Quantitative X-ray µCT Measurement of the Effect of Ore Characteristics on Non-Surface Mineral Grain Leaching
Abstract
1. Introduction
2. Experimental
2.1. Ores
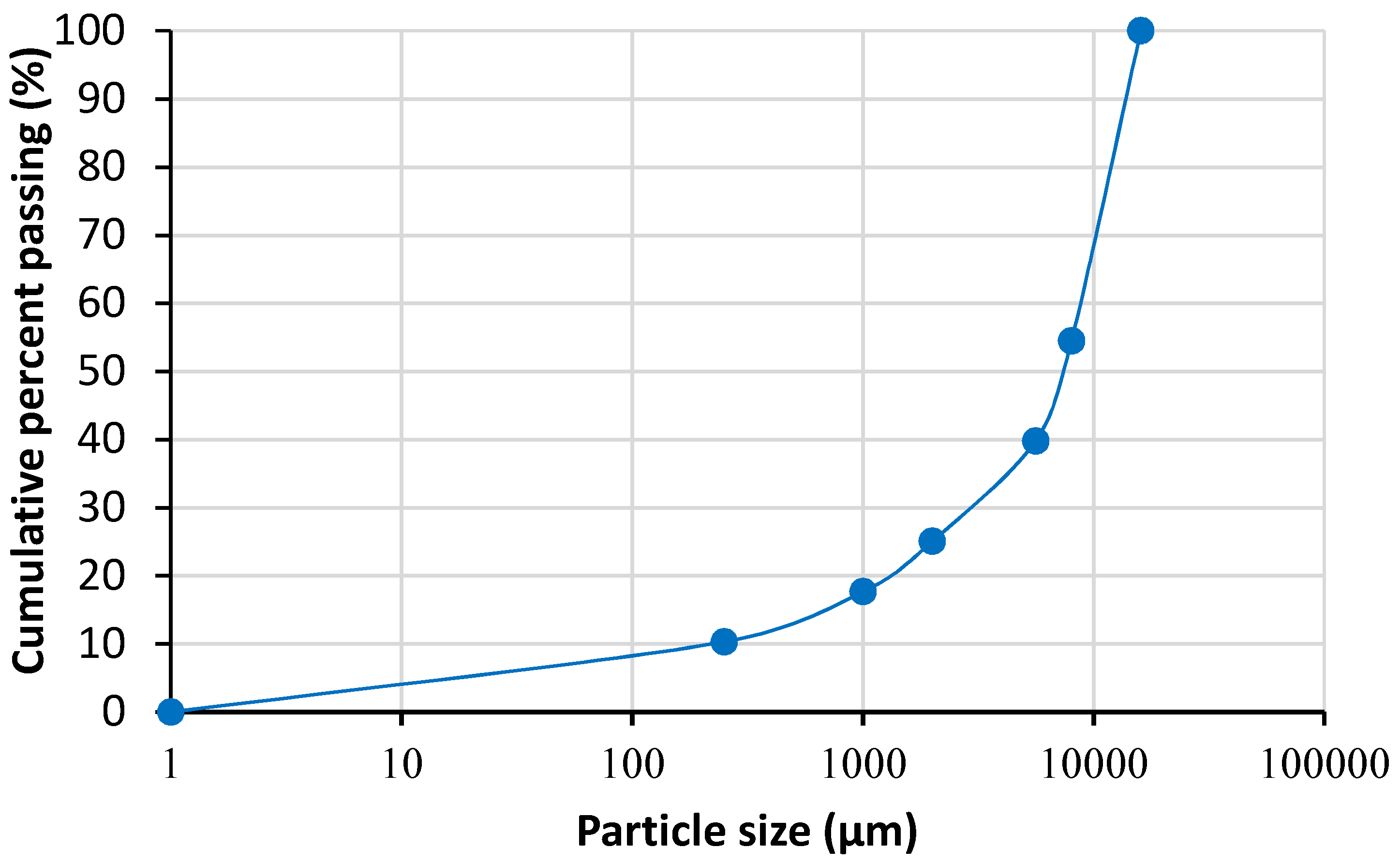
2.1.1. Ore Particle Size Distribution
2.1.2. Ore Agglomeration
2.2. Experimental Setup
2.2.1. Mini-Leaching Columns
2.2.2. Incubators
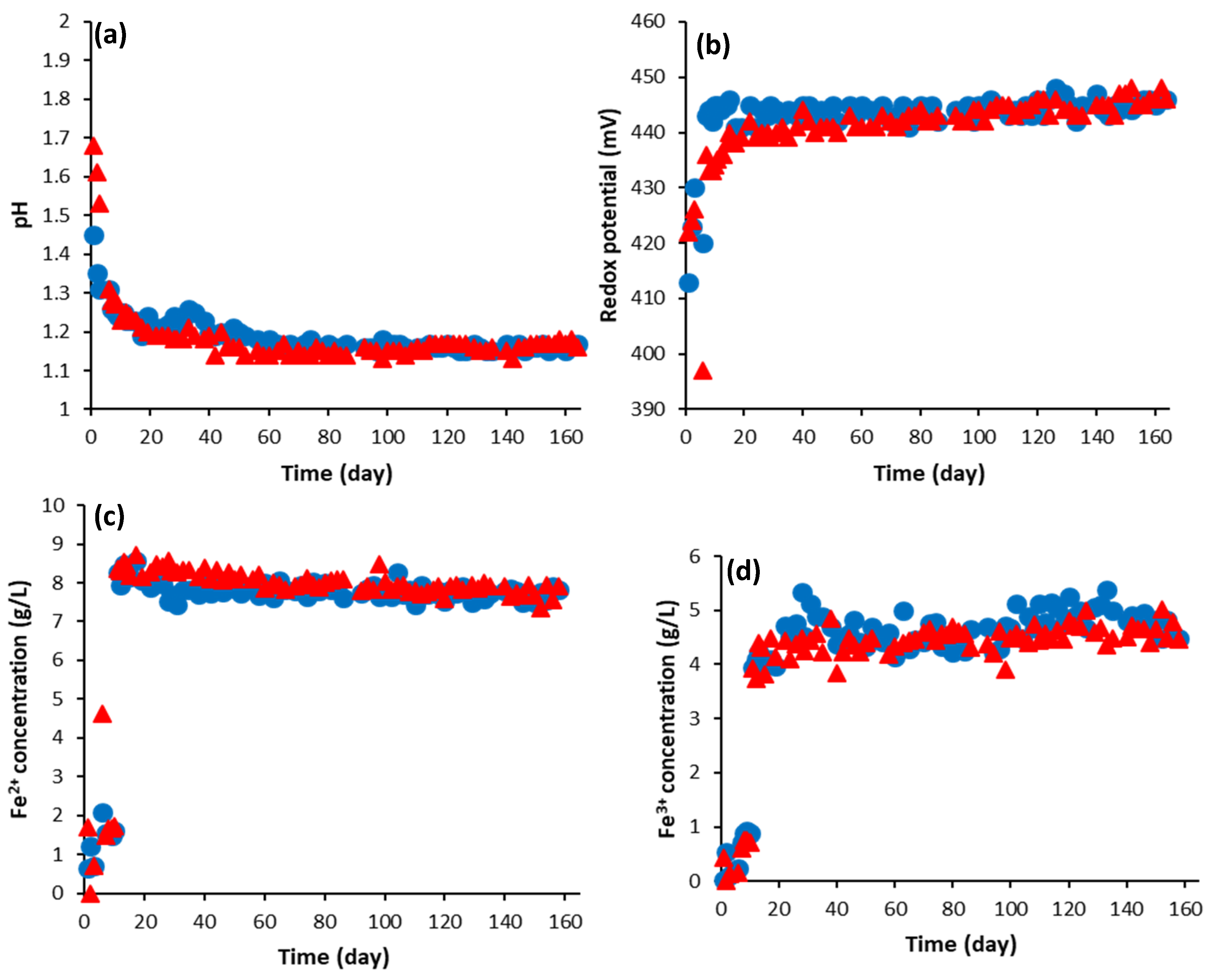
2.3. Solution Chemistry
2.4. Flow-Through Leaching Experiments
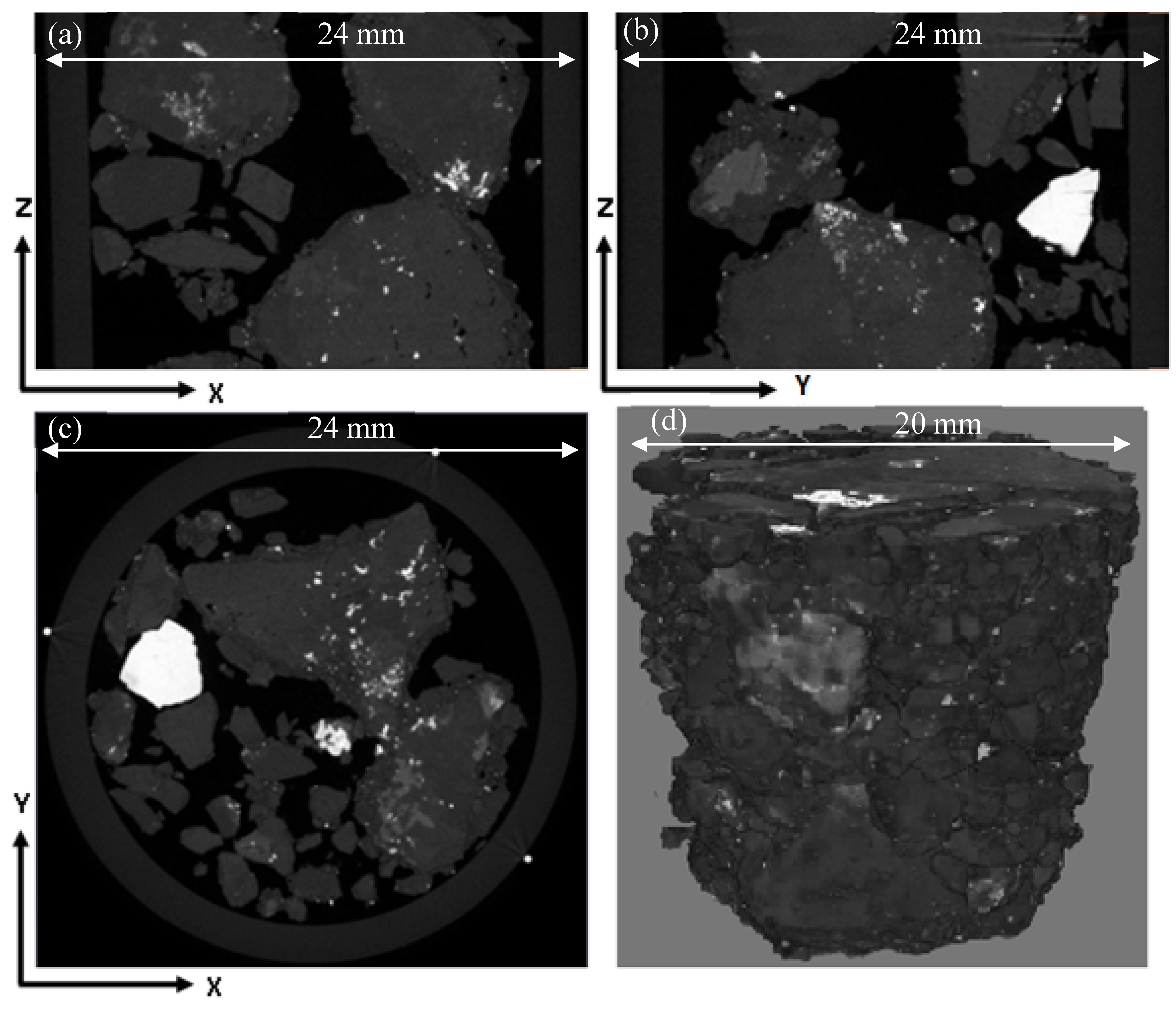
2.5. X-ray µCT Image Acquisition
3. Image Processing Design
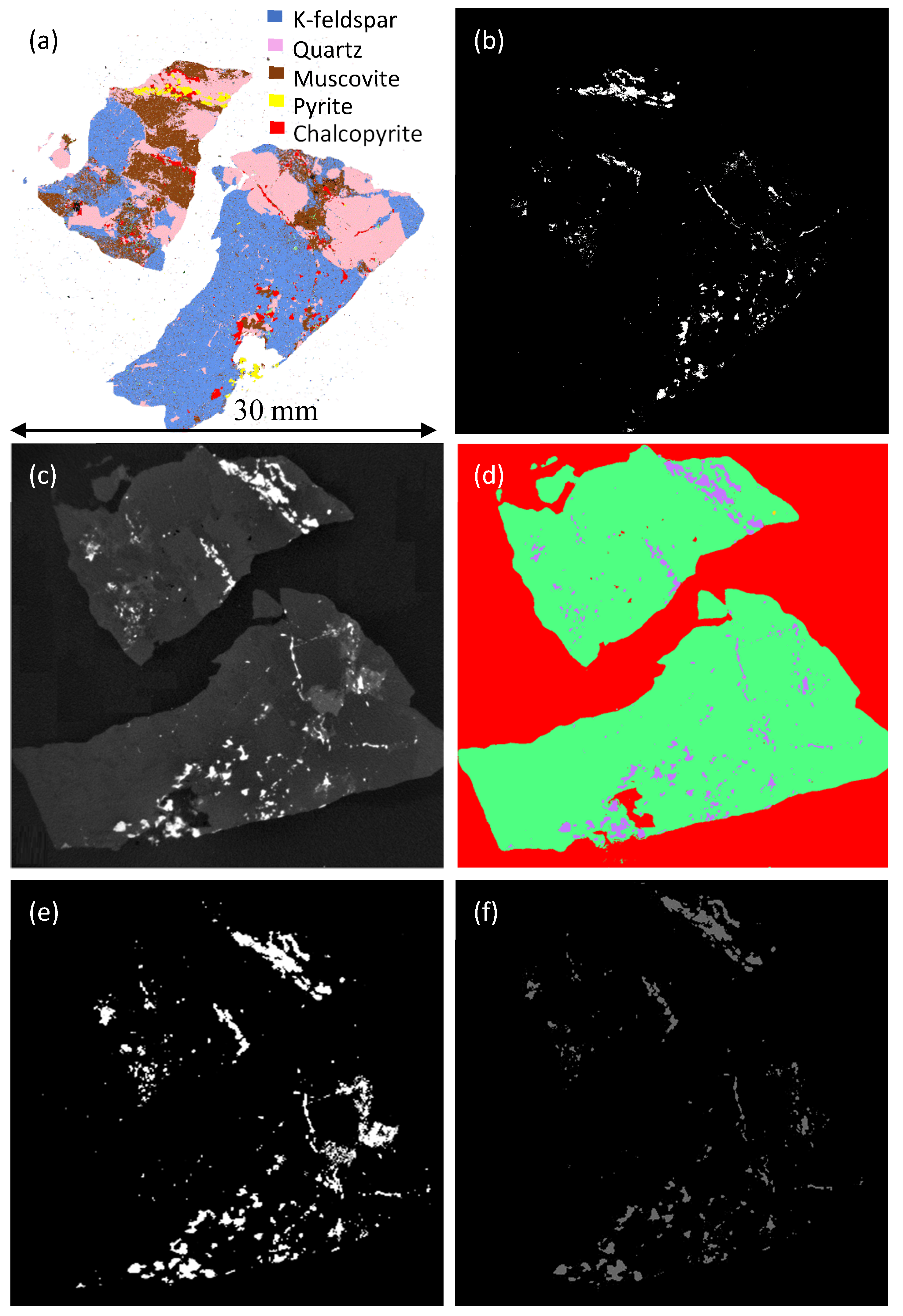
3.1. Mineral Grain Segmentation
3.2. Mineral Grain Distance Mapping
4. Results and Discussion
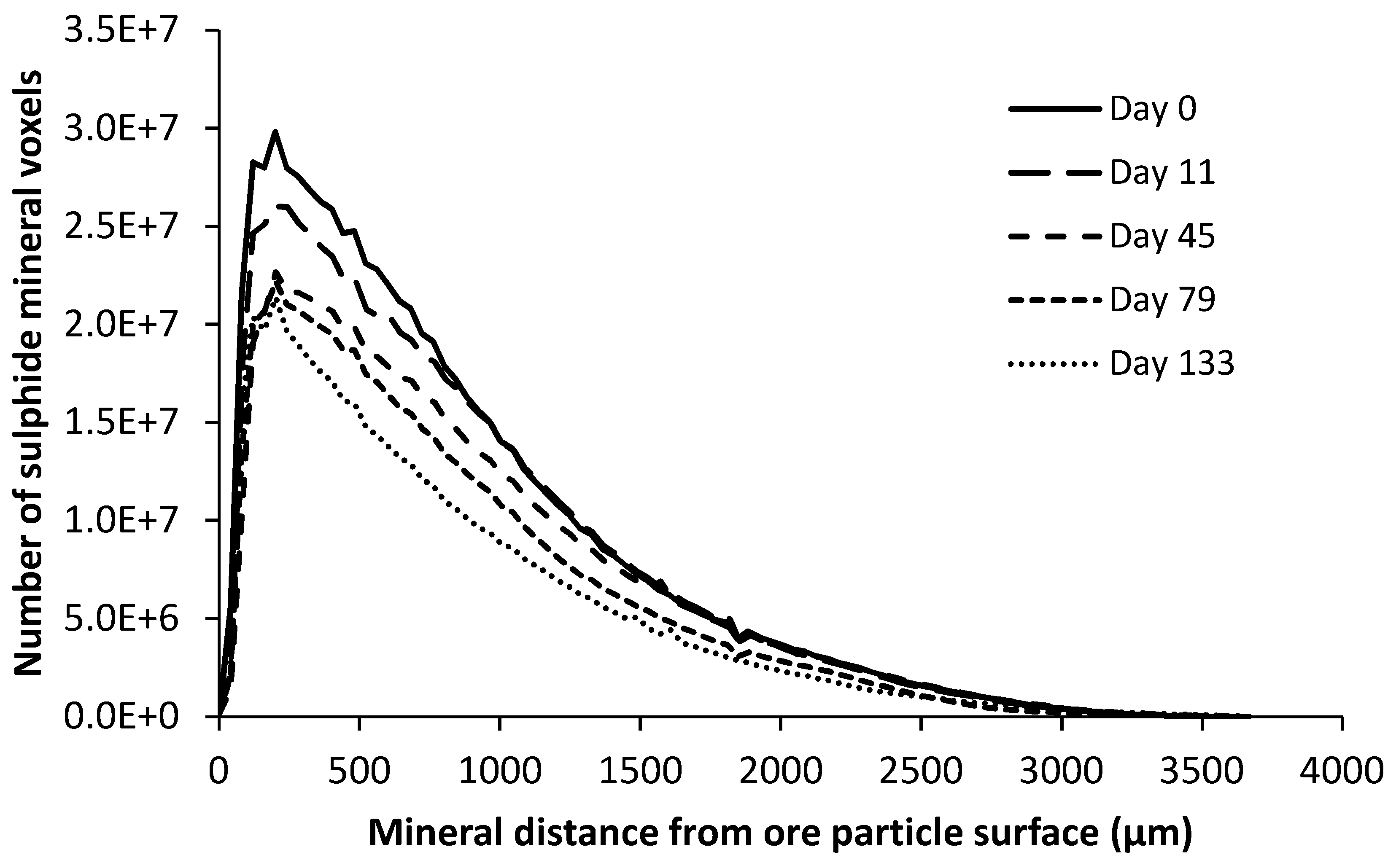
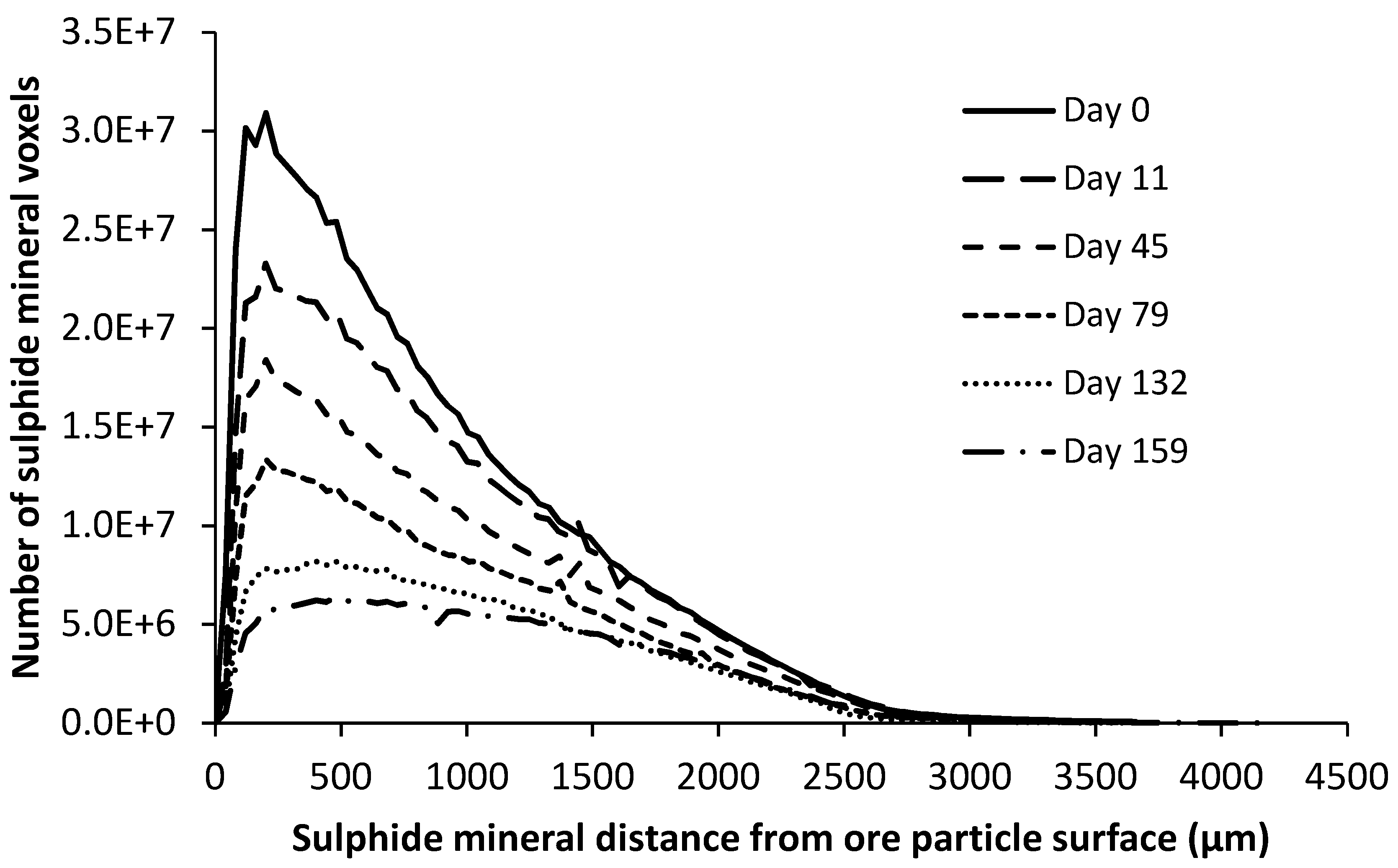
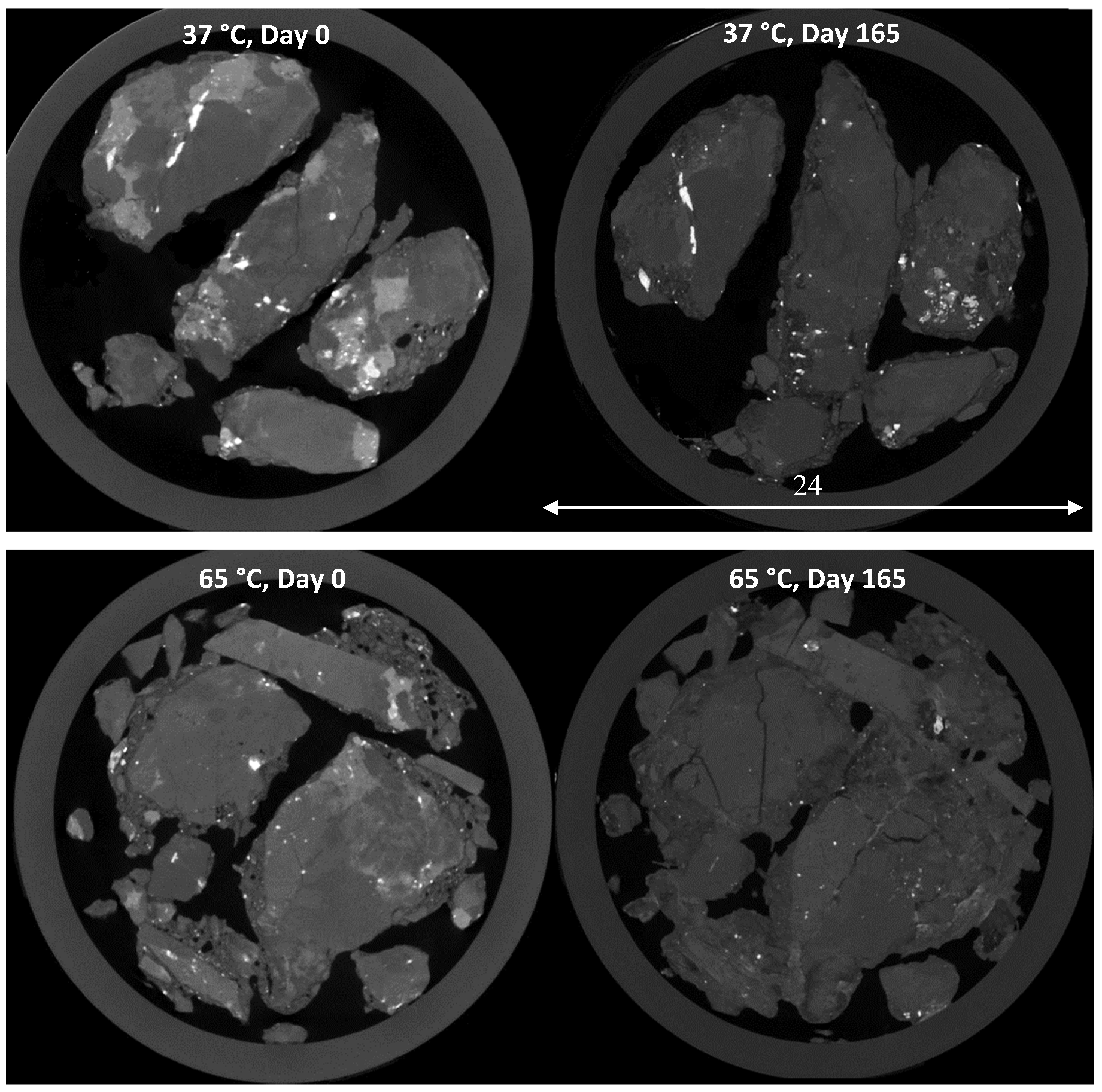
4.1. Ore Structure Characterisation
4.2. Leaching Results
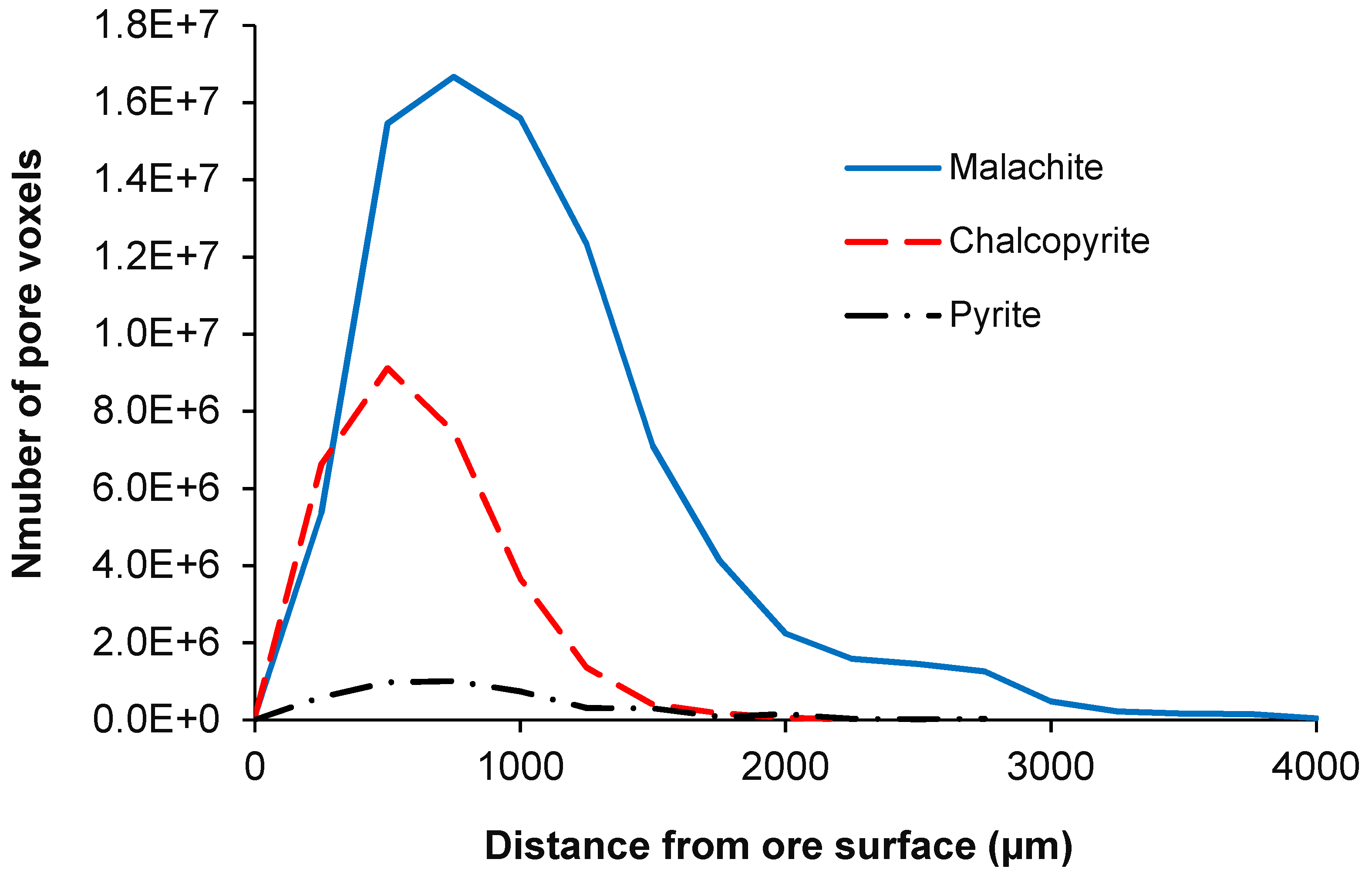
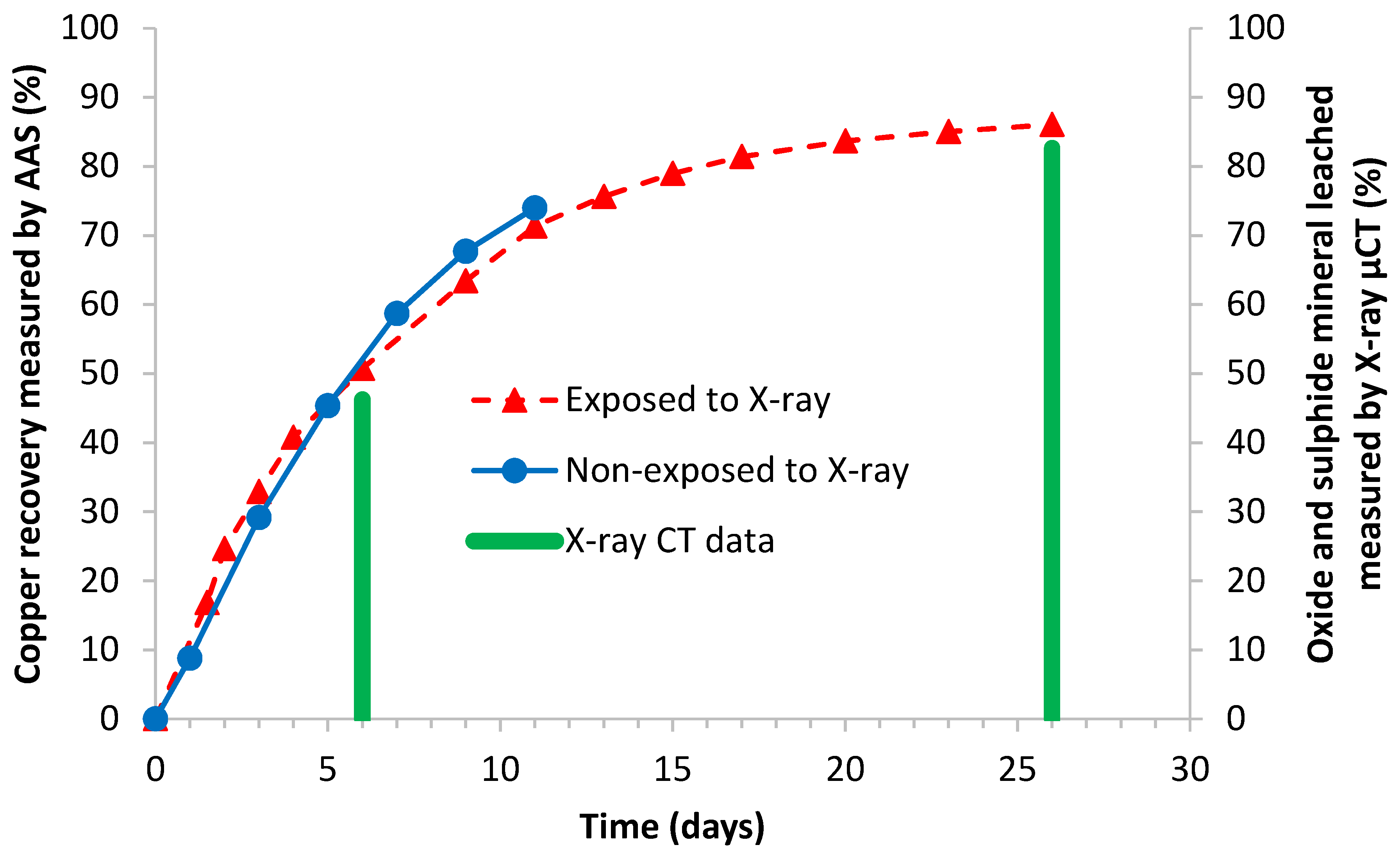
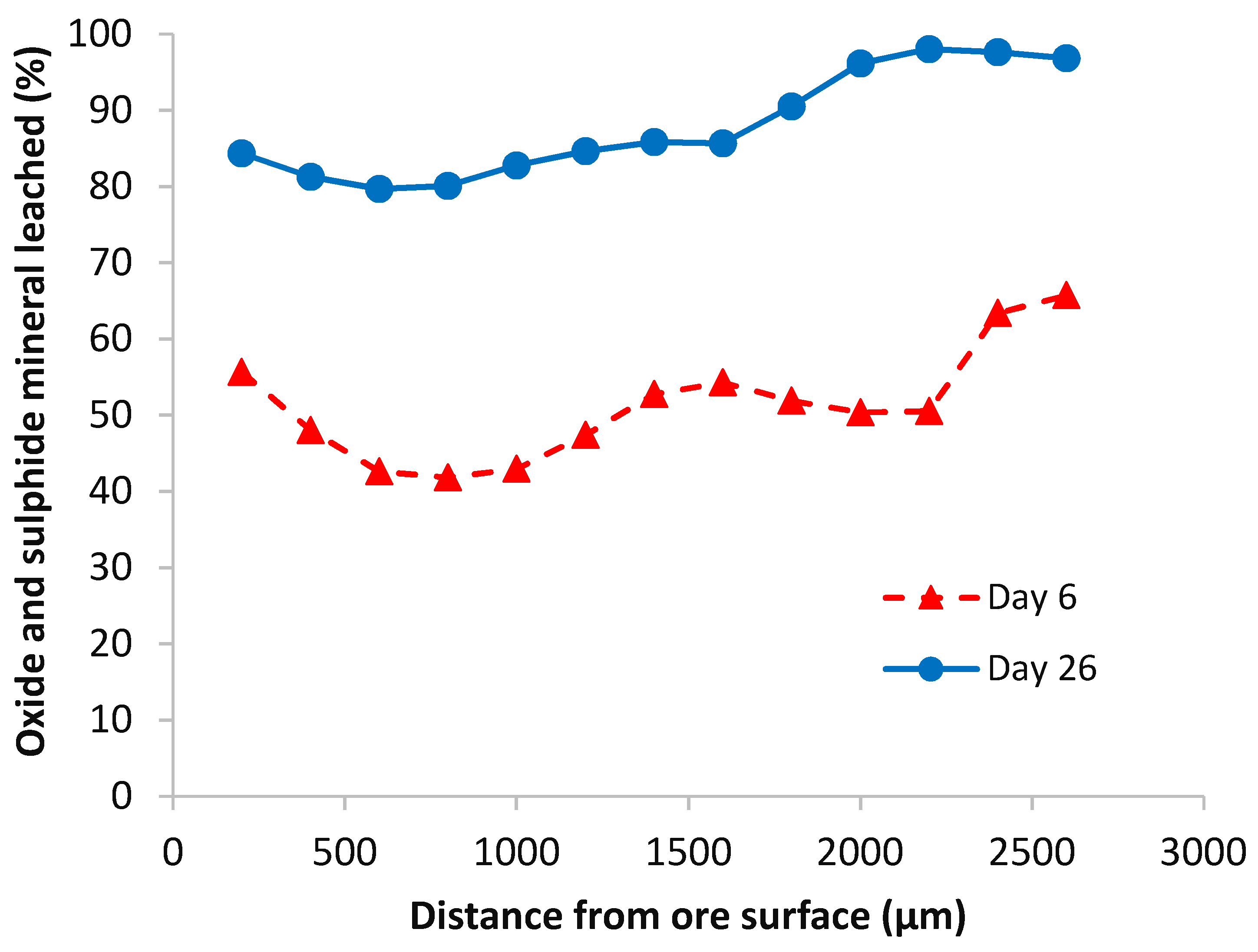
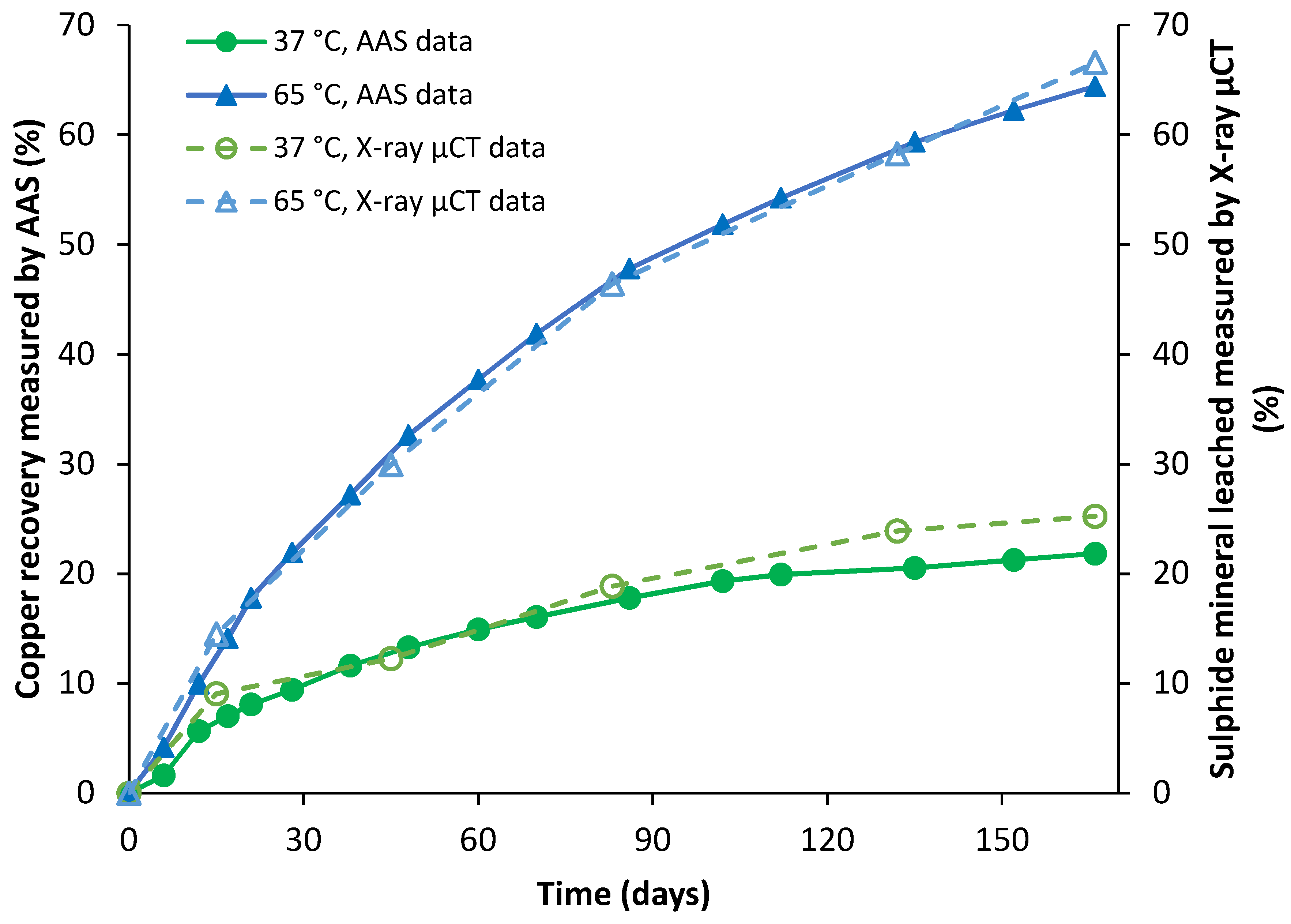
4.2.1. Malachite
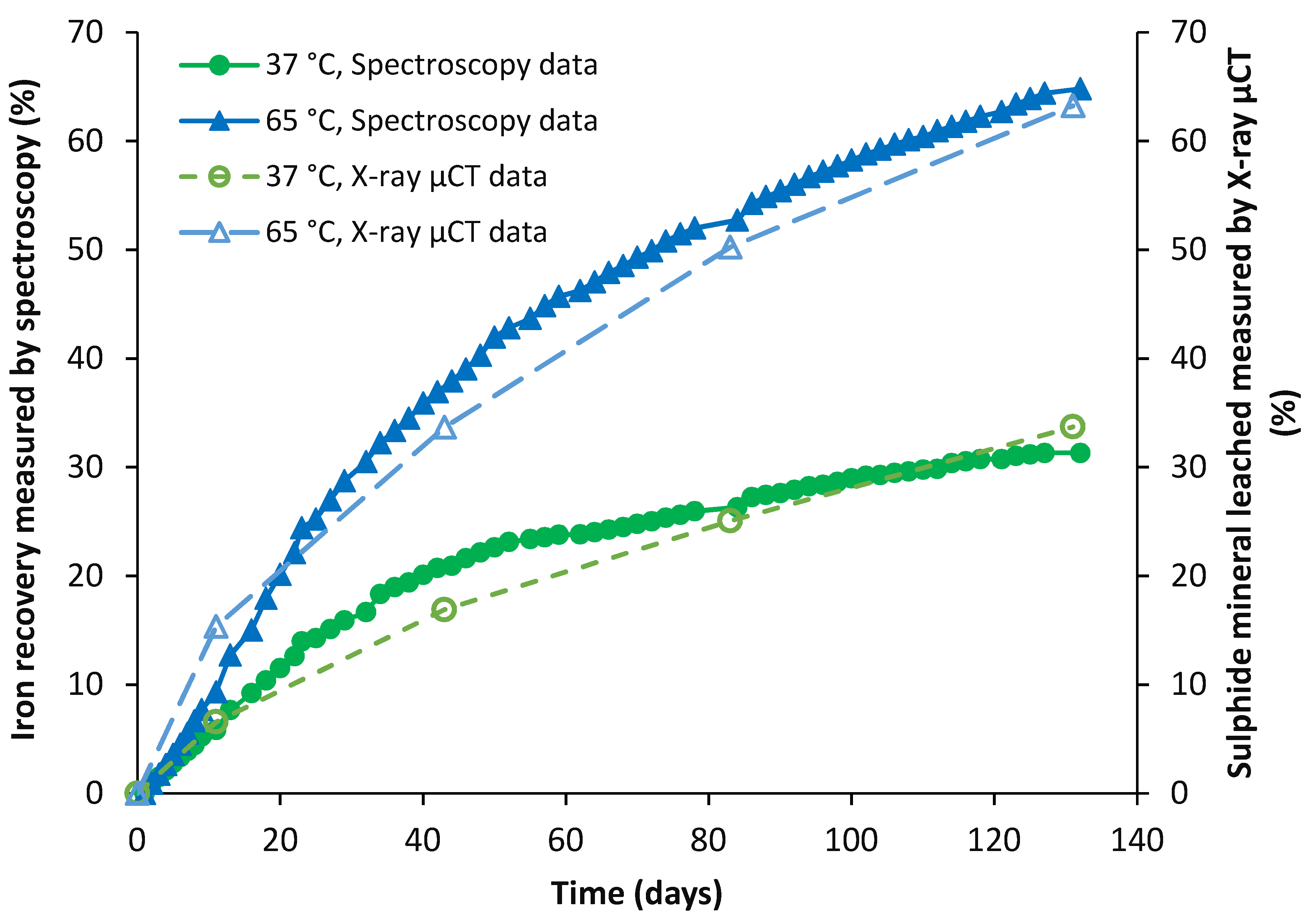
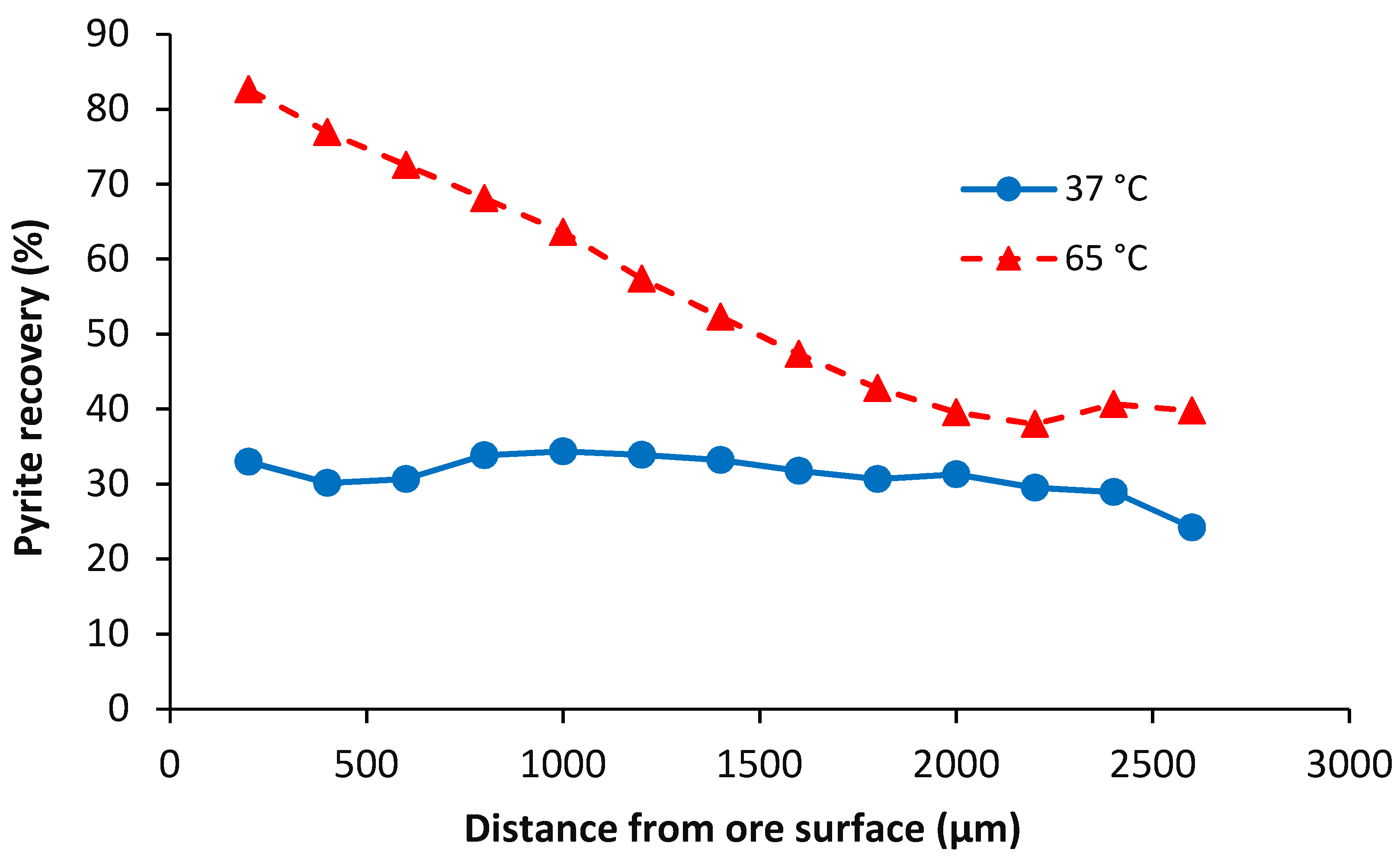
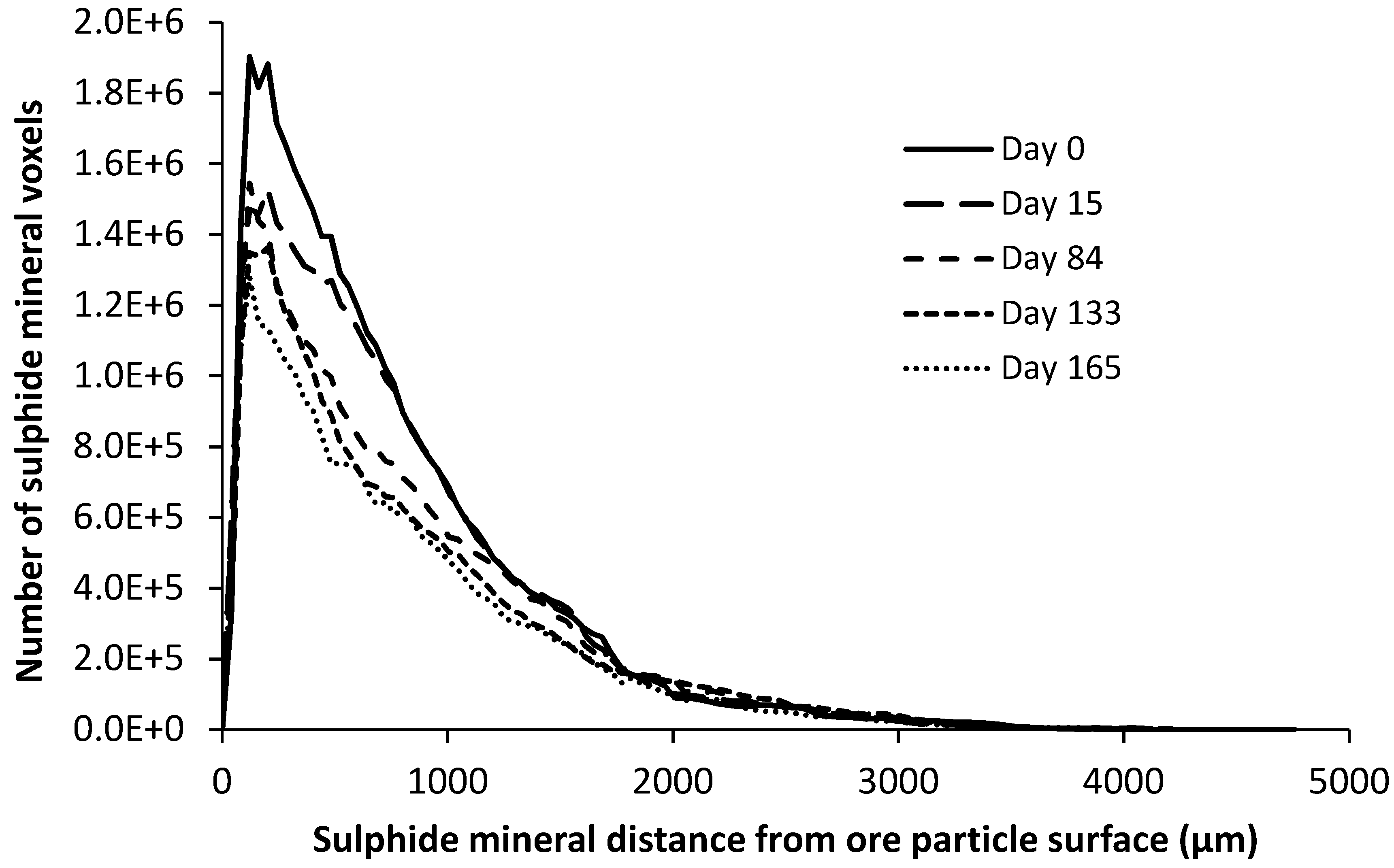
4.2.2. Pyrite
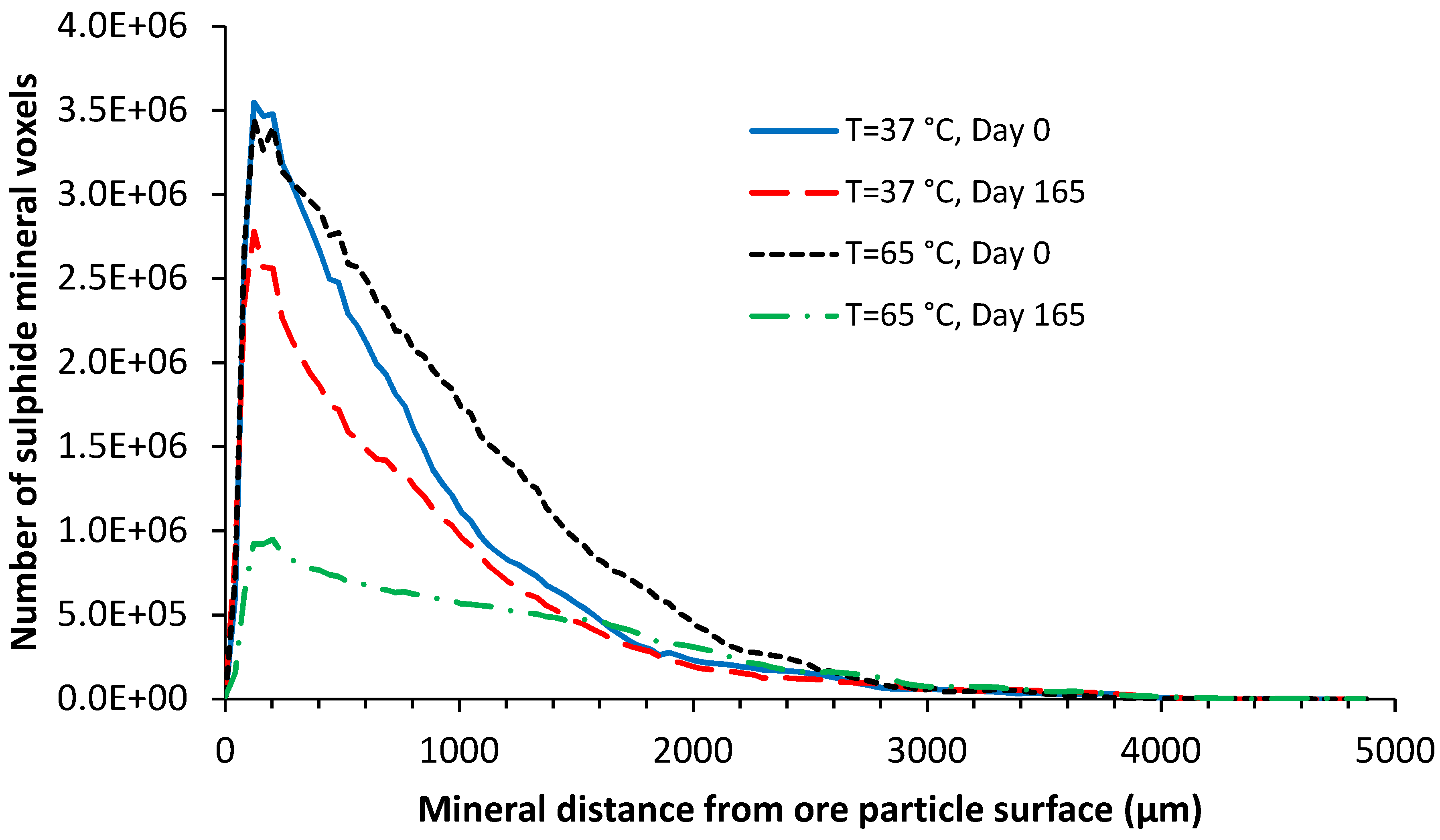
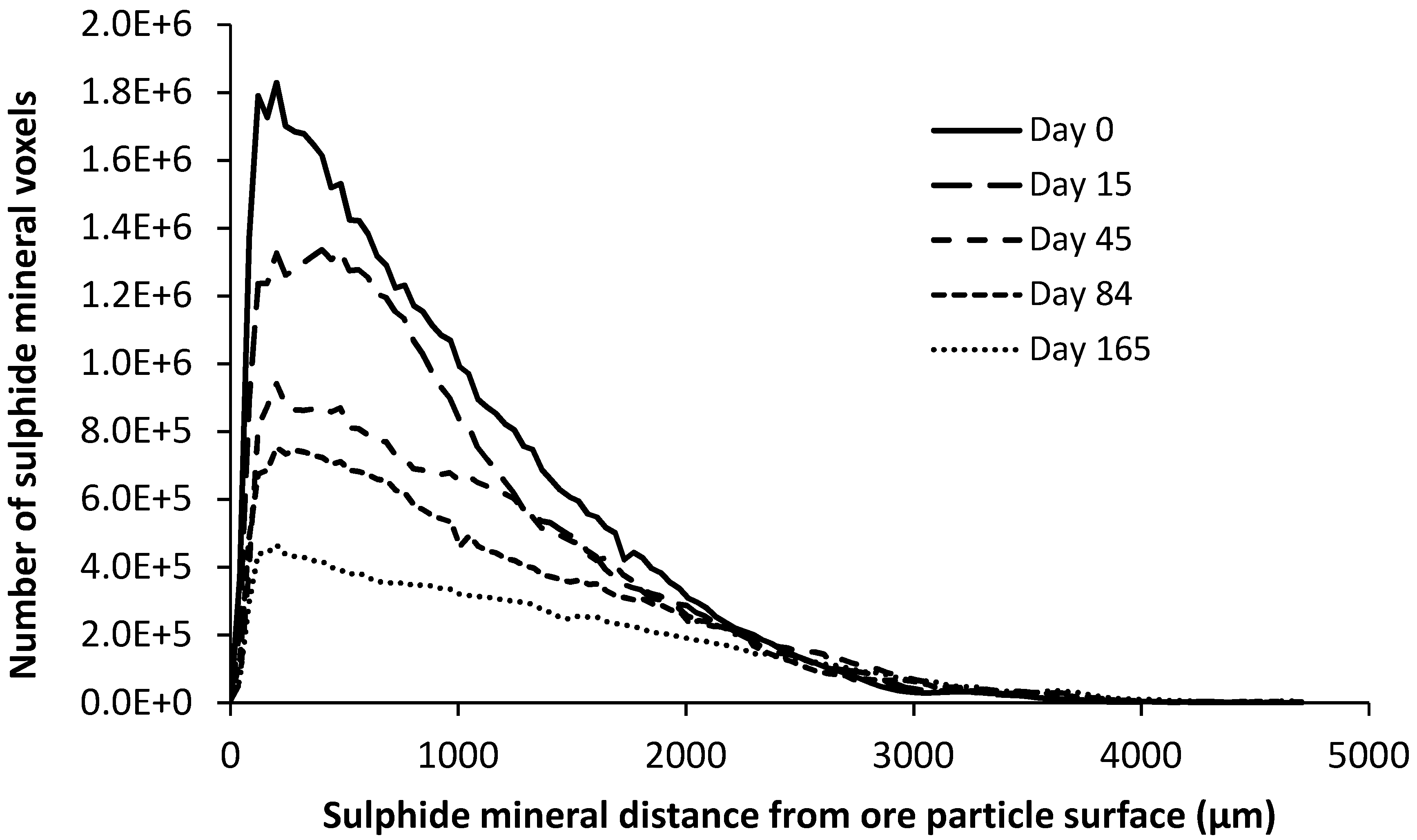
4.2.3. Chalcopyrite
