Cement Render and Mortar and Their Damages Due to Salt Crystallization in the Holy Trinity Church, Dominicans Monastery in Cracow, Poland
Abstract
:1. Introduction
2. Materials and Methods
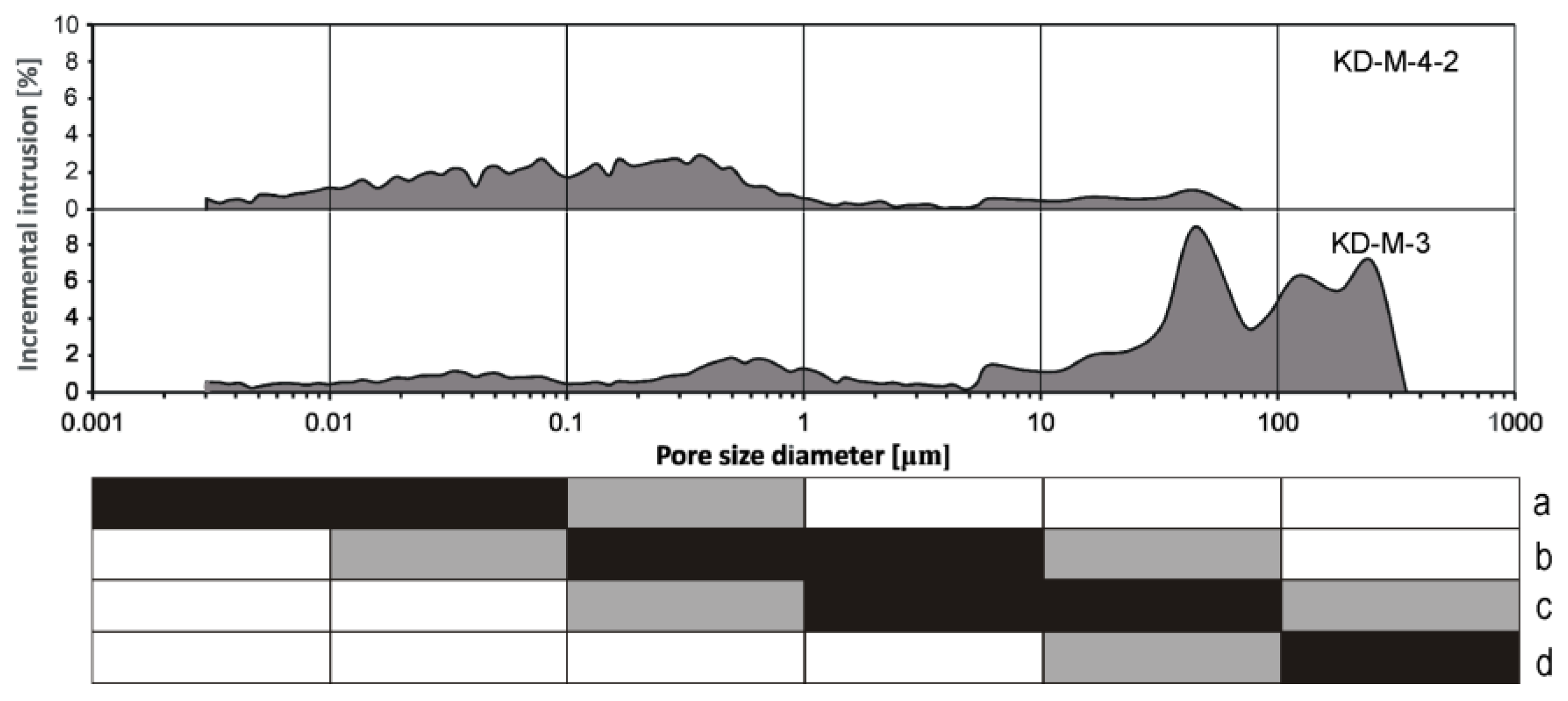
3. Results and Discussion
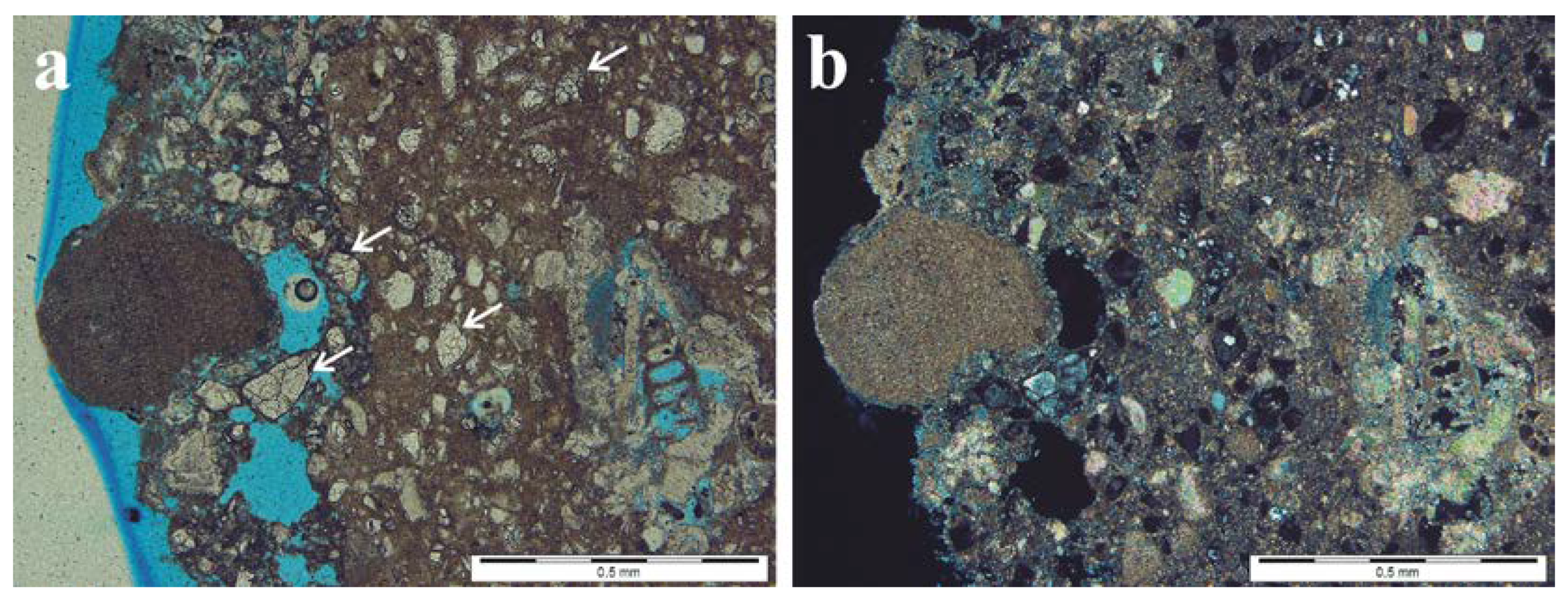
3.1. Bright Cement Render at Limestone Blocks above the Pavement Level
3.2. Cement Mortar between Stony Blocks of the Subwall below the Pavement Level
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snethlage, R.; Sterflinger, K. Stone Conservation. In Stone in Architecture; Siegesmund, S., Snethlage, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 411–544. [Google Scholar] [CrossRef]
- Adamski, G.; Bratasz, L.; Kozlowski, R.; Mayr, N.; Mucha, D.; Stilhammerova, M.; Weber, J. Roman cement—Key historic material to cover the Exteriors of buildings. In RILEM Workshop Repair Mortars for Historic Masonry; Groot, C., Ed.; RILEM Publications SARL: Paris, France, 2009; pp. 2–11. [Google Scholar]
- Gosselin, C.; Verges-Belmin, V.; Royer, A.; Martinet, G. Natural cement and monumental restoration. Mater. Struct. 2009, 42, 749–763. [Google Scholar] [CrossRef] [Green Version]
- Kozłowski, R.; Hughes, D.; Weber, J. Roman Cements: Key Materials of the Built Heritage of the 19th Century. In Materials, Technologies and Practice in Historic Heritage Structures; Bostenaru Dan, M., Přikryl, R., Török, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 259–277. [Google Scholar] [CrossRef]
- Pintér, F.; Gosselin, C. The origin, composition and early age hydration mechanisms of Austrian natural Portland cement. Cem. Concr. Res. 2018, 110, 1–12. [Google Scholar] [CrossRef]
- Bartz, W.; Filar, T. Mineralogical characterization of rendering mortars from decorative details of a baroque building in Kożuchów (SW Poland). Mater. Charact. 2010, 61, 105–115. [Google Scholar] [CrossRef]
- Szeląg, H.; Garbacik, A.; Pichniarczyk, P.; Baran, T. Roman cement and its properties. Surow. Masz. Bud. 2008, 1, 74–78. (In Polish) [Google Scholar]
- Szeląg, H.; Garbacik, A.; Pichniarczyk, P.; Baran, T. Contemporary Roman cement and its properties. Bud. Czas. Tech. Wydaw. Politech. Krak. 2009, 109, 337–345, (In Polish with English Abstract). [Google Scholar]
- Weber, J.; Gadermayr, N.; Kozłowski, R.; Mucha, D.; Hughes, D.; Jaglin, D.; Schwarz, W. Microstructure and mineral composition of Roman cements produced at defined calcination conditions. Mater. Charact. 2007, 58, 1217–1228. [Google Scholar] [CrossRef]
