Worth a Closer Look: Raman Spectra of Lead-Pipe Scale
Abstract
1. Introduction
2. Methods
2.1. Instrumentation
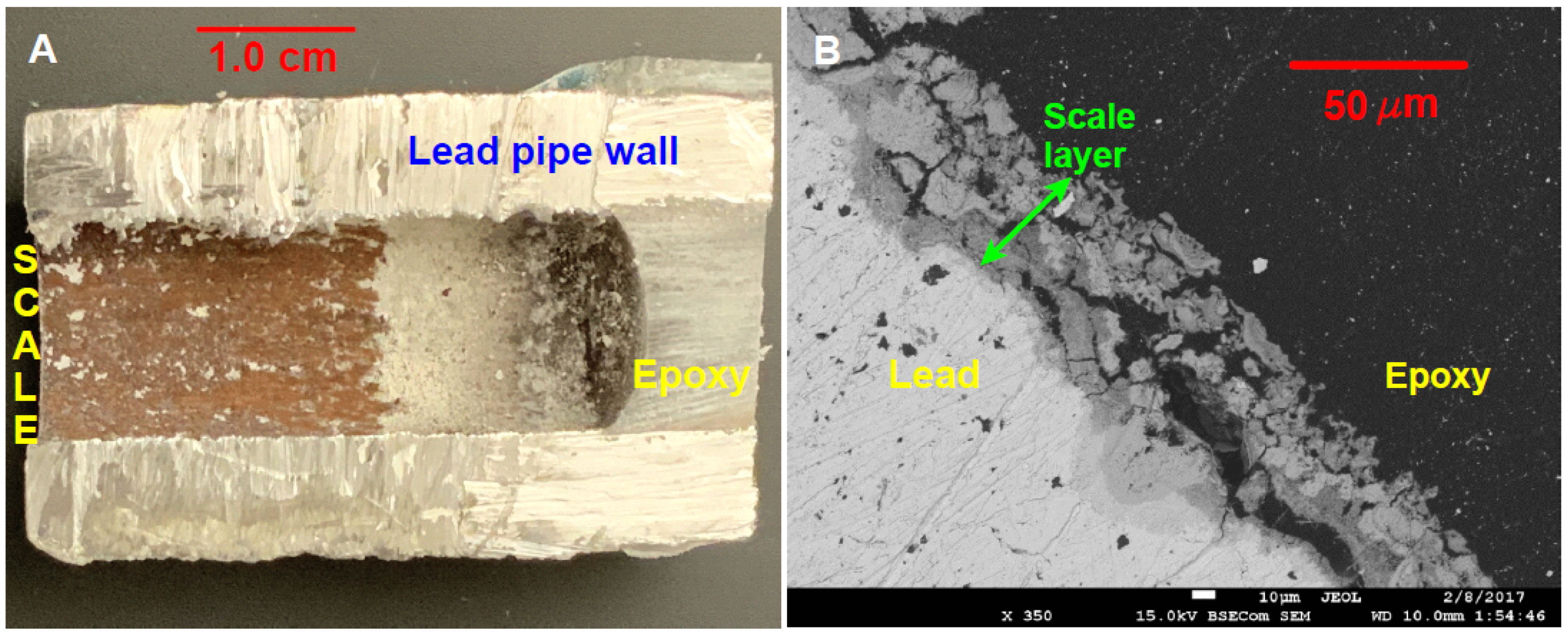
2.2. Samples
2.3. Preparation of Samples for Raman Analysis
3. Results and Discussion
3.1. Why Consider Using Raman Spectroscopy?
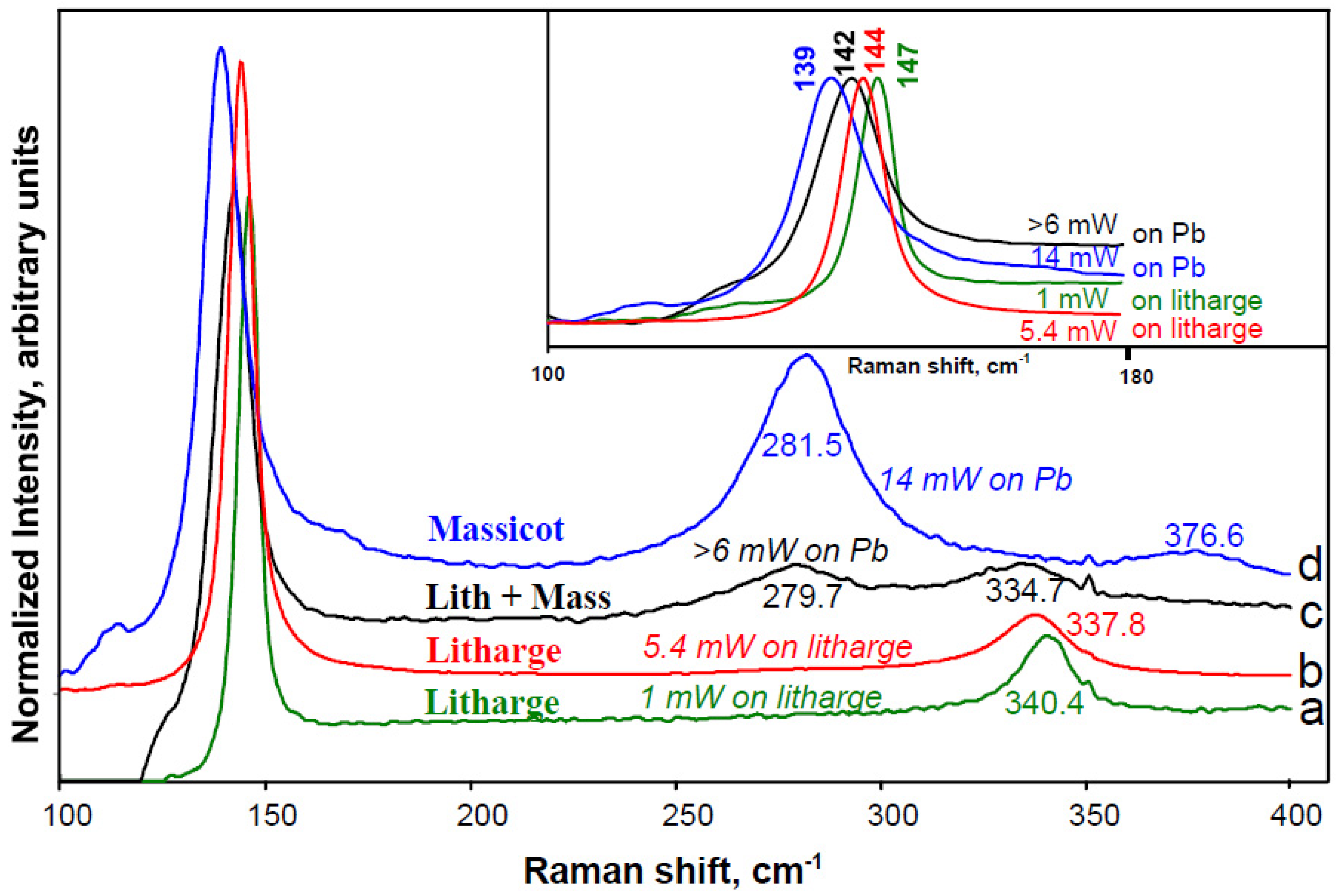
3.2. Detecting and Dealing with the Effects of Laser Heating
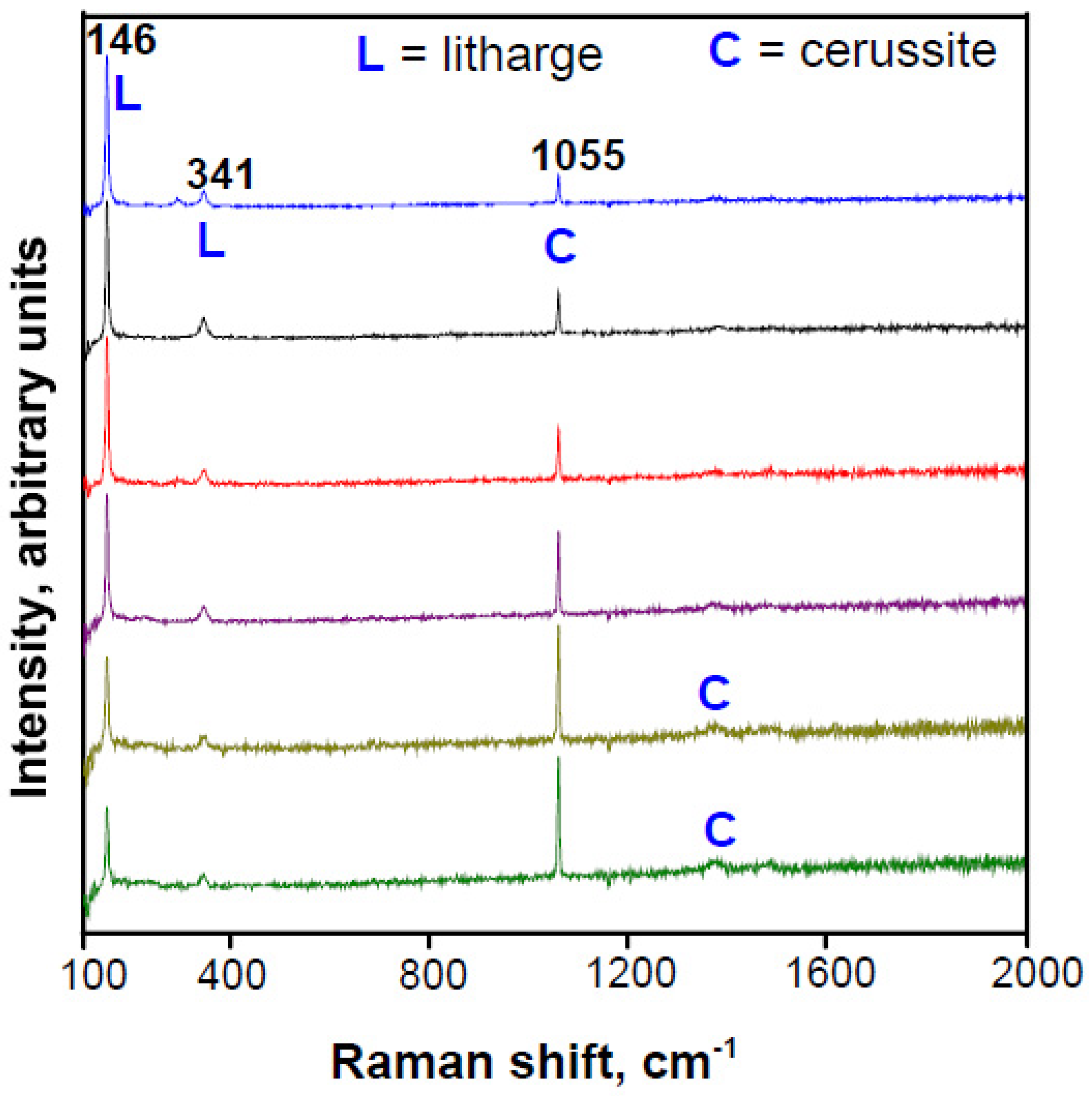
3.3. Raman Spectroscopy (RS) as a Compositional–Structural Probe
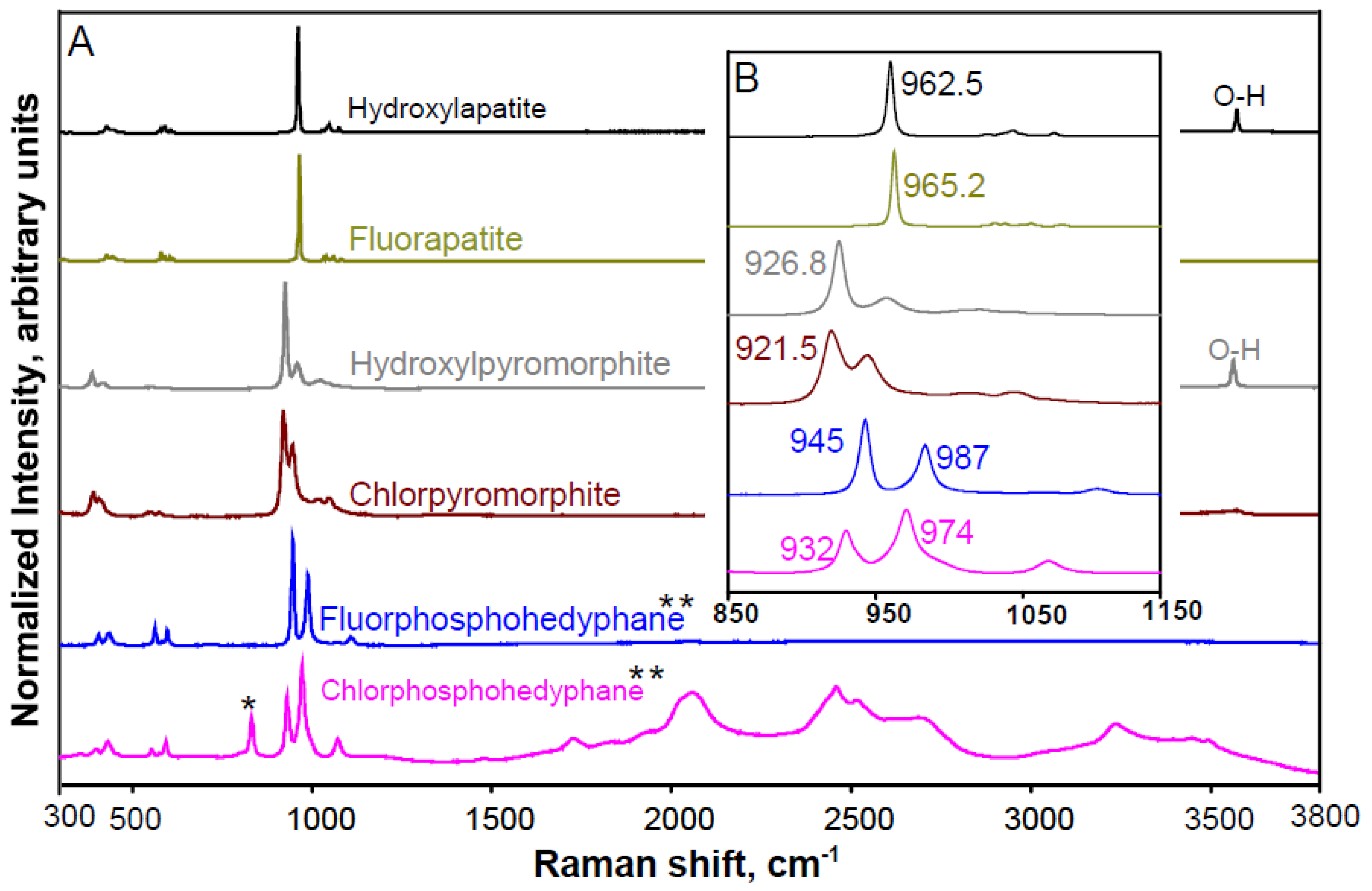
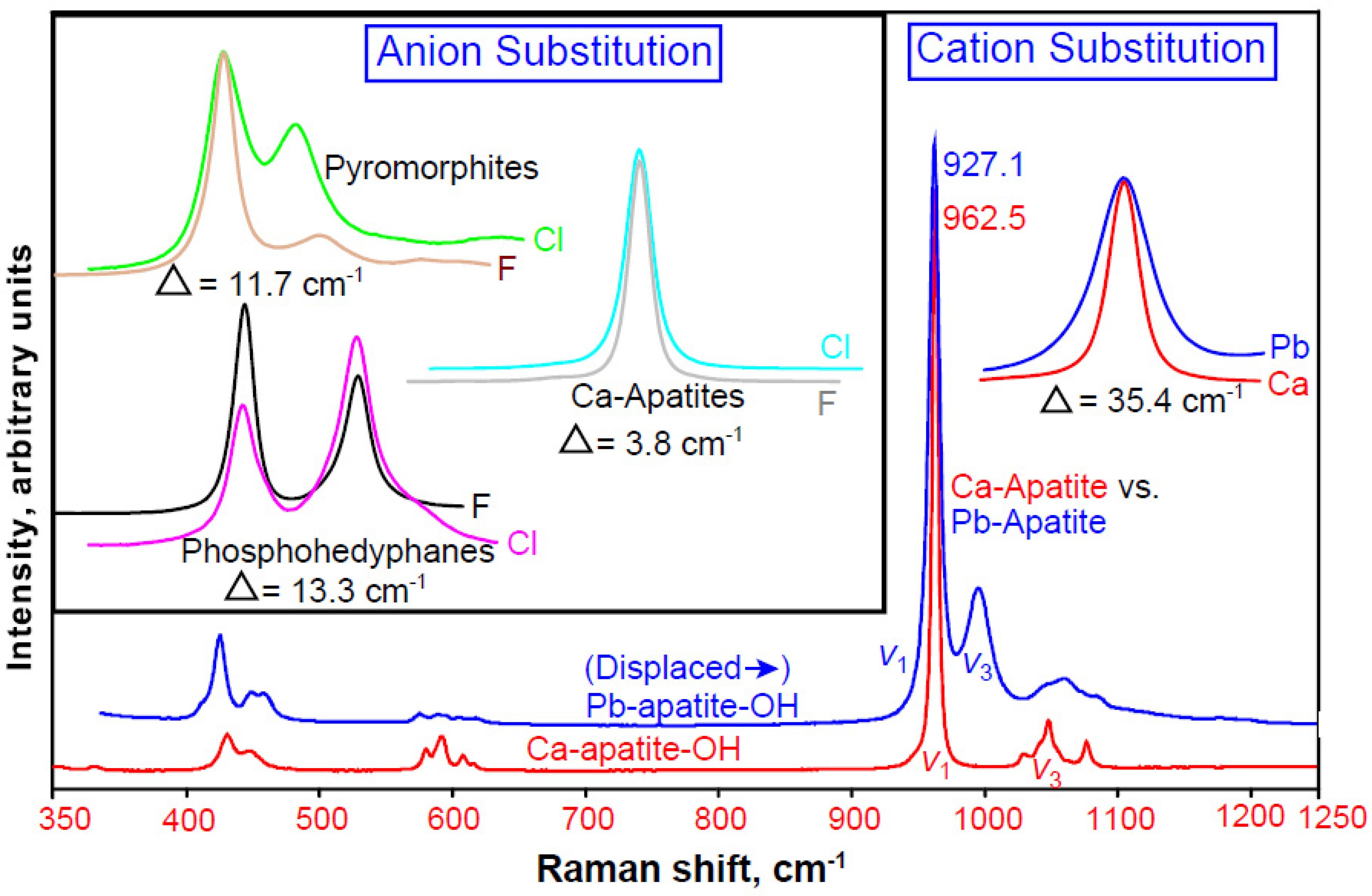
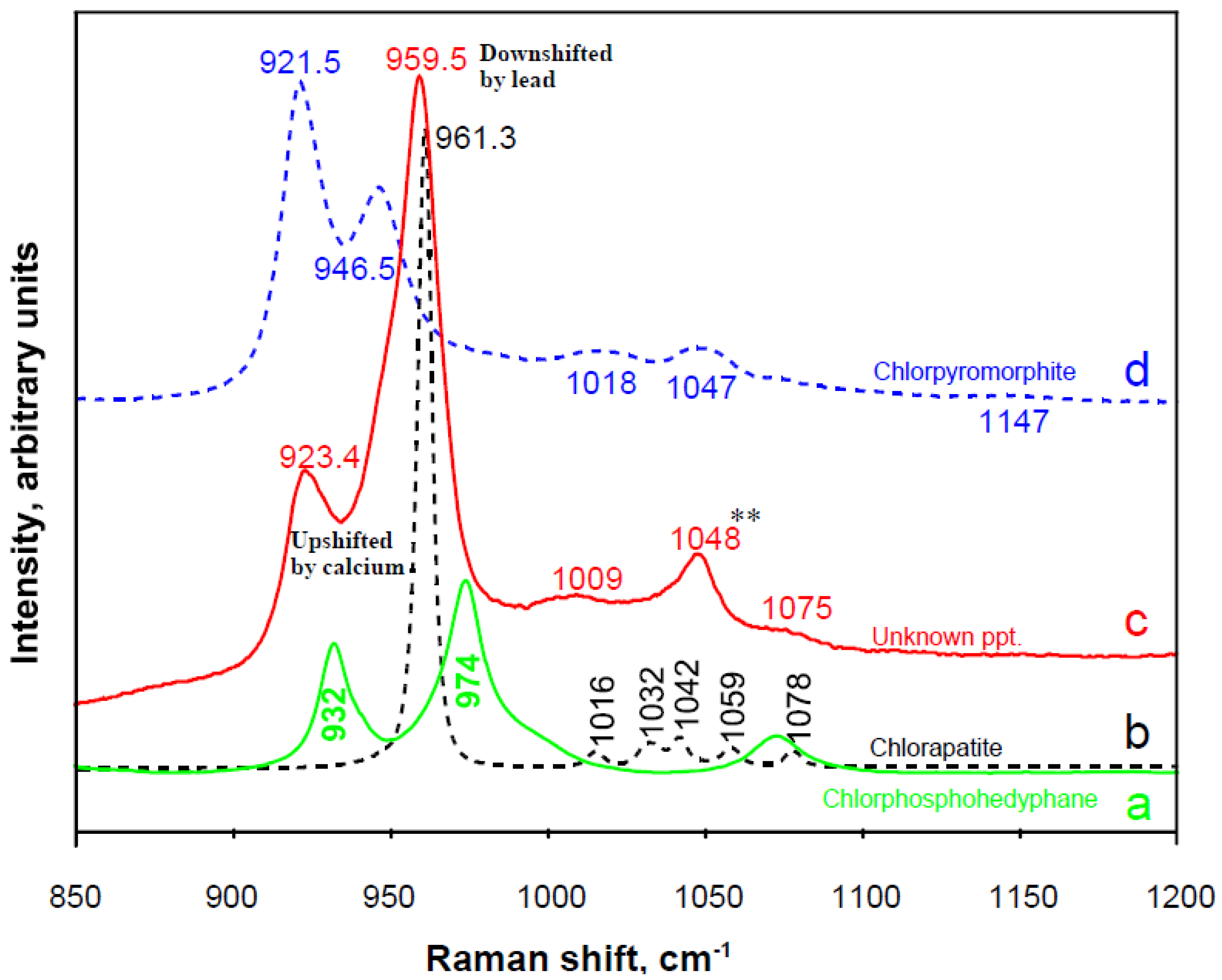
3.3.1. Phosphate Phases
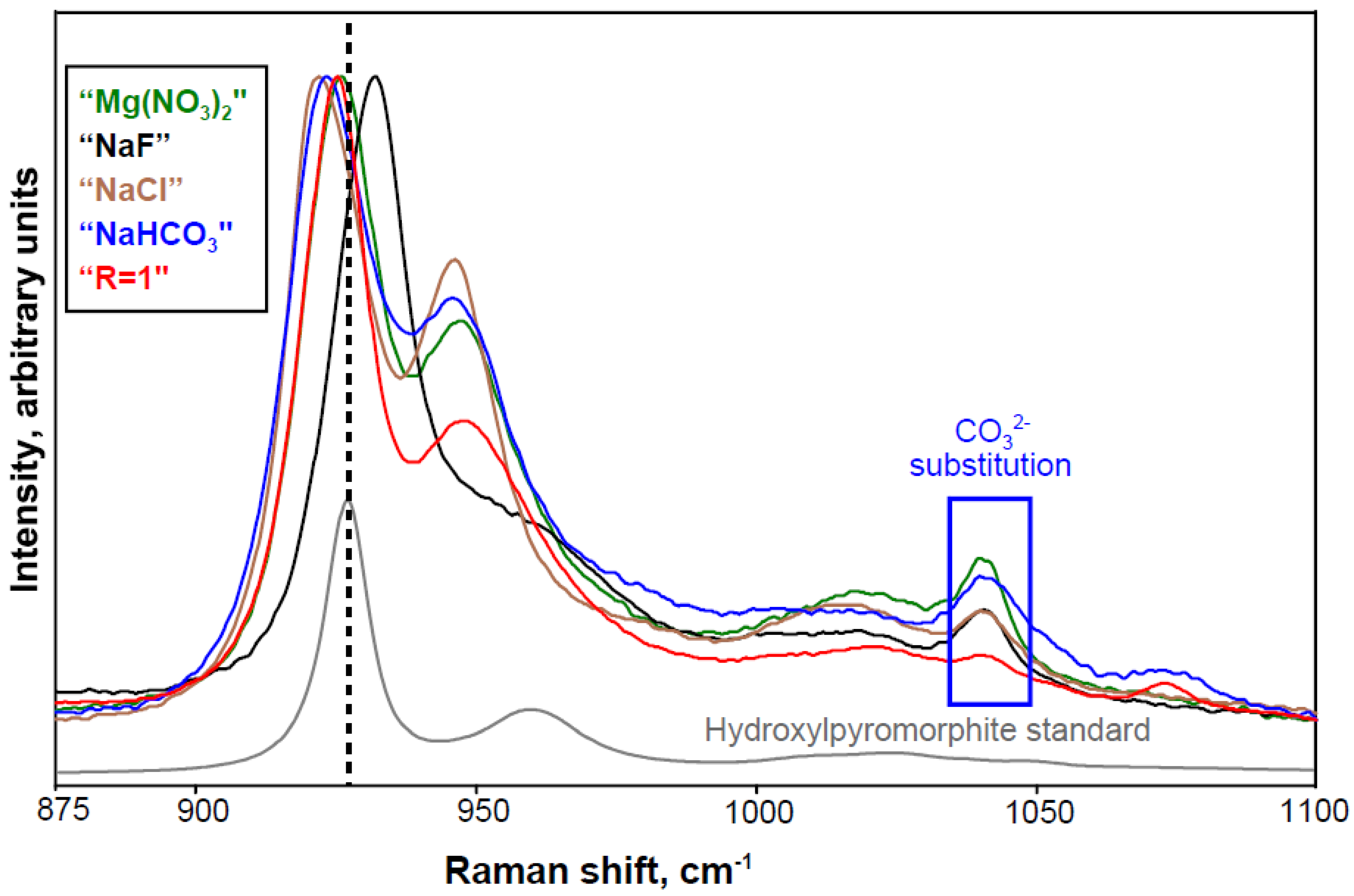
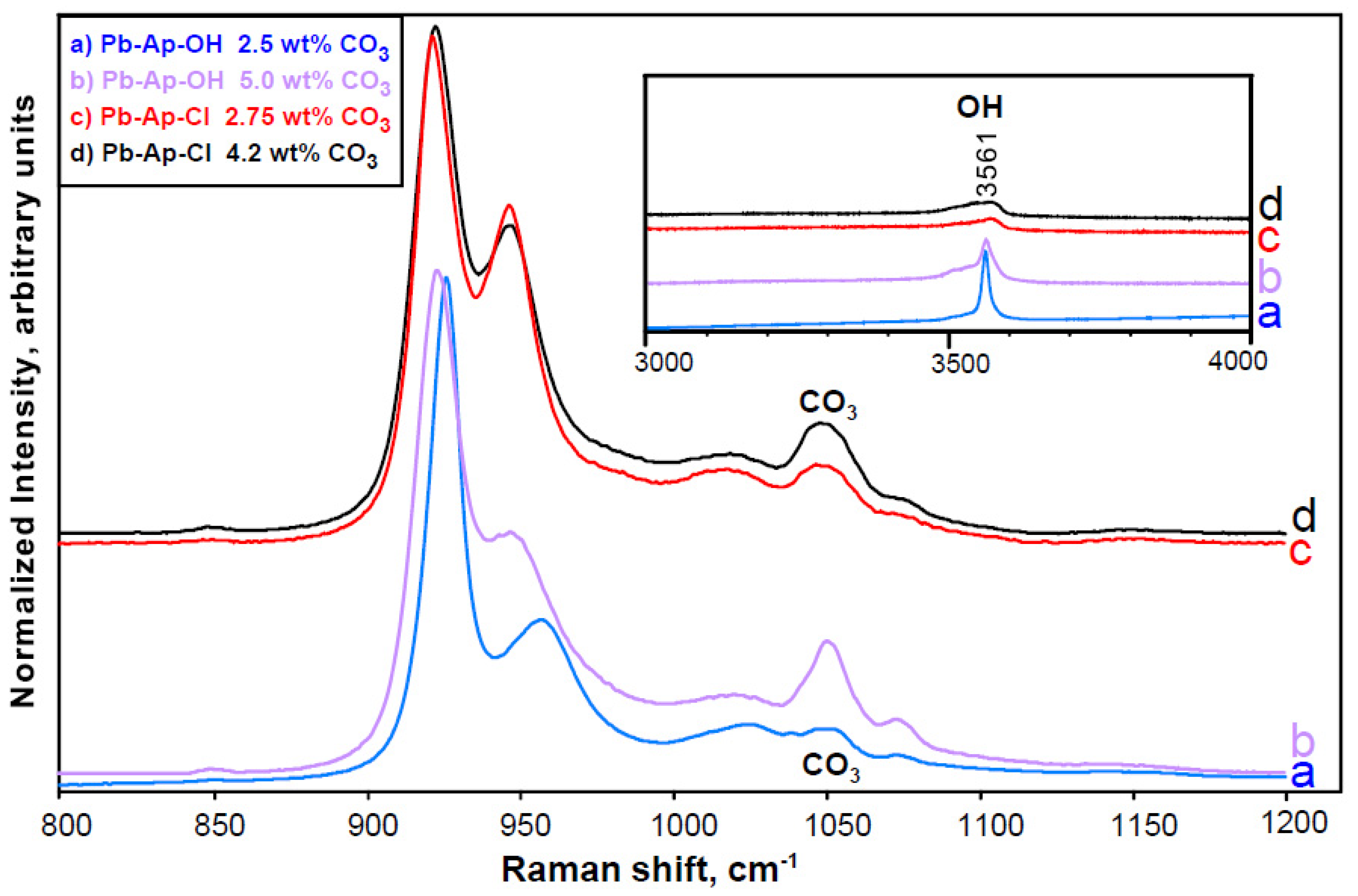
3.3.2. Other Polyatomic Ions
3.4. Detecting More Than Meets the Eye
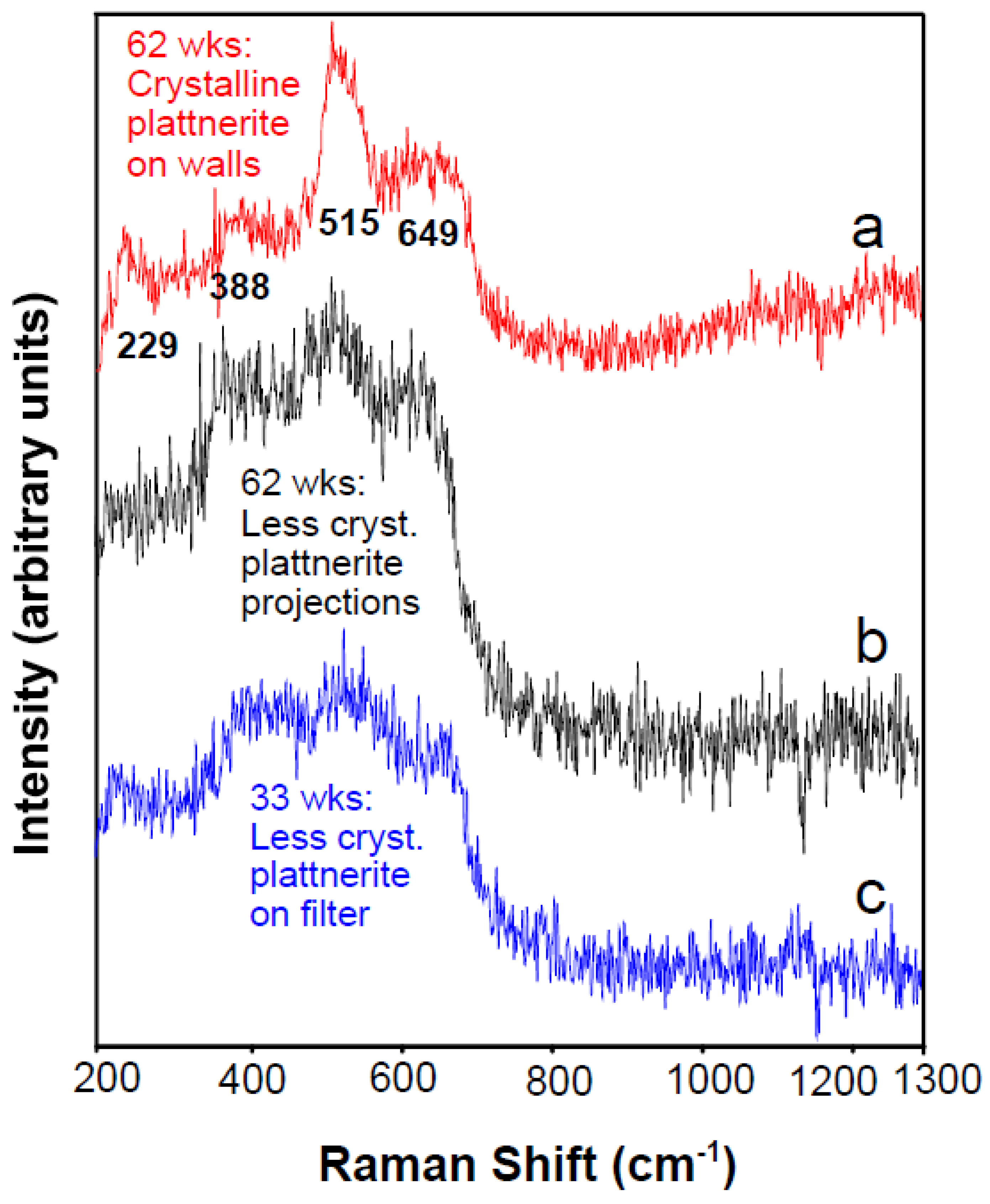
3.5. Assessing the Most Recent Precipitate; Dealing with Amorphous Material
4. Conclusions and Future Raman Application to Lead-Pipe Scales
4.1. Conclusions
4.2. Moving Forward with Raman Spectroscopy on Lead Issues
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabin, R. The lead industry and lead water pipes: “A modest campaign”. Am. J. Public Health 2008, 98, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Schock, M.R. Understanding corrosion control strategies for lead. J. AWWA 1989, 81, 88–100. [Google Scholar] [CrossRef]
- Santucci, R.J.; Scully, J.R. The pervasive threat of lead (Pb) in drinking water: Unmasking and pursuing scientific factors that govern lead release. Proc. Natl. Acad. Sci. USA 2020, 117, 23211–23218. [Google Scholar] [CrossRef]
- Zhao, J.; Giammar, D.E.; Pasteris, J.D.; Dai, C.; Bae, Y.; Hu, Y. Formation and aggregation of lead phosphate particles: Implications for lead immobilization in water supply systems. Environ. Sci. Technol. 2018, 52, 12612–12623. [Google Scholar] [CrossRef] [PubMed]
- Renner, R. Plumbing the depths of D.C.’s drinking water crisis. Environ. Sci. Technol. 2004, 38, 224A–227A. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olson, T.M.; Wax, M.; Yonts, J.; Heidecorn, K.; Haig, S.-J.; Yeoman, D.; Hayes, Z.; Raskin, L.; Ellis, B.R. Forensic estimates of lead release from lead service lines during the water crisis in Flint, Michigan. Environ. Sci. Technol. Lett. 2017, 4, 356–361. [Google Scholar] [CrossRef]
- Pieper, K.J.; Tang, M.; Edwards, M.A. Flint water crisis caused by interrupted corrosion control: Investigating “ground zero” home. Environ. Sci. Technol. 2017, 51, 2007–2014. [Google Scholar] [CrossRef]
