Ecotoxicity of Pore Water in Meadow Soils Affected by Historical Spills of Arsenic-Rich Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Properties
2.3. Fertilizers
2.4. Chemistry of Soil Pore Water
2.5. Bioassays
2.6. Statistics
3. Results
3.1. Soil Properties
3.2. Pore Water Concentrations of As and Other Potentially Toxic Elements
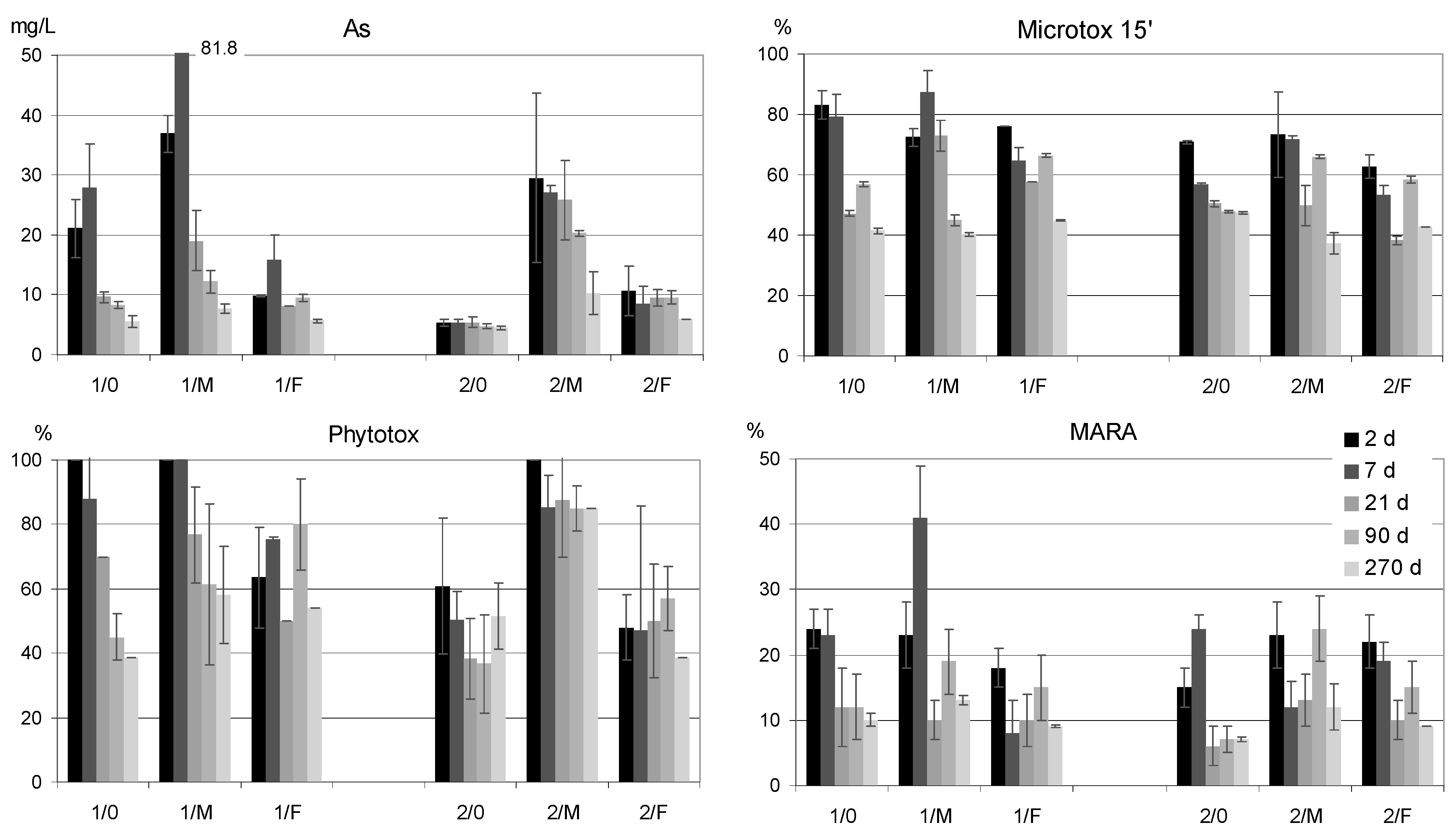
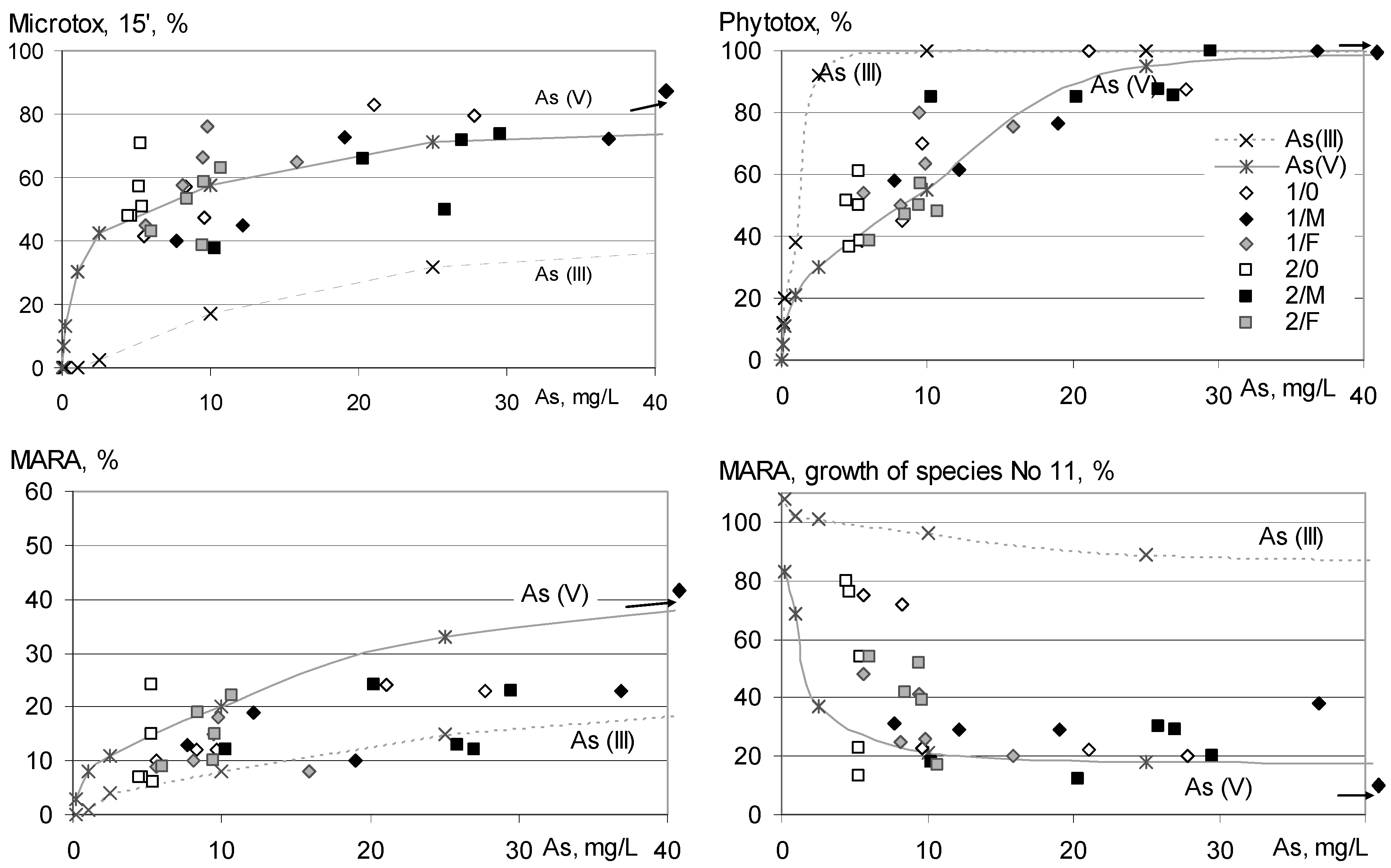
3.3. Bioassays
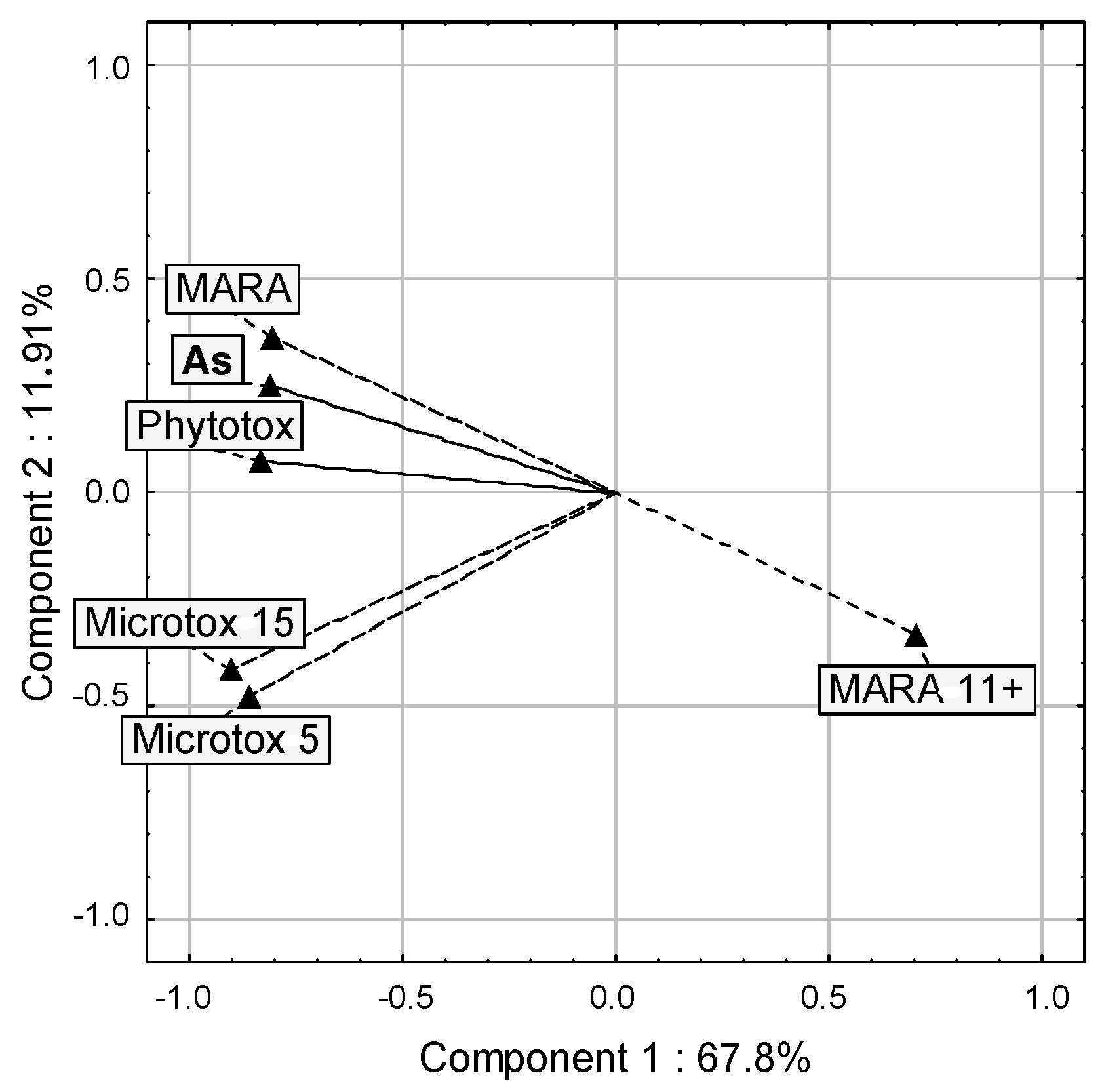
3.4. General Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karczewska, A.; Krysiak, A.; Mokrzycka, D.; Jezierski, P.; Szopka, K. Arsenic distribution in soils of former as mining area and processing. Pol. J. Environ. St. 2013, 22, 175–181. [Google Scholar]
- Kaźmierczak, U.; Strzałkowski, P.; Lorenc, M.W.; Szumska, E.; Sánchez, A.A.P.; Baker, K.A. Post-mining Remnants and Revitalization. Geoheritage 2019, 11, 2025–2044. [Google Scholar] [CrossRef] [Green Version]
- Dradrach, A.; Karczewska, A.; Szopka, K.; Lewińska, K. Accumulation of arsenic by plants growing in the sites strongly contaminated by historical mining in the Sudetes region of Poland. Int. J. Environ. Res. Public Health 2020, 17, 3342. [Google Scholar] [CrossRef]
- Wenzel, W.W. Arsenic. In Heavy Metals in Soils. Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Berlin, Germany, 2013; pp. 241–282. [Google Scholar]
- Lewińska, K.; Karczewska, A.; Siepak, M.; Gałka, B. Potential of Fe-Mn wastes produced by a water treatment plant for arsenic immobilization in contaminated soils. J. Geochem. Explor. 2018, 184, 226–231. [Google Scholar] [CrossRef]
- Bolan, N.S.; Mahimairaja, S.; Kunhikrishnan, A.; Choppala, G. Phosphorus–arsenic interactions in variable-charge soils in relation to arsenic mobility and bioavailability. Sci. Total Environ. 2013, 463, 1154–1162. [Google Scholar] [CrossRef]
