Abstract
Transformation of aragonite, a mineral phase metastable at Earth’s surface, to calcite widely occurs in both sedimentary and metamorphic systems with the presence of an aqueous phase. The transformation process can affect geochemical signatures of aragonite (a protolith). This study focused on quantification of the retention of Mg/Ca and Sr/Ca ratios, and δ18O during the transformation process as well as evaluation of the transformation rate. To investigate the effect of transformation from aragonite to calcite on elemental and stable isotope ratios, we conducted a series of experiments in NaCl solutions at temperatures between 120 and 184 °C. Two additional experiments at 250 °C were conducted to estimate the transformation rate of aragonite to calcite. Protolith materials consist of (1) synthetic (Mg; Sr-bearing or non-Mg; Sr bearing) needle-shaped microcrystals of aragonite (<5 µm in size) and (2) larger chips (>100 µm in size) of natural aragonite. X-ray diffraction (XRD) showed that microcrystals successfully transformed to calcite within 30 h and scanning electron microscopy (SEM) yielded a change in the crystal size to >10 µm in rhombohedral shape. Electron backscatter diffraction (EBSD) of the larger aragonite chips showed that transformation to randomly oriented calcite occurred at the rims and along the cracks while the core retained an aragonite crystal structure. Isotope-ratio mass spectrometry (IRMS) analyses showed that calcite δ18O was controlled by temperature and δ18O of the solution. The obtained calibration curve of isotope fractionation factor versus temperature is consistent with other studies. Inductively coupled plasma optical emission spectroscopy (ICP-OES) analyses showed that calcite partially or completely retained Mg/Ca and Sr/Ca ratios through the transformation.
1. Introduction
Aragonite and calcite are widespread calcium carbonate (CaCO3) polymorphic minerals that transform their crystalline structure but retain the same composition under different conditions within the Earth’s crust. Calcite is thermodynamically stable at lower pressures and higher temperatures compared to aragonite [1,2]. The transformation of aragonite, which is metastable at standard conditions (25 °C, 1 bar), to calcite can be accelerated by heating at temperatures that are common for the diagenesis and metamorphism of limestone [2]. As marine carbonates are widely used in the reconstruction of the paleoenvironment, the effect of metamorphism on trace element and isotopic signatures in the transformation product (e.g., calcite) needs to be evaluated. It has been reported that the transformation process causes textural changes, as micritic limestone experienced inversion from acicular crystals, probably aragonite, to equant crystals of calcite [3]. The mechanism of the transformation between polymorphs was proposed to be dissolution coupled with precipitation [4,5], which helps to explain the observed textural changes. Alternatively, the transformation of large aragonite chips (>100 µm in size) showed aragonite partially replaced by calcite in hydrothermal experiments [5]. No systematic orientational relationship between the calcite and the aragonite crystallographic directions was identified, and calcite nucleated in apparently random crystallographic orientations [5].
The stable isotopic composition of oxygen in carbonate minerals (usually expressed as δ18O) is widely used when studying paleoclimate [6,7,8,9]. It is also used in mineral deposits (magmatic, hydrothermal, and metamorphic) to evaluate the source of the mineralizing fluid [10,11,12]. The strong effect of temperature on the fractionation of oxygen isotopes allowed for the development of δ18O-T °C calibrations using isotope data of inorganically precipitated calcite as well as of various marine calcifying organisms of both calcitic and aragonitic mineralogy. Foraminifera shells are the most widely used carbonate materials in the reconstruction of paleotemperature [9,13,14]. Magnesium content (expressed as millimole of Mg per mole of Ca) in calcifying organisms is sensitive to temperature [14,15,16], but is not as useful in aragonitic organisms [17]. Ref. [18] suggested that the skeletal Sr/Ca ratio be used as a proxy record of seawater temperature and salinity. The Sr/Ca ratio is used in coral palaeothermometry [19,20,21].
The growth of amorphous calcium carbonate (ACC) as a precursor for crystalline forms of CaCO3 was reported by a number of experimental studies conducted at room temperature [22,23,24]. They observed that ACC was the first phase precipitated before being transformed to calcite. The Mg/Ca ratio of ACC was partially retained during transformation to calcite and strongly affected the stability of ACC. For example, the Mg/Ca ratio of ACC increased when the Mg/Ca ratio of the solution increased. Whereas the experiments with Mg/Ca = 2 yielded ACC containing 8 mol% Mg, which was stable for under 30 min, and ACC containing 24 mol% Mg was stable for up to 14 h in the experiments with Mg/Ca = 10 [25]. It was shown that trace elements play an important role in the stabilization of an amorphous precursor phase prior to complete transformation into calcite. The mechanisms of formation of Mg-calcite were also investigated. In the work, experiments were performed at 25 °C and a pH of 8.3, and the data showed an ACC formed in the aqueous solutions with a high Mg/Ca ratio. Two mechanisms of Mg-calcite formation were revealed: (i) At initial aqueous Mg/Ca ≤ 1:6, Mg-calcite forms directly from the solution, and (ii) at higher initial aqueous Mg/Ca = 1:4 and 1:5, Mg-calcite forms via an intermediate Mg-rich ACC phase [26]. The effect of Mg2+ on Sr2+ retention during ACC transformation to calcite was observed by [27]. It was suggested that Mg2+ ions stabilize the ACC phase, which promotes rapid nucleation-mediated growth of calcite and causes Sr2+ sorption and incorporation [27].
