Indium and Antimony Distribution in a Sphalerite from the “Burgstaetter Gangzug” of the Upper Harz Mountains Pb-Zn Mineralization
Abstract
1. Introduction
1.1. Overview and Motivation
1.2. Overview of the Geological Setting of the Ore Body
1.3. Short Review of In and Sb Incorporation in Sphalerite
2. Material and Methods
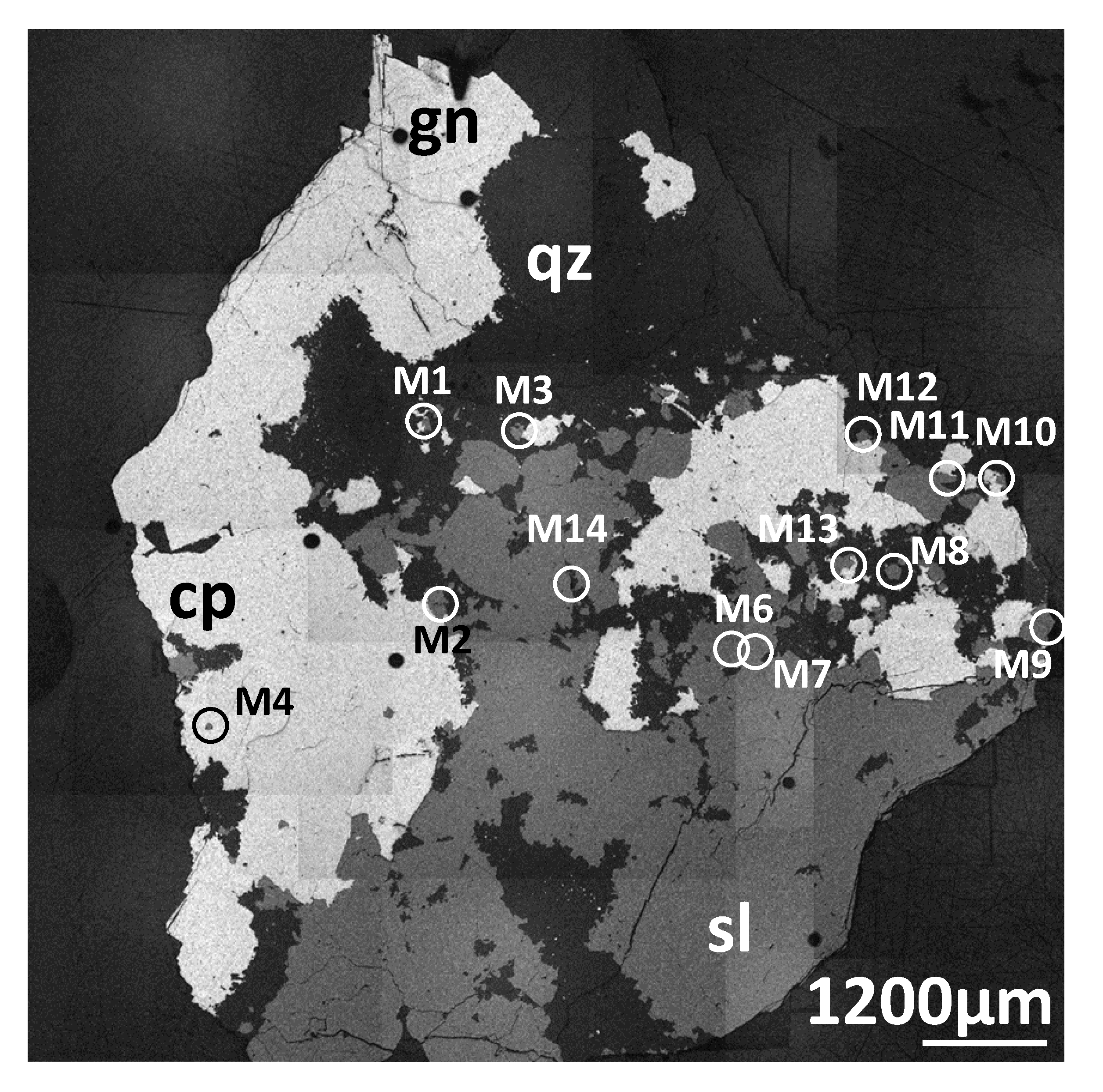
2.1. Material
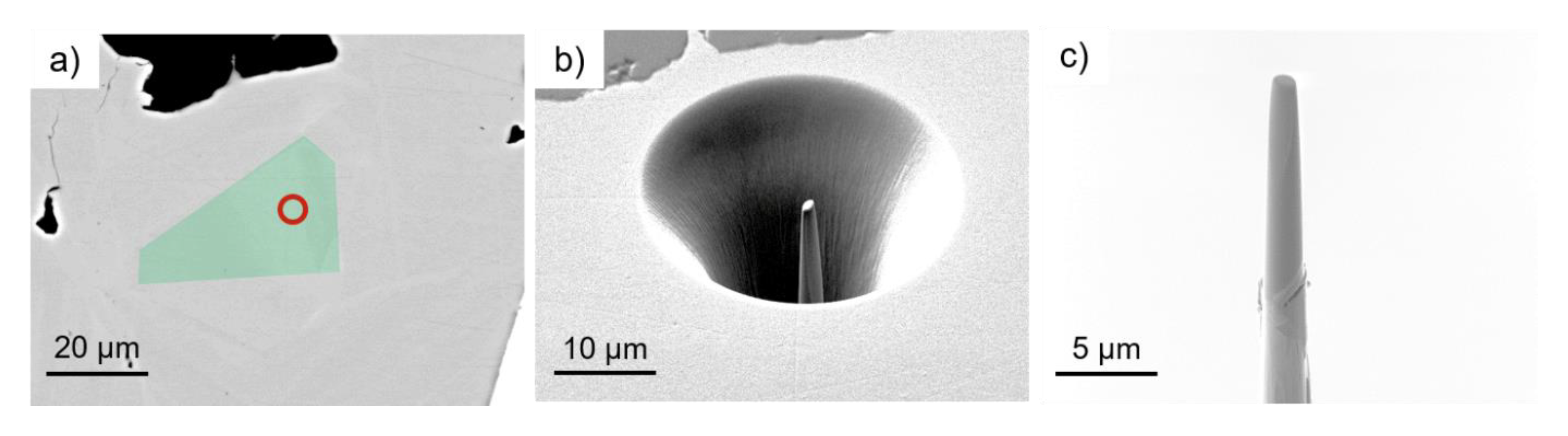
2.2. Methods
3. Results
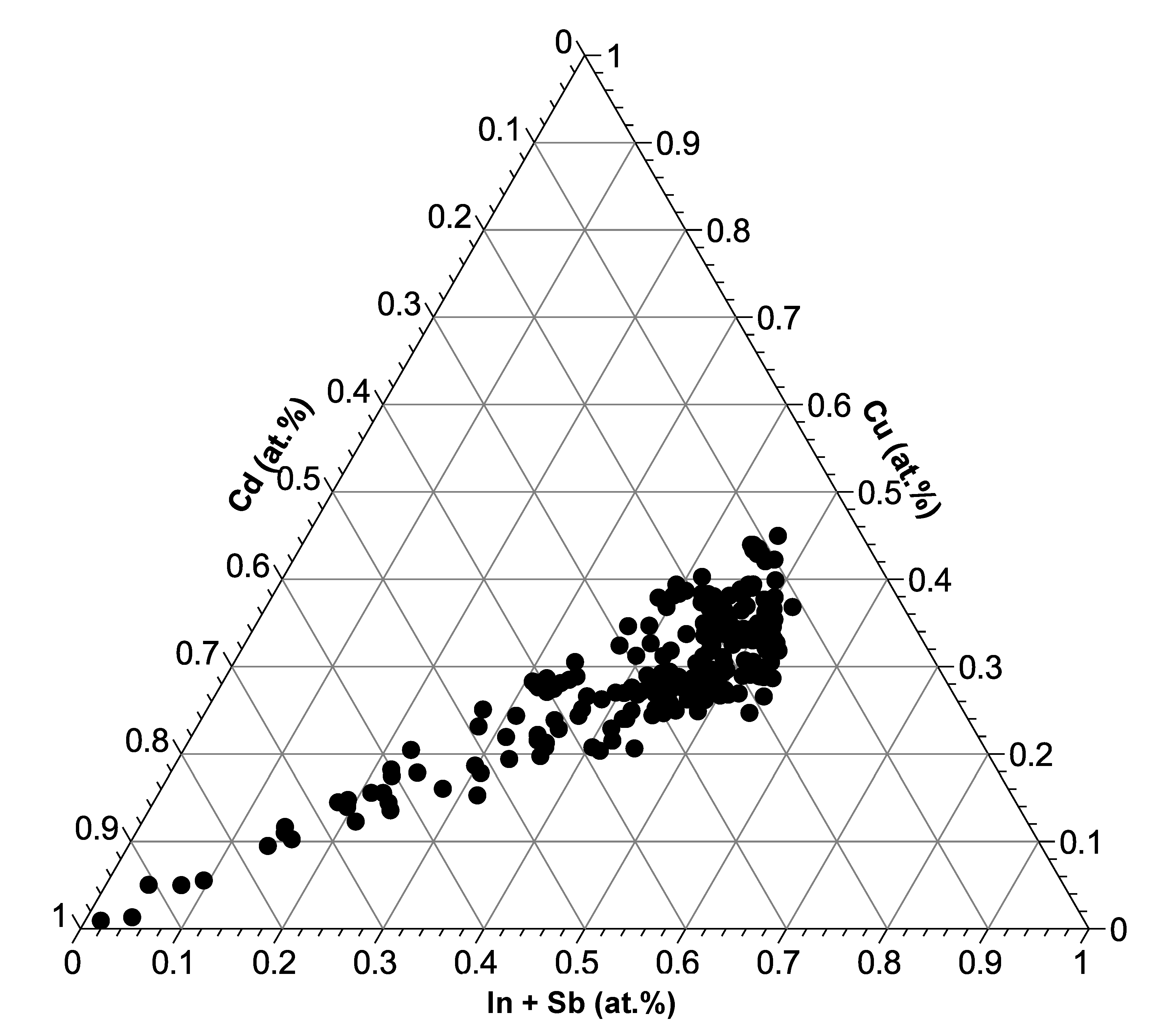
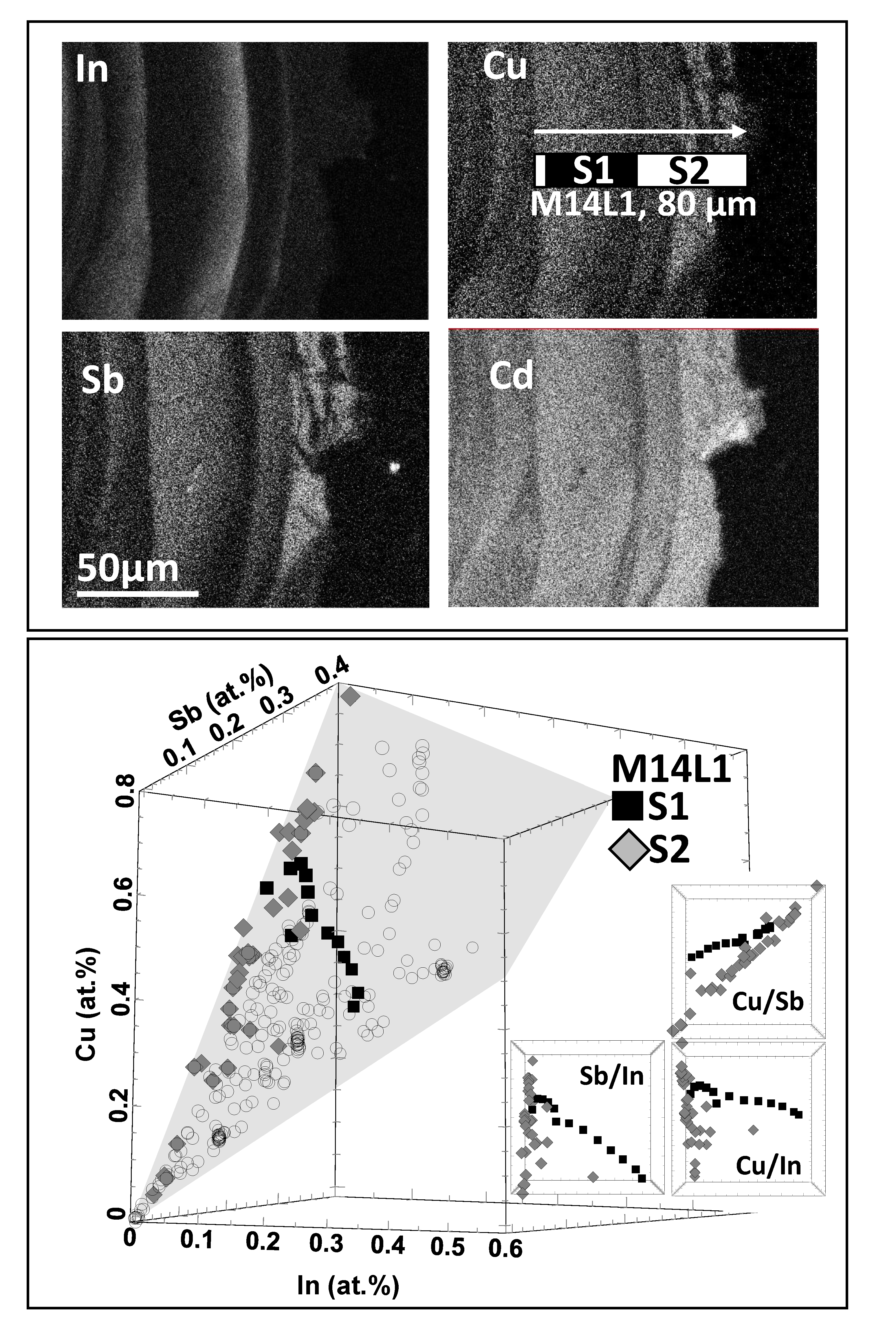
3.1. Compilation of All Element Distribution Profiles
3.2. Selected Sphalerite Element Maps and Scans
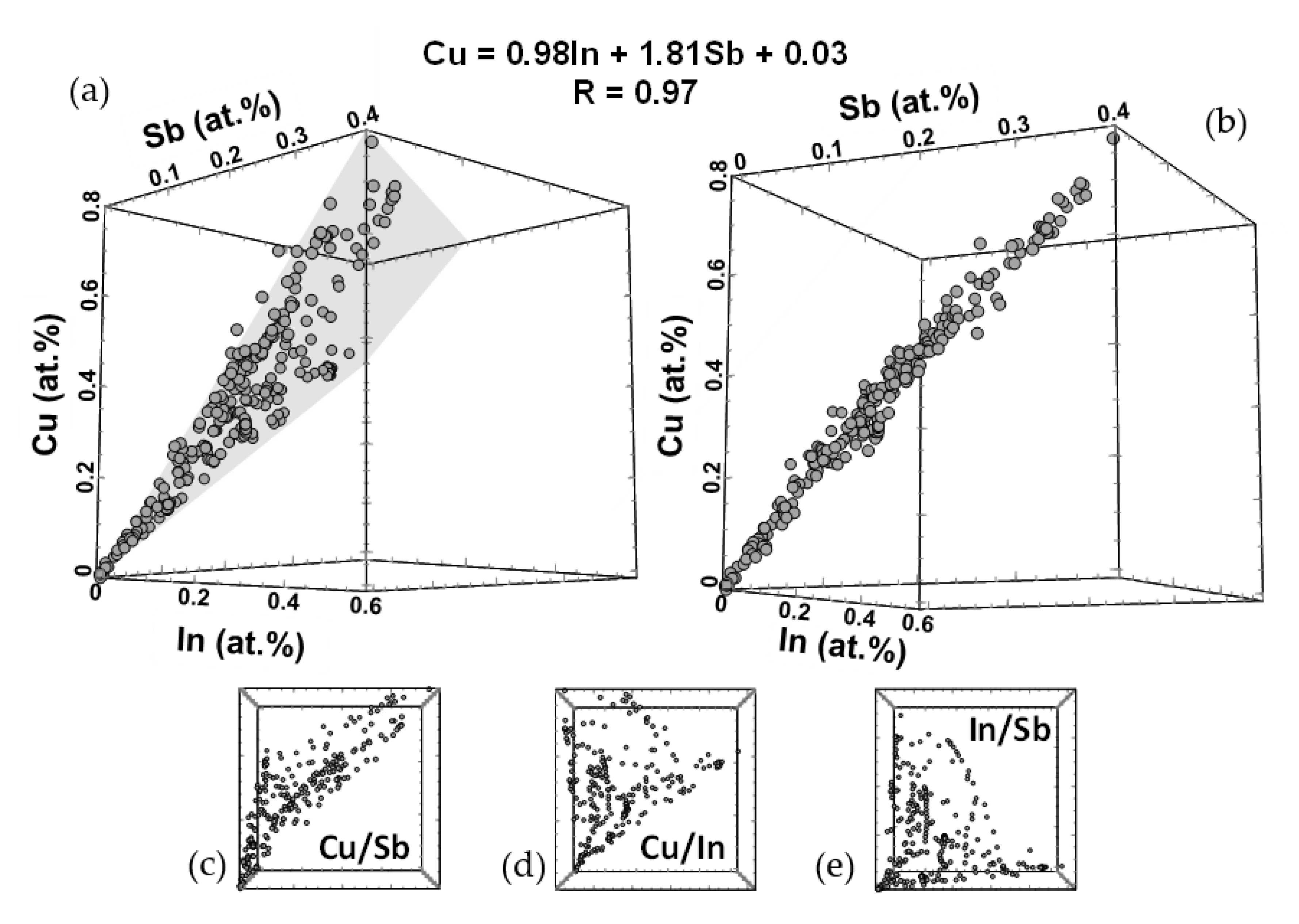
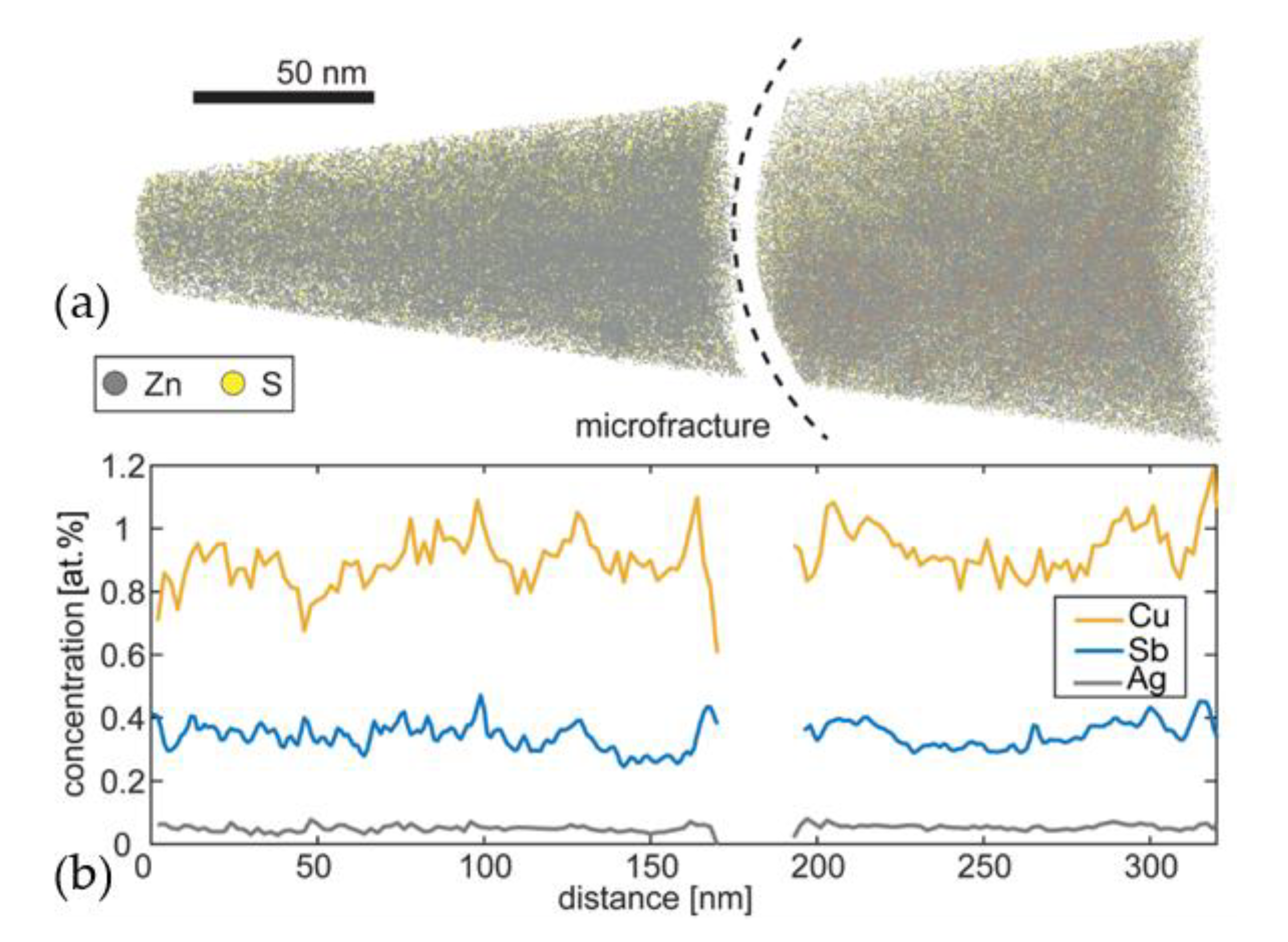
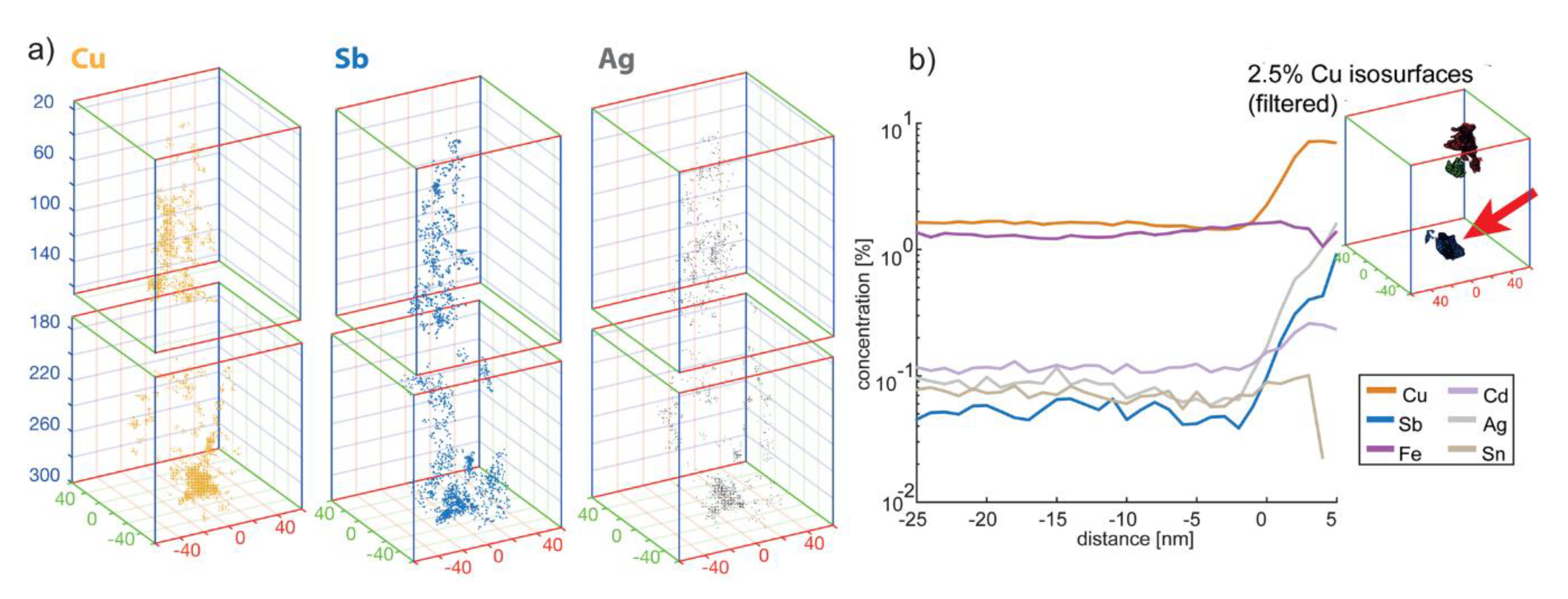
3.3. Atom Probe Tomography Results
4. Discussion
4.1. General Implications
4.2. Crystal Structural Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Distance (µ) | Zn | S | Fe | Cd | Cu | In | Sb |
|---|---|---|---|---|---|---|---|
| 0 | 48.7 | 50.0 | 1.01 | 0.11 | 0.08 | 0.07 | <0.002 |
| 1.86 | 48.4 | 50.2 | 0.75 | 0.09 | 0.26 | 0.22 | <0.002 |
| 3.71 | 48.5 | 50.3 | 0.68 | 0.07 | 0.22 | 0.18 | <0.002 |
| 5.57 | 48.8 | 50.3 | 0.57 | 0.08 | 0.16 | 0.12 | <0.002 |
| 7.42 | 48.8 | 50.0 | 0.72 | 0.08 | 0.20 | 0.14 | 0.011 |
| 9.28 | 48.6 | 50.2 | 0.67 | 0.07 | 0.25 | 0.17 | <0.002 |
| 11.14 | 48.6 | 50.0 | 0.65 | 0.08 | 0.38 | 0.26 | 0.01 |
| 12.99 | 48.4 | 50.3 | 0.53 | 0.11 | 0.41 | 0.23 | 0.07 |
| 14.85 | 48.0 | 49.9 | 0.78 | 0.20 | 0.65 | 0.09 | 0.31 |
| 16.7 | 47.3 | 50.2 | 1.07 | 0.21 | 0.74 | 0.11 | 0.33 |
| 18.56 | 47.4 | 50.3 | 0.87 | 0.20 | 0.73 | 0.15 | 0.31 |
| 20.42 | 47.4 | 50.4 | 0.81 | 0.20 | 0.70 | 0.19 | 0.27 |
| 22.27 | 47.5 | 50.4 | 0.82 | 0.19 | 0.68 | 0.24 | 0.22 |
| 24.13 | 47.4 | 50.1 | 1.20 | 0.17 | 0.64 | 0.29 | 0.16 |
| 25.98 | 47.7 | 50.1 | 1.06 | 0.16 | 0.58 | 0.30 | 0.13 |
| 27.84 | 47.6 | 50.4 | 0.93 | 0.14 | 0.53 | 0.32 | 0.09 |
| 29.69 | 47.9 | 50.1 | 0.97 | 0.13 | 0.51 | 0.33 | 0.06 |
| 31.55 | 48.0 | 50.1 | 0.99 | 0.11 | 0.44 | 0.31 | 0.02 |
| 33.41 | 48.4 | 50.2 | 0.65 | 0.10 | 0.36 | 0.23 | 0.03 |
| 35.26 | 48.3 | 50.1 | 0.74 | 0.10 | 0.43 | 0.34 | 0.01 |
| 37.12 | 48.1 | 50.1 | 0.60 | 0.10 | 0.55 | 0.52 | 0.01 |
| 38.97 | 48.1 | 50.2 | 0.60 | 0.11 | 0.54 | 0.43 | 0.05 |
| 40.83 | 48.0 | 50.1 | 0.83 | 0.18 | 0.54 | 0.11 | 0.25 |
