Leaching Kinetics and Mechanism of Laterite with NH4Cl-HCl Solution
Abstract
:1. Introduction
2. Experimental and Analytical Methods
2.1. Materials
2.2. Methods
2.3. Analytical Methods
3. Results and Discussion
3.1. Leaching Mechanism
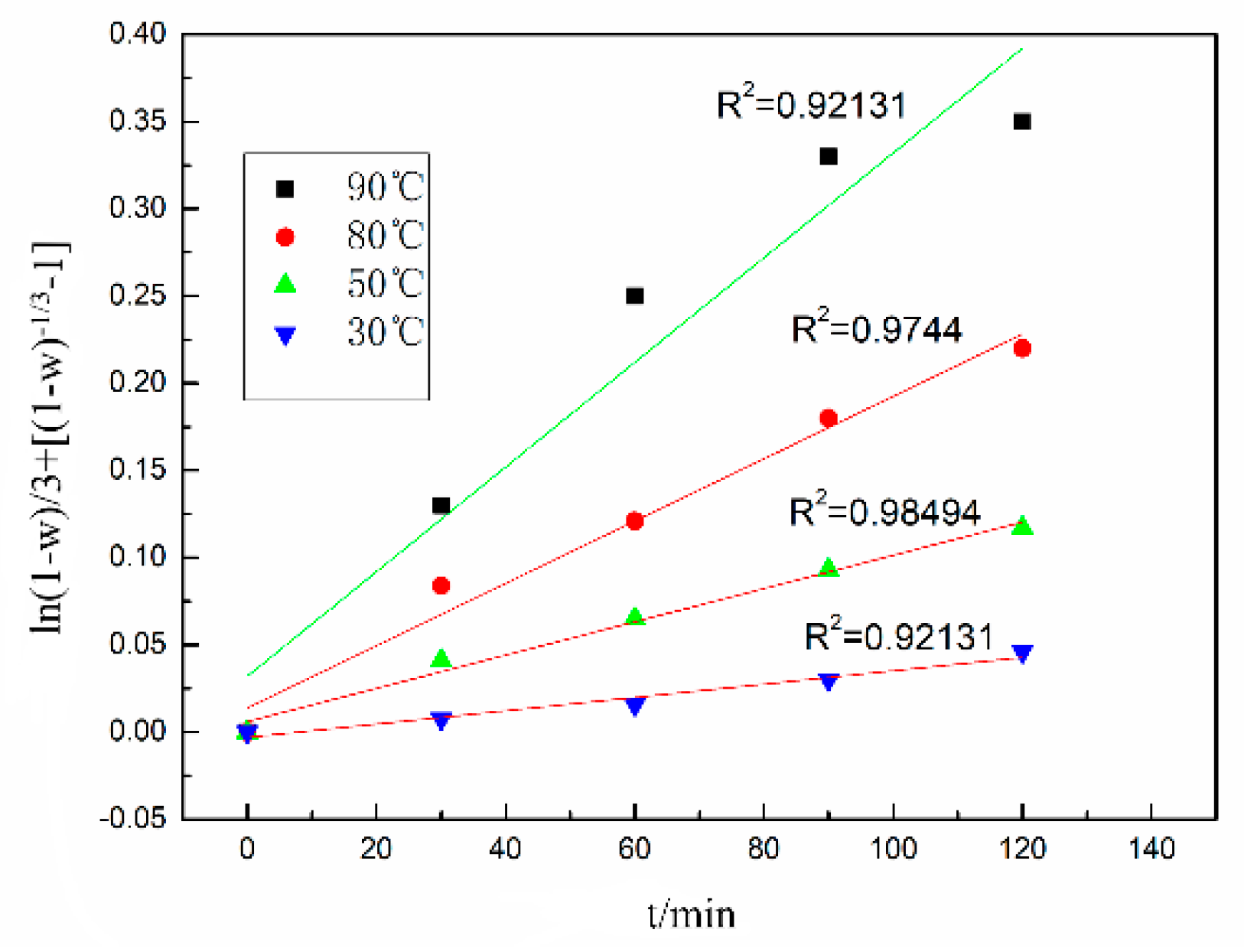
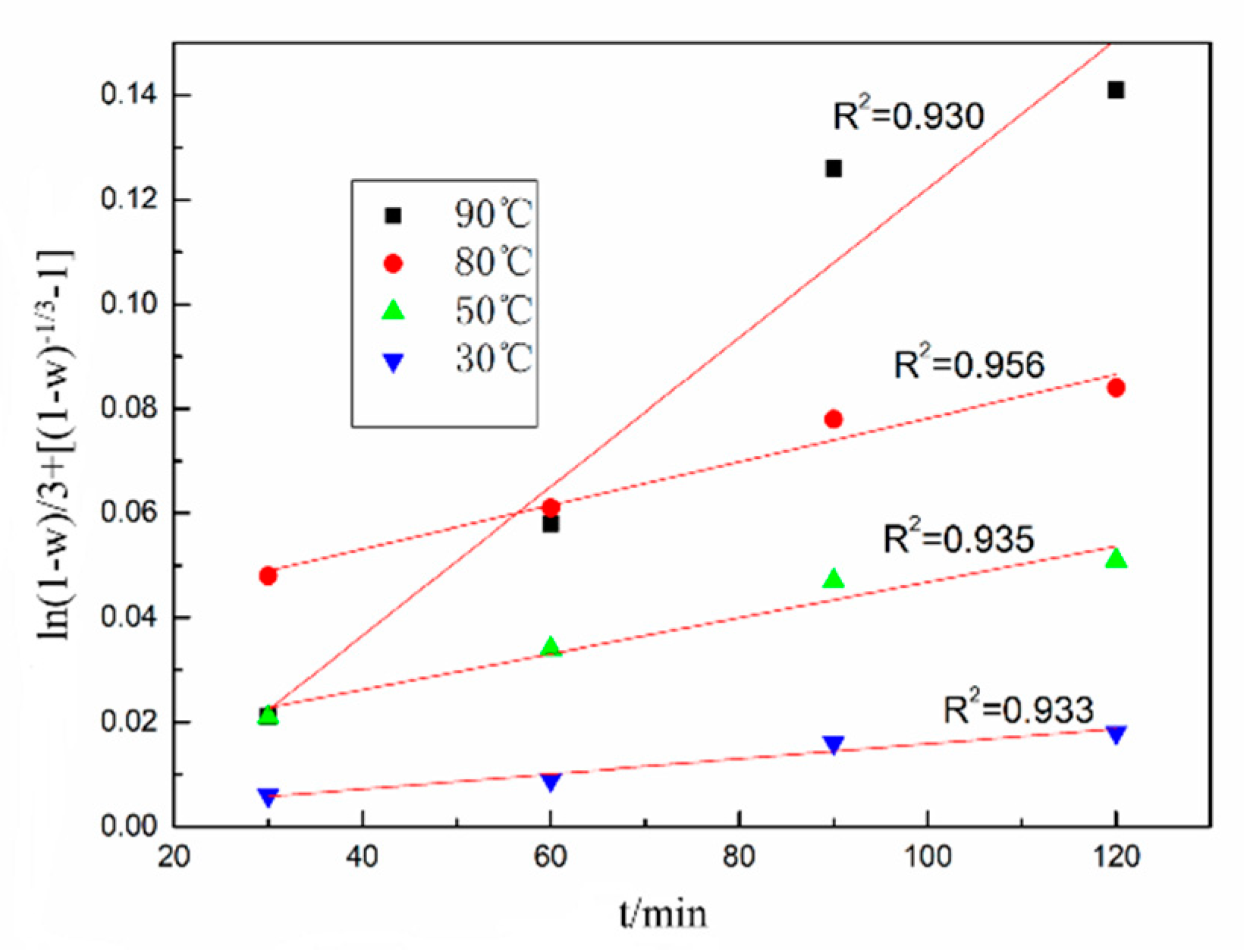
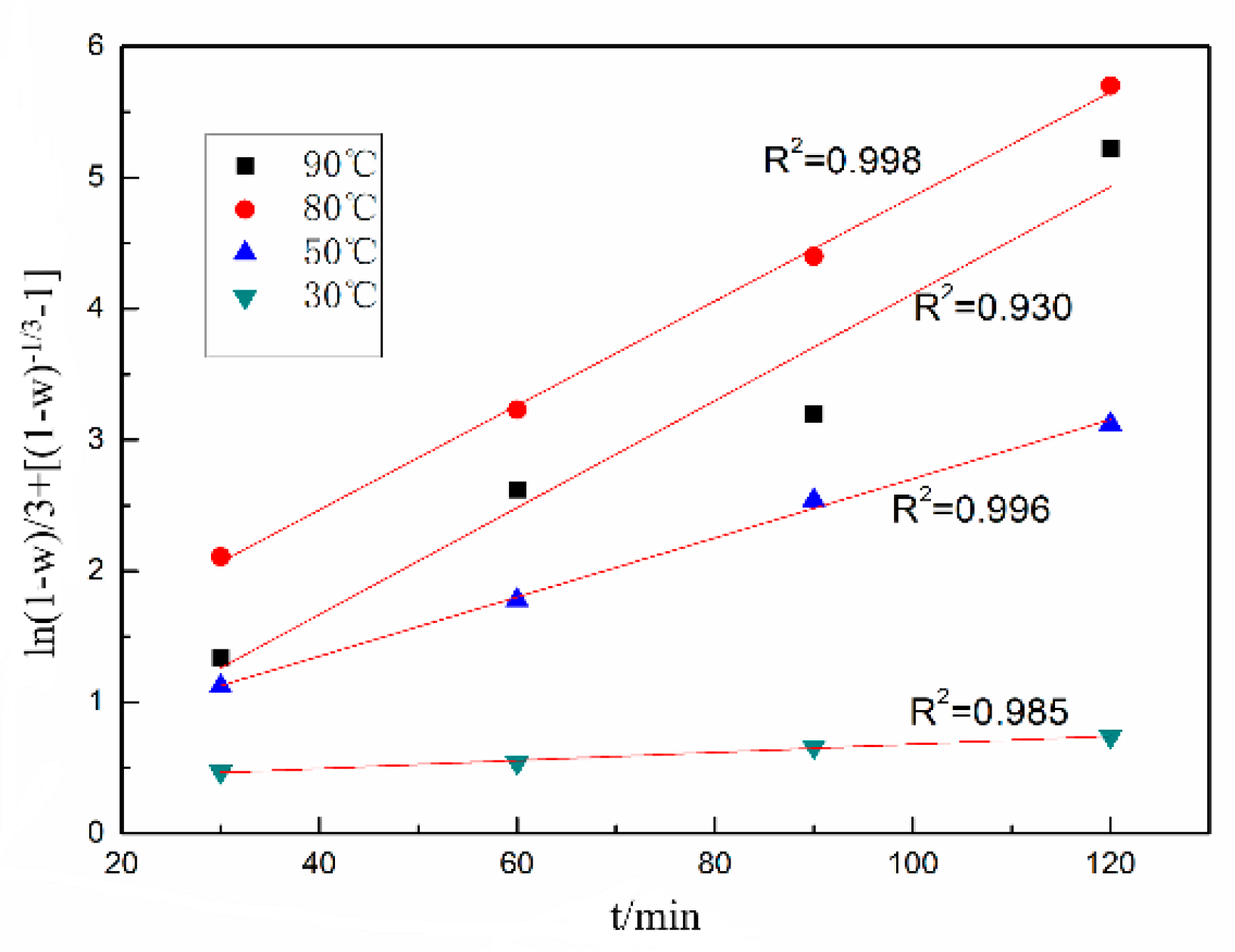
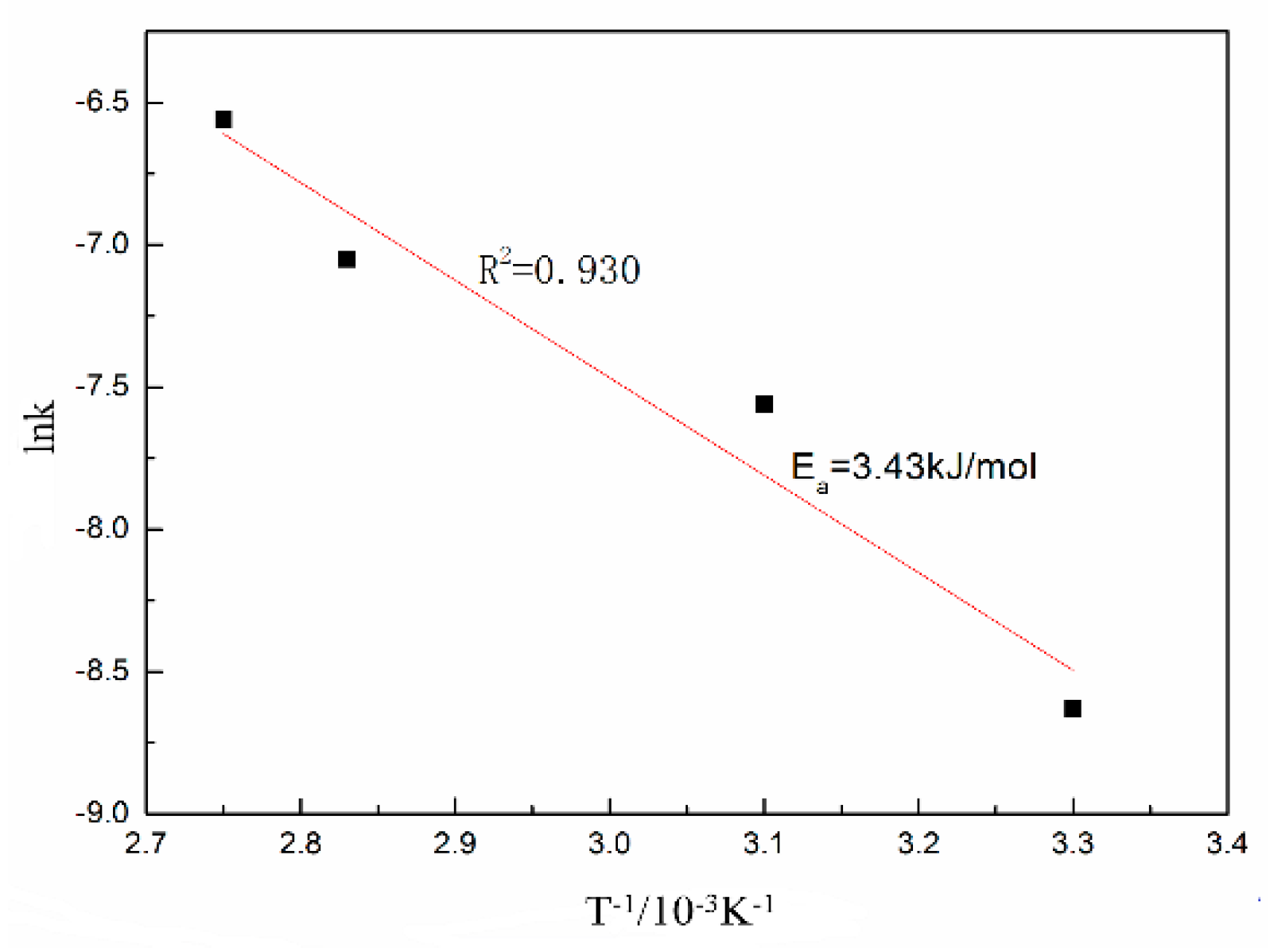
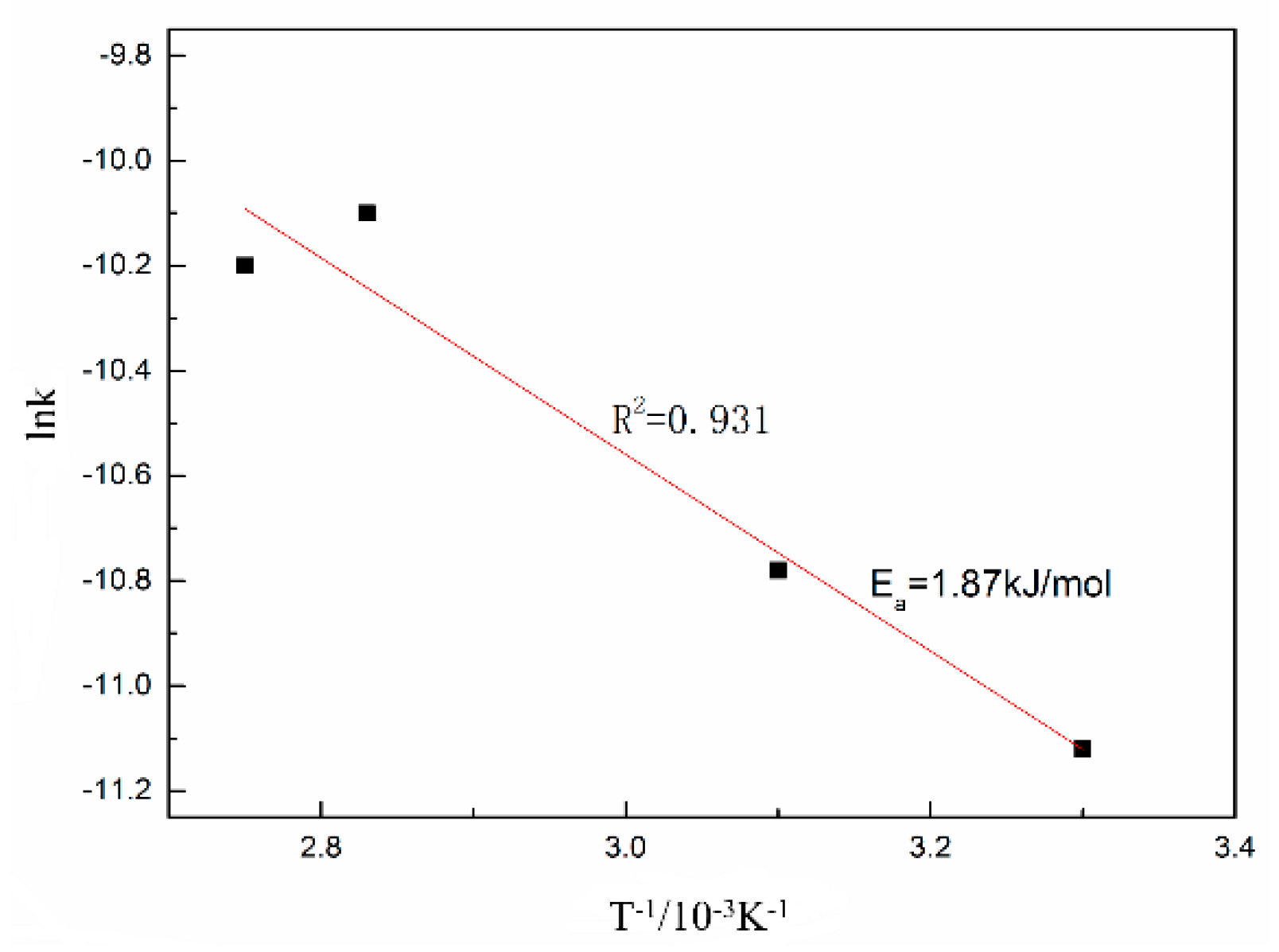
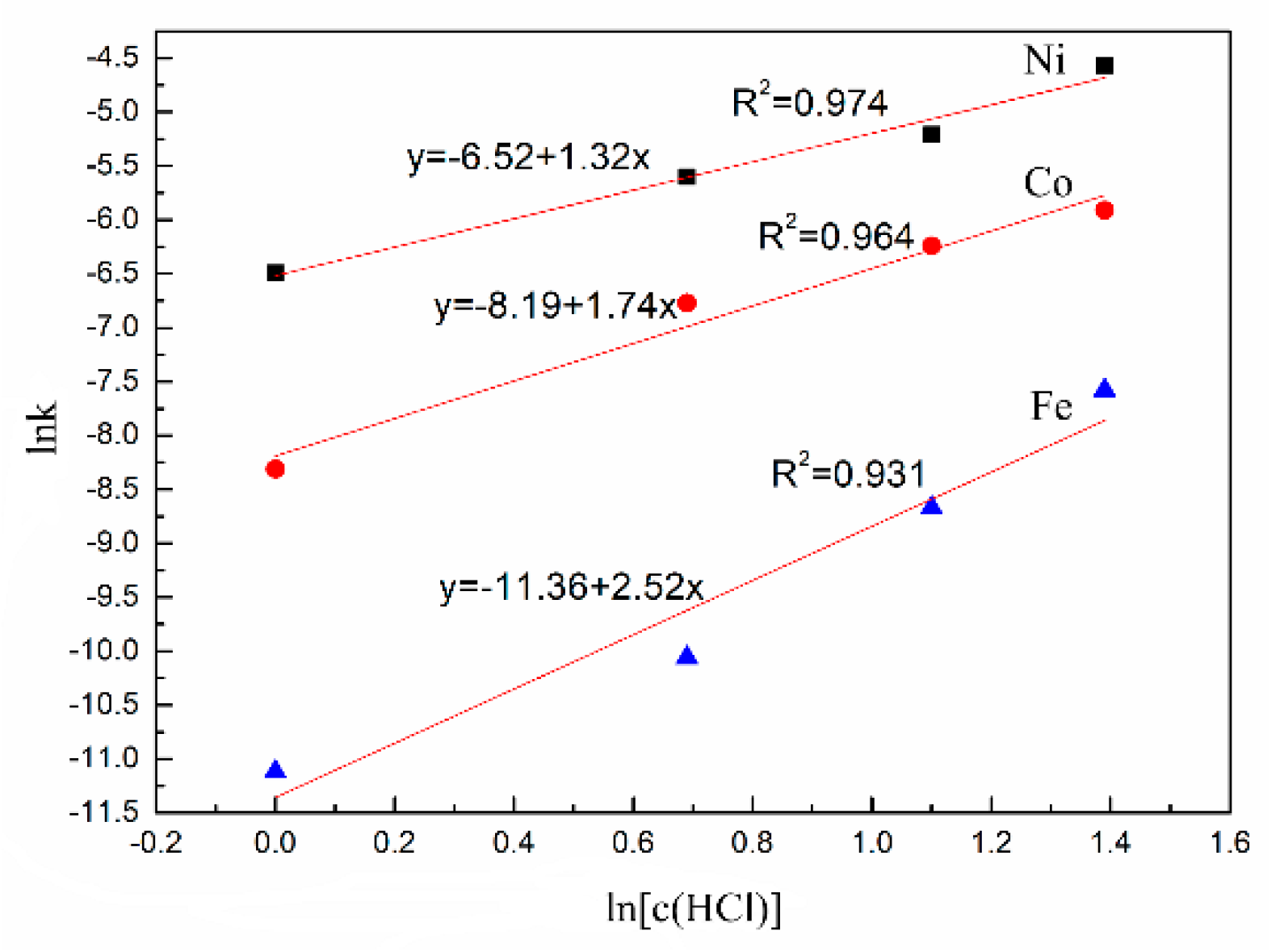
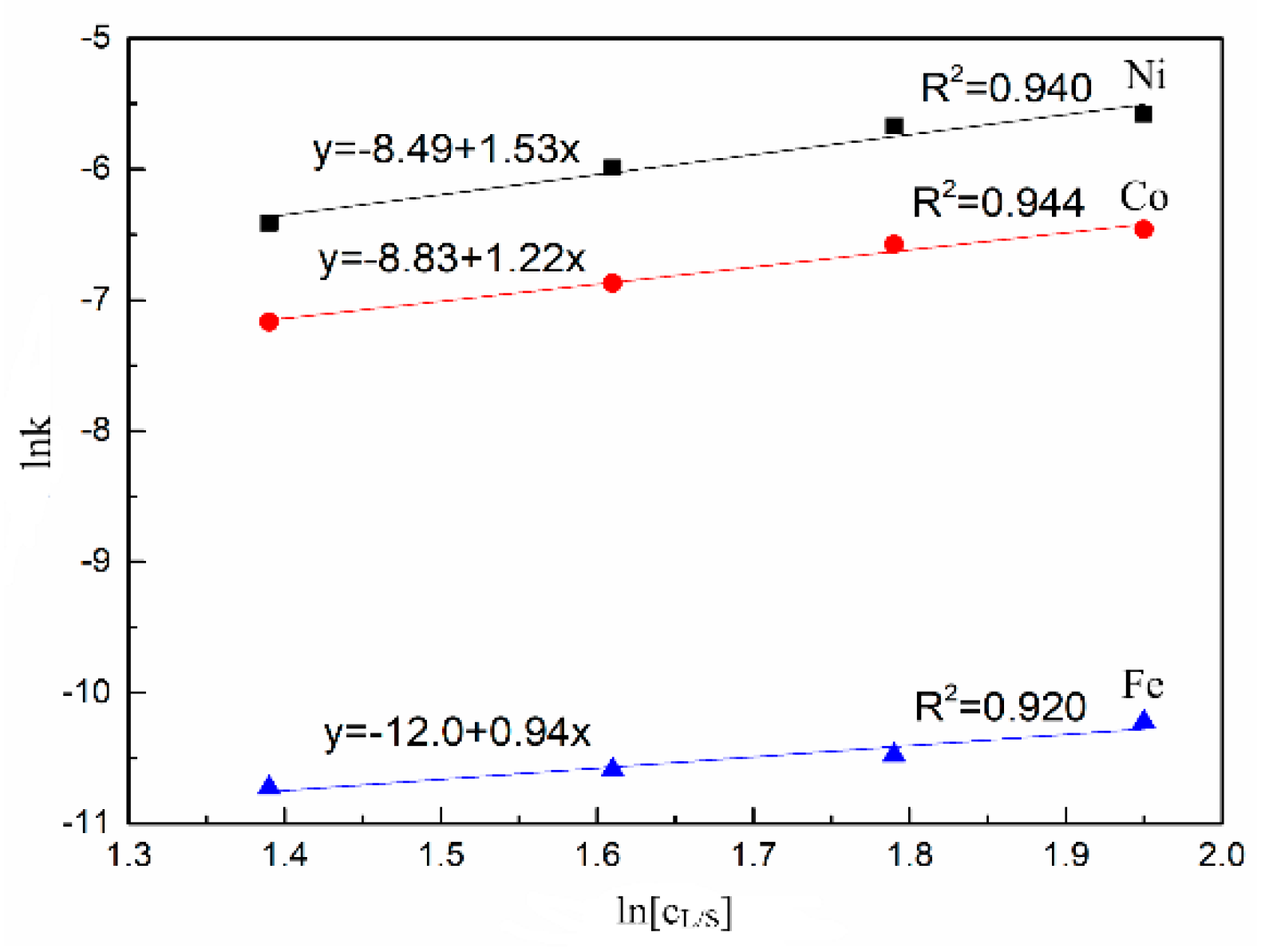
3.2. Leaching Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moskalyk, R.; Alfantazi, A. Nickel laterite processing and electrowinning practice. Miner. Eng. 2002, 15, 593–605. [Google Scholar] [CrossRef]
- McDonald, R.G.; Whittington, B. Atmospheric acid leaching of nickel laterites review: Part I. Sulphuric acid technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.-M.; Yue, Y.; Peng, B.; Que, Z.