- Pieper, K.J.; Martin, R.; Tang, M.; Walters, L.A.; Parks, J.; Roy, S.; Devine, C.; Edwards, M.A. Evaluating water lead levels during the Flint water crisis. Environ. Sci. Technol. 2018, 52, 8124–8132. [Google Scholar] [CrossRef]
- Lytle, D.A.; Schock, M.R.; Formal, C.; Bennett-Stamper, C.; Harmon, S.; Nadagouda, M.N.; Williams, D.; DeSantis, M.K.; Tully, J.; Pham, M. Lead particle size fractionation and identification in Newark, New Jersey’s drinking water. Environ. Sci. Technol. 2020, 54, 13672–13679. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; White, C.; Lytle, D. Lead pipe scale analysis using broad-beam argon ion milling to elucidate drinking water corrosion. Microsc. Microanal. 2011, 17, 284–291. [Google Scholar] [CrossRef]
- Tully, J.; DeSantis, M.K.; Schock, M.R. Water quality--pipe deposit relationships in Midwestern lead pipes. AWWA Water Sci. 2019, 2019, e1127. [Google Scholar] [CrossRef]
- DeSantis, M.K.; Schock, M.R.; Tully, J.; Bennett-Stamper, C. Orthophosphate interactions with destabilized PbO2 scales. Environ. Sci. Technol. 2020, 54, 14302–14311. [Google Scholar] [CrossRef] [PubMed]
- Gerke, T.L.; Scheckel, K.G.; Schock, M.R. Identification and distribution of vanadinite (Pb5(V5+O4)3Cl) in lead pipe corrosion by-products. Environ. Sci. Technol. 2009, 43, 4412–4418. [Google Scholar] [CrossRef] [PubMed]
- Avasarala, S.; Orta, J.; Schaefer, M.; Abernathy, M.; Ying, S.; Liu, H. Effects of residual disinfectants on the redox speciation of lead(II)/(IV) minerals in drinking water distribution systems. Environ. Sci. Water Res. 2021, 7, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.J.; Davidson, C.M.; Britton, A.; Robertson, S.J. The nature of corrosion products in lead pipes used to supply drinking water to the city of Glasgow, Scotland, UK. Fresen. J. Anal. Chem. 1999, 363, 562–565. [Google Scholar] [CrossRef]
- Kim, E.J.; Herrera, J.E. Characteristics of lead corrosion scales formed during drinking water distribution and their potential influence on the release of lead and other contaminants. Environ. Sci. Technol. 2010, 44, 6054–6061. [Google Scholar] [CrossRef]
- Hopwood, J.D.; Derrick, G.R.; Brown, D.R.; Newman, C.D.; Haley, J.; Kershaw, R.; Collinge, M. The identification and synthesis of lead apatite minerals formed in lead water pipes. J. Chem. 2016, 2016, 9074062. [Google Scholar] [CrossRef]
