Abstract
This work studies the removal of uranium ions from chemically leached solutions by sorption using two weak and two strong base anionites. Batch sorption experiments were performed to evaluate the optimum conditions at pH 1.2–2.2, 1.0 g resin dose for 1–12 h contact time at room temperature. These experiments addressed sorption kinetics and sorption isotherm. The maximum sorption capacity reached 55.8 mg/g at room temperature. The kinetics data are well described by the pseudo-second-order kinetic model at initial uranium concentration of 0.62 mg·L−1. To describe sorption kinetics pseudo-first-order, pseudo-second-order and intraparticle diffusion models were proposed. Studies indicated that the sorption of uranium can be fitted by a pseudo-second-order kinetic model very well. Equilibria were described by Langmuir, Freundlich, and Dubinin–Radushkevich equations. The experimental sorption isotherm is successfully described by the Langmuir model.
1. Introduction
The sorption of uranium ions from aqueous solution plays an important role in the nuclear power industry [1,2,3]. The development of this industry is determined by the growing energy consumption and causes an increase in demand for uranium raw materials. The main method of uranium mining is currently in-situ leaching (ISL), which requires not only a careful study of the reagent leaching scheme, but also a reasonable choice of technology for further processing of productive solutions.
Uranium is widely distributed in different rocks, especially in black shale ores. Recently, uranium extraction from black shale ore has gained importance [4,5]. All of these black shale processing technologies can be classified as pyrometallurgical and hydrometallurgical methods. The main process of recovering uranium in the hydrometallurgical method is sorption [6,7,8].
In the practice of uranium extraction, various types of ionites are used: Strong-base, weak base, strong acid, and weak acid. Strong base ionites have found their application in the processing of ISL solutions in China [9]. Weak base ionites are often used for sorption of uranium from sulfuric acid solutions [10]. Since uranium in the pregnant leach solution of black shale ore exists in anionic form, cation-exchange resins are not considered in this article.
The ion exchange mechanism of uranium recovery from solution loaded with uranium is shown in Figure 1.
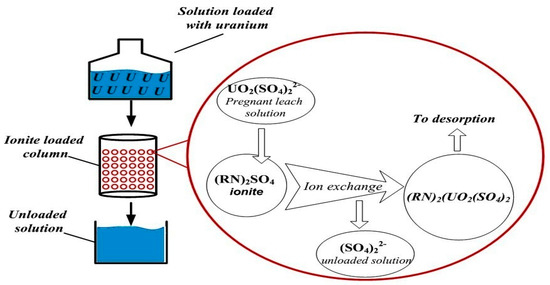
Figure 1.
Ion exchange mechanism of uranium recovery.
It is known that uranium is represented as ions in acidic sulfuric acid solutions, such as UO22+, UO2SO4 and [UO2(SO4)2]2 [10,11].
In most circumstances, uranium is extracted from solutions with application of solvent extraction [12], chemical precipitation [13], and adsorption processes [14,15]. Due to the benefits of adsorption processes like low cost and high efficiency, ion exchange process becomes a suitable technique for the recovery of uranium from pregnant leach solutions [16,17,18,19]. To extract uranium in the form of [UO2(SO4)2]2–, anionites have to be used. However, uranium extraction is carried out according to the following Equation (1):
where RN is the matrix of the anionite.
(RN)2SO4 + [UO2(SO4)2]2− = (RN)2(UO2(SO4)2) + (SO4)2−
In this regard, it is necessary to observe and monitor pH and redox potential of the solution, so that they directly affect the ionic state of uranium [20,21].
Most studies on uranium sorption have focused mainly on the model solutions [22,23,24,25,26]. Many of these studies have limited industrial application because industrial leach liquors are more complex, containing several metal ions and other contaminants [27,28]. For this reason, more research is needed using real pregnant leach solutions to demonstrate the applicability of a sorption process for the recovery of uranium. The most important factors determining the course and result of the sorption process, are the capacity of the sorbent and the nature of sorption. Data definition using kinetic models make it possible to draw conclusions about the nature of sorption and identify the limiting stages of the process, which can also be used to solve a number of practical issues for optimizing ion exchange processes [29].
The present work has been oriented towards studying the recovery of uranium (VI) from the chemically leached solutions. For sorption process of uranium, weak and strong base anionite ion exchange resins were used.
2. Experiment
2.1. Materials
In this study, samples of black shale ores (100 g) were ground to a size of 0.2 mm, thoroughly mixed with ammonium hydrosulfate (NH4)HSO4 in a ratio of 1:0.75 (75 g of ammonium hydrosulfate) and sintered in a muffle electroheating furnace SNOL 40/1200 at 350 °C for 2 h. Furthermore, pregnant leach solutions were obtained after leaching sintered black shale ores with sulfuric acid at a concentration of 40 g·L−1.