-Q.; Guo, M.; Zhang, M. Extraction and separation of nickel and cobalt from saprolite laterite ore by microwave-assisted hydrothermal leaching and chemical deposition. Int. J. Miner. Met. Mater. 2013, 20, 612–619. [Google Scholar] [CrossRef]
- MacCarthy, J.; Nosrati, A.; Skinner, W.; Addai-Mensah, J. Atmospheric acid leaching mechanisms and kinetics and rheological studies of a low grade saprolitic nickel laterite ore. Hydrometallurgy 2016, 160, 26–37. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, M.-S. Separation of Co and Ni from a chloride leach solutions of laterite ore by solvent extraction with extractant mixtures. J. Ind. Eng. Chem. 2015, 28, 322–327. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Q.; Hu, H. Comparative leaching of minerals by sulphuric acid in a Chinese ferruginous nickel laterite ore. Hydrometallurgy 2009, 98, 281–286. [Google Scholar] [CrossRef]
- Marrero, J.; Coto, O.; Goldmann, S.; Graupner, T.; Schippers, A. Recovery of nickel and cobalt from laterite tailings by reductive dissolution under aerobic conditions using Acidithiobacillus species. Environ. Sci. Technol. 2015, 49, 6674–6682. [Google Scholar] [CrossRef]
- Ma, B.; Yang, W.; Yang, B.; Wang, C.; Chen, Y.; Zhang, Y. Pilot-scale plant study on the innovative nitric acid pressure leaching technology for laterite ores. Hydrometallurgy 2015, 155, 88–94. [Google Scholar] [CrossRef]
- Xu, D.; Liu, L.; Quast, K.; Addai-Mensah, J.; Robinson, D.J. Effect of nickel laterite agglomerate properties on their leaching performance. Adv. Powder Technol. 2013, 24, 750–756. [Google Scholar] [CrossRef]
- Tang, X.-H.; Liu, R.-Z.; Yao, L.; Ji, Z.-J.; Zhang, Y.-T.; Li, S.-Q. Ferronickel enrichment by fine particle reduction and magnetic separation from nickel laterite ore. Int. J. Miner. Met. Mater. 2014, 21, 955–961. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Zhao, H.-L.; Wang, C. Two-stage reduction for the preparation of ferronickel alloy from nickel laterite ore with low Co and high MgO contents. Int. J. Miner. Met. Mater. 2017, 24, 512–522. [Google Scholar] [CrossRef] [Green Version]
- McDonald, R.G.; Whittington, B. Atmospheric acid leaching of nickel laterites review. Part II. Chloride and bio-technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Whittington, B.I.; Muir, D. Pressure acid leaching of nickel laterites: A review. Miner. Process. Extr. Met. Rev. 2000, 21, 527–599. [Google Scholar] [CrossRef]
- Guo, X.; Shi, W.-T.; Li, N.; Tian, Q. Leaching behavior of metals from limonitic laterite ore by high pressure acid leaching. Trans. Nonferrous Met. Soc. China 2011, 21, 191–195. [Google Scholar] [CrossRef]
- Büyükakinci, E.; Topkaya, Y. Extraction of nickel from lateritic ores at atmospheric pressure with agitation leaching. Hydrometallurgy 2009, 97, 33–38. [Google Scholar] [CrossRef]
- Rice, N.M. A hydrochloric acid process for nickeliferous laterites. Miner. Eng. 2016, 88, 28–52. [Google Scholar] [CrossRef]
- Luo, J.; Li, G.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Atmospheric leaching characteristics of nickel and iron in limonitic laterite with sulfuric acid in the presence of sodium sulfite. Miner. Eng. 2015, 78, 38–44. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Q.; Wei, G.; Zhang, P.; Qu, J.; Qi, T. Characterization and atmospheric hydrochloric acid leaching of a limonitic laterite from Indonesia. Hydrometallurgy 2012, 129, 7–13. [Google Scholar] [CrossRef]
- Da Costa, G.M.; Couto, D.J.F.; De Castro, F.P.M. Existence of maghemite and trevorite in nickel laterites. Miner. Process. Extr. Met. Rev. 2013, 34, 304–319. [Google Scholar] [CrossRef]