- Grimes, S.M.; Johnston, S.R.; Batchelder, D.N. Lead carbonate-phosphate system: Solid-dilute solution exchange reactions in aqueous systems. Analyst 1995, 120, 2741–2746. [Google Scholar] [CrossRef]
- Guo, D.; Robinson, C.; Herrera, J.E. Mechanism of dissolution of minium (Pb3O4) in water under depleting chlorine conditions. Corros. Sci. 2016, 103, 42–49. [Google Scholar] [CrossRef]
- Dai, C.; Zhao, J.; Giammar, D.E.; Pasteris, J.D.; Zuo, X.; Hu, Y. Heterogeneous lead phosphate nucleation at organic-water interfaces: Implications for lead immobilization. ACS Earth Space Chem. 2018, 2, 869–877. [Google Scholar] [CrossRef]
- Bae, Y.; Pasteris, J.D.; Giammar, D.E. The ability of phosphate to prevent lead release from pipe scale when switching from free chlorine to monochloramine. Environ. Sci. Technol. 2020, 54, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Pasteris, J.D.; Giammar, D.E. Impact of orthophosphate on lead release from pipe scale in high pH, low alkalinity water. Water Res. 2020, 177, 115764. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Pasteris, J.D.; Giammar, D.E. Impact of iron-rich scale in service lines on lead release to water. AWWA Water Sci. 2020, 2020, e1188. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Li, W.; Wang, Z.; Giammar, D.E. Formation of lead (IV) oxides from lead (II) compounds. Environ. Sci. Technol. 2010, 44, 8950–8956. [Google Scholar] [CrossRef]
- Li, X.; Azimzadeh, B.; Martinez, C.E.; McBride, M.B. Pb mineral precipitation in solutions of sulfate, carbonate and phosphate: Measured and modeled Pb solubility and Pb2+ activity. Minerals 2021, 11, 620. [Google Scholar] [CrossRef]
- Nasdala, L.; Smith, D.C.; Kaindl, R.; Ziemann, M.A. Raman spectroscopy: Analytical perspectives in mineralogical research. In Spectroscopic Methods in Mineralogy; Beran, A., Libowitziky, E., Eds.; Eotvos University Press: Budapest, Hungary, 2004; pp. 281–343. [Google Scholar]
- Nasdala, L.; Beyssac, O.; Schopf, J.W.; Bleisteiner, B. Application of Raman-based images in the Earth sciences. In Raman Imaging; Zoubir, A., Ed.; Springer Nature: Cham, Switzerland, 2012; pp. 145–187. [Google Scholar]
- Fritsch, E.; Rondeau, B.; Hainschwang, T.; Karampelas, S. Raman spectroscopy applied to gemology. In Applications of Raman Spectroscopy to Earth Sciences and Cultural Heritage; Dubessy, J., Caumon, M.-C., Rull, F., Eds.; European Mineralogical Society of Great Britain and Ireland: Cambridge, UK, 2012; pp. 455–489. [Google Scholar]