All leaching tests were carried out in a temperature-controlled three-necked flat bottom glass flask (cap. 250 mL) on hot-plate cum magnetic stirrer at fixed rpm (400) and a reflux condenser to avoid the loss, due to evaporation. The leaching time was recorded after successive addition of the sintered sample and leaching agent solution to the reaction vessel, and then this vessel was put in an oil bath maintained at a preset temperature (temperature range is 20–90 °C). The analysis of leaching solutions showed the following composition, g/L: Fe2+—2.52; Fe3+—12.7; Al—13.9; V—2.89; Мo—0.08; U—0.062; Σ REE—0.05.
In all sorption experiments, the concentration of uranium amounted 62 mg·L−1. The characteristic properties of anion-exchange resins that were used in the experiments are shown in Table 1.

Table 1.
Technical characteristics of applied ion-exchange resins.
The major valuable elements present in the black shale ores is shown 0.68% V, 0.33% U, 0.26% Mo, 0.01% REE along with other minor impurity and the results, as shown in Table 2.

Table 2.
Chemical composition of black shale ore.
2.2. Sorption Studies
The sorption experiments were carried out using the batch method. Experiments were conducted using the strong base anionites Ambersep 900 SO4 and Puromet MTA4601PF, as well as with weak base anionites Dowex Marathon WBA and Diaion WA30. The scheme of this investigation is presented in Figure 2.
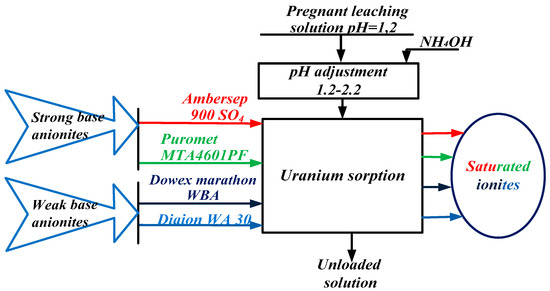
Figure 2.
The scheme of investigation of the process of sorption and desorption of uranium from pregnant leaching solution.
All anionites were preconditioned prior to contact with pregnant leach solutions by mixing with five bed volumes of H2SO4 (1 M) for 24 h followed by washing with two bed volumes of deionized water. The solution pH (pH = 1.2 of the feed solution) was adjusted by dropwise addition of ammonia solution while stirring. Experiments were conducted using the leach solution with various adsorption conditions, including contact time and solution pH values. A 100 mL pregnant leach solution and 1.0 g of resin were added to a beaker with a magnetic stirrer and agitated at 180 rpm at room temperature (25 ± 1 °C). After a period of sorption, the solution was subjected to uranium analysis, which provided elemental sorption data for these conditions.
The sorption efficiency (%) was calculated based on Equation (2):
where Ci and Cf are the initial and final concentrations of uranium in the solution phase, respectively.
2.3. Sorption Kinetic and Equilibrium Studies
Sorption kinetic and equilibrium studies were carried out under batch experiment conditions: Sorbent mass 1.0 g, the volume of contacting solution 100.0 mL at an initial uranium concentration of 62.0 mg·L−1, stirring at (25 ± 1) °C.
2.3.1. The Sorption Kinetic Equations
The process kinetics was described by three models: Pseudo-first order, pseudo-second-order and intraparticle diffusion model equations [30,31]. These equations are generally used to describe theoretical sorption kinetics on the solid/liquid interface.
The pseudo-first-order Lagergren model. In differential form, the Lagergren pseudo-first order equation can be presented as follows:
After integration and applying boundary conditions t = 0 to t = t and qt = 0 to qt = qt, the integrated form of the Lagergren equation becomes:
qe = equilibrium concentration of the sorbed ion (mg∙g−1); qt = concentration of the sorbed ion at time t (min); k1 is the pseudo-first order rate constant (min−1).
The pseudo second-order sorption kinetic equation is expressed by the following equation:
After integration and applying boundary conditions t = 0 to t = t and qt = 0 to qt = qt, the integrated form of the Lagergren equation becomes:
which an be rearranged to obtain the following linear form:
where qe and qt (mg∙g−1) are the amount of uranium sorbed at equilibrium and at time t, k2 (g∙mg−1∙min−1) is the pseudo-second-order rate constant and t (min) is the time.
For pseudo-second-order model, the initial adsorption rate, h (mg/mg min), is expressed by the following equation [32]:
The kinetic experimental results were also fitted to the intra-particle diffusion model. It is expressed by the following equation [33]:
where C is the intercept (mg/g) which is related to the boundary layer thickness and kid is the slope which represents the intra-particle diffusion rate constant (mg/g h1/2).
qt = kidt½ + C
2.3.2. The Sorption Equilibrium (Isotherm) Equations
The sorption of uranium on anionites were fitted by three typical models: Langmuir (10,11), Freundlich (12) and Dubinin–Radushkevich (13,14,15), which are described as equations [34,35]:
where Ce (mg/L) is the equilibrium concentration, qe (mg/g) is the amount of uranium adsorbed at equilibrium and qmax, b are Langmuir constants related to maximum uptake capacity (mg/g) and adsorption energy (L/mg).
where b is the Langmuir constant and C0 (mg/L) is the initial U(VI) concentration. The value of RL indicates the nature of isotherm to be irreversible (RL = 0), favorable (0 < RL < 1), linear (RL = 1) or unfavorable (RL > 1).
qe is equilibrium sorbent-phase concentration of adsorbate (mg uranium/g anionite), Ce is equilibrium aqueous-phase concentration of uranium (mg L−1), KF is a constant indicative of the relative adsorption capacity of the anionite (mg g−1), 1/n is the intensity of the adsorption.