- Anawar, H.M.; Rengel, Z.; Damon, P.; Tibbett, M. Arsenic-phosphorus interactions in the soil-plant-microbe system: Dynamics of uptake, suppression and toxicity to plants. Environ. Pollut. 2018, 233, 1003–1012. [Google Scholar] [CrossRef]
- Li, S.W.; Liu, X.; Sun, H.J.; Li, M.Y.; Zhao, D.; Luo, J.; Li, H.B.; Ma, L.Q. Effect of phosphate amendment on relative bioavailability and bioaccessibility of lead and arsenic in contaminated soils. J. Hazard. Mater. 2017, 339, 256–263. [Google Scholar] [CrossRef]
- Lewińska, K.; Karczewska, A. Influence of soil properties and phosphate addition on arsenic uptake from polluted soils by velvetgrass (Holcus lanatus). Int. J. Phytoremediat. 2013, 15, 91–104. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, X.; Zhang, H.; Li, Y.; Zhao, S.; Su, S.; Zhang, T. Effect of exogenous phosphate on the lability and phytoavailability of arsenic in soils. Chemosphere 2018, 196, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef]
- Xie, H.; Han, D.; Cheng, J.; Zhou, P.; Wang, W. Fate and risk assessment of arsenic compounds in soil amended with poultry litter under aerobic and anaerobic circumstances. Water Air Soil Pollut. 2015, 226, 1–11. [Google Scholar] [CrossRef]
- Karczewska, A.; Gałka, B.; Dradrach, A.; Lewińska, K.; Mołczan, M.; Cuske, M.; Gersztyn, L.; Litak, K. Solubility of arsenic and its uptake by ryegrass from polluted soils amended with organic matter. J. Geochem. Explor. 2017, 182, 193–200. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Clemente, R.; Mestrot, A.; Meharg, A.A. Arsenic and selenium mobilisation from organic matter treated mine spoil with and without inorganic fertilization. Environ. Pollut. 2013, 173, 238–244. [Google Scholar] [CrossRef]