| 42.69 | 47.6 | 50.4 | 0.88 | 0.20 | 0.63 | 0.03 | 0.32 |
| 44.54 | 47.6 | 50.3 | 0.93 | 0.20 | 0.65 | 0.06 | 0.31 |
| 46.4 | 47.5 | 50.3 | 1.00 | 0.19 | 0.64 | 0.12 | 0.27 |
| 48.25 | 47.7 | 50.2 | 1.09 | 0.17 | 0.56 | 0.18 | 0.17 |
| 50.11 | 48.2 | 50.3 | 1.03 | 0.15 | 0.21 | 0.16 | 0.01 |
| 51.97 | 48.5 | 50.0 | 1.16 | 0.15 | 0.12 | 0.12 | <0.002 |
| 53.82 | 48.6 | 50.2 | 0.97 | 0.15 | 0.09 | 0.09 | <0.002 |
| Distance (µ) | Zn | S | Fe | Cd | Cu | In | Sb |
|---|---|---|---|---|---|---|---|
| 0 | 47.6 | 50.2 | 1.09 | 0.21 | 0.63 | 0.01 | 0.33 |
| 1.64 | 47.4 | 50.3 | 1.22 | 0.21 | 0.63 | 0.02 | 0.33 |
| 3.29 | 47.2 | 50.4 | 1.21 | 0.21 | 0.61 | 0.02 | 0.32 |
| 4.93 | 47.3 | 50.6 | 0.96 | 0.20 | 0.59 | 0.02 | 0.31 |
| 6.57 | 47.4 | 50.3 | 1.21 | 0.20 | 0.57 | 0.02 | 0.29 |
| 8.21 | 47.6 | 50.1 | 1.33 | 0.19 | 0.49 | 0.02 | 0.25 |
| 9.86 | 47.9 | 49.0 | 2.03 | 0.18 | 0.53 | 0.03 | 0.23 |
| 11.5 | 47.5 | 50.2 | 1.33 | 0.19 | 0.56 | 0.04 | 0.26 |
| 13.14 | 47.7 | 50.1 | 1.14 | 0.19 | 0.57 | 0.06 | 0.26 |
| 14.78 | 47.7 | 50.0 | 1.24 | 0.19 | 0.55 | 0.07 | 0.25 |
| 16.43 | 47.8 | 49.9 | 1.20 | 0.19 | 0.53 | 0.09 | 0.23 |
| 18.07 | 47.9 | 49.8 | 1.34 | 0.20 | 0.47 | 0.09 | 0.19 |
| 19.71 | 47.8 | 49.9 | 1.31 | 0.19 | 0.51 | 0.13 | 0.18 |
| 21.36 | 47.9 | 50.0 | 1.10 | 0.18 | 0.49 | 0.16 | 0.17 |
| 23 | 47.9 | 49.9 | 1.16 | 0.18 | 0.49 | 0.20 | 0.13 |
| 24.64 | 47.9 | 49.7 | 1.42 | 0.18 | 0.47 | 0.24 | 0.10 |
| 26.28 | 47.8 | 49.9 | 1.22 | 0.18 | 0.46 | 0.27 | 0.08 |
| 27.93 | 48.0 | 49.9 | 1.12 | 0.17 | 0.43 | 0.30 | 0.05 |
| 29.57 | 48.2 | 49.7 | 1.21 | 0.13 | 0.41 | 0.32 | 0.02 |
| 31.21 | 48.6 | 50.0 | 0.72 | 0.10 | 0.32 | 0.19 | 0.03 |
| 32.85 | 48.8 | 49.8 | 0.89 | 0.12 | 0.23 | 0.06 | 0.07 |
| 34.5 | 48.7 | 50.2 | 0.49 | 0.15 | 0.31 | 0.04 | 0.15 |
| 36.14 | 48.5 | 50.0 | 0.74 | 0.19 | 0.42 | 0.01 | 0.21 |
| 37.78 | 48.5 | 50.0 | 0.71 | 0.18 | 0.42 | 0.01 | 0.21 |
| 39.43 | 48.4 | 49.8 | 1.03 | 0.18 | 0.39 | 0.02 | 0.20 |
| 41.07 | 48.4 | 50.2 | 0.63 | 0.18 | 0.38 | 0.02 | 0.19 |
| 42.71 | 48.4 | 50.1 | 0.74 | 0.17 | 0.37 | 0.02 | 0.18 |
| 44.35 | 48.4 | 50.2 | 0.66 | 0.17 | 0.34 | 0.03 | 0.16 |
| 46 | 48.5 | 49.8 | 1.05 | 0.16 | 0.32 | 0.05 | 0.13 |
| 47.64 | 48.4 | 50.2 | 0.75 | 0.16 | 0.31 | 0.08 | 0.13 |
| 49.28 | 47.9 | 50.4 | 0.73 | 0.18 | 0.46 | 0.07 | 0.24 |