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghorbani, Y.; Franzidis, J.-P.; Petersen, J. Heap leaching technology—current state, innovations, and future directions: A review. Miner. Process. Extract. Metallur. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Ruan, R.; Zou, G.; Zhong, S.; Wu, Z.; Chan, B.; Wang, D. Why Zijinshan copper bioheapleaching plant works efficiently at low microbial activity—Study on leaching kinetics of copper sulfides and its implications. Miner. Eng. 2013, 48, 36–43. [Google Scholar] [CrossRef]
- Jansen, M.; Taylor, A. Overview of gangue mineralogy issues in oxide copper heap leaching. In Proceedings of the ALTA Conference, Perth, Australia, 19–24 May 2003; p. 32. [Google Scholar]
- Khoshkhoo, M.; Dopson, M.; Shchukarev, A.; Sandström, Å. Chalcopyrite leaching and bioleaching: An X-ray photoelectron spectroscopic (XPS) investigation on the nature of hindered dissolution. Hydrometallurgy 2014, 149, 220–227. [Google Scholar] [CrossRef]
- Berry, V.K.; Murr, L.E.; Hiskey, J.B. Galvanic interaction between chalcopyrite and pyrite during bacterial leaching of low-grade waste. Hydrometallurgy 1978, 3, 309–326. [Google Scholar] [CrossRef]