- Chou, I.-M.; Wang, A. Application of laser Raman micro-analyses to Earth and planetary materials. J. Asian Earth Sci. 2017, 145, 309–333. [Google Scholar] [CrossRef]
- Gazquez, F.; Rull, F.; Sanz-Arranz, A.; Medina, J.; Calaforra, J.M.; de las Heras, C.; Lasheras, J.A. In situ Raman characterization of minerals and degradation processes in a variety of cultural and geological heritage sites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 172, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Pasteris, J.D.; Beyssac, O. Welcome to Raman spectroscopy: Successes, challenges, and pitfalls. Elements 2020, 16, 87–92. [Google Scholar] [CrossRef]
- Simard, S.; Odziemkowski, M.; Irish, D.E.; Brossard, L.; Menard, H. In situ micro-Raman spectroscopy to investigate pitting corrosion product of 1024 mild steel in phosphate and bicarbonate solutions containing chloride and sulfate ions. J. Appl. Electrochem. 2001, 31, 913–920. [Google Scholar] [CrossRef]
- Noel, J.D.; Nading, T.M.; Pasteris, J.D.; Shah, V.; Suresh, A.K.; Giammar, D.E. The influence of water chemistry on lead release rates of lead (II) carbonate solids found in water distribution systems. In Proceedings of the 2009 Water Quality Technology Conference (WQTC), Seattle, WA, USA, 15 November 2009; American Water Works Association: Denver, CO, USA, 2009. [Google Scholar]
- Pasteris, J.D.; Ding, D.Y. Experimental fluoridation of nanocrystalline apatite. Am. Mineral. 2009, 94, 53–63. [Google Scholar] [CrossRef]
- Beyssac, O. New trends in Raman spectroscopy: From high-resolution geochemistry to planetary exploration. Elements 2020, 16, 117–122. [Google Scholar] [CrossRef]
- Tarr, J. Visual Characterization, Raman Spectroscopy, and Electron Probe Microanalysis of Lead Pipe Scale from Providence, Rhode Island. Bachelor’s Thesis, Washington University in St. Louis, Saint Louis, MO, USA, 2019. [Google Scholar]
- Rakovan, J.; Waychunas, G. Luminescence in minerals. Mineral. Rec. 1996, 27, 7–19. [Google Scholar]
- Burgio, L.; Clark, R.J.H.; Firth, S. Raman spectroscopy as a means for the identification of plattnerite (PbO2), of lead pigments and of their degradation products. Analyst 2001, 126, 222–227. [Google Scholar] [CrossRef]
- Marucci, G.; Beeby, A.; Parker, A.W.; Nicholson, C.E. Raman spectroscopic library of medieval pigments collected with five different wavelengths for investigation of illuminated manuscripts. Anal. Methods UK 2018, 10, 1219–1236. [Google Scholar] [CrossRef]
