Crystallinity and Play-of-Colour in Gem Opal with Digit Patterns from Wegel Tena, Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
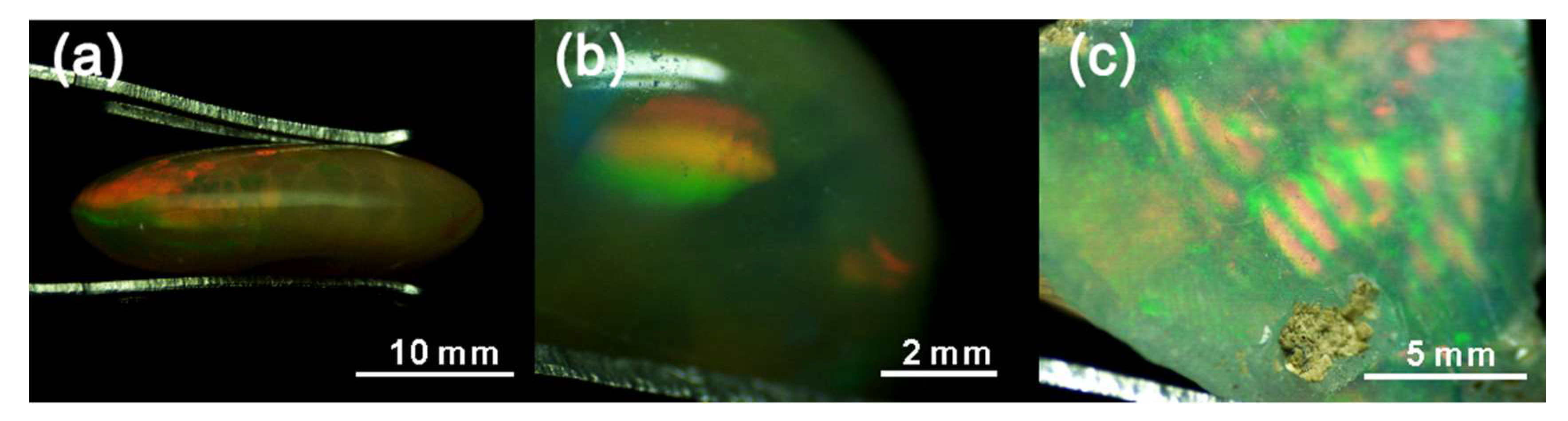
3.1. Appearance and Gemmological Properties
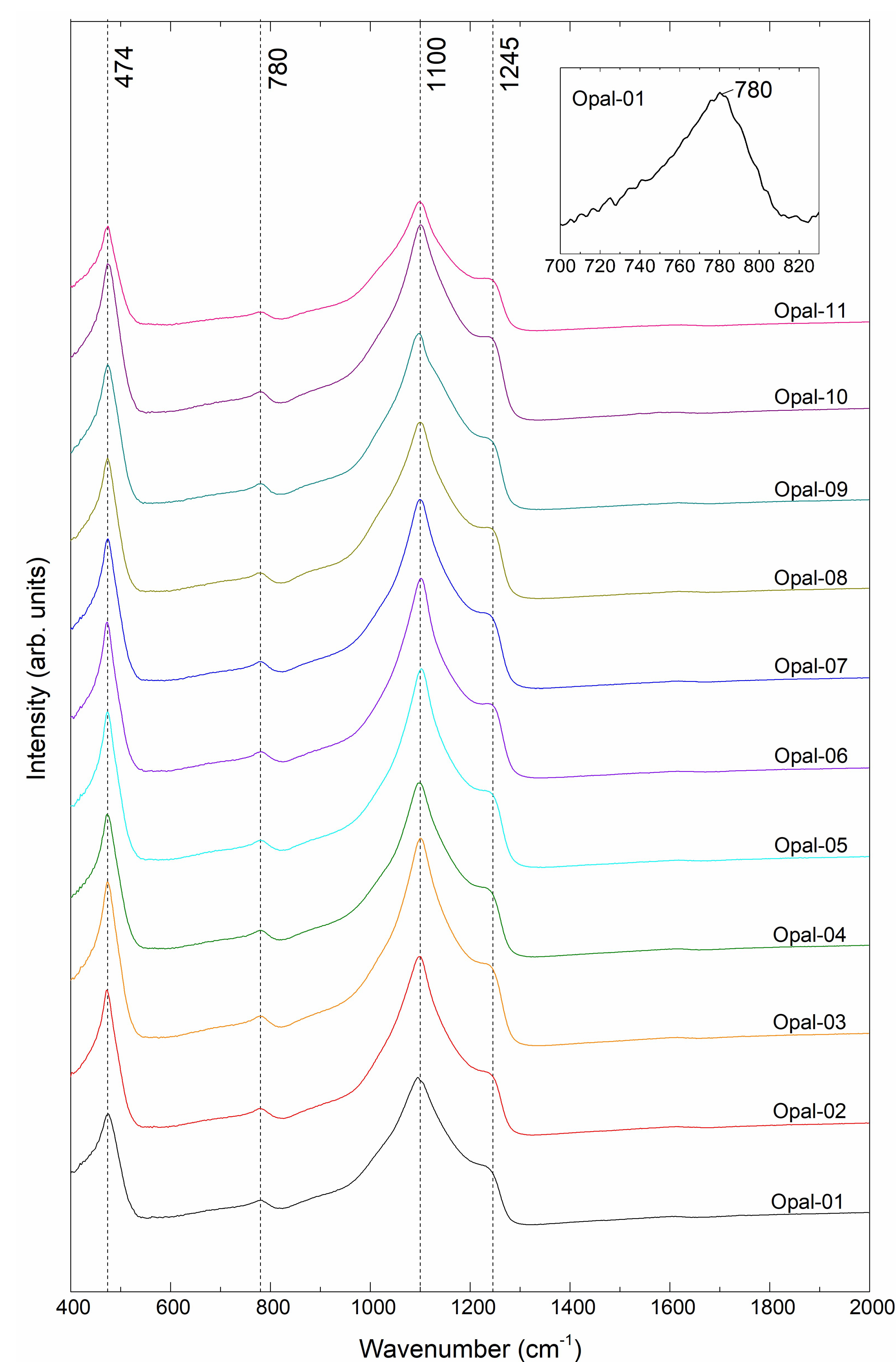
3.2. FTIR Spectroscopy
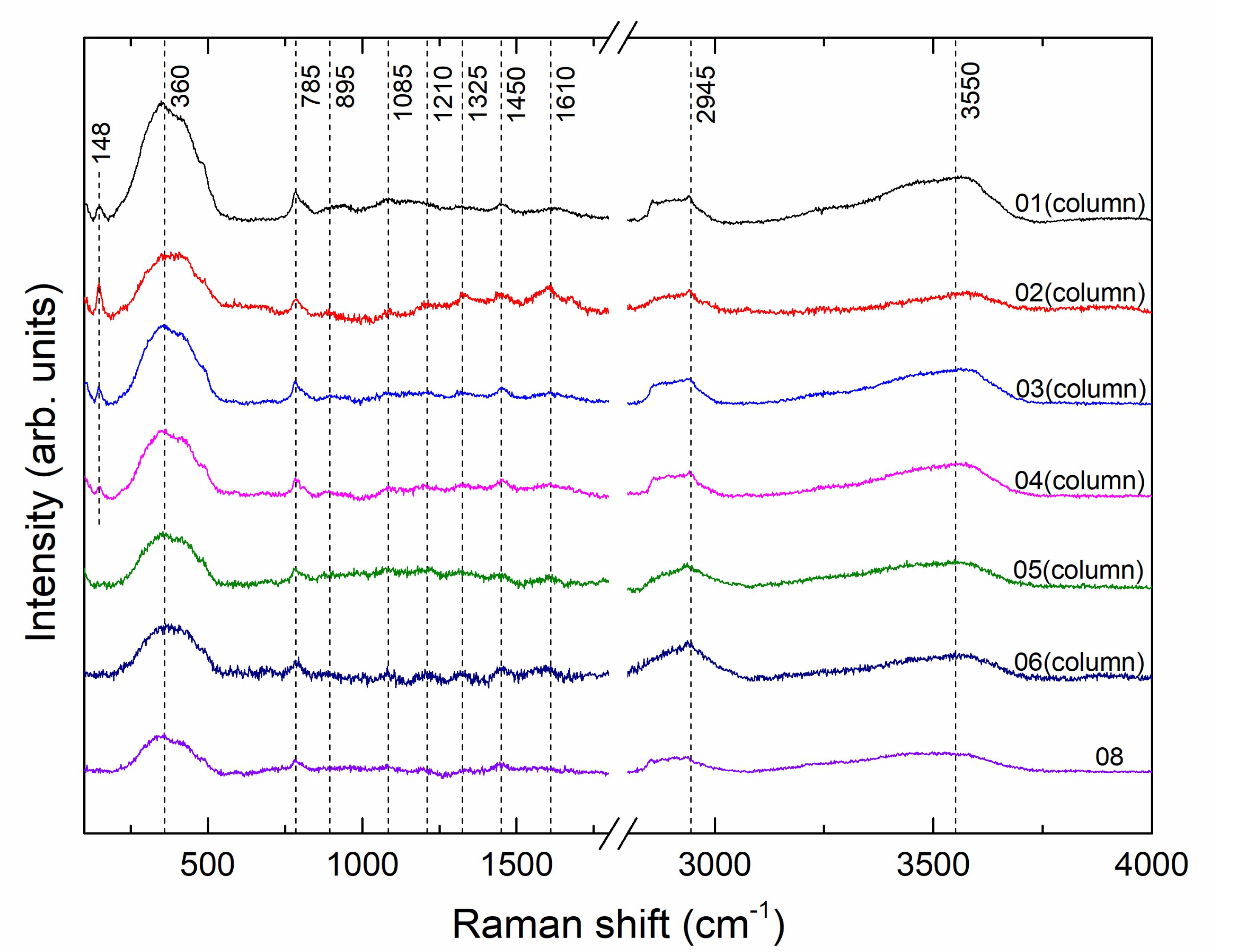
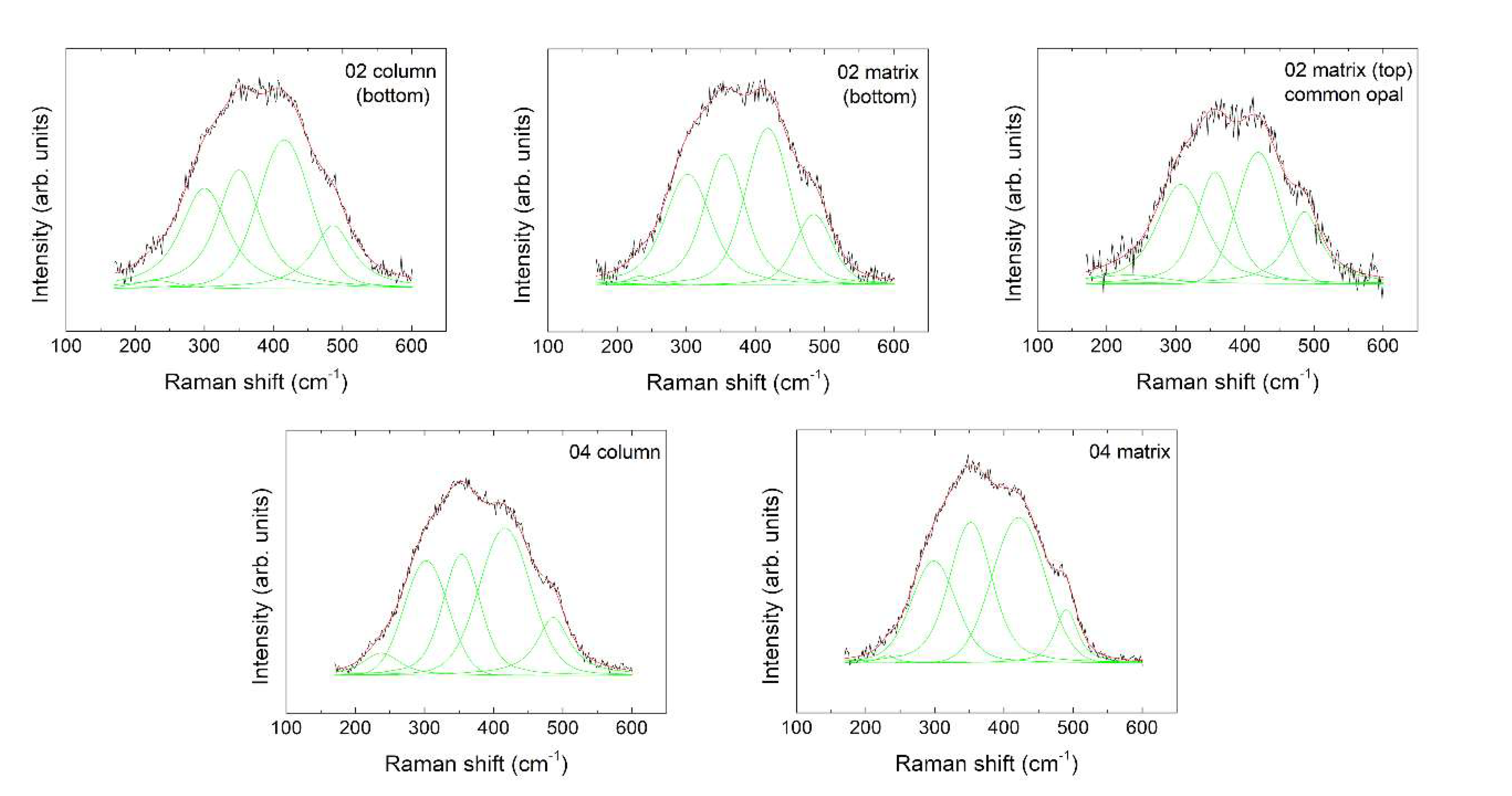
3.3. Micro-Raman Spectroscopy
3.4. Microstructure
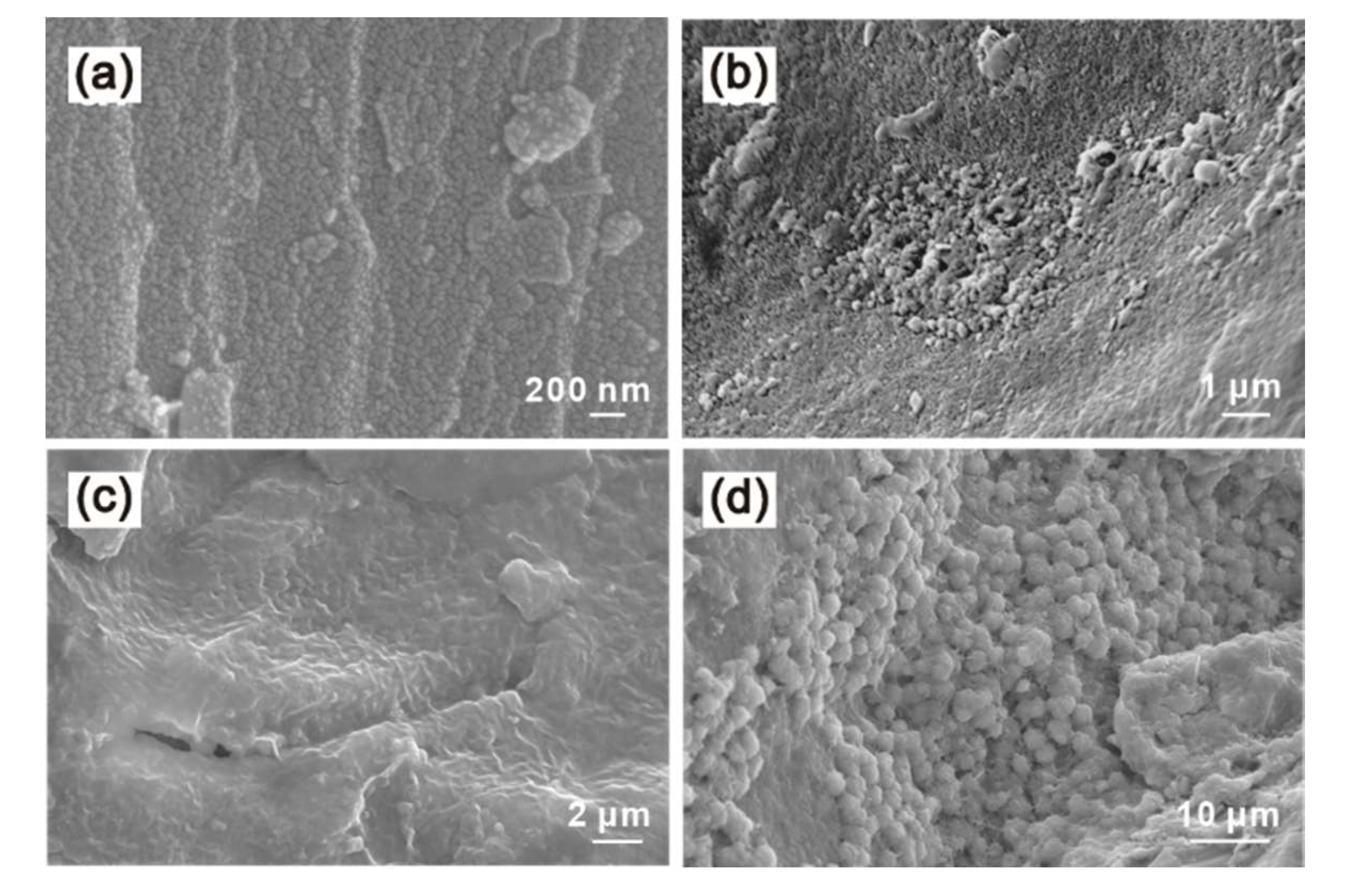
3.4.1. SEM Observations
3.4.2. TEM Observations
4. Discussion
4.1. Differences Between Columns and Matrix in Microcrystalline Opal, and Steps of Digit Formation
4.2. The Cause of Play-of-Colour and Iridescence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, J.B.; Segnit, E.R. The nature of opal I. nomenclature and constituent phases. J. Geol. Soc. Austral. 1971, 18, 57–68. [Google Scholar] [CrossRef]
- Yu, X.Y. Colored Gemmology, 2nd ed.; Geological Publishing House: Beijing, China, 2009; p. 10. [Google Scholar]
- Wilson, J. The structure of opal-CT revisited. J. Non-Cryst. Solids 2014, 405, 68–75. [Google Scholar] [CrossRef]
- Curtis, N.J.; Gascooke, J.R.; Johnston, M.R.; Pring, A. A review of the classification of opal with reference to recent new localities. Mineral 2019, 9, 299. [Google Scholar] [CrossRef] [Green Version]
- Adamo, I.; Ghisoli, C.; Caucia, F. A contribution to the study of ftir spectra of opals. Neues Jahrb. Mineral. Abh. J. Mineral. Geochem. 2010, 187, 63–68. [Google Scholar] [CrossRef]
- Graetsch, H.; Flörke, O.W.; Miehe, G. The nature of water in chalcedony and opal-c from brazilian agate geodes. Phys. Chem. Miner. 1985, 12, 300–306. [Google Scholar] [CrossRef]