The Dubinin–Radushkevich equation can be represented as follows:
where qe (mg/g) is the adsorbed value of the uranium ions at equilibrium concentration, qD (mg/g) is the theoretical saturation capacity, BD (mol2/KJ2) is related to the free energy of sorption per mole of the uranium, R (8.314 J mol−1 K−1) is the gas constant, T (K) is the temperature, Ce (mol L−1) is the equilibrium concentration.
The mean sorption energy (E, J mol−1), which was calculated as follow:
The magnitude of E can give an idea about the type of sorption process, whether it is physical or chemical. If the magnitude of E is between 8 and 16 kJ/mol, the sorption process is supposed to proceed via chemisorption, but if E is less than 8 kJ/mol, the sorption process is of physical nature [36,37].
2.4. Analytical Procedure
All solutions were analyzed by ICP-OES (model 8300 DV) (Perkin Elmer, Waltham, MA, USA) for uranium. The pH of solutions were controlled by the ionometer I-150. The tests were repeated three times to obtain reproducible results with an accuracy of 0.5%.
3. Results and Discussion
3.1. Sorption of Uranium (VI) by Weak Base Anionites
The uranium sorption process was carried out using a weak base anionite–Dowex Marathon WBA and Diaion WA30. The uranium removal from aqueous solutions is very much dependent on solution pH. So a pH study on removal of uranium ions is significant. Danko and coworkers revealed in their study that at different pH values, uranium forms different anionic species and these influence sorptions [10]. We evaluated the sorption efficiency of weak base anionites for the sorption of U(VI) at different pH values ranging from 1.2 to 2.2 and process duration from 1.0 h to 12.0. The results are presented in Figure 3.
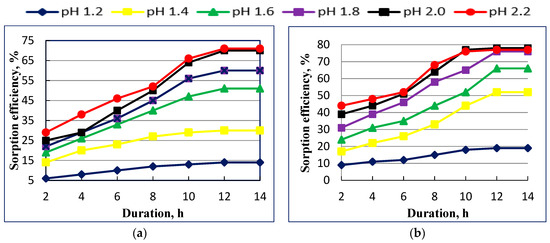
Figure 3.
Effect of contact time and рН on uranium (VI) sorption by Dowex Marathon WBA (a) and Diaion WA30 (b) anionites.
It was found that the sorption of U(VI) increases as the pH and duration increases (Figure 3a,b). This feature can be explained by the fact that uranium in this pH range exists mostly in the form of UO2(SO4)22− anion [10]. The maximum degree of uranium recovery for Dowex Marathon WBA anionite is 71% with a process duration of 12 h. The sorption reaches equilibrium, and further extension in the duration of sorption does not give any effect in increasing the degree of sorption. The obtained results for Diaion WA30 anionite, shown in Figure 3b, indicates that uranium (VI) sorption efficiency increases with increasing the contact time from 1 to 10 and the best results of sorption efficiency corresponds to pH 2.2 and reaches 77%.
3.2. Sorption of Uranium (VI) by Strong Base Anionites
In order to investigate the effect of pH on the sorption efficiency of strong base anionites as Ambersep 900 SO4 and Puromet MTA4601PF, several experiments were conducted at different pH values of 1.2, 1.4, 1.6, 1.8, 2.0, and 2.2. The impact of pH and process duration on the sorption of U(VI) by strong base anionites is shown in Figure 4.
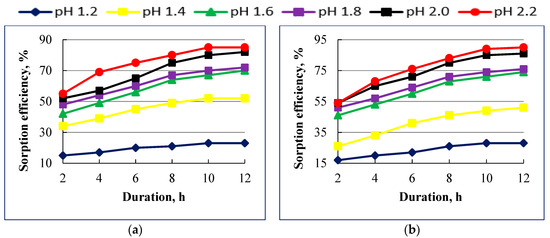
Figure 4.
Effect of contact time and рН on uranium (VI) sorption by Ambersep 900 SO4 (a) and Puromet MTA4601PF (b) anionites.
Figure 4 reveals that the uranium sorption with the usage of strong base anionite Ambersep 900 SO4 is much more efficient compared to weak base ionite Dowex Marathon WBA and Diaion WA30. Maximum sorption is observed in 10 h and reaches 85%, beyond which there is almost no further increase in the sorption. The best results for uranium sorption on this anionite were obtained at pH 2.2.
Results also showed a pronounced pH effect on U(VI) sorption with the strong base anionites. These two ionites show that U(VI) sorption increases to 88% and 90%, respectively, as pH increases from 1.2 to 2.2, which is 13% more than with weak base anionites. The results of the investigation showed that the best results were achieved at a pH of 2.2, with the duration of the process is 12 h.