- Lakshmanan, V.I.; Sridhar, R.; Chen, J.; Halim, M.A. Development of mixed-chloride hydrometallurgical processes for the recovery of value metals from various resources. Trans. Indian Inst. Met. 2016, 69, 39–50. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Q.; Wei, G.; Meng, L.; Han, L.; Qu, J.; Qi, T. Leaching metals from saprolitic laterite ore using a ferric chloride solution. J. Clean. Prod. 2016, 112, 3531–3539. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Xu, Z.; Liao, C.; Liu, Y.; Zhong, B. Selective leaching of valuable metals from laterite nickel ore with ammonium chloride-hydrochloric acid solution. J. Clean. Prod. 2018, 179, 24–30. [Google Scholar] [CrossRef]
- Li, J.; Xiong, D.; Chen, H.; Wang, R.; Liang, Y. Physicochemical factors affecting leaching of laterite ore in hydrochloric acid. Hydrometallurgy 2012, 129, 14–18. [Google Scholar] [CrossRef]
- Ma, B.; Yang, W.; Xing, P.; Wang, C.; Chen, Y.; Lv, D. Pilot-scale plant study on solid-state metalized reduction–magnetic separation for magnesium-rich nickel oxide ores. Int. J. Miner. Process. 2017, 169, 99–105. [Google Scholar] [CrossRef]
- Thubakgale, C.; Mbaya, R.; Kabongo, K. A study of atmospheric acid leaching of a South African nickel laterite. Miner. Eng. 2013, 54, 79–81. [Google Scholar] [CrossRef]
- Dickinson, C.; Heal, G. Solid–liquid diffusion controlled rate equations. Thermochim. Acta 1999, 340, 89–103. [Google Scholar] [CrossRef]
- Dehghan, R.; Noaparast, M.; Kolahdoozan, M. Leaching and kinetic modelling of low-grade calcareous sphalerite in acidic ferric chloride solution. Hydrometallurgy 2009, 96, 275–282. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zheng, S.; Xiong, D.; Chen, H.; Zhang, Y. Research review of laterite nickel ore metallurgy. Nonferrous Met. Sci. Eng. 2015, 6, 35–40. [Google Scholar]
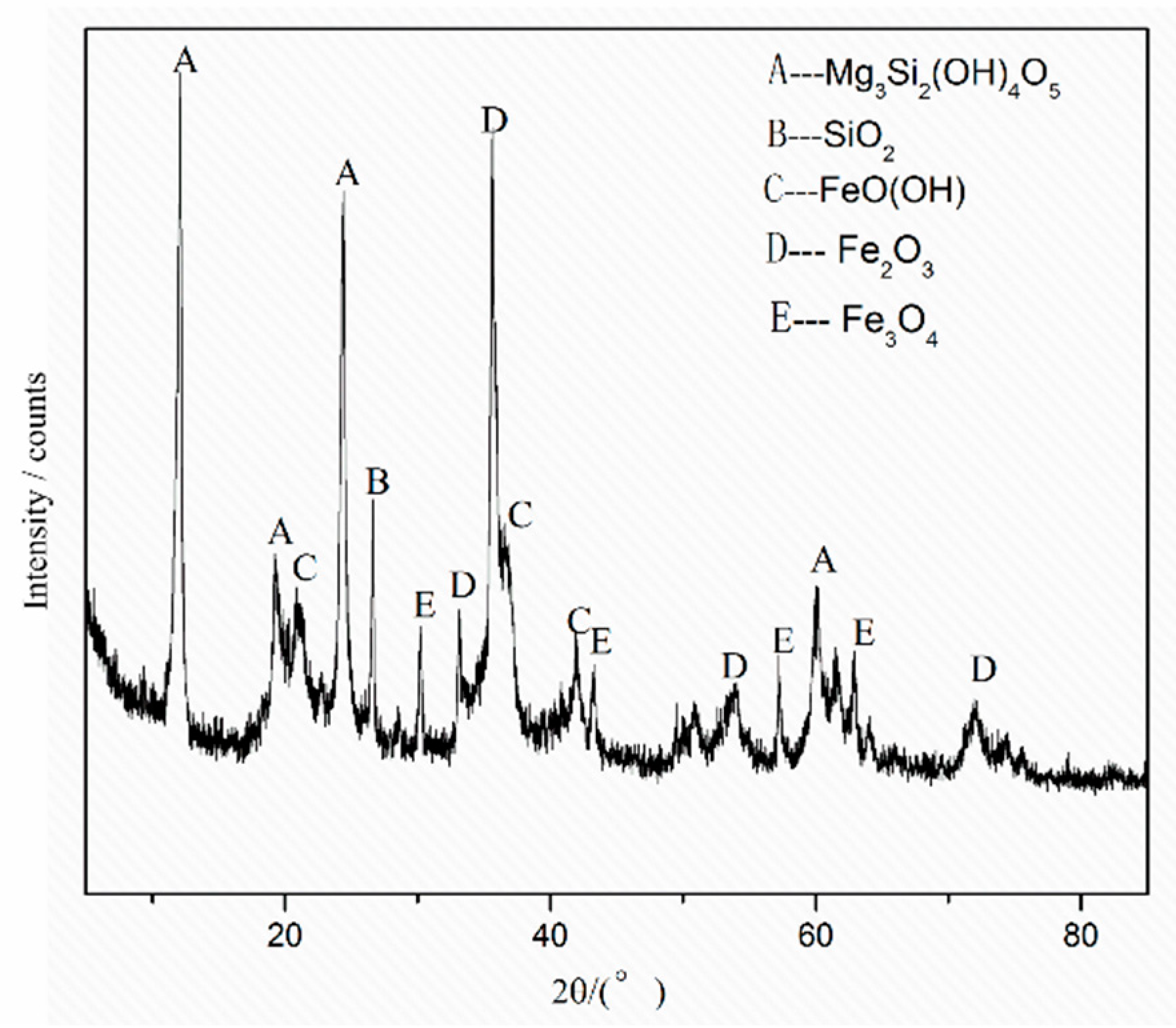
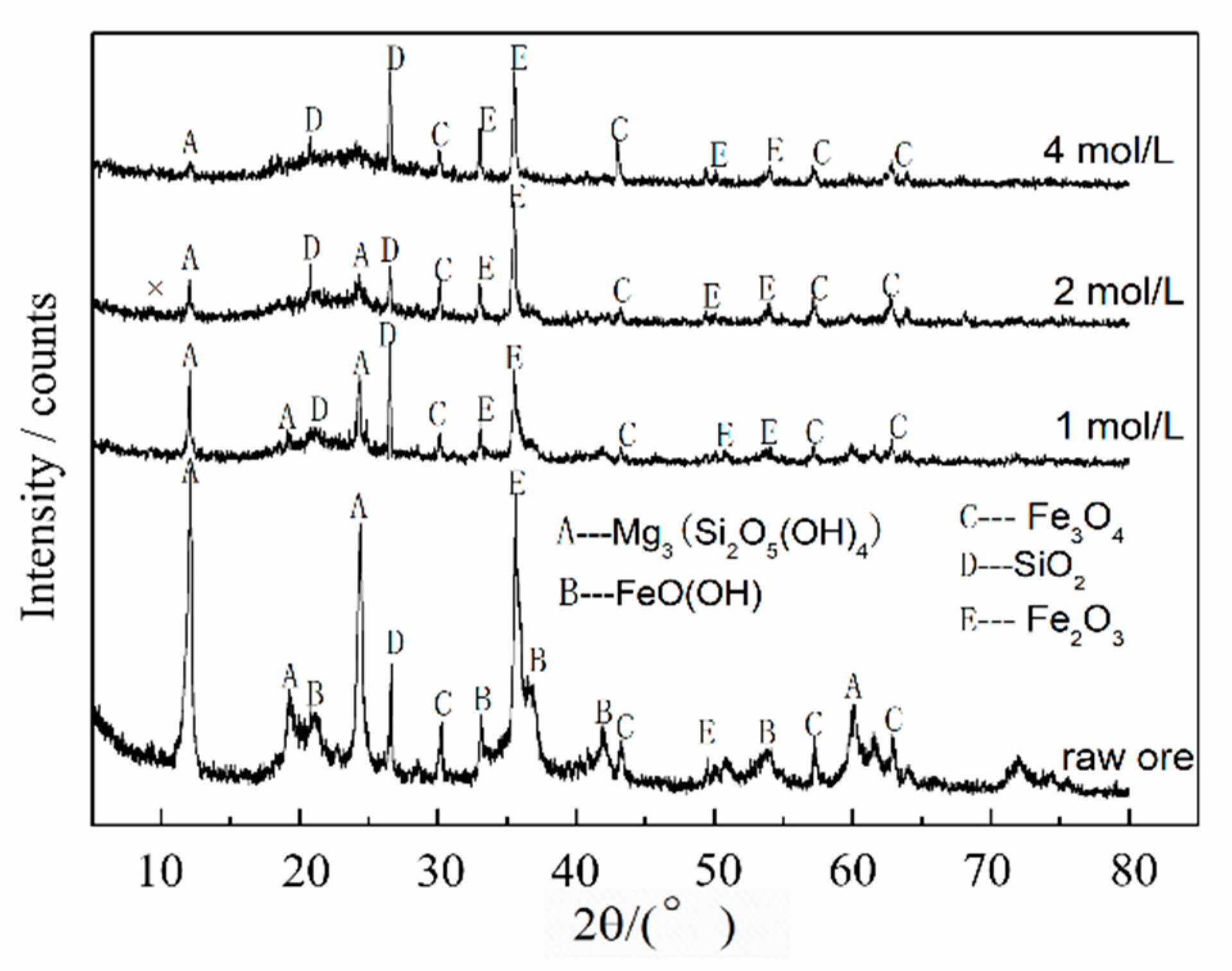