- Kloppmann, W.; Bromblet, P.; Vallet, J.M.; Vergès-Belmin, V.; Rolland, O.; Guerrot, C.; Gosselin, C. Building materials as intrinsic sources of sulphate: A hidden face of salt weathering of historical monuments investigated through multi-isotope tracing (B, O, S). Sci. Total Environ. 2011, 409, 1658–1669. [Google Scholar] [CrossRef]
- Prikryl, R.; Weishauptova, Z.; Novotna, M.; Prikrylova, J.; St’astna, A. Physical and mechanical properties of the repaired sandstone ashlars in the facing masonry of the Charles Bridge in Prague (Czech Republic) and an analytical study for the causes of its rapid decay. Environ. Earth Sci. 2011, 63, 1623–1639. [Google Scholar] [CrossRef]
- Morillas, H.; Maguregui, M.; Trebolazabala, J.; Madariaga, J.M. Nature and origin of white efflorescence on bricks, artificial stones, and joint mortars of modern houses evaluated by portable Raman spectroscopy and laboratory analyses. Spectrochim. Acta Part A 2015, 136, 1195–1203. [Google Scholar] [CrossRef]
- Flatt, R.J.; Steiger, M.; Scherer, G.W. A commented translation of the paper by C.W. Correns and W. Steinborn on crystallization pressure. Environ. Geol. 2007, 52, 187–203. [Google Scholar] [CrossRef]
- Bieniarzówna, J.; Małecki, J.M. The History of Cracow, Volume 2, Cracow in the 16th–18th Centuries; Wydawnictwo Literackie: Kraków, Poland, 1984; 670p. (In Polish) [Google Scholar]
- Dobrowolski, T. Art of Cracow, 5th ed.; Wydawnictwo Literackie: Kraków, Poland, 1978; 623p. (In Polish) [Google Scholar]
- Rożek, M. Urbs Celeberrima. Cracow Monuments Guide; WAM: Kraków, Poland, 2009; 624p. (In Polish) [Google Scholar]
- Grabski, W.; Nowak, J. Technological and Mineralogical-Petrographic Studies on the Pińczów Limestone (Symptoms and Causes of Stone Destruction at the Dome of the Myszkowskis Chapel of the Dominican Order Church in Cracow); No inv.3126/77; Archive of Cracow City Office, Department of Conservation of Monuments: Kraków, Poland, 1960; p. 26, (In Polish, Manuscript). [Google Scholar]
- Rajchel, J. Stony Cracow; Uczelniane Wydawnictwa Naukowo-Dydaktyczne AGH: Kraków, Poland, 2004; p. 235. (In Polish) [Google Scholar]
- Grabski, W.; Nowak, J. The Problem of stone destruction in the historic buildings of Cracow. Mater. Bud. 1957, 1, 33–39. (In Polish) [Google Scholar]