This study investigates the retention of δ18O, and Sr/Ca and Mg/Ca ratios of aragonite during its transformation into calcite under the laboratory conditions. The transformation occurred in an aqueous solution under a sustained saturated water vapor pressure maintained at 120–250 °C, which is the lower temperature limit for metamorphism.
2. Materials and Methods
The crystallization method was based on the transformation of metastable aragonite to calcite under hydrothermal conditions at a range of temperatures. There are three types of aragonites used in this study, including Mg-Sr bearing and non-Mg-Sr bearing (needle-shaped crystals radiating from the nucleation center, size about 5 µm), and natural aragonite chips (size about 0.1–1 mm, originated from Sefrou, Morocco).
2.1. Precipitation of Aragonite
The initial material for the aragonite–calcite transformation experiments was prepared by mixing Ca- and carbonate-bearing solutions via two methods to obtain Mg Sr aragonite and non-Mg Sr aragonite. The first method yielded aragonite doped with Mg and Sr. The aragonite was precipitated from an artificial seawater solution that was prepared by mixing NaCl, MgCl2⋅6H2O, CaCl2⋅2H2O, and SrCl2⋅6H2O salts (Fisher Scientific, Hampton, NH, USA, A.C.S.) in 1 L of deionized (DI) water. The resulting solution (solution-1) consisted of 0.49 mol/kg NaCl, 0.05 mol/kg MgCl2, 0.01 mol/kg CaCl2, and 0.0001 mol/kg SrCl2. To promote aragonite crystallization, 400 mL of 0.01 mol/kg Na2CO3 solution was slowly added to 1000 mL of solution-1 at an injection rate of 5 mL/min and at room temperature. In the second method, precipitation of Mg-free aragonite was based on mixing 0.1 mol/kg Na2CO3 and 0.86 mol/kg Ca(NO3)2 (ACROS Organics, Geel, Belgium, A.C.S.) solutions at 57 °C. Five hundred ml of Na2CO3 solution (growth media) was placed in the constant temperature batch with a submersed magnetic stirrer. Fifty mL of Ca(NO3)2 solution was added into continuously stirred growth media at a rate of 5 mL/min and a constant temperature at 57 °C. In both methods, crystalline powder was collected, rinsed with deionized water, and dried at room temperature.
2.2. Crystallization Experiments
Aragonite (0.1 g) was placed on the bottom of a 100 mL Teflon (polytetrafluoroethylene, PTFE)-lined autoclave (Figure 1a,c), then 40 mL of the 0.49 mol/kg NaCl solution was poured into the autoclave, followed by immediate closure of the autoclave. Two other experiments were conducted in titanium autoclaves (40 mL in volume) with a Teflon lining at 250 °C. In these two experiments, 10 mL of a solution of 0.48 mol/kg NaHCO3 and 0.51 mol/kg Na2SO4 or 0.01 mol/kg Na2SO4 (Table 1) was added to each 40 mL titanium autoclave. Quartz tubes (5 mm ID, Figure 1b) containing aragonite were immersed in the brine. The pressure in the autoclave was maintained as saturated water vapor. After the furnace was set to 250, 184, 180, 162, 150, or 120 °C, autoclaves were placed inside and run for different durations (Table 1). Following pre-determined time intervals of reaction, the autoclaves were quenched by 20–30 min of cool airflow. Following completion of the incubations, fluids and crystals were extracted from autoclaves to conduct mineralogical, morphological, chemical, and stable isotopic analyses.