- Acosta, M.; Galleguillos, P.; Ghorbani, Y.; Tapia, P.; Contador, Y.; Velásquez, A.; Espoz, C.; Pinilla, C.; Demergasso, C. Variation in microbial community from predominantly mesophilic to thermotolerant and moderately thermophilic species in an industrial copper heap bioleaching operation. Hydrometallurgy 2014, 150, 281–289. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Justel, F.J.; Claros, M.; Taboada, M.E. Solubilities and physical properties of saturated solutions in th copper sulphate+sulfuric acid+seawater system at different temperatures. Braz. J. Chem. Eng. 2015, 32, 629–635. [Google Scholar] [CrossRef]
- Petersen, J. Determination of oxygen gas–liquid mass transfer rates in heap bioleach reactors. Miner. Eng. 2010, 23, 504–510. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Becker, M.; Mainza, A.; Franzidis, J.-P.; Petersen, J. Large particle effects in chemical/biochemical heap leach processes—A review. Miner. Eng. 2011, 24, 1172–1184. [Google Scholar] [CrossRef]
- Dhawan, N.; Safarzadeh, M.S.; Miller, J.D.; Moats, M.S.; Rajamani, R.K.; Lin, C.-L. Recent advances in the application of X-ray computed tomography in the analysis of heap leaching systems. Miner. Eng. 2012, 35, 75–86. [Google Scholar] [CrossRef]