In addition to uranium, the content of vanadium, molybdenum, iron and aluminum in the initial solution and in the solution at pH 1.2–2.2 after sorption were analyzed. The analysis results show that the sorption rate of uranium with different anionites is from 71% to 90%, and the sorption rate of vanadium is from 21% to 25%, molybdenum is from 8.0% to 11.5%, iron is from 3.0% to 5%, and aluminum is from 2.5% to 4.5%. It is known that vanadium is better sorbed in the five-valent state, so the four-valent vanadium contained in the initial solution is poorly sorbed [38], as well as molybdenum, iron and aluminum at pH 1.2–2.2 in the cationic state [11,30].
Due to the fact that the concentration of uranium in the solution after sorption is less than 0.01 g/L, its further processing for objective reasons is not of practical interest. Solutions containing valuable components, such as vanadium, molybdenum and REE, were sent for selective-stage extraction by sorption methods. In view of the fact that these components were recovered using different technological conditions (different pH values, redox potential, cation exchange resin, etc.), the presence of low concentrations of uranium did not affect the technological processes.
Results of uranium sorption efficiency of the anionites present increase according to:
Dowex Marathon WBA ˃ Diaion WA30 ˃ Ambersep 900 SO4 ˃ Puromet MTA4601PF.
3.3. Sorption Kinetics Studies
The kinetic study was carried out with leaching solution with uranium concentration of 62.0 mg·L−1, and other parameters are kept constant at pH 2.2 at room temperature.
The effect of contact time on the sorption capacity of Dowex Marathon WBA, Diaion WA30, Ambersep 900 SO4, Puromet MTA4601PF anionites was investigated, and the Pseudo-first-order, Pseudo-second-order and intraparticle diffusion models were used to interpret the experimental data. The linear form of the pseudo-first-order model can be represented by Lagergren Equation (4). As it is seen in Figure 5, linear plots are obtained.
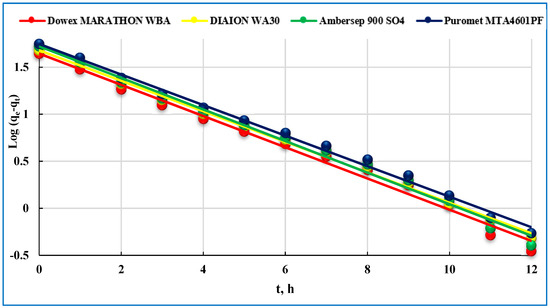
Figure 5.
Pseudo-first-order model of U(VI) adsorption on studied anionites.
The parameters for pseudo-first-order, pseudo- second-order and intraparticle diffusion models are listed in Figure 6.
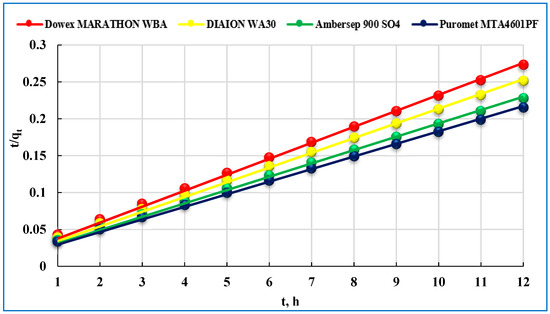
Figure 6.
Pseudo-second-order model of U(VI) adsorption on studied anionites.
As it is seen in Table 2, the sorption capacities are determined from the intercept of the plots and are found to be 43.53, 47.17, 52.11 and 55.17 mg/g, besides that the R2 values for this model were 0.989, 0.986, 0.981 and 0.984 for Dowex Marathon WBA, Diaion WA30, Ambersep 900 SO4 and Puromet MTA4601PF anionites, respectively. Pseudo-first-order model does not accurately describe the kinetics of U(VI) sorption for these anionites as evidenced by the poor model fit.
Studies have revealed that the pseudo second-order model is based on the assumption that the rate-limiting step may be chemisorption, which involves valence forces by sharing or electron exchange between the adsorbent and the adsorbate [39]. The pseudo-second-order kinetics isotherm has been used to describe and explain the sorption mechanism of the studied ionites. The Equations (5)–(7) are given in the Section 2.3.1. It could be found that the calculated value of qe from the pseudo-second-order kinetic model was in good agreement with the experimental data. The correlation coefficients (R2) from the Pseudo-second-order model exceeded 99%, which were higher than that from the Pseudo-first-order model. The results suggested that a good linear correlation existed between Pseudo-second-order model and the adsorption data.
Table 3 also demonstrates the initial adsorption rate values (h), g/mg·min: 0.022 for Dowex Marathon WBA; 0.020 for Diaion WA30; 0.018 for Ambersep 900 SO4 and 0.017 for Puromet MTA4601PF.

Table 3.
Kinetic parameters for U(VI) adsorption of onto studied anionites.