| 50.92 | 47.7 | 50.2 | 0.87 | 0.23 | 0.62 | 0.03 | 0.32 |
| 52.57 | 47.3 | 50.4 | 1.12 | 0.24 | 0.62 | 0.03 | 0.25 |
| 54.21 | 47.3 | 50.4 | 0.85 | 0.25 | 0.79 | 0.02 | 0.39 |
| 55.85 | 48.0 | 50.2 | 0.82 | 0.22 | 0.50 | 0.03 | 0.28 |
| 57.5 | 48.4 | 50.6 | 0.71 | 0.17 | 0.06 | 0.03 | 0.02 |
| 59.14 | 48.3 | 50.7 | 0.80 | 0.18 | 0.03 | 0.03 | <0.002 |
| 60.78 | 47.9 | 50.4 | 1.04 | 0.22 | 0.26 | 0.02 | 0.11 |
| 62.42 | 47.8 | 50.8 | 0.96 | 0.21 | 0.12 | 0.03 | 0.05 |
| 64.07 | 47.2 | 51.0 | 0.92 | 0.22 | 0.42 | 0.03 | 0.20 |
| 65.71 | 47.4 | 50.8 | 1.18 | 0.24 | 0.24 | 0.05 | 0.13 |
| 67.35 | 47.0 | 50.7 | 1.39 | 0.28 | 0.43 | 0.03 | 0.20 |
| 68.99 | 46.6 | 50.7 | 1.66 | 0.37 | 0.47 | 0.02 | 0.20 |
| 70.64 | 46.4 | 51.0 | 1.30 | 0.33 | 0.60 | 0.02 | 0.29 |
| 72.28 | 46.3 | 51.4 | 0.96 | 0.28 | 0.68 | 0.02 | 0.34 |
| 73.92 | 46.6 | 51.1 | 1.28 | 0.39 | 0.42 | 0.01 | 0.19 |
| 75.57 | 47.0 | 50.9 | 1.39 | 0.42 | 0.25 | 0.01 | 0.11 |
| 77.21 | 47.2 | 51.6 | 0.90 | 0.23 | 0.07 | 0.03 | 0.02 |
| 78.85 | 47.8 | 51.1 | 0.86 | 0.18 | <0.01 | 0.01 | <0.002 |
| 80.49 | 47.9 | 51.5 | 0.46 | 0.15 | <0.01 | 0.01 | <0.002 |
References
- Stedingk, K. Geologie und Erzlagerstätten im Oberharz. Exkursf. Und Veröfftl. D Ges. Geowiss. 2012, 247, 9–81. [Google Scholar]
- Sperling, H.; Stoppel, D. Monographien der deutschen Blei—Zink Erzlagerstätten Die Blei—Zink Erzgänge des Oberharzes—Beschreibung der Oberharzer Erzgänge (einschliesslich der Neuaufschlüsse im Erzbergwerk Grund seit Erscheinen der Lieferung 2). Geol. Jahrb. 1979, 34, 5–345. [Google Scholar]
- Möller, P.; Lüders, V. Formation of Hydrothermal Vein Deposits, a Case Study of the Pb-Zn, Barite and Fluorite Deposits of the Harz Mountains; Monograph Series on Mineral Deposits; Gebrüder Bornträger: Berlin, Stuttgart, 1993; p. 30. [Google Scholar]
- Schwarz-Schampera, U.; Herzig, P.M. Indium: Geology, Mineralogy, and Economics; Springer: Heidelberg/Berlin, Germany, 2002. [Google Scholar]
- Briskey, J.A. Indium in Zinc-Lead and Other Mineral Deposits—A Reconnaissance Survey of 1118 Indium Analyses Published Before 1985; US Geological Survey: Reston, VA, USA, 2005.
- Miroshnichenko, L.A. New data on distribution of indium in ore deposits of Central Kazakhstan. Int. Geol. Rev. 1965, 7, 233–240. [Google Scholar] [CrossRef]