- Marszałek, M.; Dudek, K.; Gaweł, A.; Czerny, J. Mineralogical and geochemical studies of secondary mineral assemblages related to deterioration of building materials. Geol. Q. 2019, 63, 683–698. [Google Scholar] [CrossRef]
- Górecki, J.; Sermet, E. Quarries of Cracow—The heritage underestimated. In Mining History—An Element of the European Cultural Heritage; Zagożdżon, P.P., Madziarz, M., Eds.; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Poland, 2010; Volume 3, pp. 123–138. (In Polish) [Google Scholar]
- Bochnak, A.; Samek, J. Katalog Zabytków Sztuki w Polsce. Tom IV Miasto Kraków. Kościoły i Klasztory Śródmieścia, 2; Instytut Sztuki Polskiej Akademii Nauk: Warszawa, Poland, 1978; 256p. [Google Scholar]
- Hansteen, E.T.; Burke, E. Aphthitalite in hight-temperature fluid inclusions in quartz from Eikeren-Skrim granite complex, the Oslo paleorift. Nor. Geol. Tidsskr. 1994, 74, 238–240. [Google Scholar]
- Buzgar, N.; Buzatu, A.; Sanislav, I.V. The Raman study on certain sulfates. An. Științ. Univ. “Al.I. Cuza” 2009, 55, 5–23. [Google Scholar]
- Hamilton, A.; Menzies, R.I. Raman spectra of mirabilite, Na2SO4·10H2O and the rediscovered metastable heptahydrate, Na2SO4·7H2O. J. Raman Spectrosc. 2010, 41, 1014–1020. [Google Scholar] [CrossRef]
- Jentzsch, P.V.; Ciobota, V.; Kampe, B.; Rösch, P.; Popp, J. Origin of salt mixtures and mixed salts in atmospheric particulate matter. J. Raman Spectrosc. 2012, 43, 514–519. [Google Scholar] [CrossRef]
- Prieto-Taboada, N.; Gómez-Laserna, O.; Martínez-Arkarazo, I.; Olazabal, Á.M.; Madariaga, J.M. Raman Spectra of the Different Phases in the CaSO4–H2O System. Anal. Chem. 2014, 86, 10131–10137. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Taboada, N.; Fdez-Ortiz de Vallejuelo, S.; Veneranda, M.; Lama, E.; Castro, K.; Arana, G.; Larrañaga, A.; Madariaga, J.M. The Raman spectra of the Na2SO4 -K2SO4 system: Applicability to soluble salts studies in built heritage. J. Raman Spectrosc. 2019, 50, 175–183. [Google Scholar] [CrossRef]
- Pintér, F.; Vidovszky, I.; Weber, J.; Bayer, K. Mineralogical and microstructural characteristics of historic Roman cement renders from Budapest, Hungary. J. Cult. Herit. 2014, 15, 219–226. [Google Scholar] [CrossRef]
- Ando, Y.; Hirono, S.; Sawaki, D.; Katayama, T. Microscopy to evaluate the properties of cement and alterations in historic mortar/concrete of old Nobiru Port project, Northeast Japan. In Proceedings of the 36th ICMA Conference, Milan, Italy, 13–17 April 2014; pp. 212–233. [Google Scholar]