It is known that the sorption of uranium ions from its solutions onto the anionites is a multistep process, including transport of uranium ions from the aqueous phase to the solid surface of anionite particles as a first step (bulk diffusion). Further, a second step occurs by diffusion from the film to the particle surface (film diffusion). At the last stage, transport of uranium ions from the surface to the internal sites (surface diffusion or pore diffusion) occurs, and this happens at a much slower pace in comparison to other steps. Moreover, adsorption of uranium ions by an active site on the solid phase surface could also occur through chemical processes [40].
The intraparticle diffusion model assumes that the effect of the diffusion of radionuclide is the rate-controlling step for the sorption process. The intraparticle diffusivity is constant, and the direction of the diffusion is radial. This model was used to calculate the intraparticle diffusion rate constant and the intercept of the linear plots as expressed in Figure 7.
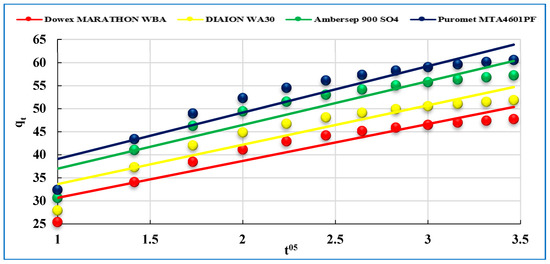
Figure 7.
Intra-particle diffusion model of U(VI) sorption on studied anionites.
The plots of qt vs. t0.5 show that the obtained straight lines do not pass through the origin (C > 0). The obtained data in Table 2, indicates that the values of correlation coefficient R2 are 0.876, 0.873, 0.874 and 0.875, while the intraparticle diffusion rate constants kid are 7.99, 8.54, 9.49 and 10.07 mg/g·min0.5 and the thickness of the boundary layer C is 22.63, 25.05, 27.44 and 28.98 (mg/g) for Dowex Marathon WBA, Diaion WA30, Ambersep 900 SO4 and Puromet MTA4601PF anionites, respectively. The obtained data confirm that this model is not suitable for characterization of the sorption kinetics. Lastly, from the kinetic parameters of three kinetic models, as seen in Table 2, the adsorption kinetics is estimated and fitted well by the pseudo-second-order kinetic model.
3.4. Adsorption Equilibrium Studies
The amount of ion adsorbed is determined as a function of the concentration at a constant temperature that could be explained in adsorption isotherms. Equations that are often used to describe the experimental isotherm data were developed by, namely, Freundlich, Langmuir and the Dubinin–Redushkevich isotherm. The equilibrium studies can also be used for both the design of sorption process and understanding the sorption mechanism. The mechanism of sorption depends on the nature of ionites, surface properties, affinities of the sorbent, and the bulk properties of the aqueous solution [41]. To investigate the best fitting isotherm model, the sorption experiments are conducted at the conditions described in Section 2.3.
The Langmuir adsorption isotherm is used to describe the equilibrium between adsorbate and adsorbent system, where the adsorbate adsorption is limited to one molecular layer at or before a relative pressure of unity is reached.
The linear plots of Ce/qe versus Ce for Langmuir isotherm model for the sorption of uranium onto the studied anionites is shown in Figure 8. The parameters of the kinetic models and the regression correlation coefficients (R2) are listed in Table 3. From the R2 value, it was found that the Langmuir isotherm model fitted the kinetic data of the anionites better than that of the Freundlich and Dubinin–Redushkevich models. The confirmation of Langmuir isotherm model isotherms indicates that the concentrations of both adsorbate (uranium) and sorbent (studied anionites) are involved in the rate-determining step of the sorption process.
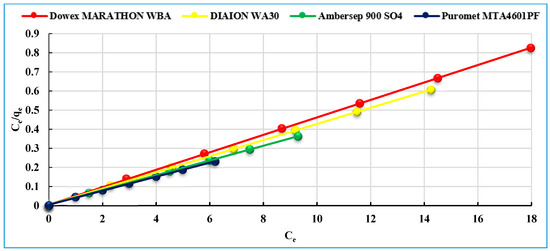
Figure 8.
The Langmuir isotherm model of uranium (VI) sorption on studied anionites.
The Freundlich isotherm model is based on the assumption that adsorption occurs on a heterogenous surface. It proposes that the sorption carries out with a heterogenous energetic distribution of active sites, accompanied by interactions between adsorbed molecules. The adsorption parameters are shown in Table 3. A 1/n value of less than 1.0 indicates that U(VI) is favorably adsorbed by studied anionites. From the obtained data, it is shown that the Freundlich isotherm does not fit the experimental data (Figure 9).
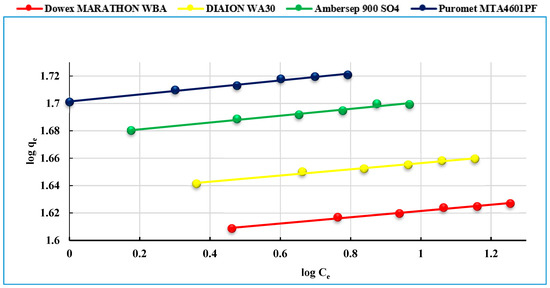
Figure 9.
The Freundlich isotherm model of of uranium (VI) sorption on the studied anionites.
