influence of Genetic Processes on Geochemistry of Fe-oxy-hydroxides in Supergene Zn Non-Sulfide Deposits. Minerals. 2020; 10(7):602. https://doi.org/10.3390/min10070602
Chicago/Turabian StyleSantoro, Licia, Francesco Putzolu, Nicola Mondillo, Maria Boni, and Richard Herrington. 2020. "Influence of Genetic Processes on Geochemistry of Fe-oxy-hydroxides in Supergene Zn Non-Sulfide Deposits" Minerals 10, no. 7: 602. https://doi.org/10.3390/min10070602
APA StyleSantoro, L., Putzolu, F., Mondillo, N., Boni, M., & Herrington, R. (2020). Influence of Genetic Processes on Geochemistry of Fe-oxy-hydroxides in Supergene Zn Non-Sulfide Deposits. Minerals, 10(7), 602. https://doi.org/10.3390/min10070602