- Awadh, S.M.; Yaseen, Z.M. Investigation of silica polymorphs stratified in siliceous geode using ftir and xrd methods. Mater. Chem. Phys. 2019, 228, 45–50. [Google Scholar] [CrossRef]
- Smallwood, A.; Thomas, P.S.; Ray, A.S. Characterisation of sedimentary opals by fourier transform raman spectroscopy. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 1997, 53, 2341–2345. [Google Scholar] [CrossRef]
- Fritsch, E.; Rondeau, B.; Ostrooumov, M.; Lasnier, B.; Marie, A.M.; Barreau, A.; Wery, J.; Connoué, J.; Lefrant, S. Découvertes récentes sur l’opale [recent discoveries about opal]. Rev. Gemmol. AFG 1999, 138/139, 34–40. [Google Scholar]
- Ostrooumov, M.; Fritsch, E.; Lasnier, B.; Lefrant, S. Spectres raman des opales: Aspect diagnostic et aide à la classification. Eur. J. Miner. 1999, 11, 899–908. [Google Scholar] [CrossRef]
- Rodgers, K.A.; Hampton, W.A. Laser raman identification of silica phases comprising microtextural components of sinters. Miner. Mag. 2003, 67, 1–13. [Google Scholar] [CrossRef]
- Ilieva, A.; Mihailova, B.; Tsintsov, Z.; Petrov, O. Structural state of microcrystalline opals: A Raman spectroscopic study. Am. Miner. 2007, 92, 1325–1333. [Google Scholar] [CrossRef]
- Sodo, A.; Municchia, A.C.; Barucca, S.; Bellatreccia, F.; Della-Ventura, G.; Butini, F.; Ricci, M.A. Raman, FT-IR and XRD investigation of natural opals. J. Raman Spectrosc. 2016, 47, 1444–1451. [Google Scholar] [CrossRef]
- Rondeau, B.; Fritsch, E.; Guiraud, M.; Renac, C. Opals from slovakia (˝hungarian˝ opals): A re-assessment of the conditions of formation. Eur. J. Miner. 2004, 16, 789–799. [Google Scholar] [CrossRef]
- Koivula, J.I.; Kammerling, R.C.; Fritsch, E. Gem news: Opal from ethiopia. Gems Gemol. 1994, 30, 52–53. [Google Scholar]