- Katayama, T.; Ando, Y.; Hirono, S.; Sawaki, D.; Itoga, H. Relicts of unhydrated cement clinker in a historic concrete from the 19th century—Microscopy with EDS analysis of old training dyke at Yokohama port, Japan. In Proceedings of the 36th ICMA Conference, Milan, Italy, 13–17 April 2014; pp. 432–458. [Google Scholar]
- Insley, H. Structural characteristics of some constituents of Portland cement clinker. J. Res. Natl. Bur. Stand. 1936, 17, 353–361. [Google Scholar] [CrossRef]
- Campbell, D.H. Microscopical Examination and Interpretation of Portland Cement and Clinker, 2nd ed.; Portland Cement Association: Skokie, IL, USA, 1999; p. 214. [Google Scholar]
- Sabbioni, C.; Zappia, G.; Riontino, C.; Blanco-Varela, M.T.; Aguilera, J.; Puertas, F.; Van Balen, K.; Toumbakari, E.E. Atmospheric deterioration of ancient and modern hydraulic mortars. Atmos. Environ. 2001, 35, 539–548. [Google Scholar] [CrossRef]
- Gosselin, C.; Girardet, F.; Feldman, S.B. Compatibility of Roman cement mortars with gypsum stones and anhydrite mortars: The example of Valère Castle (Sion, Switzerland). In Proceedings of the 12th International Congress on the Deterioration and Conservation of Stone, New York, NY, USA, 21–25 October 2012; Columbia University: New York, NY, USA, 2012; pp. 1–11. [Google Scholar]
- Gosselin, C.; Scrivener, K.L.; Feldman, S.B.; Schwarz, W. The hydration of modern roman cements used for current architectural restoration. In Historic Mortars: Characterisation, Assessment and Repair; RILEM Book Series, 7, Valek, J., Hughes, J.J., Groot, C.J.W.P., Eds.; Springer: Dordrecht, Holland, 2012; pp. 297–308. [Google Scholar] [CrossRef]
- Łagosz, A.; Małolepszy, J. Tricalcium aluminate hydration in the presence of calcium sulfite hemihydrates. Cem. Concr. Res. 2003, 33, 333–339. [Google Scholar] [CrossRef]
- Suh, J.; Sung Yumb, W.; Jeong, Y.; Park, H.G.; Eun Oh, J. The cation-dependent effects of formate salt additives on the strength and microstructure of CaO-activated fly ash binders. Constr. Build. Mater. 2019, 194, 92–101. [Google Scholar] [CrossRef]
- Benavente, D. Why pore size is important in the deterioration of porous stones used in the built heritage. MACLA 2011, 15, 41–42. [Google Scholar]