- Karczewska, A.; Lewińska, K.; Siepak, M.; Gałka, B.; Dradrach, A.; Szopka, K. Transformation of beech forest litter as a factor that triggers arsenic solubility in soils developed on historical mine dumps. J. Soils Sed. 2018, 18, 2749–2758. [Google Scholar] [CrossRef] [Green Version]
- Lewińska, K.; Karczewska, A.; Siepak, M.; Szopka, K.; Gałka, B.; Iqbal, M. Effects of waterlogging on the solubility of antimony and arsenic in variously treated shooting range soils. Appl. Geochem. 2019, 105, 7–16. [Google Scholar] [CrossRef]
- Pigna, M.; Cozzolino, V.; Violante, A.; Meharg, A.A. Influence of phosphate on the arsenic uptake by wheat (Triticum durum L.) irrigated with arsenic solutions at three different concentrations. Water Air Soil Pollut. 2009, 197, 371–380. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Effect of natural organic matter on arsenic release from soil and sediments into groundwater. Environ. Geochem. Health 2006, 28, 197–214. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef]
- Rocha, L.; Rodrigues, S.M.; Lopes, I.; Soares, A.M.V.M.; Duarte, A.C.; Pereira, E. The water-soluble fraction of potentially toxic elements in contaminated soils: Relationships between ecotoxicity, solubility and geochemical reactivity. Chemosphere 2011, 84, 1495–1505. [Google Scholar] [CrossRef]
- Fulladosa, E.; Murat, J.C.; Martínez, M.; Villaescusa, I. Patterns of metals and arsenic poisoning in Vibrio fischeri bacteria. Chemosphere 2005, 60, 43–48. [Google Scholar] [CrossRef]
- Villaescusa, I.; Bollinger, J.C. Arsenic in drinking water: Sources, occurrence and health effects (a review). Rev. Environ. Sci. Bio/Technol. 2008, 7, 307–323. [Google Scholar] [CrossRef]
- Paustenbach, D. (Ed.) Human and Ecological Risk Assessment: Theory and Practice (Wiley Classics Library); Wiley: Hoboken, NY, USA, 2015. [Google Scholar]