- Di Benedetto, F. Compositional Zoning in Sphalerite Crystals. Am. Mineral. 2005, 90, 1384–1392. [Google Scholar] [CrossRef]
- Ishihara, S.; Murakami, H.; Li, X. Indium concentration in zinc ores in plutonic and volcanic environments: Examples at the Dulong and Dachang mines. South China Bull. Geol. Surv. Jpn. 2011, 62, 259–272. [Google Scholar] [CrossRef]
- Ye, L.; Cook, N.J.; Ciobanu, C.L.; Yuping, L.; Qian, Z.; Tiegeng, L.; Wei, G.; Yulong, Y.; Danyushevskiy, L. Trace and minor elements in sphalerite from base metal deposits in South China: A LA-ICPMS study. Ore Geol. Rev. 2011, 39, 188–217. [Google Scholar] [CrossRef]
- Lockington, J.A.; Cook, N.J.; Ciobanu, C.L. Trace and minor elements in sphalerite from metamorphosed sulphide deposits. Mineral. Petrol. 2014, 108, 873–890. [Google Scholar] [CrossRef]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Seifert, T.; Sandmann, D. Mineralogy and geochemistry of indium-bearing polymetallic vein-type deposits: Implications for host minerals from the Freiberg district, Eastern Erzgebirge, Germany. Ore Geol. Rev. 2006, 28, 1–31. [Google Scholar] [CrossRef]
- Sahlström, F.; Arribas, A.; Dirks, P.; Corral, I.; Chang, Z. Mineralogical Distribution of Germanium, Gallium and Indium at the Mt Carlton High-Sulfidation Epithermal Deposit, NE Australia, and Comparison with Similar Deposits Worldwide. Minerals 2017, 7, 213. [Google Scholar] [CrossRef]
- Johan, Z. Indium and germanium in the structure of sphalerite: An example of coupled substitution with Copper. Mineral. Petrol. 1988, 39, 211–229. [Google Scholar] [CrossRef]
- Wiggins, L.B.; Craig, J.R. Reconnaissance of the Cu-Fe-Zn-S system; sphalerite phase relationships. Econ. Geol. 1980, 75, 742–751. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Brugger, J.; Etschmann, B.; Howard, D.L.; de Jonge, M.D.; Ryan, C.; Paterson, D. Determination of the oxidation state of Cu in substituted Cu-In-Fe-bearing sphalerite via -XANES spectroscopy. Am. Mineral. 2012, 97, 476–479. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Marion, P.; Pinto, A.; Migeon, H.; Wagner, F.E. Tin and indium mineralogy within selected samples from the Neves Corvo ore deposit (Portugal): A multidisciplinary study. Mineral. Eng. 2003, 16, 1291–1302. [Google Scholar] [CrossRef]