- Tao, Y.; Zhang, W.; Li, N.; Wang, F.; Hu, S. Atomic occupancy mechanism in brownmillerite Ca2FeAlO5 from a thermodynamic perspective. J. Am. Ceram. Soc. 2020, 103, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Jupe, A.C.; Cockroft, J.K.; Barnes, P.; Colston, S.L.; Sankar, G.; Hall, C. The site occupancy of Mg in the brownmillerite structure and its effect on hydration properties: An X-ray/neutron diffraction and EXAFS study. J. Appl. Cryst. 2001, 34, 55–61. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, H.; Jovanović, M.; Linnow, K.; Steiger, M. Performance of limestones laden with mixed salt solutions of Na2SO4–NaNO3 and Na2SO4–K2SO4. Environ. Earth Sci. 2013, 69, 1751–1761. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Doehne, E. Salt weathering: Influence of evaporation rate, supersaturation and crystallization pattern. Earth Surf. Process. Landf. 1999, 24, 191–209. [Google Scholar] [CrossRef]
- Linnow, K.; Steiger, M.; Lemster, C.; De Clercq, H.; Jovanović, M. In situ Raman observation of the crystallization in NaNO3–Na2SO4–H2O solution droplets. Environ. Earth Sci. 2013, 69, 1609–1620. [Google Scholar] [CrossRef]
- Lindström, N.; Heitmann, N.; Linnow, K.; Steiger, M. Crystallization behavior of NaNO3 -Na2SO4 salt mixtures in sandstone and comparison to single salt behavior. Appl. Geochem. 2015, 63, 116–132. [Google Scholar] [CrossRef]
- Silcock, H.L. Solubilities of inorganic and organic compounds. In Ternary and Multicomponent Systems of Inorganic Substances; Pergamon Press: Oxford, UK, 1979; Volume 3. [Google Scholar]
- Holtkamp, M.; Heijnen, W. The mineral darapskite in the efflorescence on two dutch churches. Stud. Conserv. 1991, 36, 175–178. [Google Scholar] [CrossRef]
- Puşcaş, C.M.; Onac, B.P.; Tămas, T. The mineral assemblage of caves within Şălitrari Mountain (Cerna Valley, SW Romania): Depositional environment and speleogenetic implications. Carbonates Evaporites 2010, 25, 107–115. [Google Scholar] [CrossRef]
- Domasłowski, W. Preventive Conservation of Stone Historic Objects; Wydawnictwo Uniwersytetu Mikołaja Kopernika: Toruń, Poland, 1993. (In Polish) [Google Scholar]
- Doehne, E.; Price, C.A. Stone Conservation: An Overview of Current Research, 2nd ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2010; p. 175. [Google Scholar]
- Reports on the state of the environment in the Małopolska province (In Polish) 2010–2017. Wojewódzki Inspektorat Ochrony Środowiska w Krakowie: Kraków, Poland. Available online: http://www.krakow.pios.gov.pl (accessed on 10 December 2019).