- Johnson, M.L.; Kammerling, R.C.; DeGhionno, D.G.; Koivula, J.I. Opal from the shewa province, ethiopia. Gems Gemol. 1996, 32, 112–120. [Google Scholar] [CrossRef]
- Gauthier, J.P.; Mazzero, F.; Mandaba, Y.; Fritsch, E. Opal from ethiopia: Usual gemology and unusual characteristics. Rev. Gemmol. AFG 2004, 149, 15–23. [Google Scholar]
- Rondeau, B.; Fritsch, E.; Mazzero, F.; Gauthier, J.P.; Cenki-Tok, B.; Bekele, E.; Gaillou, E. Play-of-color opal from wegel tena, wollo province, ethiopia. Gems Gemol. 2010, 46, 90–105. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, E.; Rondeau, B. Gem news international: Rare optical phenomenon in play-of-color opal. Gems Gemol. 2009, 45, 147–148. [Google Scholar]
- Mazzero, F.; Gauthier, J.P.; Rondeau, B.; Fritsch, E.; Bekele, E. Nouveau gisement d’opales d’ethiopie dans la province du welo: Premières informations [New deposit of Ethiopian opals in Welo Province: Early information]. Rev. Gemmol. AFG 2009, 167, 4–5. [Google Scholar]
- Rondeau, B.; Mazzero, F.; Bekele, E.; Gauthier, F.P.; Fritsch, E. Gem news international: New play-of-color opal from welo, ethiopia. Gems Gemol. 2009, 45, 59–60. [Google Scholar]
- Rondeau, B.; Cenki-Tok, B.; Fritsch, E.; Mazzero, F.; Gauthier, J.P.; Bodeur, Y.; Bekele, E.; Gaillou, E.; Ayalew, D. Geochemical and petrological characterization of gem opals from wegel tena, wollo, ethiopia: Opal formation in an oligocene soil. Geochem. Explor. Environ. Anal. 2012, 12, 93–104. [Google Scholar] [CrossRef]
- Rondeau, B.; Gauthier, J.P.; Mazzero, F.; Bodeur, Y.; Chauvire, B. On the origin of digit patterns in gem opal. Gems Gemol. 2013, 49, 138–146. [Google Scholar] [CrossRef]