- Lin, C.L.; Garcia, C. Microscale characterization and analysis of particulate systems via cone-beam X-ray microtomography (XMT). In Innovations in Natural Resource Processing: Proceedings of the Jan D. Miller Symposium; Young, C.A., Kellar, J.J., Free, M.L., Drelich, J., King, R.P., Eds.; Society for Mining, Metallurgy and Exploration: Englewood, CO, USA, 2005; pp. 421–432. [Google Scholar]
- Ghorbani, Y.; Becker, M.; Petersen, J.; Mainza, A.N.; Franzidis, J.P. Investigation of the effect of mineralogy as rate-limiting factors in large particle leaching. Miner. Eng. 2013, 52, 38–51. [Google Scholar] [CrossRef]
- Chiume, R.; Minnaar, S.H.; Ngoma, I.E.; Bryan, C.G.; Harrison, S.T.L. Microbial colonisation in heaps for mineral bioleaching and the influence of irrigation rate. Miner. Eng. 2012, 39, 156–164. [Google Scholar] [CrossRef]
- Van Hille, R.P.; van Zyl, A.W.; Spurr, N.R.L.; Harrison, S.T.L. Investigating heap bioleaching: Effect of feed iron concentration on bioleaching performance. Miner. Eng. 2010, 23, 518–525. [Google Scholar] [CrossRef]
- Komadel, P.; Stucki, J.W. Quantitative assay of minerals for Fe2+ and Fe3+ using 1,10 phenanthroline; III, A rapid photochemical method. Clays Clay Miner. 1988, 36, 379–381. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Miki, H.; Hirajima, T.; Tsunekawa, M. Enhancement of chalcopyrite leaching by ferrous ions in acidic ferric sulfate solutions. Hydrometallurgy 2001, 60, 185–197. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D.G. Competitive bioleaching of pyrite and chalcopyrite. Hydrometallurgy 2006, 83, 40–49. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef]
- Istiadi Guntoro, P.; Ghorbani, Y.; Koch, P.H.; Rosenkranz, J. X-ray microcomputed tomography (µCT) for mineral characterization: A review of data analysis methods. Minerals 2019, 9, 183. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, C.L.; Miller, J.D. Improved 3D image segmentation for X-ray tomographic analysis of packed particle beds. Miner. Eng. 2015, 83, 185–191. [Google Scholar] [CrossRef]
- Lin, Q.; Neethling, S.J.; Courtois, L.; Dobson, K.J.; Lee, P.D. Multi-scale quantification of leaching performance using X-ray tomography. Hydrometallurgy 2016, 164, 265–277. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Schindelin, J.; Cardona, A.; Seung, H.S. Trainable Weka Segmentation: A machine learning tool for microscopy image segmentation. In Neuroscience 2014 Short Course 2; Sebastian Seung: Princeton, NJ, USA, 2014; pp. 73–80. [Google Scholar]
- Kapur, J.N.; Sahoo, P.K.; Wong, A.K.C. A new method for gray-level picture thresholding using the entropy of the histogram. Comput. Vis. Graph. Image Process. 1985, 29, 273–285. [Google Scholar] [CrossRef]