- Marszałek, M. Identification of secondary salts and their sources in deteriorated stone monuments using micro-Raman spectroscopy, SEM-EDS and XRD. J. Raman Spectrosc. 2016, 47, 1473–1485. [Google Scholar] [CrossRef]
- Matuszko, D.; Piotrowicz, K.; Kowanetz, L. Components of the natural environment—Climate (in Polish with English summary). In Natural Environment of Krakow. Resources—Protection—Management, 2nd ed.; Baścik, M., Degórska, B., Eds.; Uniwersytet Jagielloński w Krakowie: Kraków, Poland, 2015; pp. 81–108. [Google Scholar]


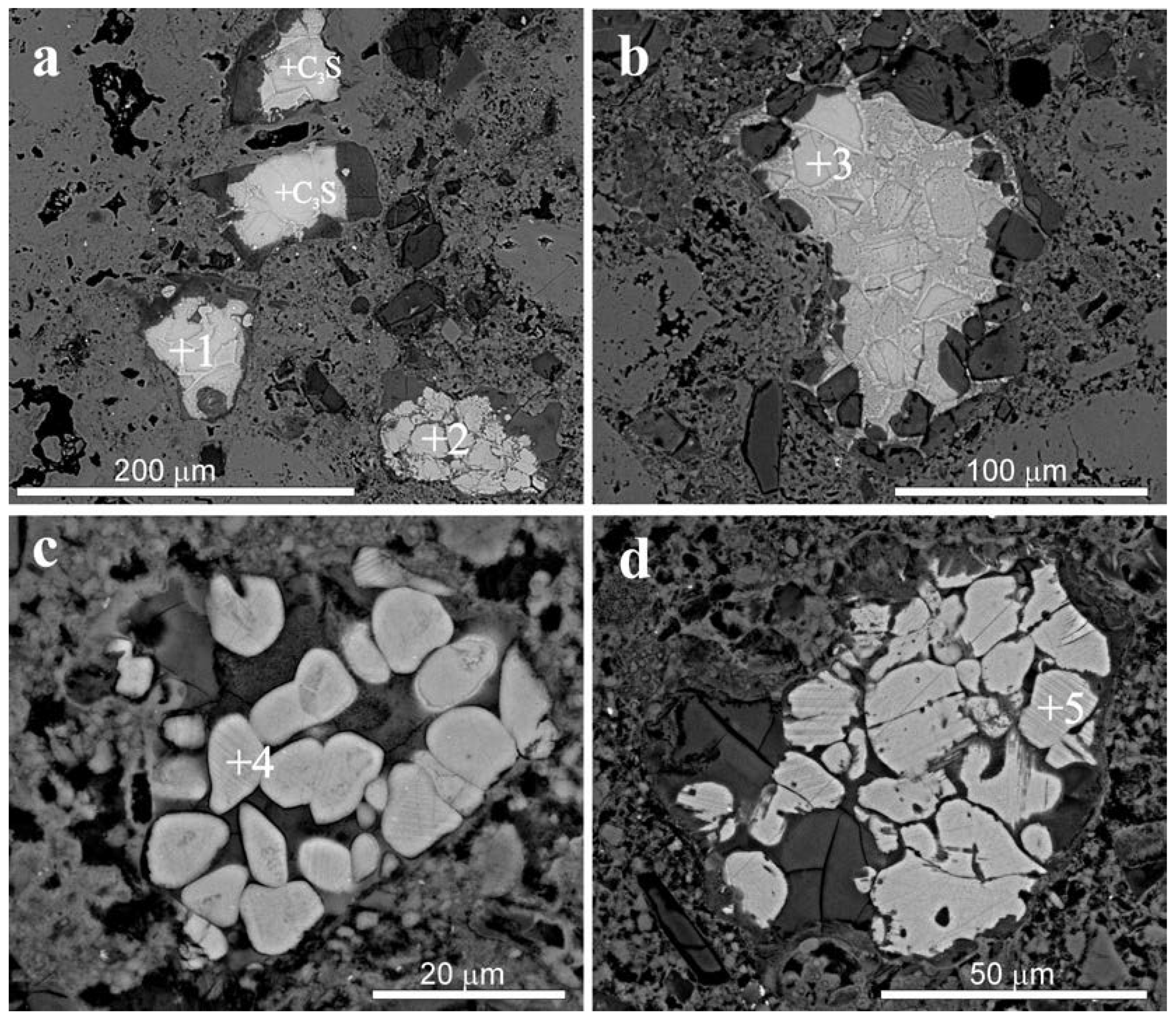








| Sample | Phase | Analyzed Point | Element | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Na | Mg | Al | Si | Ca | Fe | P | |||
| KD-M-4-2 | ||||||||||
| C3S | +1 | 55.52 | 0.00 | 0.47 | 1.80 | 10.87 | 31.34 | |||
| C2S | +2 | 56.64 | 0.00 | 0.00 | 0.83 | 14.33 | 28.19 | |||
| C3S | +3 | 55.86 | 0.00 | 0.00 | 2.14 | 10.71 | 31.29 | |||
| C2S | +4 | 54.39 | 0.00 | 0.00 | 0.69 | 14.37 | 29.95 | 0.25 | ||
| C2S | +5 | 56.42 | 0.00 | 0.00 | 0.90 | 14.04 | 28.64 | |||
| KD-M-3 | ||||||||||
| C3S | +6 | 56.98 | 0.00 | 0.98 | 1.88 | 12.40 | 27.76 | |||
| C3A | +7 | 54.12 | 1.07 | 0.00 | 14.33 | 5.73 | 23.29 | 1.46 | ||