- Lippincott, E.R.; Vanvalkenburg, A.; Weir, C.E.; Bunting, E.N. Infrared studies on polymorphs of silicon dioxide and germanium dioxide. J. Res. Natl. Bur. Stand. 1958, 61, 61–70. [Google Scholar] [CrossRef]
- Rice, S.B.; Freund, H.; Huang, W.L.; Clouse, J.A.; Isaacs, C.M. Application of fourier transform infrared spectroscopy to silica diagenesis: The opal-A to opal-CT transformation. J. Sediment. Res. 1995, 65, 639–647. [Google Scholar] [CrossRef]
- Schmidt, P.; Fröhlich, F. Temperature dependent crystallographic transformations in chalcedony, SiO2, assessed in mid infrared spectroscopy. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2011, 78, 1476–1481. [Google Scholar] [CrossRef]
- Moenke, H.H.W. Silica, the three-dimensional silicates, borosilicates and beryllium silicates. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Science Press: Beijing, China, 1982; pp. 289–303. [Google Scholar]
- Wu, J.G. Fourier Transform. Infrared Spectroscopy Technology and Application; Scientific and Technology Documentation Press: Beijing, China, 1994; Volume 2, p. 722. [Google Scholar]
- Hu, Y.; Fan, L.W.; Xue, Q.F. Study on filling treatment and identification characteristics of opal from ethiopian. J. Gems Gemmol. 2013, 15, 32–37. [Google Scholar] [CrossRef]
- Zhong, Q.; Qi, L.J.; Shao, H.J.; Zhou, Y. Gemmological characteristics of green opals from brazil. J. Gems Gemmol. 2013, 15, 29–36. [Google Scholar] [CrossRef]
- Yan, J.; Hu, X.C.; Fang, B.; Tao, J.B.; Peng, Q.J.; Zhang, J. Study on the mineralogical and optical characteristics of blue opal from lishui investigated by XRF-SEM-XRD-FTIR. J. Rock Miner. Anal. 2014, 33, 795–801. [Google Scholar] [CrossRef]