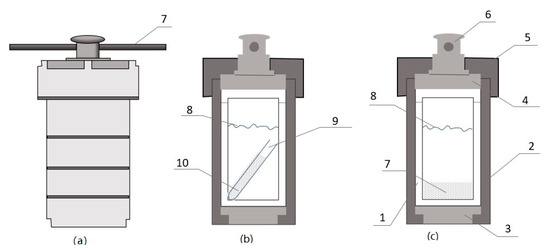
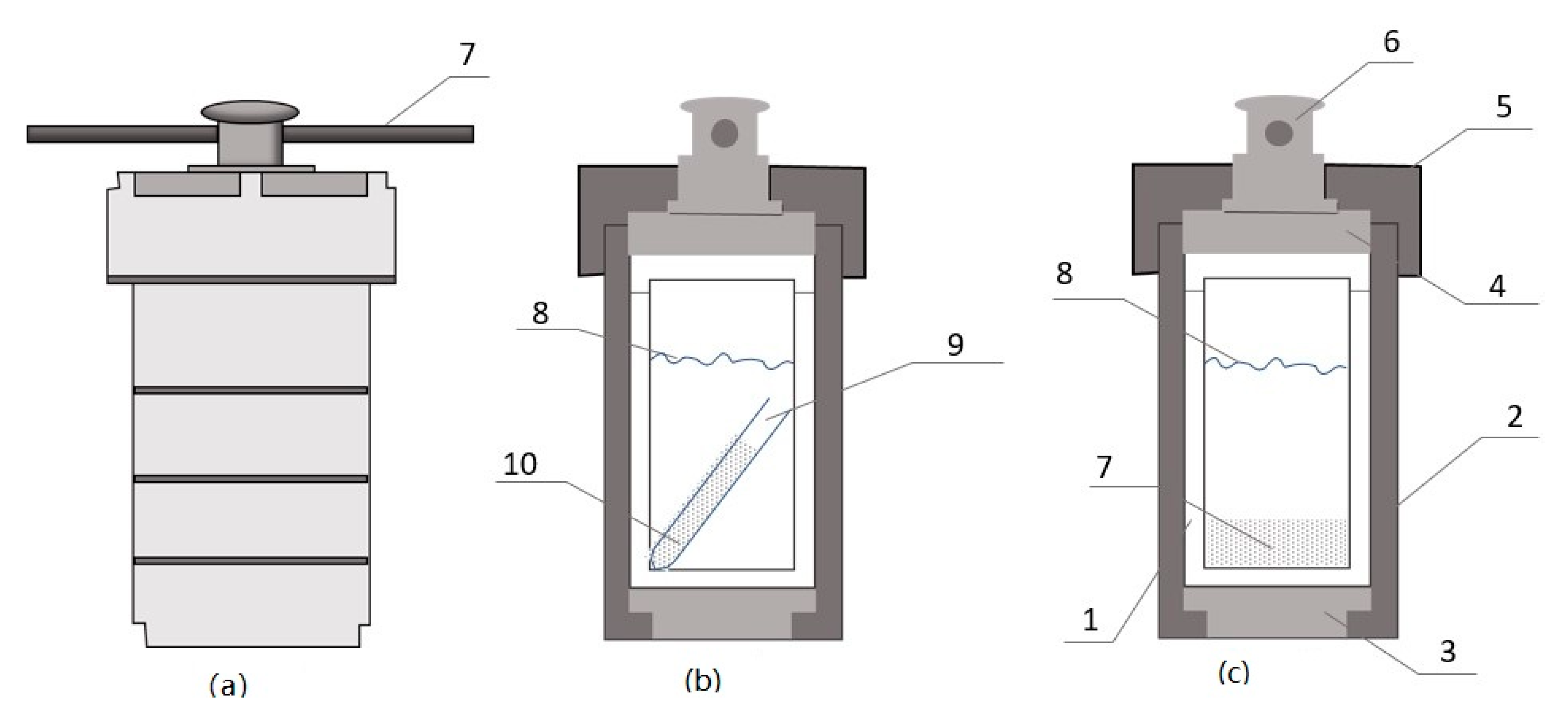
Figure 1.
Teflon-lined autoclave. (a) Stainless steel body, (b) experimental design 1, (c) experimental design 2. The structure of the autoclave contains the following: (1) Teflon chamber, (2) sleeve body, (3) bottom disc, (4) rupture disc, (5) Lid, (6) locking screw, (7) synthetic aragonite powder placed at the bottom of the Teflon chamber, (8) brine level, (9) Teflon tube, and (10) aragonite powder placed in the Teflon tube. All experiments that were conducted at 120°, 150°, 162°, 180°, 184 °C used design 1c. 52 *# at 250 °C used design (c) and 03 & at 250 °C used design (b).

Table 1.
Experimental conditions.
2.3. Mineralogical Analysis with X-ray Diffraction Method (XRD), Electron Backscatter Diffraction (EBSD), and Scanning Electron Microscopy (SEM)
The solid experimental products were examined with XRD to confirm the complete transformation of aragonite to calcite. XRD was conducted on a Rigaku Ultima III X-ray Diffraction System at the Institute for Imaging & Analytical Technologies (I2AT), Mississippi State University (MSU), Starkville, MS, USA. The machine was operated at 40 kV and 44 mA. Each sample was scanned at a scanning speed of 1°/min. The morphology of the initial and final products was verified by SEM at MSU. The samples were coated with 30 nm platinum before being placed in a JEOL JSM-6500F FE-SEM for SEM analysis. The instrument was operated at a voltage of 5 KV, with a desired probe current between 6 and 18 and a working distance of 17.4 mm. Selected samples were mounted in epoxy. The sample was left overnight for solidification, then polished wet with silicon carbide (SiC, Buehler) with abrasive SiC papers of 600, 800, and 1200 grit designation followed by polishing with 1 μm alumina powder on the cloth. Large calcite and aragonite grains were examined with EBSD in a JEOL 7000 SEM instrument housed at Alabama Analytical Research Center (AARC) of the University of Alabama, following previously reported analytical conditions [28].
2.4. Elemental and Isotope Analyses
Crystalline powders and fluid samples were analyzed by an Avio 200 ICP-OES (Perkin Elmer, Shelton, CT, USA) in the Department of Plant and Soil Sciences at MSU. Standard solutions of 1000 ppm each of Mg, Sr, and Ca were used to make instrument calibrant solutions of 0.1, 1, 10, and 100 ppm. Crystalline samples were prepared by dissolving about 10 mg of powder (Table A1) in 10 mL of 3% trace metal-grade nitric acid to make a homogeneous mixture.