- Andersen, J.C.Ø.; Stickland, R.J.; Rollinson, G.K.; Shail, R.K. Indium mineralisation in SW England: Host parageneses and mineralogical relations. Ore Geol. Rev. 2016, 78, 213–238. [Google Scholar] [CrossRef]
- Valkama, M.; Sundblad, K.; Cook, N.J.; Ivashchenko, V.I. Geochemistry and petrology of the indium-bearing polymetallic skarn ores at Pitkäranta, Ladoga Karelia. Russ. Mineral. Depos. 2016, 51, 823–839. [Google Scholar] [CrossRef]
- Bauer, M.E.; Seifert, T.; Burisch, M.; Krause, J.; Richter, N.; Gutzmer, J. Indium-bearing sulfides from the Hämmerlein skarn deposit, Erzgebirge, Germany: Evidence for late-stage diffusion of indium into sphalerite. Mineral. Depos. 2017, 54, 175–192. [Google Scholar] [CrossRef]
- Kieft, K. Indium-Bearing Chalcopyrite and Sphalerite from the Gåsborn Area, West Bergslagen, Central Sweden. Mineral. Mag. 1990, 54, 109–112. [Google Scholar] [CrossRef][Green Version]
- Shimizu, T.; Morishita, Y. Petrography, chemistry, and near-infrared microthermometry of indium-bearing sphalerite from the Toyoha polymetallic deposit. Jpn. Econ. Geol. 2012, 107, 723–735. [Google Scholar] [CrossRef]
- Sahlström, F.; Blake, K.; Corral, I.; Chang, Z. Hyperspectral cathodoluminescence study of indium-bearing sphalerite from the Mt Carlton high-sulphidation epithermal deposit, Queensland, Australia. Eur. J. Mineral. 2017, 29, 985–993. [Google Scholar] [CrossRef]
- Ohta, E. Occurrence and Chemistry of Indium-containing Minerals from the Toyoha Mine, Hokkaido, Japan. Min. Geol. 1989, 39, 355–372. [Google Scholar]
- Schorr, S.; Wagner, G. Structure and phase relations of the Zn2x(CuIn)1−xS2 solid solution series. J. Alloys Compd. 2005, 396, 202–207. [Google Scholar] [CrossRef]
- Reiser, F.K.M.; Diogo, R.N.; Rosa, Á.M.M.; Pinto, J.R.S.; Carvalho, J.X.; Matos, F.M.G.; Guimarães, L.C.A.; de Oliveira, D.P.S. Mineralogy and Geochemistry of Tin- and Germanium-Bearing Copper Ore, Barrigão Re-Mobilized Vein Deposit, Iberian Pyrite Belt, Portugal. Int. Geol. Rev. 2011, 53, 1212–1238. [Google Scholar] [CrossRef]
- Qian, Z.; Xinzhi, Z.; Jiayong, P.; Shuxun, S. Geochemical enrichment and mineralization of indium. Chin. J. Geochem. 1998, 17, 221–225. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Murakami, H.; Ishihara, S. Trace elements of Indium-bearing sphalerite from tin-polymetallic deposits in Bolivia, China and Japan: A femto-second LA-ICPMS study. Ore Geol. Rev. 2013, 53, 223–243. [Google Scholar] [CrossRef]