- Eckert, J.; Gourdon, O.; Jacob, D.E.; Meral, C.; Monteiro, P.J.M.; Vogel, S.C.; Wirth, R.; Wenk, H. Ordering of water in opals with different microstructures. Eur. J. Miner. 2015, 27, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.J.; Li, L.P. Chemistry of opal from ethiopia. J. Gems Gemmol. 2015, 17, 23–28. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, X.W.; Wang, Y.; Fan, C.X.; Ye, H.; Cheng, Y.F.; Liu, D.Z. Infrared spectral features of SiO2 with different crystallinity and their implications. Infrared 2010, 31, 31–35. [Google Scholar] [CrossRef]
- Chen, H.S.; Sun, Z.Y.; Shao, J.C. Investigation on FT-IR spectroscopy for eight different sources of SiO2. Bull. Chin. Ceram. Soc. 2011, 30, 934–937. [Google Scholar] [CrossRef]
- Splett, A.; Splett, C.; Pilz, W. Dynamics of the raman background decay. J. Raman Spectrosc. 1997, 28, 481–485. [Google Scholar] [CrossRef]
- Zu, E.D.; Li, M.C.; Zhang, P.X. Study on jades of SiO2 by Raman spectroscopy. J. Kunming Univ. Sci. Technol. Nat. Sci. Ed. 2000, 35, 77–78. [Google Scholar] [CrossRef]
- Zarubin, D.P. The two-component bands at about 4500 and 800 cm−1 in infrared spectra of hydroxyl-containing silicas. Interpretation in terms of fermi resonance. J. Non-Cryst. Solids 2001, 286, 80–88. [Google Scholar] [CrossRef]
- Gaillou, E.; Mocquet, B.; Fritsch, E. Gem news international: A new gem material from Madagascar: A mixture of cristobalite and opal. Gems Gemol. 2004, 40, 339–340. [Google Scholar]
- Aguilar-Reyes, B.O.; Ostrooumov, M.; Fritsch, E. Estudio mineralógico de la desestabilización de ópalos Mexicanos. Rev. Mex. Cienc. Geol. 2005, 22, 391–400. [Google Scholar]