In addition to elemental analyses, δ18O values were determined in selected calcite and fluid samples. Analyses were conducted using an automated carbonate preparation device (KIEL-III) combined with the isotope-ratio mass spectrometer (IRMS) Finnigan MAT 252 in the Environmental Isotope Laboratory at the University of Arizona. Powdered samples were reacted with dehydrated phosphoric acid under vacuum at 70 °C. The isotope-ratio measurement was calibrated based on repeated measurements of NBS-19 (δ18OVPDB = −2.20 VPDB) and NBS-18 (δ18OVPDB = −23.2) with a precision of ±0.1 (1σ). Calibration on the VPDB scale was based on the principle of identical treatment of standards and unknowns, thus the δ18O value of CO2 derived from NBS-19 and NBS-18 formed the basis of the VPDB scale for all unknowns.
3. Results
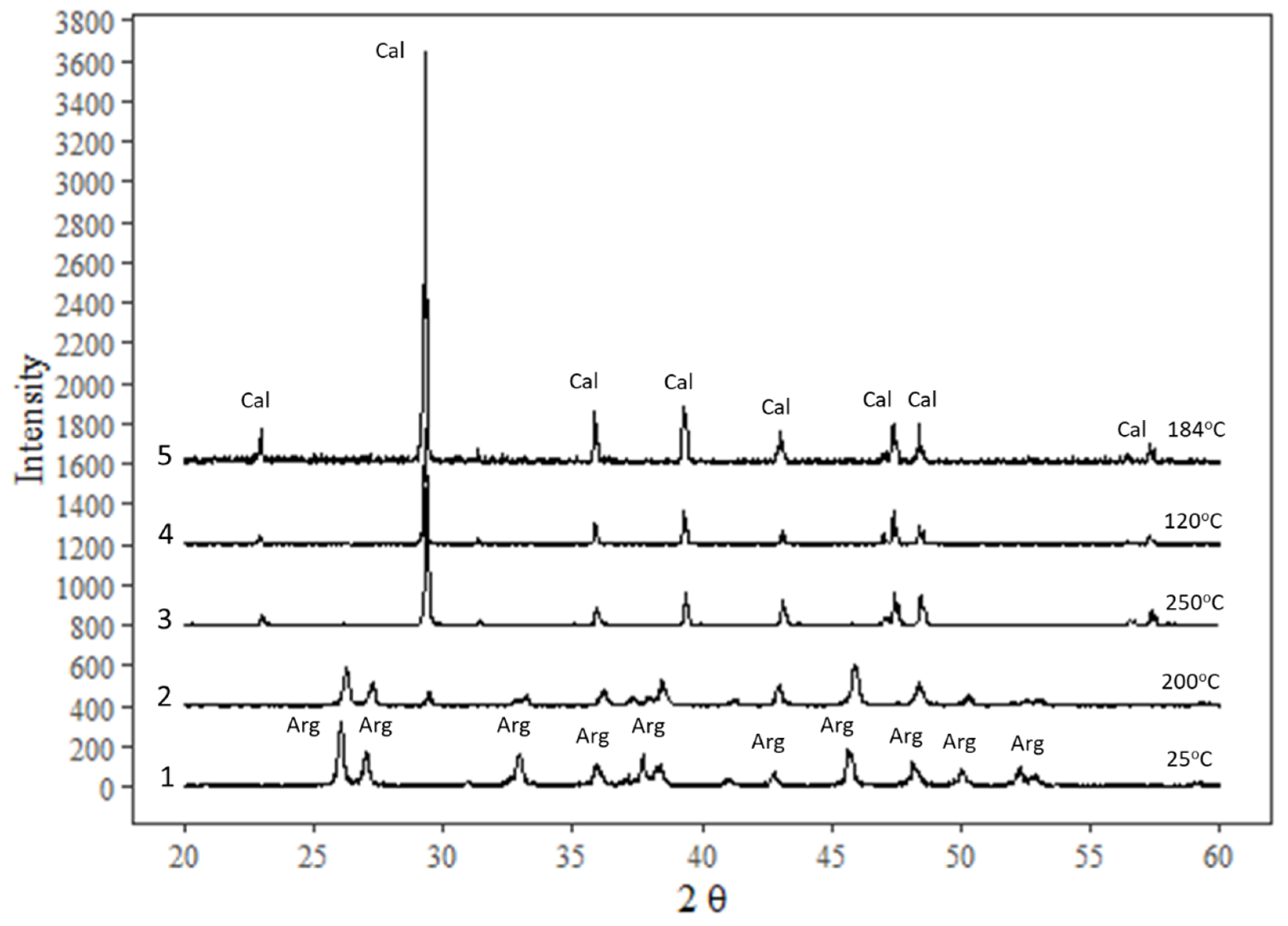
The crystal structure of calcite was confirmed in experimental products by XRD; aragonite completely transformed to calcite within 30 h at 120 °C as well as at higher temperatures (Figure 2). The XRD results that were chosen from experiments at the same temperature but of the lowest incubation time (30 h) are shown here, in addition to an initial sample and a test sample at 200 °C. Complete transformation of aragonite to calcite is expected in longer experiments because of metastability of aragonite, which is consistent with the findings of Ukita et al. [22].