- Kocabag, D.; Shergold, H.L.; Kelsall, G.H. Natural oleophilicity/hydrophobicity of sulphide minerals, II. Pyrite. Int. J. Miner. Process. 1990, 29, 211–219. [Google Scholar] [CrossRef]
- Nicol, M.J. The kinetics of the dissolution of malachite in acid solutions. Hydrometallurgy 2018, 17, 214–217. [Google Scholar] [CrossRef]
- Pesic, B.; Olson, F.A. Dissolution of bornite in sulfuric acid using oxygen as oxidant. Hydrometallurgy 1984, 12, 195–215. [Google Scholar] [CrossRef]
- Li, L.; Bergeron, I.; Ghahreman, A. The effect of temperature on the kinetics of the ferric-ferrous redox couple on pyrite. Electrochim. Acta 2017, 245, 814–828. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Dimitrijević, M.; Janković, Z. Leaching of pyrite with hydrogen peroxide in sulphuric acid. Hydrometallurgy 1997, 46, 71–83. [Google Scholar] [CrossRef]
- Ahonen, L.; Tuovinen, O.H. Temperature effects on bacterial leaching of sulfide minerals in shake flask experiments. Appl. Environ. Microbiol. 1991, 57, 138. [Google Scholar] [CrossRef]
- Dew, D.W.; Van Buuren, C.; McEwan, K.; Bowker, C. Bioleaching of base metal sulphide concentrates: A comparison of high and low temperature bioleaching. J. S. Afr. Inst. Min. Metall. 2000, 409, 31. [Google Scholar]