- Everall, N.J.; Lumsdon, J.; Christopher, D.J. The effect of laser-induced heating upon the vibrational Raman-spectra of graphites and carbon-fibers. Carbon 1991, 29, 133–137. [Google Scholar] [CrossRef]
- Bryant, R.N.; Pasteris, J.D.; Fike, D.A. Variability in the Raman spectrum of unpolished growth and fracture surfaces of pyrite due to laser heating and crystal orientation. Appl. Spectrosc. 2018, 72, 37–47. [Google Scholar] [CrossRef]
- Hopwood, J.D.; Davey, R.J.; Jones, M.O.; Pritchard, R.G.; Cardew, P.T.; Booth, A. Development of chloropyromorphite coatings for lead water pipes. J. Mater. Chem. 2002, 12, 1717–1723. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, B.; Zhu, Z.; Liu, H.; Huang, Y.; Zhao, X.; Liang, M. Characterization, dissolution and solubility of the hydroxypyromorphite-hydroxyapatite solid solution [(PbxCa1-x)5(PO4)3OH] at 25 °C and pH 2–9. Geochem. Trans. 2016, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Kampf, A.R.; Steele, I.M.; Jenkins, R.A. Phosphohedyphane, Ca2Pb3(PO4)3Cl, the phosphate analog of hedyphane: Description and crystal structure. Am. Mineral. 2006, 91, 1909–1917. [Google Scholar] [CrossRef]
- Kampf, A.R.; Housley, R.M. Fluorphosphohedyphane, Ca2Pb3(PO4)3F, the first apatite supergroup mineral with essential Pb and F. Am. Mineral. 2011, 96, 423–429. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S. A Raman spectroscopic study of the phosphate mineral pyromorphite Pb5(PO4)3Cl. Polyhedron 2007, 26, 4533–4541. [Google Scholar] [CrossRef][Green Version]
- Frost, R.L.; Bouzaid, J.M.; Palmer, S. The structure of mimetite, arsenian pyromorphite and hedyphane—A Raman spectroscopic study. Polyhedron 2007, 26, 2964–2970. [Google Scholar] [CrossRef][Green Version]
- Frost, R.L.; Scholz, R.; Lopez, A.; Firmino, B.E.; Lana, C.; Xi, Y. A Raman and infrared spectroscopic characterisation of the phosphate mineral phosphohedyphane, Ca2Pb3(PO4)3Cl from the Roote mine, Nevada, USA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 237–242. [Google Scholar] [CrossRef]
- Olds, T.A.; Kampf, A.R.; Rakovan, J.F.; Burns, P.C.; Mills, O.P.; Laughlin-Yurs, C. Hydroxylpyromorphite, a mineral important to lead remediation: Modern description and characterization. Am. Mineral. 2021, 106, 922–929. [Google Scholar] [CrossRef]
- Sternlieb, M.; Pasteris, J.D.; Williams, B.R.; Krol, K.A.; Yoder, C.H. The structure and solubility of carbonated hydroxyl and chloro lead apatites. Polyhedron 2010, 29, 2364–2372. [Google Scholar] [CrossRef]