- Jiang, Y.; Cao, Q. Raman spectroscopic investigation of magnetized water, raw water and distilled water. Chin. J. Light Scatting 1992, 2, 102–106. [Google Scholar]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Li, Y.; Duan, Y.; Li, W. Study on raman spectrum of nanophase anatase. Spectrosc. Spectr. Anal. 2000, 6, 699–702. [Google Scholar] [CrossRef]
- RRUFF. Anatase R070582. Available online: Rruff.info/R070582 (accessed on 11 April 2020).
- Ku, H.H. Notes on the Use of Propagation of Error Formulas. J. Res. Nat. Bur. Stand. Sec. C: Eng. Inst. 1966, 70, 263–273. [Google Scholar] [CrossRef]
- Luo, J.; Liu, X.F.; Yan, X.X. Comparative analysis of the characteristics of australian opal and ethiopian opal. Superhard Mater. Eng. 2016, 28, 50–57. [Google Scholar] [CrossRef]
- Dong, Z.C. Discussion on the morphology of cristobalite and tridymite. Bull. Chin. Ceram. Soc. 1984, 6, 45–50. [Google Scholar] [CrossRef]
- Liesegang, M.; Tomaschek, F. Tracing the continental diagenetic loop of the opal-A to opal-CT transformation with X-ray diffraction. Sediment. Geol. 2020, 398, 105603. [Google Scholar] [CrossRef]
- Sanders, J.V. Diffraction of light by opals. Acta Cryst. Sect. A Found. Cryst. 1968, 24, 427–434. [Google Scholar] [CrossRef]
- Elzea, J.M.; Rice, S.B. TEM and X-ray diffraction evidence for cristobalite and tridymite stacking sequences in opal. Clays Clay Min. 1996, 44, 492–500. [Google Scholar] [CrossRef]
- Gaillou, E.; Fritsch, E.; Rondeau, B.; Aguilar-Reyes, B.; Barreau, A.; Ostroumov, M.; Post, J. Common gem opal: An investigation of micro- to nano-structure. Am. Miner. 2008, 93, 1865–1873. [Google Scholar] [CrossRef]
- Liang, Q. Physical Optics, 1st ed.; China Machine Press: Beijing, China, 1987; p. 345. [Google Scholar]