- Nemati, M.; Harrison, S.T.L. A comparative study on thermophilic and mesophilic biooxidation of ferrous iron. Miner. Eng. 2000, 13, 19–24. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Petersen, J.; Becker, M.; Mainza, A.N.; Franzidis, J.-P. Investigation and modelling of the progression of zinc leaching from large sphalerite ore particles. Hydrometallurgy 2013, 131–132, 8–23. [Google Scholar] [CrossRef]
- Dixon, D.G.; Hendrix, J.L. A general model for leaching of one or more solid reactants from porous ore particles. Metallur. Trans. B 1993, 24, 157–169. [Google Scholar] [CrossRef]








| Chalcopyrite | Pyrite | Malachite | |||
|---|---|---|---|---|---|
| Mineral | Amount (wt%) | Mineral | Amount (wt%) | Mineral | Amount (wt%) |
| Quartz | 37.6 | Quartz | 16.5 | Quartz | 76.8 |
| Muscovite | 19.1 | Muscovite | 29.6 | Muscovite | 3.6 |
| K-feldspar | 37.5 | Limonite | 5.0 | Fe–Ti oxide | 4.5 |
| Other | 3.3 | Other | 9.7 | Other | 5.9 |
| Ore Type | Feed | pH | Temperature |
|---|---|---|---|
| Malachite | Acidified water | 1.20 | 30 °C |
| Pyrite | Fe3+ solution | 1.15 | 37 °C |
| Pyrite | Fe3+ solution | 1.15 | 65 °C |
| Chalcopyrite | Fe3+ and Fe2+ solution | 1.15 | 37 °C |
| Chalcopyrite | Fe3+ and Fe2+ solution | 1.15 | 65 °C |