Although the Langmuir and Freundlich isotherm models are widely used, they do not give information on the adsorption mechanism. To this end, the equilibrium data were tested with the Dubinin–Radushkevich (D–R) isotherm model. This isotherm model predicts the nature of the adsorbate sorption onto the anionite, and it is used to calculate the mean free energy of sorption [42,43].
The D–R constants are calculated from the linear plots of ln qe versus ε2∙105 from the intercept and slope (Figure 10 and Table 4).
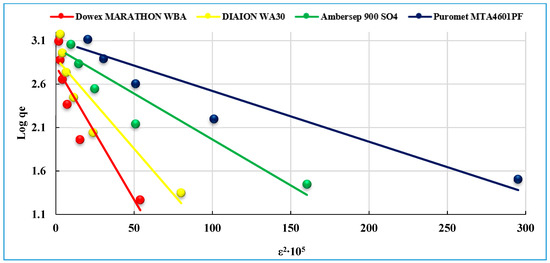
Figure 10.
The Dubinin–Radushkevich isotherm model of uranium (VI) sorption on the studied anionites.

Table 4.
Isotherms parameters of different models for the adsorption of U(VI) ion onto Dowex Marathon WBA, Diaion WA30, Ambersep 900 SO4 and Puromet MTA4601PF surface. D–R, Dubinin–Radushkevich.
It is known, that the sorption process proceeds via physical and chemical nature. From the obtained data in Table 4, the value of E for Dowex Marathon WBA, Diaion WA30 and Ambersep 900 SO4 is less than 8 k/Jmol for uranium ions indicating that the U(VI) sorption process proceeds via physisorption. The value of E for Puromet MTA4601PF corresponds to 9.28 kJ/mol, so the sorption process is supposed to proceed via chemisorption [33]. The values of correlation coefficient (R2) are 0.849, 0.854, 0.866 and 0.880 for Dowex Marathon WBA, Diaion WA30, Ambersep 900 SO4 and Puromet MTA4601PF anionites, respectively. Therefore, the Dubinin–Radushkevich (D–R) isotherm models do not fit the adsorption processes of the uranium ions on the studied anionites.
4. Conclusions
In this study, usage of various anionites for uranium (VI) sorption was obtained from leaching solution. The important findings are listed below:
- It was found that the sorption efficiency of anionites increases in the following order: Dowex Marathon WBA < Diaion WA30 < Ambersep 900 SO4 < Puromet MTA4601PF.
- The best results for the sorption of uranium were obtained on strong-base anionites Ambersep 900 SO4 and Puromet MTA4601PF. The uranium sorption on the strong-base anionite Puromet MTA4601PF increases with the increase of pH and reaches 90%, which is 13% more than on weak base anionites. The results of the investigation showed that the best results were achieved at a pH of 2.2 and a duration of 12 h.
- The kinetics study demonstrated that the kinetic mechanism for the sorption of uranium (VI) ions followed a pseudo-second-order model, which provided the best experimental data correlation.
- The Langmuir isotherm model provided the best fit for the experimental sorption data for uranium.
- From the Dubinin–Radushkevich isotherm, the values of the mean free energy of adsorption revealed that the sorption mechanism for Dowex Marathon WBA, Diaion WA30 and Ambersep 900 SO4 was predominantly, due to a physical nature (E < 8.0 KJ/mo1). However, the data obtained for free energy for Puromet MTA4601PF (E = 9.28 KJ/mo1) indicated that the sorption mechanism proceeded via chemisorption.
Author Contributions
Data curation, A.K.; Investigation, O.B. and M.A.; Methodology, M.D.T.; Supervision, B.M. and T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the grant of the Ministry of Education and Science of the Republic of Kazakhstan (Grant2018/AP05134773).