- Wilt, Z.; Fuller, C.; Bachman, T.; Weidner, V.; Pasteris, J.D.; Yoder, C.H. Synthesis and structure of carbonated barium and lead fluorapatites: Effect of cation size on A-type carbonate substitution. Am. Mineral. 2014, 99, 2176–2186. [Google Scholar] [CrossRef]
- Yoder, C.H.; Pasteris, J.D.; Worcester, K.N.; Schermerhorn, D.V. Structural water in carbonated hydroxylapatite and fluorapatite: Confirmation by solid state 2H NMR. Calcif. Tissue Int. 2012, 90, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.A.; Fox, J.L.; Young, R.A.; Wang, Z.; Hsu, J.; Higuchi, W.I.; Chhettry, A.; Zhuang, H.; Otsuka, M. Relationships among carbonated apatite solubility, crystallite size, and microstrain parameters. Calcif. Tissue Int. 1999, 64, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Deymier, A.C.; Nair, A.K.; Depalle, B.; Qin, Z.; Arcot, K.; Yoder, C.H.; Buehler, M.J.; Thomopoulos, S.; Genin, G.M.; Pasteris, J.D. Protein-free formation of bone-like apatite: New insights into the key role of carbonation. Biomaterials 2017, 127, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Stalder, M.; Rozendaal, A. Graftonite in phosphatic iron formations associated with the mid-Proterozoic Gamsberg Zn-Pb deposit, Namaqua Province, South Africa. Mineral. Mag. 2002, 66, 915–927. [Google Scholar] [CrossRef]
- Mavropoulos, E.; Rocha, N.C.C.; Soares, G.A.; Moreira, J.C.; Rossi, A.M. Effects on calcium phosphate ceramics stability. Key Eng. Mater. 2004, 254–256, 123–126. [Google Scholar] [CrossRef]
- Ondrejka, M.; Bacik, P.; Putis, M.; Uher, P.; Mikus, T.; Luptakova, J.; Ferenc, S.; Smirnov, A. Carbonate-bearing phosphohedyphane-“hydroxylphosphohedyphane” and cerussite: Supergene products of galena alteration in Permian aplite (Western Carpathians, Slovakia). Can. Mineral. 2020, 58, 347–365. [Google Scholar] [CrossRef]
- Davidson, C.M. Surface analysis and depth profiling of corrosion products formed in lead pipes used to supply low alkalinity drinking water. Water Sci. Technol. 2004, 49, 49–54. [Google Scholar] [CrossRef]
- Markl, G.; Marks, M.A.W.; Holzapfel, J.; Wenzel, T. Major, minor, and trace element composition of pyromorphite-group minerals as recorders of supergene weathering processes from the Schwarzwald mining district, SW Germany. Am. Mineral. 2014, 99, 1133–1146. [Google Scholar] [CrossRef]
- Yoder, C.H.; Havlusch, M.D.; Dudrick, R.N.; Schermerhorn, J.T.; Tran, L.K.; Deymier, A.C. The synthesis of phosphate and vanadate apatites using an aqueous one-step method. Polyhedron 2017, 127, 403–409. [Google Scholar] [CrossRef]