| Thresholding Technique | Area (µm2) | Number of Pixels | Error (%) |
|---|---|---|---|
| QEMSCAN | 10,490,424 | 49895 | - |
| Weka segmentation | 10,764,534 | 38,598 | 2.62 |
| Max Entropy | 12,727,447 | 45,636 | 21.32 |
| Interactive Thresholding | 10,839,488 | 38,867 | 3.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghadiri, M.; Harrison, S.T.L.; Fagan-Endres, M.A. Quantitative X-ray µCT Measurement of the Effect of Ore Characteristics on Non-Surface Mineral Grain Leaching. Minerals 2020, 10, 746. https://doi.org/10.3390/min10090746
Ghadiri M, Harrison STL, Fagan-Endres MA. Quantitative X-ray µCT Measurement of the Effect of Ore Characteristics on Non-Surface Mineral Grain Leaching. Minerals. 2020; 10(9):746. https://doi.org/10.3390/min10090746
Chicago/Turabian StyleGhadiri, Mahdi, Susan T.L. Harrison, and Marijke A. Fagan-Endres. 2020. "Quantitative X-ray µCT Measurement of the Effect of Ore Characteristics on Non-Surface Mineral Grain Leaching" Minerals 10, no. 9: 746. https://doi.org/10.3390/min10090746
APA StyleGhadiri, M., Harrison, S. T. L., & Fagan-Endres, M. A. (2020). Quantitative X-ray µCT Measurement of the Effect of Ore Characteristics on Non-Surface Mineral Grain Leaching. Minerals, 10(9), 746. https://doi.org/10.3390/min10090746