- Doherty, F. A review of the Microtox® toxicity test system for assessing the toxicity of sediments and soils. Water Qual. Res. J. 2001, 36, 475–518. [Google Scholar] [CrossRef]
- Kokkali, V.; van Delft, W. Overview of commercially available bioassays for assessing chemical toxicity in aqueous samples. TrAC Trends Anal. Chem. 2014, 61, 133–155. [Google Scholar] [CrossRef]
- Karczewska, A.; Kabała, C. Environmental risk assessment as a new basis for evaluation of soil contamination in Polish law. Soil Sci. Ann. 2017, 68, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Parvez, S.; Venkataraman, C.; Mukherji, S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 2006, 32, 265–268. [Google Scholar] [CrossRef]
- Rubinos, D.A.; Calvo, V.; Iglesias, L.; Barral, M.T. Acute toxicity of arsenic to Aliivibrio fischeri (Microtox® bioassay) as influenced by potential competitive–protective agents. Environ. Sci. Pollut. Res. 2014, 21, 8631–8644. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef]
- Urbanczyk, H.; Ast, J.C.; Higgins, M.J.; Carson, J.; Dunlap, P.V. Reclassification of Vibrio fischeri, Vibrio logei, Vibrio salmonicida and Vibrio wodanis as Aliivibrio fischeri gen. nov., comb. nov., Aliivibrio logei comb. nov., Aliivibrio salmonicida comb. nov. and Aliivibrio wodanis comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2823–2829. [Google Scholar] [CrossRef] [Green Version]
- Dradrach, A.; Szopka, K.; Karczewska, A. Ecotoxicity of soil pore water on historical arsenic mine dumps—The effects of forest litter. Ecotoxicol. Environ. Saf. 2019, 181, 202–213. [Google Scholar] [CrossRef]