- Li, Y.; Tao, Y.; Zhu, F.; Liao, M.; Xiong, F.; Deng, X. Distribution and existing state of indium in the Gejiu Tin polymetallic deposit, Yunnan Province, SW China. Chin. J. Geochem. 2015, 34, 469–483. [Google Scholar] [CrossRef]
- Beaudoin, G. Acicular sphalerite enriched in Ag, Sb, and Su embedded within color-banded sphalerite from the Kokanee range, British Columbia, Canada. Can. Mineral. 2000, 38, 1387–1398. [Google Scholar] [CrossRef]
- Kelley, K.D. Textural, Compositional, and Sulfur Isotope Variations of Sulfide Minerals in the Red Dog Zn-Pb-Ag Deposits, Brooks Range, Alaska: Implications for Ore Formation. Econ. Geol. 2004, 99, 1509–1532. [Google Scholar] [CrossRef]
- More, A.P.; Vaughan, D.J.; Ashworth, J.R. Banded Sphalerite from the North Pennine Orefield. Mineral. Mag. 1991, 55, 409–416. [Google Scholar] [CrossRef]
- Jercinovic, M.J.; Williams, M.L.; Allaz, J.; Donovan, J.J. Trace analysis in EPMA. IOP Conf. Ser. Mater. Sci. Eng. 2012, 32, 12012. [Google Scholar] [CrossRef]
- Merlet, C. Quantitative Electron Probe Microanalysis: New Accurate Φ (ρz) Description. In Electron Microbeam Analysis; Boekestein, A., Pavićević, M.K., Eds.; Springer: Vienna, Austria, 1992; pp. 107–115. [Google Scholar]
- Cerezo, A.; Godfrey, T.J.; Sijbrandij, S.J.; Smith, G.D.W.; Warren, P.J. Performance of an Energy-Compensated Three-Dimensional Atom Probe. Rev. Sci. Instrum. 1998, 69, 49–58. [Google Scholar] [CrossRef]
- Peterman, E.M.; Reddy, S.M.; Saxey, D.W.; Snoeyenbos, D.R.; Rickard, W.D.; Fougerouse, D.; Kylander-Clark, A.R. Nanogeochronology of Discordant Zircon Measured by Atom Probe Microscopy of Pb-Enriched Dislocation Loops. Sci. Adv. 2016, 2, e1601318. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.M.; van Riessen, A.; Saxey, D.W.; Johnson, T.E.; Rickard, W.D.A.; Fougerouse, D.; Fischer, S. Mechanisms of Deformation-Induced Trace Element Migration in Zircon Resolved by Atom Probe and Correlative Microscopy. Geochim. Cosmochim. Acta 2016, 195, 158–170. [Google Scholar] [CrossRef]
- Stephenson, L.T.; Michael, P.M.; und Ringer, S.P. Theory of Solute Clustering in Materials for Atom Probe. Philos. Mag. 2011, 91, 2200–2215. [Google Scholar] [CrossRef]