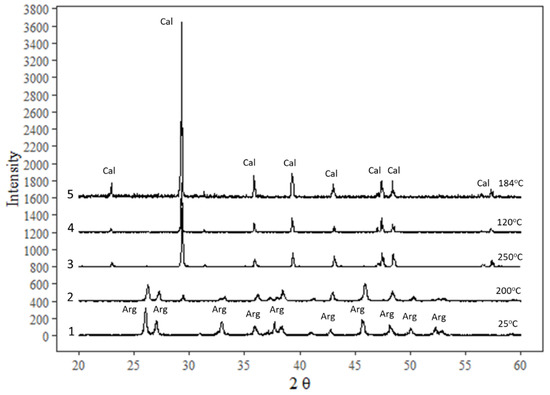
Figure 2.
XRD diffractograms of some samples in different range of temperature from 25 °C: (1) initial aragonite precipitated at 25 °C; (2) a test of aragonite run at 200 °C in open atmosphere; (3) aragonite run in water vapor at saturated pressure at 250 °C; (4) aragonite run in NaCl brine at 120 °C for 30 h; (5) aragonite run in NaCl brine at 184 °C.
Transformation of the two aragonite samples was tested by high temperature treatment in open air at 200 °C and under saturated vapor pressure at a temperature of 250 °C for a few days. XRD analysis showed that the solid product remained aragonite at 200 °C but transformed completely to calcite under water vapor pressure at 250 °C. In Figure 2, two minerals, calcite and aragonite, have their highest-intensity peaks at different positions, as shown in the diffractograms that were compared to the RRuff (https://rruff.info/ accessed on 21 December 2020) spectral database to confirm matching peaks of calcite or aragonite.
The complete transformation of aragonite to calcite was reflected by the change in morphology and crystal structure. Figure 3 shows a distinct difference in the morphology of the initial aragonite and the mineral formed as a result of the experiment. Initially, the aragonite (Figure 3a) consisted of individual needle-like crystals and crystal bundles radiating from a center (spherulites) with a diameter of ~5 µm. All crystals cluster together to form floral-like spheres and are referred to as spherulites. Figure 3b presents a completed transformation of aragonite to calcite. The SEM image shows calcite