- Cotter-Howells, J. Lead phosphate formation in soils. Environ. Pollut. 1996, 93, 9–16. [Google Scholar] [CrossRef]
- Orta, J.E. The Effects of Changing Water Chemistry on the Stability of Lead Service Lines. Master’s Thesis, University of California at Riverside, Riverside, CA, USA, 2019. [Google Scholar]
- Wasserstrom, L.; Miller, S.A.; Triantafyllidou, S.; DeSantis, M.K.; Schock, M.R. Scale formation under blended phosphate treatment for a utility with lead pipes. J. AWWA 2017, 109, E464–E478. [Google Scholar] [CrossRef] [PubMed]
- Wopenka, B.; Pasteris, J.D. Structural characterization of kerogens to granulite-facies graphite: Applicability of Raman microprobe spectroscopy. Am. Mineral. 1993, 78, 533–557. [Google Scholar]
- Beyssac, O.; Goffe, B.; Chopin, C.; Rouzaud, J.N. Raman spectra of carbonaceous material in metasediments: A new geothermometer. J. Metamorph. Geol. 2002, 20, 859–871. [Google Scholar] [CrossRef]
- Stukelj, J.; Svanback, S.; Agopov, M.; Lobmann, K.; Strachan, C.J.; Rades, T.; Yliruusi, J. Direct measurement of amorphous solubility. Anal. Chem. 2019, 91, 7411–7417. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.N., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Schiavi, F.; Bolfan-Casanova, N.; Buso, R.; Laumonier, M.; Laporte, D.; Medjoubi, K.; Venugopal, S.; Gomez-Ulla, A.; Cluzel, N.; Hardiagon, M. Quantifying magmatic volatiles by Raman microtomography of glass inclusion-hosted bubbles. Geochem. Perspect. Lett. 2020, 16, 17–24. [Google Scholar] [CrossRef]
- Das Gupta, S.; Killenberger, M.; Tanner, T.; Rieppo, L.; Saarakkala, S.; Heikkila, J.; Anttonen, V.; Finnila, M.A.J. Mineralization of dental tissues and caries lesions detailed with Raman microspectroscopic imaging. Analyst 2021, 146, 1705–1713. [Google Scholar] [CrossRef]
- Leban, M.B.; Kosec, T. Characterization of corrosion products formed on mild steel in deoxygenated water by Raman spectroscopy and energy dispersive X-ray spectroscopy. Eng. Fail. Anal. 2017, 79, 940–950. [Google Scholar] [CrossRef]
- McCann, L.I.; Trentelman, K.; Possley, T.; Golding, B. Corrosion of ancient Chinese bronze money trees studied by Raman microscopy. J. Raman Spectrosc. 1999, 30, 121–132. [Google Scholar] [CrossRef]
- Sandvig, A.; Kwan, P.; Kirmeyer, G.; Maynard, B.; Mast, D.; Trussell, R.R.; Trussell, S.; Cantor, A.; Prescott, A. Contribution of Service Line and Plumbing Fixtures to Lead and Copper Rule Compliance Issues; Awwa Research Foundation: Denver, CO, USA, 2008. [Google Scholar]