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Doligez, X.; Bouneau, S.; David, S.; Ernoult, M.; Zakari-Issoufou, A.-A.; Thiollière, N.; Bidaud, A.; Méplan, O.; Nuttin, A.; Capellan, N. Fundamentals of reactor physics with a view to the (possible) futures of nuclear energy. Comptes Rendus Phys. 2017, 18, 372–380. [Google Scholar] [CrossRef]
- Liu, W.; Dai, X.; Bai, Z.; Wang, Y.; Yang, Z.; Zhang, L.; Xu, L.; Chen, L.; Li, Y.; Gui, D.; et al. Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal-organic framework equipped with abundant lewis basic sites: A combined batch, X-ray absorption spectroscopy, and first principles simulation investigation. Environ. Sci. Technol. 2017, 51, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Sheta, M.E.I.; Mahfouz, M.G.; Ahmed, S.H.; Aal, M.M.A. Uranium extraction from sulphuric acid solution using anion-exchange resin. Сhem. Technol. Indian J. 2015, 10, 88–94. [Google Scholar]
- Sanakulov, K.S.; Petukhov, O.F.; Sharafutdinov, U.Z. Extraction of vanadium and uranium from refractory black shale ores. Tsvetnye Met. 2019, 10, 46–49. [Google Scholar] [CrossRef]
- Zagorodnyaya, A.; Abisheva, Z.; Sharipova, A.; Sadykanova, S.S.; Akcil, A. Regularities of Rhenium and Uranium Sorption From Mixed Solutions with Weakly Basic Anion Exchange Resin. Miner. Process. Extr. Met. Rev. 2015, 36, 391–398. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, C. Deeply purifying and recovery uranium from the uranium mine water by ion exchange. Chem. Ind. Eng. Progr. 2011, 30, 126–129. [Google Scholar]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Zagorodnyaya, A.; Abisheva, Z.; Sharipova, A.; Sadykanova, S.; Bochevskaya, Y.; Atanova, O. Sorption of rhenium and uranium by strong base anion exchange resin from solutions with different anion compositions. Hydrometallurgy 2013, 132, 127–132. [Google Scholar] [CrossRef]
- Tan, J.; Wang, Y.; Liu, N.; Liu, M. Adsorption of uranium (VI) from aqueous solution by tetraphenylimidodiphosphinate. J. Radioanal. Nucl. Chem. 2018, 315, 119–126. [Google Scholar] [CrossRef]
- Danko, B.; Dybczyński, R.S.; Samczyński, Z.; Gajda, D.; Herdzik-Koniecko, I.; Zakrzewska-Kołtuniewicz, G.; Chajduk, E.; Kulisa, K. Ion exchange investigation for recovery of uranium from acidic pregnant leach solutions. Nukleonika 2017, 62, 213–221. [Google Scholar] [CrossRef]
- Orrego, P.; Hernández, J.; Reyes, A. Uranium and molybdenum recovery from copper leaching solutions using ion exchange. Hydrometallurgy 2019, 184, 116–122. [Google Scholar] [CrossRef]
- Ali, M.M.; Taha, M.H.; Killa, H.M.; El Wanees, S.A.; El-Maadawy, M.M. Synergistic extraction of uranium from acidic sulfate leach liquor using D2EHPA mixed with TOPO. J. Radioanal. Nucl. Chem. 2014, 300, 963–967. [Google Scholar] [CrossRef]
- Mellah, A.; Chegrouche, S.; Barkat, M. The precipitation of ammonium uranyl carbonate (AUC): Thermodynamic and kinetic investigations. Hydrometallurgy 2007, 85, 163–171. [Google Scholar] [CrossRef]
- Bayyari, M.; Nazal, M.; Khalili, F. The effect of ionic strength on the extraction of Thorium(IV) from nitrate solution by didodecylphosphoric acid (HDDPA). J. Saudi Chem. Soc. 2010, 14, 311–315. [Google Scholar] [CrossRef]
- Tyrpekl, V.; Beliš, M.; Wangle, T.; Vleugels, J.; Verwerft, M.; Belis, M. Alterations of thorium oxalate morphology by changing elementary precipitation conditions. J. Nucl. Mater. 2017, 493, 255–263. [Google Scholar] [CrossRef]
- Ang, K.L.; Li, D.; Nikoloski, A. The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 2. Chelating resins. Miner. Eng. 2018, 123, 8–15. [Google Scholar] [CrossRef]
- Sazonova, V.F.; Perlova, O.; Polikarpov, A.P. Sorption of uranium(VI) compounds on fibrous anion exchanger surface from aqueous solutions. Colloid J. 2017, 79, 270–277. [Google Scholar] [CrossRef]
- Pulhani, V.A.; Dafauti, S.; Hegde, A.G. Separation of uranium from iron in ground water samples using ion exchange resins. J. Radioanal. Nucl. Chem. 2011, 294, 299–302. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, Y.; Zhao, W.; Xu, J. Sorption behavior of U(VI) onto Chinese bentonite: Effect of pH, ionic strength, temperature and humic acid. J. Mol. Liq. 2013, 188, 178–185. [Google Scholar] [CrossRef]
- Salameh, S.I.; Khalili, F.I.; Al-Dujaili, A.H. Removal of U(VI) and Th(IV) from aqueous solutions by organically modified diatomaceous earth: Evaluation of equilibrium, kinetic and thermodynamic data. Int. J. Miner. Process. 2017, 168, 9–18. [Google Scholar] [CrossRef]
- Sohbatzadeh, H.; Keshtkar, A.; Safdari, J.; Yousefi, T.; Fatemi, F. Insights into the biosorption mechanisms of U(VI) by chitosan bead containing bacterial cells: A supplementary approach using desorption eluents, chemical pretreatment and PIXE–RBS analyses. Chem. Eng. J. 2017, 323, 492–501. [Google Scholar] [CrossRef]