grains of about 20 µm in size. The resulting crystals were either single grains or groups of crystals. The calcite crystals displayed a regular rhombohedral morphology with a relatively smooth surface. The newly formed calcite was about four times bigger than the diameter of the initial aragonite spherulites, but it was impossible to estimate the calcite crystallization rate because the timing of the completion of the transformation occurred at some point prior to the end of the experiment.

Figure 3.
Secondary electron SEM images of initial aragonite samples and solid products of (A) initial Mg-bearing aragonite grown at room temperature, aragonite spherulites about ~5 µm in diameter, and (B) rhombohedral calcite crystals about 50 µm in diameter, a transformation product after hydrothermal treatment at 184 °C for 40 h.
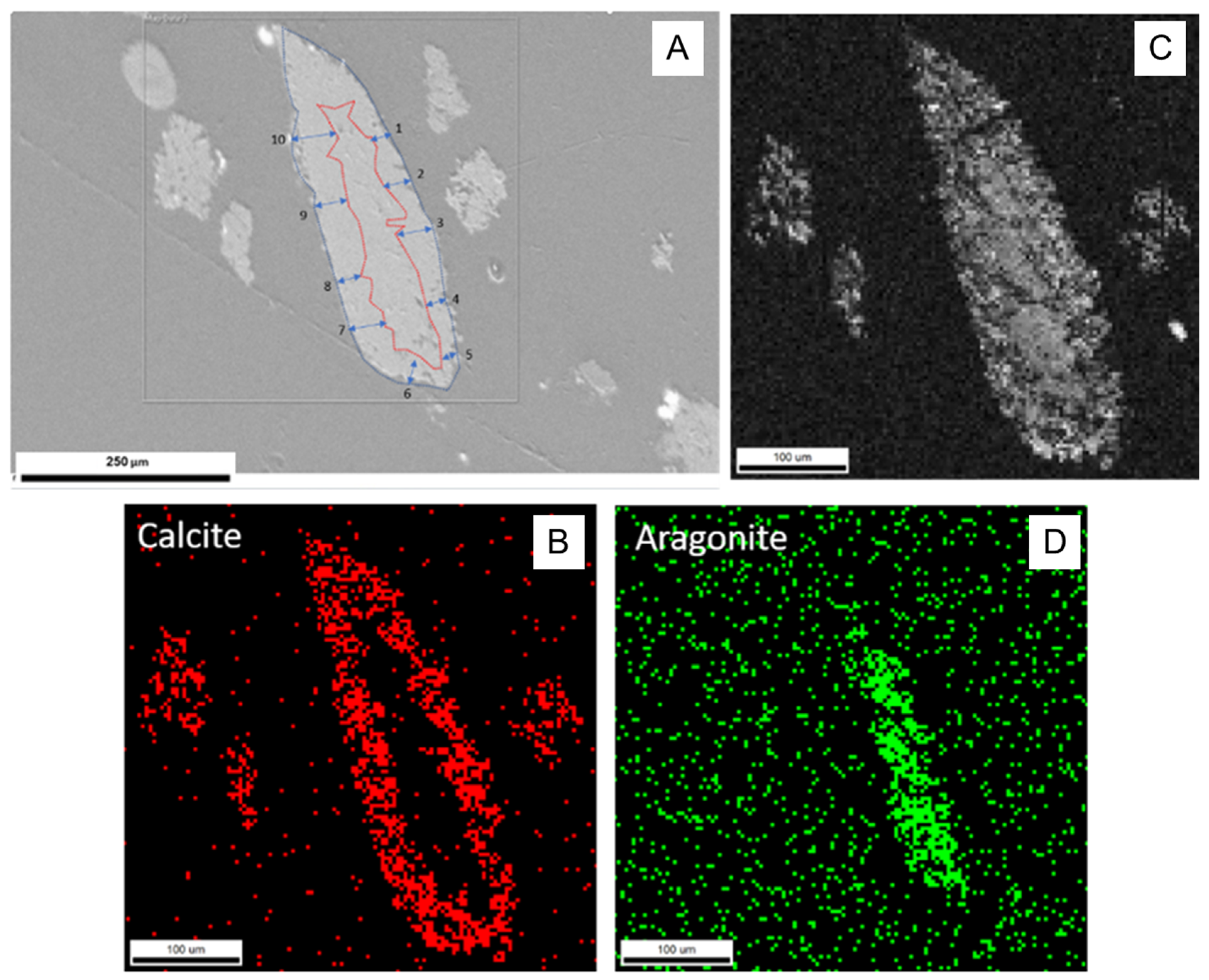
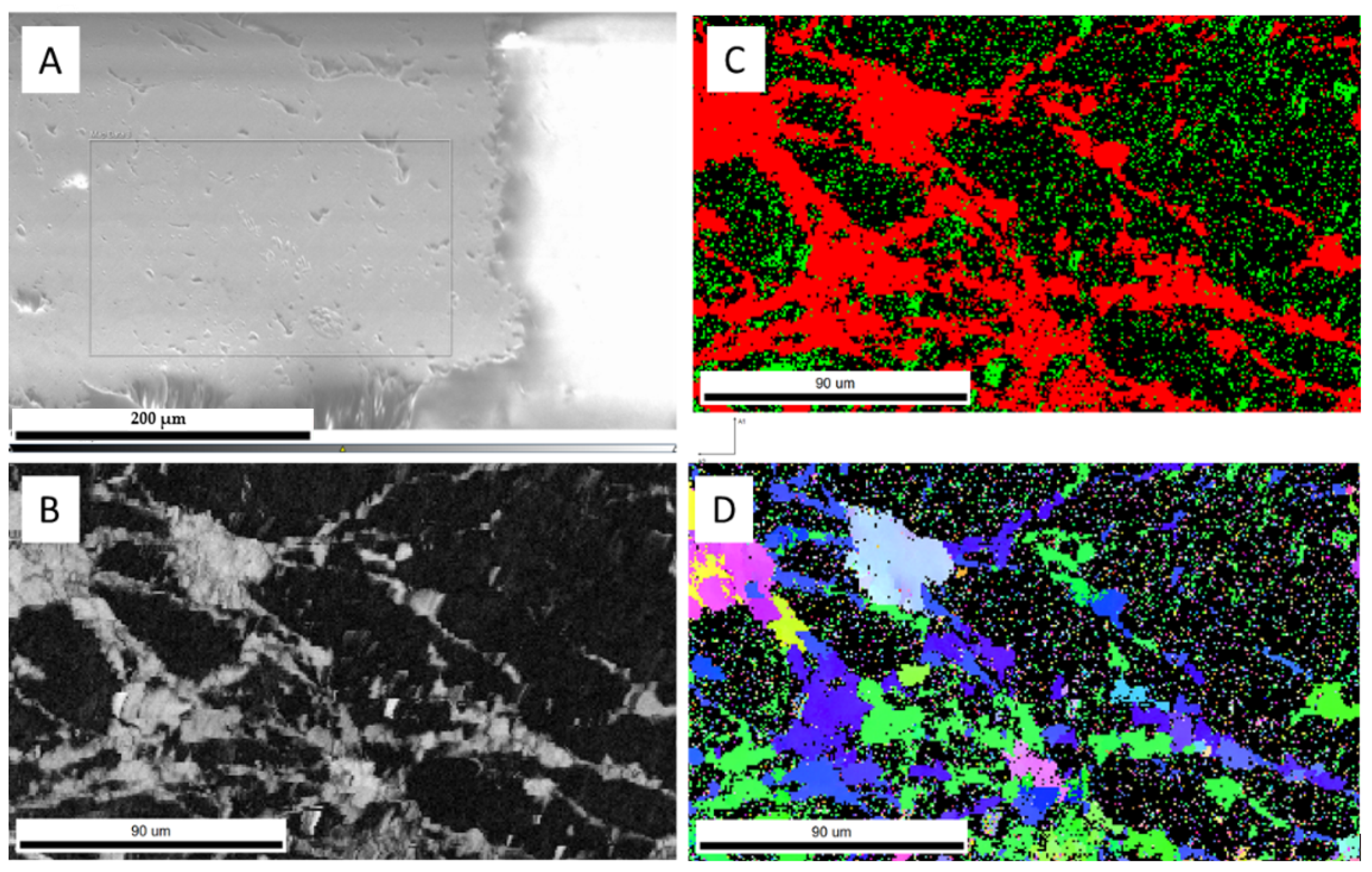
In experiment A1, the use of large chips of aragonite resulted in incomplete transformation (Figure 4). EBSD analyses of the individual chip aragonite grain showed that only the rim of the grain was transformed to calcite, as aragonite remained in the core. The incomplete transformation of aragonite to calcite was observed, suggesting that continuous recrystallization of calcite was the product of aragonite transformation. Therefore, we concluded that the transformation (recrystallization) process occurred over the duration of the experiments and the calcite growth (replacement) rate was estimated by the width of calcite rim around the untransformed aragonite core. Using ImageJ, an image analysis program, the