- Fai, P.B.; Grant, A. An assessment of the potential of the microbial assay for risk assessment (MARA) for ecotoxicological testing. Ecotoxicology 2010, 19, 1626–1633. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Kuśmierz, M.; Futa, B.; Wielgosz, E.; Ligęza, S.; Pranagal, J. Microbiological, biochemical and ecotoxicological evaluation of soils in the area of biochar production in relation to polycyclic aromatic hydrocarbon content. Geoderma 2014, 213, 502–511. [Google Scholar] [CrossRef]
- Wadhia, K.; Dando, T.; Clive-Thompson, K. Intra-laboratory evaluation of Microbial Assay for Risk Assessment (MARA) for potential application in the implementation of the Water Framework Directive (WFD). J. Environ. Monit. 2007, 9, 953–958. [Google Scholar] [CrossRef] [PubMed]
- García-Lorenzo, M.; Martinez-Sanchez, M.; Perez-Sirvent, C.; Molina, J. Ecotoxicological evaluation for the screening of areas polluted by mining activities. Ecotoxicology 2009, 18, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Antonkiewicz, J. Phytotoxicity and extractability of heavy metals from industrial wastes. Environ. Prot. Eng. 2017, 43, 143–155. [Google Scholar] [CrossRef]
- Cuske, M.; Karczewska, A.; Matyja, K.; Gałka, B. Ecotoxicity and phytotoxicity of soil solutions extracted from Cu-contaminated soils amended with organic waste materials. Fresenius Environ. Bull. 2017, 26, 1163–1173. [Google Scholar]
- Tan, K. Soil Sampling, Preparation, and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Gregorich, E.G.; Beare, M.H.; Stoklas, U.; St-Georges, P. Biodegradability of soluble organic matter in maize-cropped soils. Geoderma 2003, 113, 237–252. [Google Scholar] [CrossRef]