| C4AF | +8 | 54.99 | 0.00 | 1.30 | 11.06 | 5.76 | 20.67 | 6.23 | ||
| C2S | +9 | 57.88 | 0.00 | 0.00 | 1.62 | 16.12 | 24.38 | |||
| C2S | +10 | 58.14 | 0.00 | 0.00 | 0.70 | 13.36 | 27.79 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marszałek, M.; Dudek, K.; Gaweł, A. Cement Render and Mortar and Their Damages Due to Salt Crystallization in the Holy Trinity Church, Dominicans Monastery in Cracow, Poland. Minerals 2020, 10, 641. https://doi.org/10.3390/min10070641
Marszałek M, Dudek K, Gaweł A. Cement Render and Mortar and Their Damages Due to Salt Crystallization in the Holy Trinity Church, Dominicans Monastery in Cracow, Poland. Minerals. 2020; 10(7):641. https://doi.org/10.3390/min10070641
Chicago/Turabian StyleMarszałek, Mariola, Krzysztof Dudek, and Adam Gaweł. 2020. "Cement Render and Mortar and Their Damages Due to Salt Crystallization in the Holy Trinity Church, Dominicans Monastery in Cracow, Poland" Minerals 10, no. 7: 641. https://doi.org/10.3390/min10070641
APA StyleMarszałek, M., Dudek, K., & Gaweł, A. (2020). Cement Render and Mortar and Their Damages Due to Salt Crystallization in the Holy Trinity Church, Dominicans Monastery in Cracow, Poland. Minerals, 10(7), 641. https://doi.org/10.3390/min10070641