width of the calcite rim was estimated based on the provided scales of the image. There were 10 representative locations where calcite rim widths were chosen to measure. The error was the average number of 10 width differences to an average width. The details are presented in Figure 4 and Table A2. The average width of the calcite rim was 33.58 ± 7.88 μm. The reaction time of experiment A1 was 1152 h. Assuming that transformation time was equivalent to the time of the experiment, the rate of calcite transformation was estimated at about 0.69 ± 0.16 μm/day, which is consistent with the findings of Gabitov et al. (2021) [28] with an estimated transformation rate of about 0.64 ± 0.21 μm/day at similar experimental conditions (162 °C) [28].
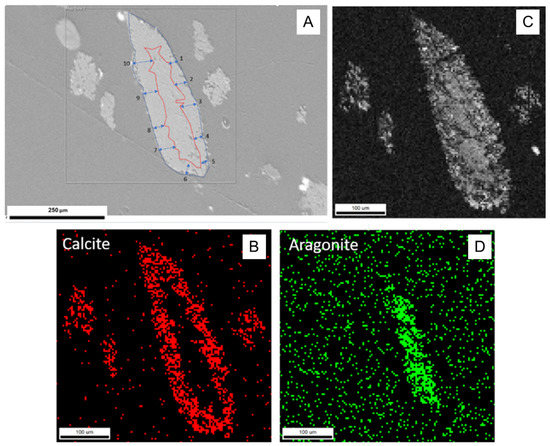
Figure 4.
EBSD data showing incomplete transformation of aragonite to calcite in sample A1 (162 °C, 1152 h): (A) SEM image of the analyzed crystal, the red line presents the boundary between aragonite and newly formed calcite, 10 locations of calcite rim widths were measured; (B) red pixels represent the phase map for calcite, showing its presence in the crystal rim; (C) diffraction intensity map; (D) green pixels represent the phase map for aragonite, showing its presence in the crystal core.
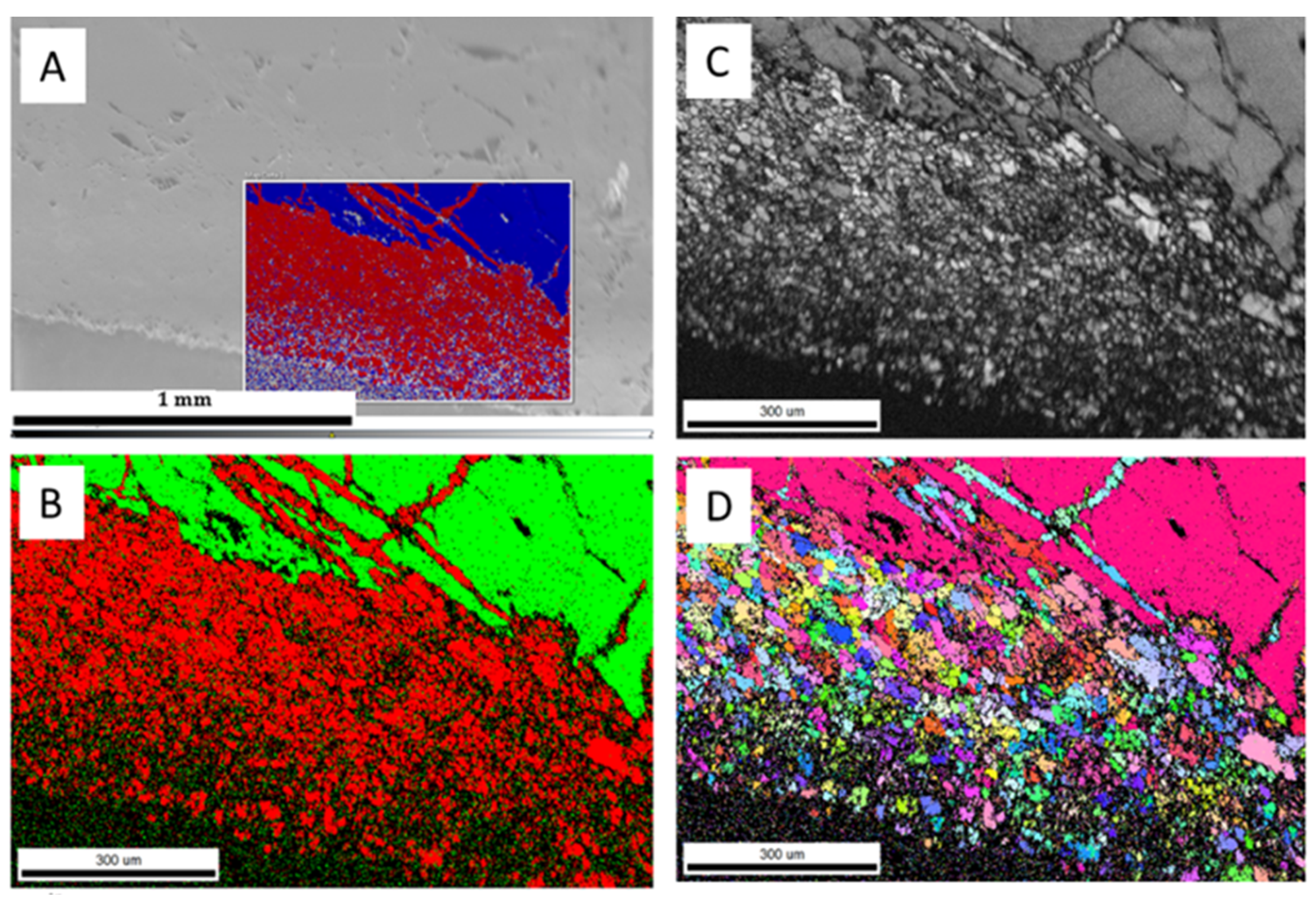
The hydrothermal treatment (sample 52*#: 250 °C for 379 h) of the larger aragonite chips (Figure 5a) yielded incomplete transformation (Figure 5b) with the formation of phases with high and low electron backscatter diffraction intensities at the rim of the chip (Figure 5c). Additional aragonite/calcite