- Zhao, J.; Fasfous, I.; Murimboh, J.; Yapici, T.; Chakraborty, P.; Boca, S.; Chakrabarti, C.L. Kinetic study of uranium speciation in model solutions and in natural waters using Competitive Ligand Exchange Method. Talanta 2009, 77, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Tournassat, C.; Tinnacher, R.; Grangeon, S.; Davis, J.A. Modeling uranium(VI) adsorption onto montmorillonite under varying carbonate concentrations: A surface complexation model accounting for the spillover effect on surface potential. Geochim. Cosmochim. Acta 2018, 220, 291–308. [Google Scholar] [CrossRef]
- Ghasemi, M.; Keshtkar, A.; Dabbagh, R.; Safdari, S.J. Biosorption of uranium(VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga: Breakthrough curves studies and modeling. J. Hazard. Mater. 2011, 189, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, X.; Wang, X.; Lu, X.; Shen, Y. Different biosorption mechanisms of Uranium(VI) by live and heat-killed Saccharomyces cerevisiae under environmentally relevant conditions. J. Environ. Radioact. 2017, 167, 92–99. [Google Scholar] [CrossRef]
- Diwan, V.; Sar, S.K.; Biswas, S.; Lalwani, R. Adsorptive extraction of uranium(VI) from aqueous phase by dolomite. Groundw. Sustain. Dev. 2020, 100424. [Google Scholar] [CrossRef]
- Quinn, J.E.; Sedger, D.; Brennan, A.T.; Ring, R.; Soldenhoff, K. Recovery of uranium from carbonate solutions using Lewatit TP 107 resin. Hydrometallurgy 2020, 194, 105360. [Google Scholar] [CrossRef]
- Ma, D.; Wei, J.; Zhao, Y.; Chen, Y.; Tang, S. The removal of uranium using novel temperature sensitive urea-formaldehyde resin: Adsorption and fast regeneration. Sci. Total Environ. 2020, 139399. [Google Scholar] [CrossRef]
- Amesh, P.; Suneesh, A.; Selvan, B.R.; Venkatesan, K.; Chandra, M. Magnetic assisted separation of uranium(VI) from aqueous phase using diethylenetriamine modified high capacity iron oxide adsorbent. J. Environ. Chem. Eng. 2020, 8, 103661. [Google Scholar] [CrossRef]
- Humelnicu, D.; Drochioiu, G.; Sturza, M.; Cecal, A.; Popa, K. Kinetic and thermodynamic aspects of U(VI) and Th(IV) sorption on a zeolitic volcanic tuff. J. Radioanal. Nucl. Chem. 2006, 270, 637–640. [Google Scholar] [CrossRef]
- Mohamud, H.; Ivanov, P.I.; Russell, B.C.; Regan, P.H.; Ward, N.I. Selective sorption of uranium from aqueous solution by graphene oxide-modified materials. J. Radioanal. Nucl. Chem. 2018, 316, 839–848. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.; Otterburn, M.S.; Aga, J.A. Intraparticle diffusion process occurring during adsorption of dyestuffs. Water Air Soil Pollut. 1987, 36, 381–390. [Google Scholar] [CrossRef]
- Eddaif, L.; Shaban, A.; Telegdi, J. Application of the Langmuir Technique to Study the Response of C-dec-9-en-1-ylcalix[4]resorcinarene and C-undecylcalix[4]resorcinarene Ultra-thin Films’ Interactions with Cd2+, Hg2+, Pb2+, and Cu2+ Cations Present in the Subphase. Water Air Soil Pollut. 2019, 230, 279. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Serpinski, V.; Jakubov, T. Dubinin–Radushkevich Equation as the Equation for the Excess Adsorption Isotherm. Adsorpt. Sci. Technol. 1993, 10, 85–92. [Google Scholar] [CrossRef]
- Tovbin, Y.K. The volume of micropores and the Dubinin—Radushkevich equation. Russ. Chem. Bull. 1998, 47, 637–643. [Google Scholar] [CrossRef]
- Luo, X.; Yu, L.; Wang, C.; Yin, X.; Mosa, A.; Lv, J.; Sun, H. Sorption of vanadium (V) onto natural soil colloids under various solution pH and ionic strength conditions. Chemosphere 2017, 169, 609–617. [Google Scholar] [CrossRef]
- Sultanbayeva, G.; Holze, R.; Chernyakova, R.; Jussipbekov, U. Removal of Fe2+-, Cu2+-, Al3+- and Pb2+-ions from phosphoric acid by sorption on carbonate-modified natural zeolite and its mixture with bentonite. Microporous Mesoporous Mater. 2013, 170, 173–180. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption properties of Congo Red from aqueous solution onto surfactant-modified montmorillonite. J. Hazard. Mater. 2008, 160, 173–180. [Google Scholar] [CrossRef]
- Parab, H.; Joshi, S.; Shenoy, N.; Verma, R.; Lali, A.; Sudersanan, M. Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies. Bioresour. Technol. 2005, 96, 1241–1248. [Google Scholar] [CrossRef]
- Cheira, M.F.; Atia, B.M.; Kouraim, M.N. Uranium(VI) recovery from acidic leach liquor by Ambersep 920U SO4 resin: Kinetic, equilibrium and thermodynamic studies. J. Radiat. Res. Appl. Sci. 2017, 10, 307–319. [Google Scholar] [CrossRef]
- Hutson, N.D.; Yang, R.T. Theoretical basis for the Dubinin-Radushkevitch (D-R) adsorption isotherm equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of the activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–337. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).