- Smolders, E.; Oorts, K.; Van Sprang, P.; Schoeters, I.; Janssen, C.R.; Mcgrath, S.P.; Mclaughlin, M.J. Toxicity of trace metals in soil as affected by soil type and aging after contamination: Using 295 calibrated bioavailability models to set ecological soil standards. Environ. Toxicol. Chem. 2009, 28, 1633. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zeng, X.B.; Lu, Y.H.; Su, S.M.; Bai, L.Y.; Li, L.F.; Wu, C.X. Effect of aging on the bioavailability and fractionation of arsenic in soils derived from five parent materials in a red soil region of Southern China. Environ. Pollut. 2015, 207, 79–87. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zeng, X.B.; Lu, Y.H.; Bai, L.Y.; Su, S.M.; Wu, C.X. Dynamic arsenic aging processes and their mechanisms in nine types of Chinese soils. Chemosphere 2017, 187, 404–412. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Smith, E.; Weber, J.; Naidu, R.; Rees, M.; Rofe, A.; Sansom, L. Effect of soil ageing on in vivo arsenic bioavailability in two dissimilar soils. Chemosphere 2008, 71, 2180–2186. [Google Scholar] [CrossRef]
- Abedin, M.J.; Meharg, A.A. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant Soil 2002, 243, 57–66. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Hurel, C.; Géret, F.; Galgani, F.; Battaglia-Brunet, F.; Marmier, N.; Roméo, M. Arsenic in marine sediments from French Mediterranean ports: Geochemical partitioning, bioavailability and ecotoxicology. Chemosphere 2013, 90, 2730–2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
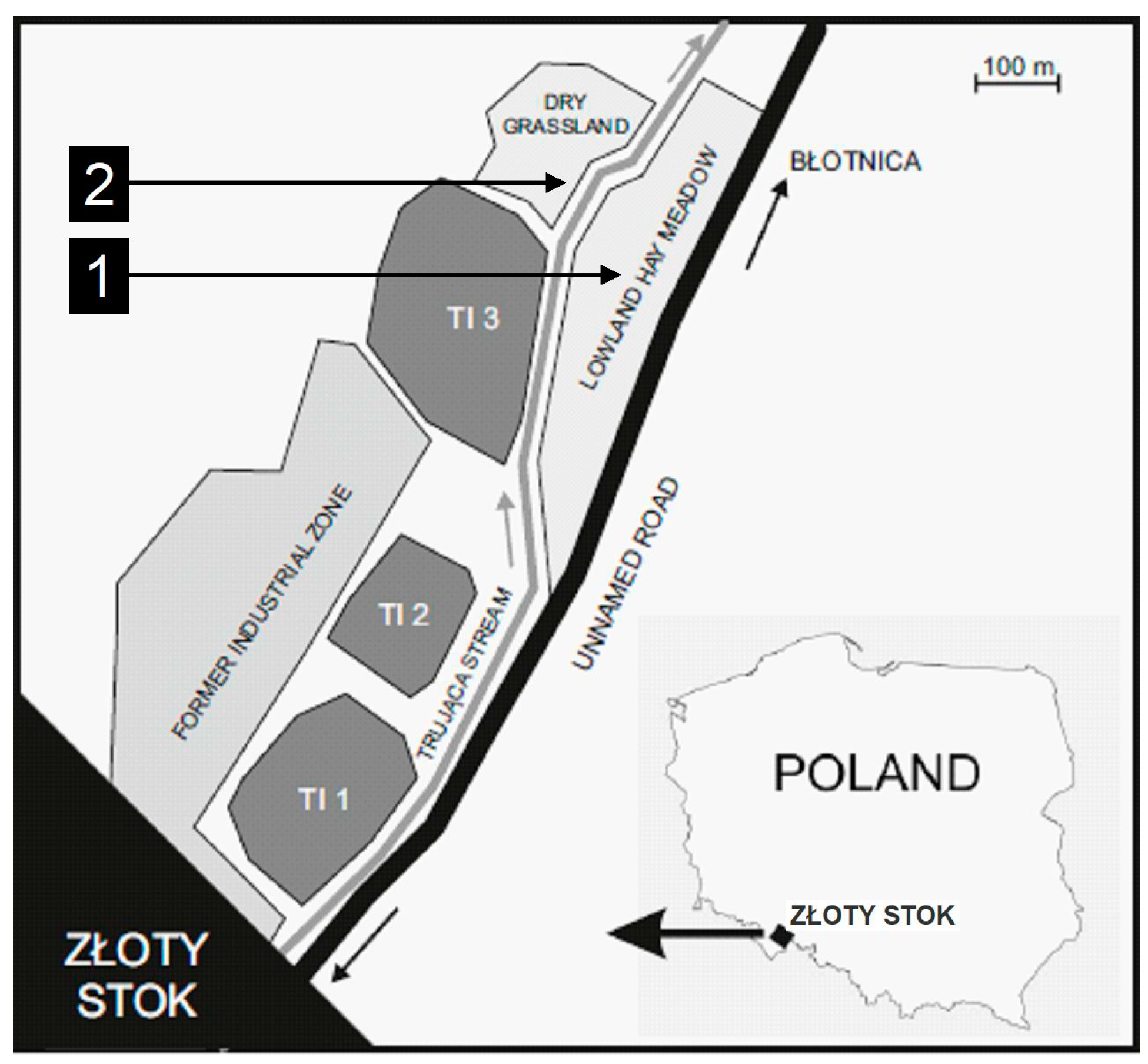
| Site No | 1 | 2 | |||
|---|---|---|---|---|---|
| Site description, settings | Fresh meadow (4 ha) within the floodplain of the Trująca river, frequently flooded in the past by stormwater mixed with tailings (the distance from tailings impoundments: 0.2–1.0 km) | Foreland of tailings impoundment, an elevated plateau (1.6 ha) built of tailings | |||
| Skeleton (>2 mm) | % | 21 | 0 | ||
| <0.002 mm | % | 6 | 3 | ||
| Textural group * (USDA) | - | SL | LS | ||