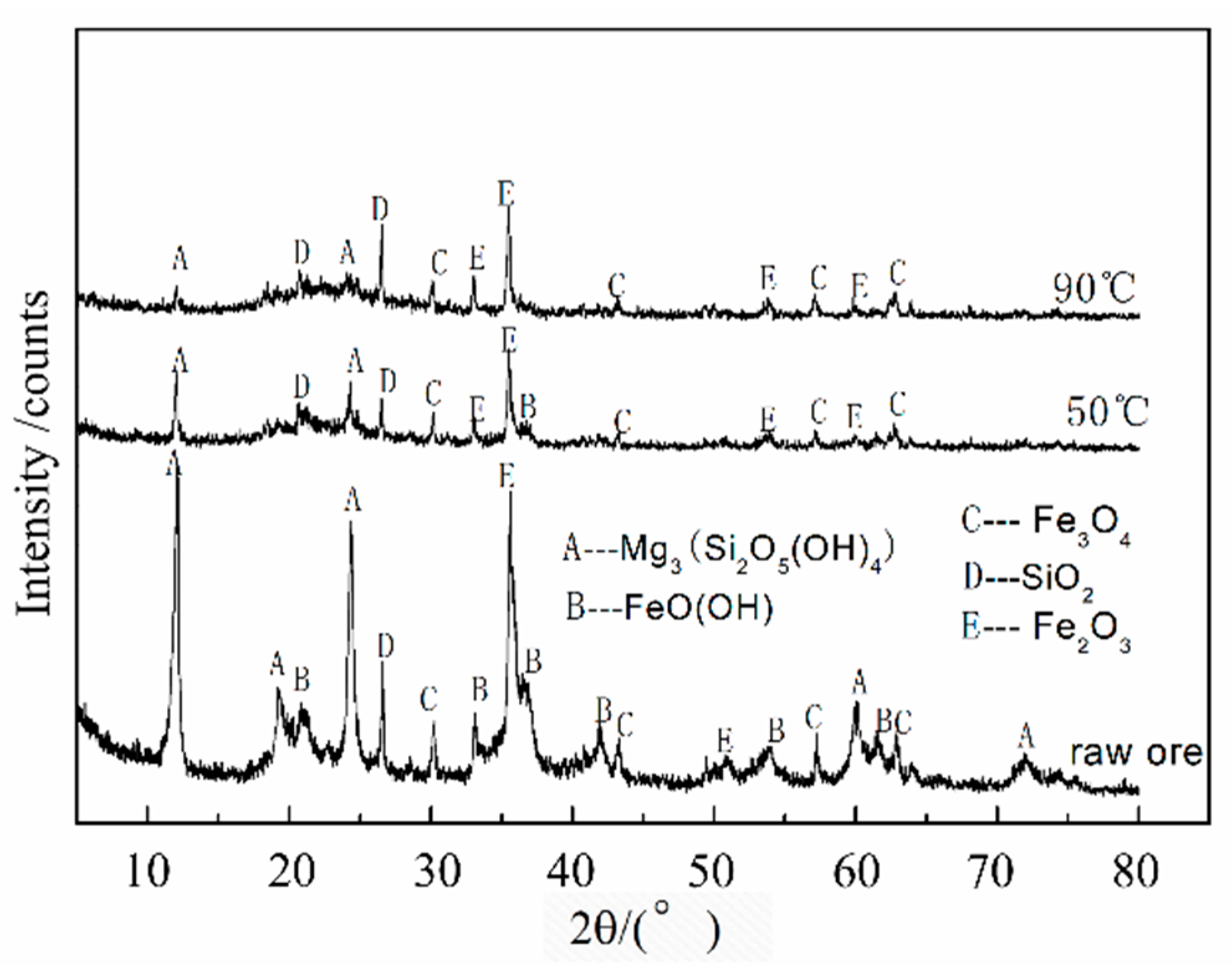
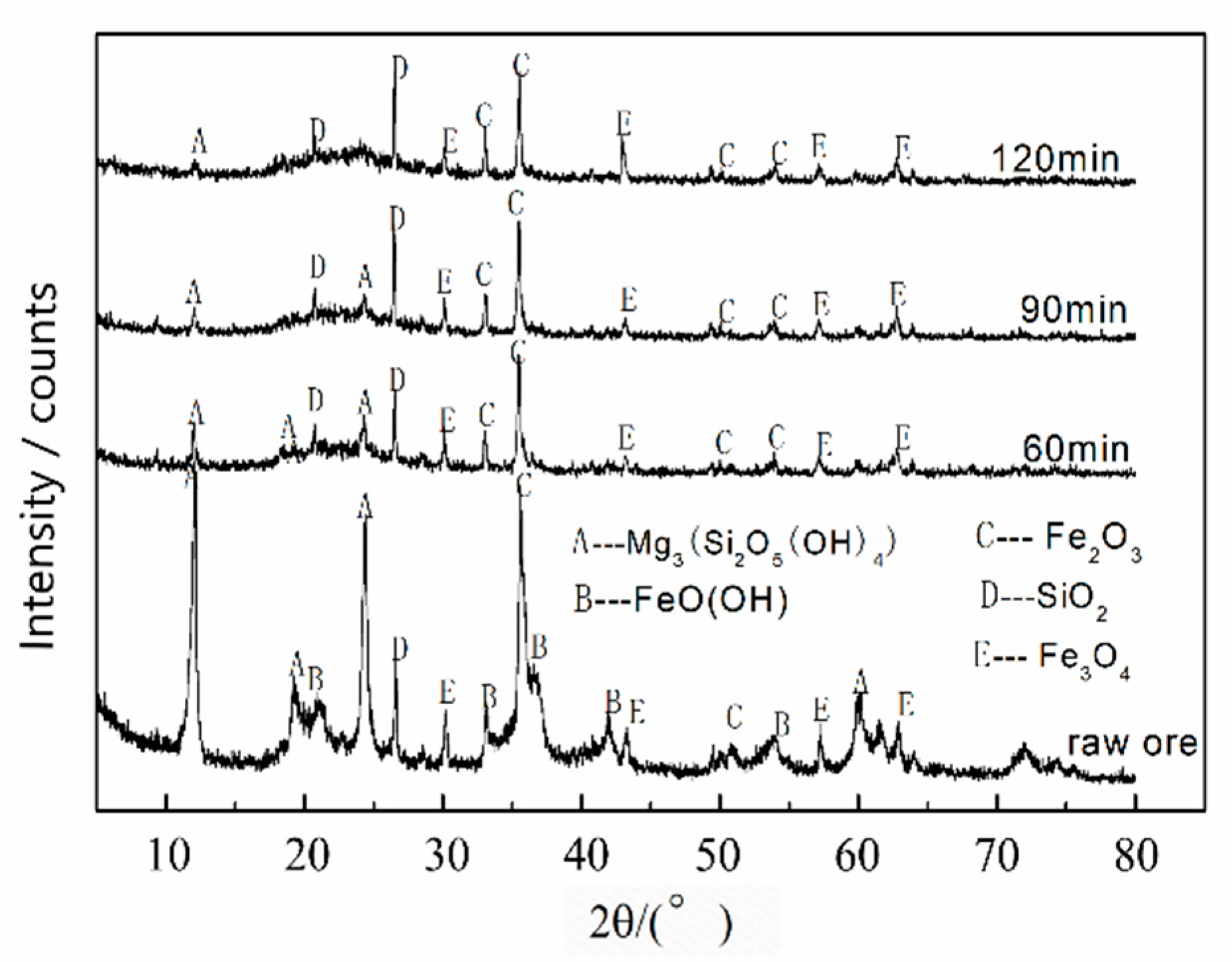


| Elements | Ni | Co | Mn | Fe | Cu | Ca | Mg | Al | Si | Na |
|---|---|---|---|---|---|---|---|---|---|---|
| Content% | 1.15 | 0.08 | 0.35 | 14.06 | 0.07 | 0.12 | 29.35 | 0.34 | 23.13 | 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, Y.; Wen, Y.; Liu, W.; Chu, Y.; Wang, R.; Xu, Z. Leaching Kinetics and Mechanism of Laterite with NH4Cl-HCl Solution. Minerals 2020, 10, 754. https://doi.org/10.3390/min10090754
Li J, Yang Y, Wen Y, Liu W, Chu Y, Wang R, Xu Z. Leaching Kinetics and Mechanism of Laterite with NH4Cl-HCl Solution. Minerals. 2020; 10(9):754. https://doi.org/10.3390/min10090754
Chicago/Turabian StyleLi, Jinhui, Yang Yang, Yaoru Wen, Wenxin Liu, Yuhang Chu, Ruixiang Wang, and Zhifeng Xu. 2020. "Leaching Kinetics and Mechanism of Laterite with NH4Cl-HCl Solution" Minerals 10, no. 9: 754. https://doi.org/10.3390/min10090754