- Gault, B.; Haley, D.; de Geuser, F.; Moody, M.P.; Marquis, E.A.; Larson, D.J.; Geiser, B.P. Advances in the Reconstruction of Atom Probe Tomography Data. Ultramicroscopy 2011, 111, 448–457. [Google Scholar] [CrossRef]
- Felfer, P.; Ceguerra, A.V.; Ringer, S.P.; Cairney, J.M. Detecting and Extracting Clusters in Atom Probe Data: A Simple, Automated Method Using Voronoi Cells. Ultramicroscopy 2015, 150, 30–36. [Google Scholar] [CrossRef]
- Lorensen, W.E.; Cline, H.E. Marching Cubes: A High Resolution 3D Surface Construction Algorithm. ACM Siggraph Comput. Graph. 1987, 21, 163–169. [Google Scholar] [CrossRef]
- Hellman, O.C.; Vandenbroucke, J.A.; Rüsing, J.; Isheim, D.; Seidman, D.N. Analysis of Three-Dimensional Atom-Probe Data by the Proximity Histogram. Microsc. Microanal. 2000, 6, 437–444. [Google Scholar] [CrossRef]
- Carrillo-Rosúa, J.; Morales-Ruano, S.; Hach-Alí, P.F. Textural and chemical features of sphalerite from the Palai-Islica deposit (SE Spain): Implications for ore genesis and color. Neues Jahrb. Mineral. 2008, 185, 63–78. [Google Scholar] [CrossRef]
- Motizuki, K.; Korenari, T.; und Shirai, M. Electronic Band Structures and Magnetism of Intermetallic Cu2Sb-Type Manganese Compounds MnAlGe and MnGaGe. J. Magn. Magn. Mater. 1992, 104–107, 1923–1924. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Bartelmy, D. Webmineral Mineralogy Database. 2010. Available online: http://webmineral.com/ (accessed on 9 May 2012).
- Sauthoff, G. Intermetallics; Whiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Nuss, J.; Wedig, U.; Prill-Diemer, S.; Muehle, K.; Wang, F. Intermetallic Phases. Available online: https://www.fkf.mpg.de/86707/Intermetallic_Phases (accessed on 1 October 2018).
- Filimonova, O.N.; Trigub, F.L.; Tonkacheev, D.E.; Nickolsky, M.S.; Kvashnina, K.O.; Chareev, D.A.; Chaplygin, I.V.; Lafuerza, S.; Tagirov, D.R. Substitution Mechanisms in In-, Au-, and Cu-Bearing Sphalerites Studied by X-Ray Absorption Spectroscopy of Synthetic Compounds and Natural Minerals. Mineral. Mag. 2019, 83, 435–451. [Google Scholar] [CrossRef]
- Mueller, G. Zur Mineralogie und Kulturgeschichte des Silbers; Jahrbuch 1997 der Braunschweigischen Wissenschaftlichen Gesellschaft: Braunschweig, Germany, 1997; pp. 67–77. [Google Scholar]