- Lanfranco, A.M.; Schofield, P.F.; Murphy, P.J.; Hodson, M.E.; Mosselmans, J.F.W.; Valsami-Jones, E. Characterization and identification of mixed-metal phosphates in soils: The application of Raman spectroscopy. Mineral. Mag. 2003, 67, 1299–1316. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.H.; Hark, R.R. Raman microscopy and x-ray fluorescence analysis of pigments on medieval and Renaissance Italian manuscript cuttings. Proc. Natl. Acad. Sci. USA 2010, 107, 5726–5731. [Google Scholar] [CrossRef]
- Stanzani, E.; Bersani, D.; Lottici, P.P.; Colomban, P. Analysis of artist’s palette on a 16th century wood panel painting by portable and laboratory Raman instruments. Vib. Spectrosc. 2016, 85, 62–70. [Google Scholar] [CrossRef]
- Boerger, A.; Ebner, E.; Ruehl, E.; Flesch, R.; Burow, D. On the use of Raman microscopy for sulfation analysis in lead-acid battery research. J. Energy Storage 2017, 12, 305–310. [Google Scholar] [CrossRef]
- Breitenfeld, L.B.; Dyar, M.D.; Carey, C.J.; Tague, T.J.; Wang, P.; Mullen, T.; Parente, M. Predicting olivine composition using Raman spectroscopy through band shift and multivariate analyses. Am. Mineral. 2018, 103, 1827–1836. [Google Scholar] [CrossRef]
- Buseck, P.R.; Beyssac, O. From organic matter to graphite: Graphitization. Elements 2014, 10, 421–426. [Google Scholar] [CrossRef]
- Liang, E.R.; Yang, Y.; Kiefer, W. Surface-enhanced Raman spectra of fulvic and humic acids adsorbed on copper electrodes. Spectrosc. Lett. 1999, 32, 689–701. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasteris, J.D.; Bae, Y.; Giammar, D.E.; Dybing, S.N.; Yoder, C.H.; Zhao, J.; Hu, Y. Worth a Closer Look: Raman Spectra of Lead-Pipe Scale. Minerals 2021, 11, 1047. https://doi.org/10.3390/min11101047
Pasteris JD, Bae Y, Giammar DE, Dybing SN, Yoder CH, Zhao J, Hu Y. Worth a Closer Look: Raman Spectra of Lead-Pipe Scale. Minerals. 2021; 11(10):1047. https://doi.org/10.3390/min11101047
Chicago/Turabian StylePasteris, Jill Dill, Yeunook Bae, Daniel E. Giammar, Sydney N. Dybing, Claude H. Yoder, Juntao Zhao, and Yandi Hu. 2021. "Worth a Closer Look: Raman Spectra of Lead-Pipe Scale" Minerals 11, no. 10: 1047. https://doi.org/10.3390/min11101047
APA StylePasteris, J. D., Bae, Y., Giammar, D. E., Dybing, S. N., Yoder, C. H., Zhao, J., & Hu, Y. (2021). Worth a Closer Look: Raman Spectra of Lead-Pipe Scale. Minerals, 11(10), 1047. https://doi.org/10.3390/min11101047