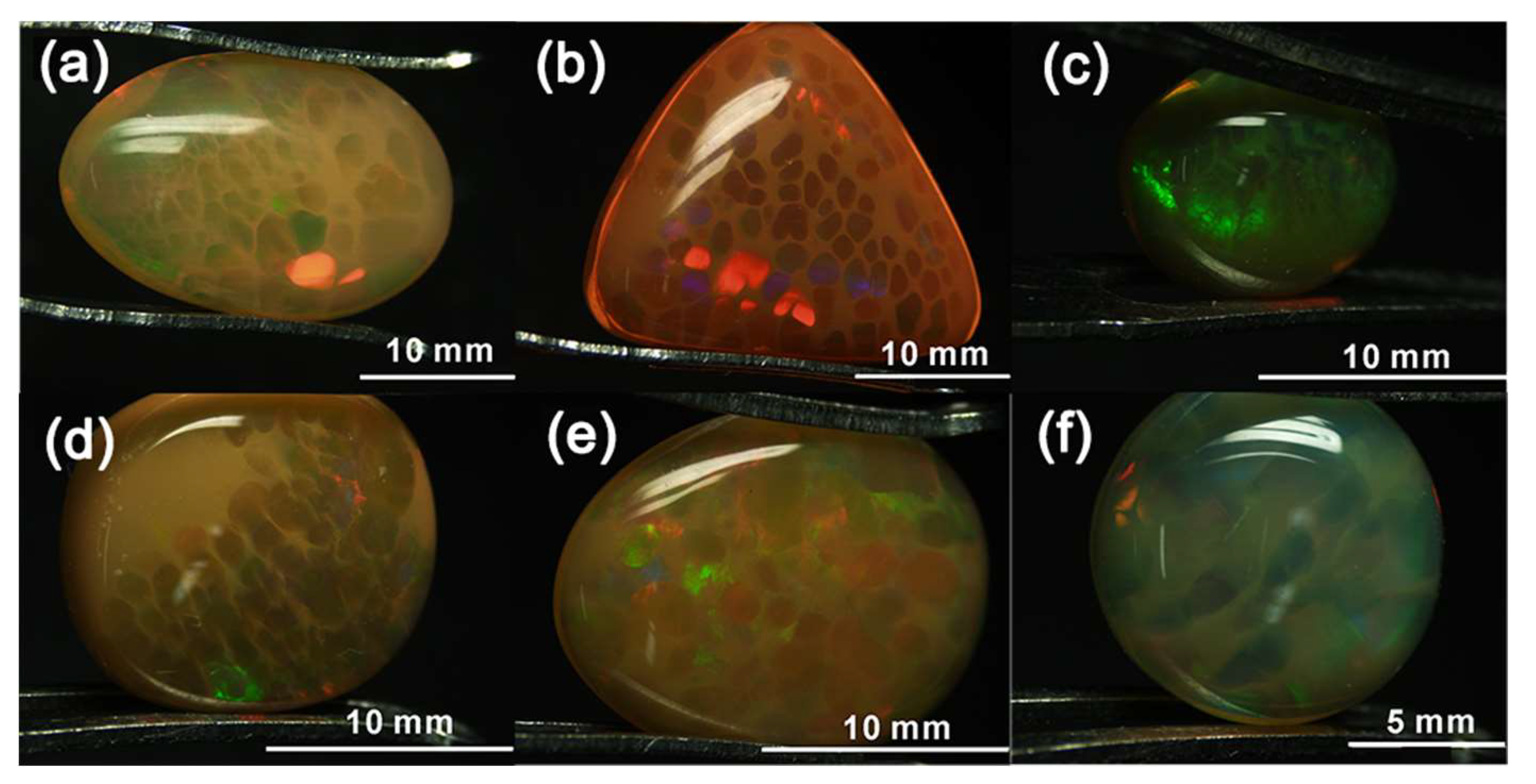
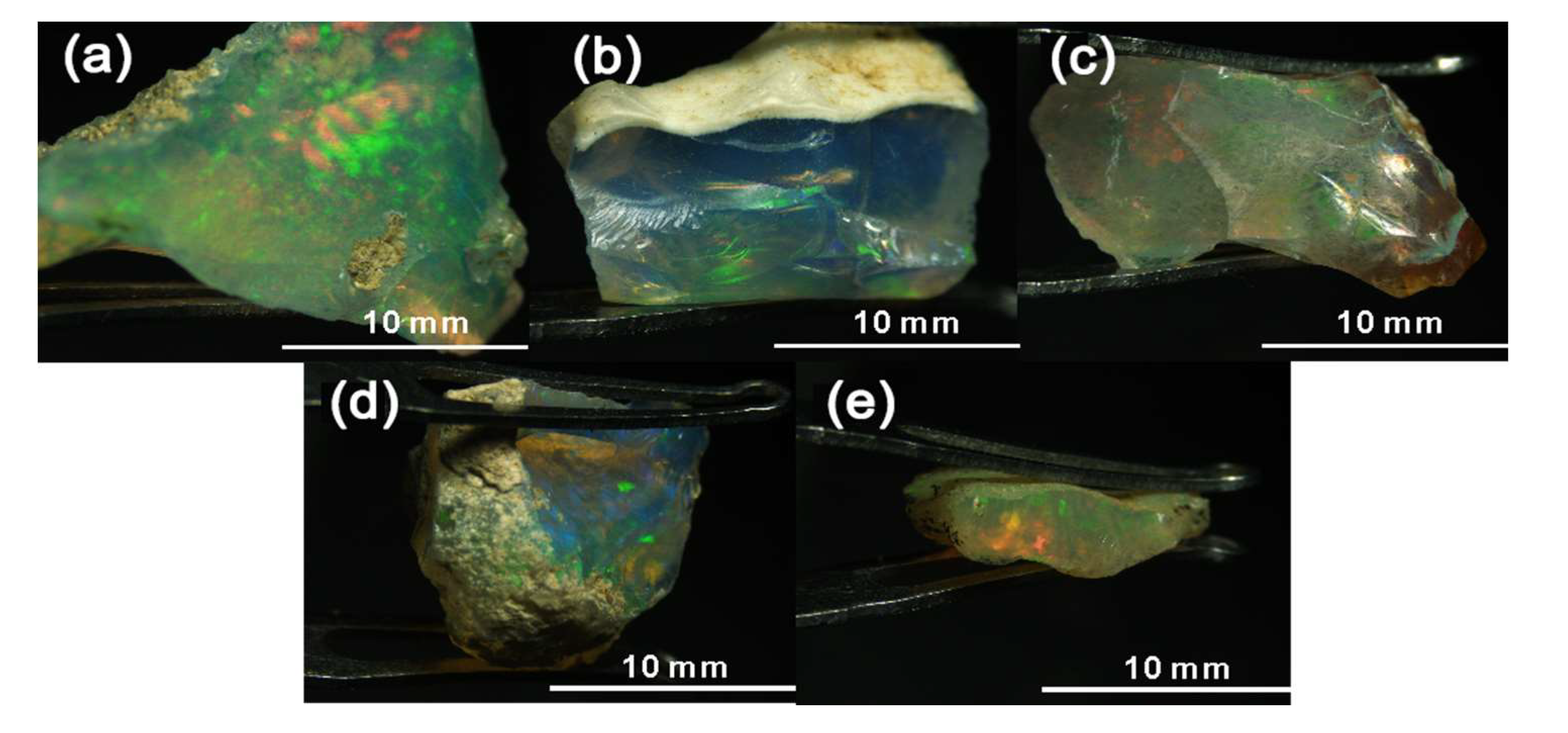

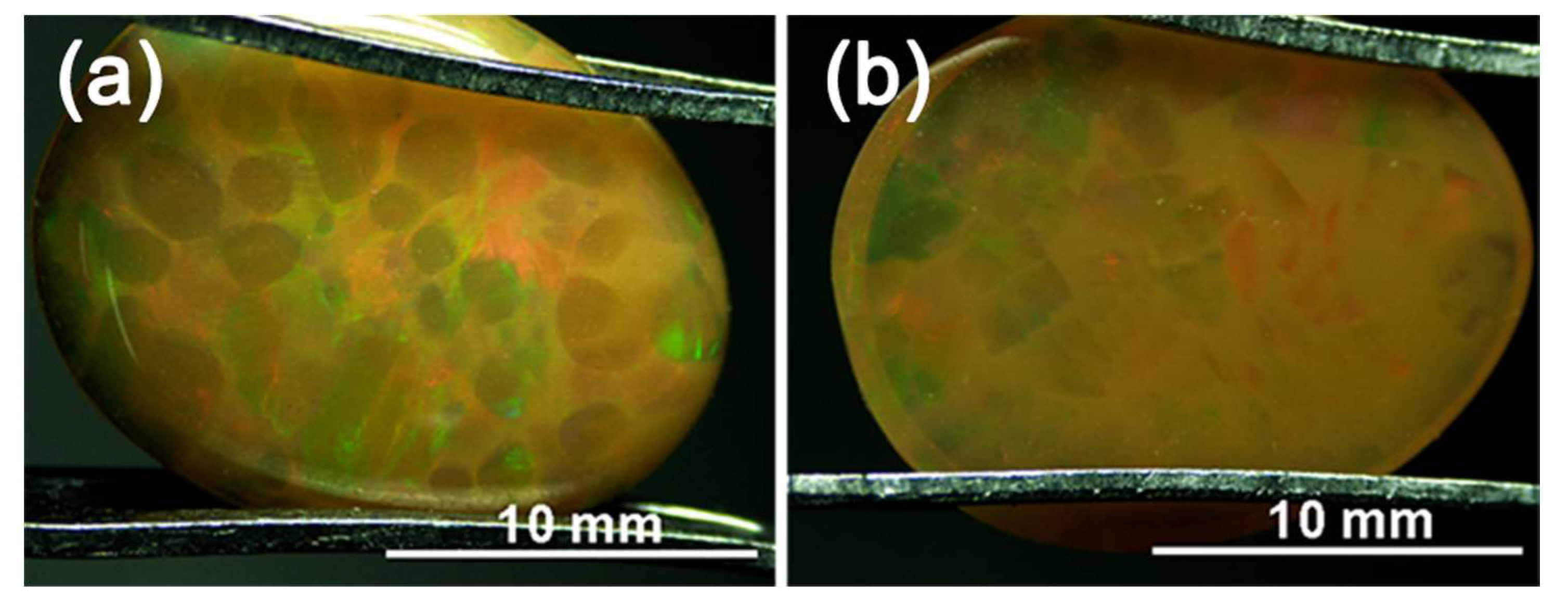

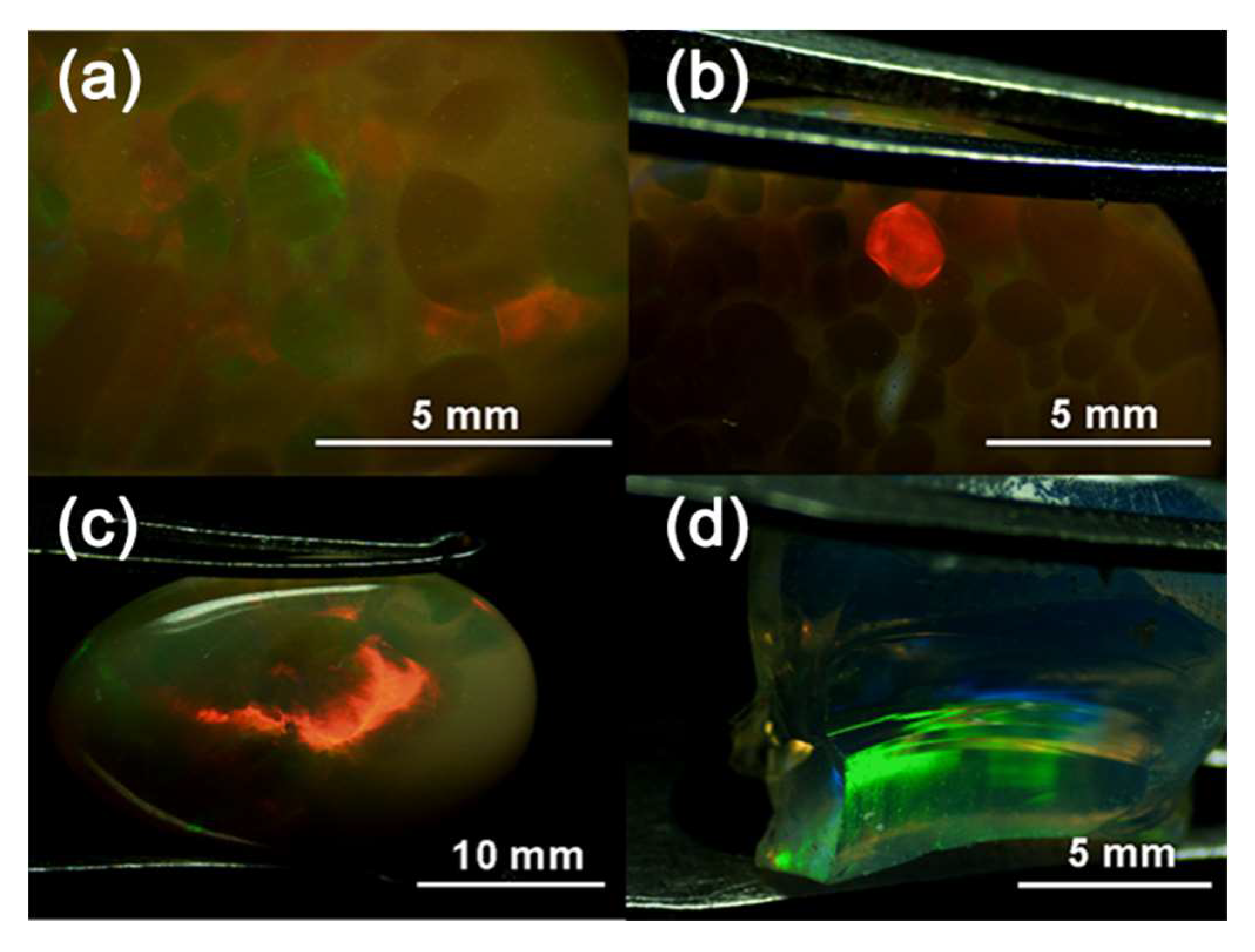

| Sample | W 1 (ct) | Colour of Columns | Transparency of Columns | Colour of Matrix | Transparency of Matrix | RI 2 | LWUV 3 Fluorescence | SWUV 4 Fluorescence |
|---|---|---|---|---|---|---|---|---|
| Opal-01 | 3.369 | Pale-yellowish-brown | Transparent | Pale-brown | Translucent | 1.430 | White, moderate | White, weak |
| Opal-02 | 4.144 | Pale-orange-brown | Transparent | Orange-brown | Semi-translucent | 1.465 | White, weak | White, very weak |
| Opal-03 | 0.537 | Yellow | Transparent | Brown | Translucent | 1.464 | White, weak | White, very weak |
| Opal-04 | 1.017 | Pale-yellowish-brown | Transparent | Pale-brown | Semi-translucent | 1.459 | Inert | Inert |
| Opal-05 | 0.909 | Pale-yellowish-brown | Transparent | Pale-brown | Translucent | 1.464 | White, weak | White, very weak |
| Opal-06 | 0.659 | Pale-brown | Transparent | Brown | Translucent | 1.460 | White, weak | White, very weak |
| Opal-07 | 2.473 | Colourless | Transparent | - | - | N.M. 5 | Bluish-white, moderate | Bluish-white, weak |
| Opal-08 | 1.214 | Colourless | Transparent | - | - | N.M. | Bluish-white, moderate | Bluish-white, weak |
| Opal-09 | 2.898 | Pale-yellow | Transparent | Pale-brown | Translucent | N.M. | Inert | Inert |
| Opal-10 | 2.093 | Pale-yellow | Transparent | - | - | N.M. | Inert | Inert |
| Opal-11 | 0.625 | Pale-brown | Transparent | Pale-brown | Translucent | N.M. | Inert | Inert |
| Test Position | R1 1 | R2 | R3 | R4 | R5 | η 5 | SE 6η | ξ 7 | SEξ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ω 2 | Ι 3 | Γ 4 | ω | Ι | Γ | ω | Ι | Γ | ω | Ι | Γ | ω | Ι | Γ | |||||