- Kammer, U.; Stöbich, J.; Zeller, T.; Klesse, L.; Goldmann, D.; Poggendorf, C.; Niewisch, T. Recycling bergbaulicher Aufbereitungsrückstände zur Gewinnung wirtschaftsstrategischer Metalle am Beispiel der Tailings am Bollrich in Goslar (REWITA): Schlussbericht: Förderschwerpunkt: “r4-Wirtschaftsstrategische Rohstoffe für Hightech-Standort Deutschland”: Laufzeit des Vorhabens: 01.05.2015–31.12.2018. Application/pdf. TU Clausthal. Available online: https://tu-freiberg.de/sites/default/files/media/ressourcenprofil/wirtschaftsstrategische_rohstoffe.pdf (accessed on 3 September 2020). [CrossRef]

| Crystal | Peak (s) | Bkg1 (s) | Bkg2 (s) | DL 25kV, wt.% | Ref | |
|---|---|---|---|---|---|---|
| SKα | LPET | 20 | 10 | 10 | 0.02 | ZnS |
| FeLl * | LTAP | 60 | 10 | 10 | 0.49 | FeS2 |
| CuLα | TAP | 180 | 90 | 90 | 0.01 | CuFeS2 |
| ZnLα | TAP | 60 | 10 | 10 | 0.03 | ZnS |
| CdLα | LPET | 30 | 15 | 15 | 0.01 | CdS |
| InLα | PET/LPET/LPET | 240/204/204 | OVL CdLβ | 120/102/102 | 0.004 | InSb |
| SbLα | PET/LPET/LPET | 240/204/204 | 120/102/102 | 120/102/102 | 0.004 | InSb |
| Ion | Coordination | Charge | Crystal Radius (Å) | % Diff (Zn) |
|---|---|---|---|---|
| Zn | IV | 2 | 0.74 | |
| Cu | IV | 1 | 0.74 | 0 |
| In | IV | 3 | 0.76 | -3 |
| Sb | IVPY | 3 | 0.9 | -22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirmer, T.; Ließmann, W.; Macauley, C.; Felfer, P. Indium and Antimony Distribution in a Sphalerite from the “Burgstaetter Gangzug” of the Upper Harz Mountains Pb-Zn Mineralization. Minerals 2020, 10, 791. https://doi.org/10.3390/min10090791
Schirmer T, Ließmann W, Macauley C, Felfer P. Indium and Antimony Distribution in a Sphalerite from the “Burgstaetter Gangzug” of the Upper Harz Mountains Pb-Zn Mineralization. Minerals. 2020; 10(9):791. https://doi.org/10.3390/min10090791
Chicago/Turabian StyleSchirmer, Thomas, Wilfried Ließmann, Chandra Macauley, and Peter Felfer. 2020. "Indium and Antimony Distribution in a Sphalerite from the “Burgstaetter Gangzug” of the Upper Harz Mountains Pb-Zn Mineralization" Minerals 10, no. 9: 791. https://doi.org/10.3390/min10090791
APA StyleSchirmer, T., Ließmann, W., Macauley, C., & Felfer, P. (2020). Indium and Antimony Distribution in a Sphalerite from the “Burgstaetter Gangzug” of the Upper Harz Mountains Pb-Zn Mineralization. Minerals, 10(9), 791. https://doi.org/10.3390/min10090791