| Soil properties (fine soil) | Corg | g/kg | 24.5 | 5.5 | |
| N total | g/kg | 3.09 | 0.38 | ||
| C:N | - | 7.9 | 14.5 | ||
| pH (1M KCl) | - | 6.32 | 7.60 | ||
| CaCO3 | % | absent | 2.1 | ||
| As total | mg/kg | 5020 | 8000 | ||
| 1M NH4NO3 -extractable As | mg/kg | 7.3 | 12.9 | ||
| 0.43 M HNO3 -extractable As | mg/kg | 2720 | 5310 | ||
| “Available” | P | mg/kg | 242 (very high) | 194 (very high) | |
| K | mg/kg | 72 (low) | 131 (medium) | ||
| Mg | mg/kg | 245 (very high) | 76 (high) | ||
| Parameter | Microtox 5′ | Microtox 15′ | Phytotox | MARA, Toxicity | MARA, Growth of Strain 11 |
|---|---|---|---|---|---|
| As in soil pore water (mg/L) | 0.593 | 0.632 | 0.716 | 0.719 | −0.447 |
| Microtox 5′ | x | 0.988 | 0.586 | 0.563 | −0.484 |
| Microtox 15′ | x | x | 0.648 | 0.599 | −0.522 |
| Phytotox | x | x | x | 0.541 | −0.552 |
| MARA, toxicity | x | x | x | x | −0.591 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dradrach, A.; Szopka, K.; Karczewska, A. Ecotoxicity of Pore Water in Meadow Soils Affected by Historical Spills of Arsenic-Rich Tailings. Minerals 2020, 10, 751. https://doi.org/10.3390/min10090751
Dradrach A, Szopka K, Karczewska A. Ecotoxicity of Pore Water in Meadow Soils Affected by Historical Spills of Arsenic-Rich Tailings. Minerals. 2020; 10(9):751. https://doi.org/10.3390/min10090751
Chicago/Turabian StyleDradrach, Agnieszka, Katarzyna Szopka, and Anna Karczewska. 2020. "Ecotoxicity of Pore Water in Meadow Soils Affected by Historical Spills of Arsenic-Rich Tailings" Minerals 10, no. 9: 751. https://doi.org/10.3390/min10090751
APA StyleDradrach, A., Szopka, K., & Karczewska, A. (2020). Ecotoxicity of Pore Water in Meadow Soils Affected by Historical Spills of Arsenic-Rich Tailings. Minerals, 10(9), 751. https://doi.org/10.3390/min10090751