| 02 column (bottom) | 230.43 | 4514 | 76.12 | 300.15 | 65,467 | 82.96 | 349.95 | 69,756 | 74.91 | 415.73 | 83,556 | 90.42 | 486.38 | 37,986 | 70.49 | 0.61 | 0.14 | 0.83 | 0.17 |
| 02 matrix (bottom) | 233.20 | 3445 | 44.26 | 302.20 | 62,437 | 79.54 | 355.50 | 64,916 | 70.84 | 418.00 | 76,706 | 76.50 | 484.40 | 29,522 | 63.40 | 0.61 | 0.17 | 0.93 | 0.20 |
| 02 matrix (top) | 230.44 | 4537 | 124.55 | 307.72 | 36,506 | 83.80 | 356.94 | 30,301 | 64.80 | 419.37 | 33,122 | 74.42 | 486.38 | 20,970 | 58.32 | 0.64 | 0.25 | 0.87 | 0.20 |
| 04 column | 237.41 | 12,841 | 64.33 | 302.43 | 56,312 | 74.68 | 353.50 | 59,810 | 64.00 | 416.36 | 91,546 | 88.38 | 486.38 | 31,884 | 56.43 | 0.53 | 0.09 | 0.72 | 0.09 |
| 04 matrix | 231.13 | 1862 | 28.77 | 298.39 | 49,522 | 76.80 | 351.78 | 67,882 | 72.95 | 421.38 | 3543 | 90.00 | 490.28 | 15,814 | 38.66 | 0.61 | 0.06 | 0.81 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Bai, F. Crystallinity and Play-of-Colour in Gem Opal with Digit Patterns from Wegel Tena, Ethiopia. Minerals 2020, 10, 625. https://doi.org/10.3390/min10070625
Zhao K, Bai F. Crystallinity and Play-of-Colour in Gem Opal with Digit Patterns from Wegel Tena, Ethiopia. Minerals. 2020; 10(7):625. https://doi.org/10.3390/min10070625
Chicago/Turabian StyleZhao, Kehan, and Feng Bai. 2020. "Crystallinity and Play-of-Colour in Gem Opal with Digit Patterns from Wegel Tena, Ethiopia" Minerals 10, no. 7: 625. https://doi.org/10.3390/min10070625
APA StyleZhao, K., & Bai, F. (2020). Crystallinity and Play-of-Colour in Gem Opal with Digit Patterns from Wegel Tena, Ethiopia. Minerals, 10(7), 625. https://doi.org/10.3390/min10070625