transformation occurred along with the fractures toward the interior of the chip (Figure 5b). Newly formed calcite rims consisted of randomly oriented calcite crystals (Figure 5d). A similar observation was reported for biogenic aragonite, where shell fragments of Arctica islandica transformed into calcite at hydrothermal conditions (175 °C for 7 and 84 days) [29]. In our experiments, the average width of the calcite rim was about 414.94 ± 23.58 μm. The estimated replacement rate was approximately 26.28 ± 0.92 μm/day. The single orientation of the interior of the aragonite chip suggests that the original orientation of the aragonite was preserved, and hence, transformation did not take place in this internal zone. A low EBSD signal was observed in the carbonates from the shorter experiment (sample 3&: 250 °C for 90 h), which suggests the presence of a non-crystalline carbonate phase (Figure 6).
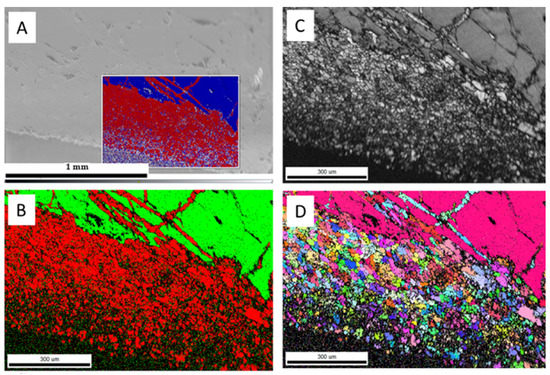
Figure 5.
SEM and EBSD images of carbonates (sample 52*#) including (A) SEM image of aragonite grain; (B) EBSD phase map (calcite is red, aragonite is green); (C) diffraction intensity map; (D) EBSD crystallographic orientation map of aragonite and calcite micro-crystals, with different-colored grains indicating the different orientations of newly formed calcite.
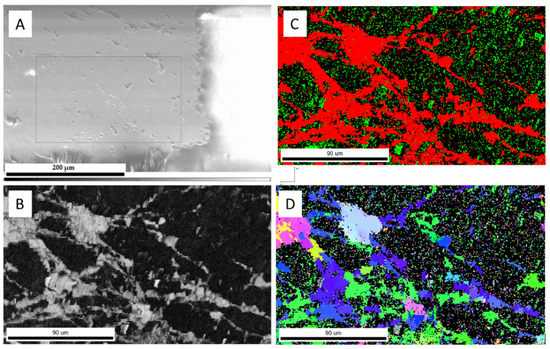
Figure 6.
The SEM and EBSD analyses (sample 3&) show the lack of crystallinity (dark areas): (A) SEM image; (B) diffraction intensity map; (C) EBSD phase map (calcite is red, aragonite is green); (D) EBSD crystallographic orientation map of aragonite and calcite crystals.
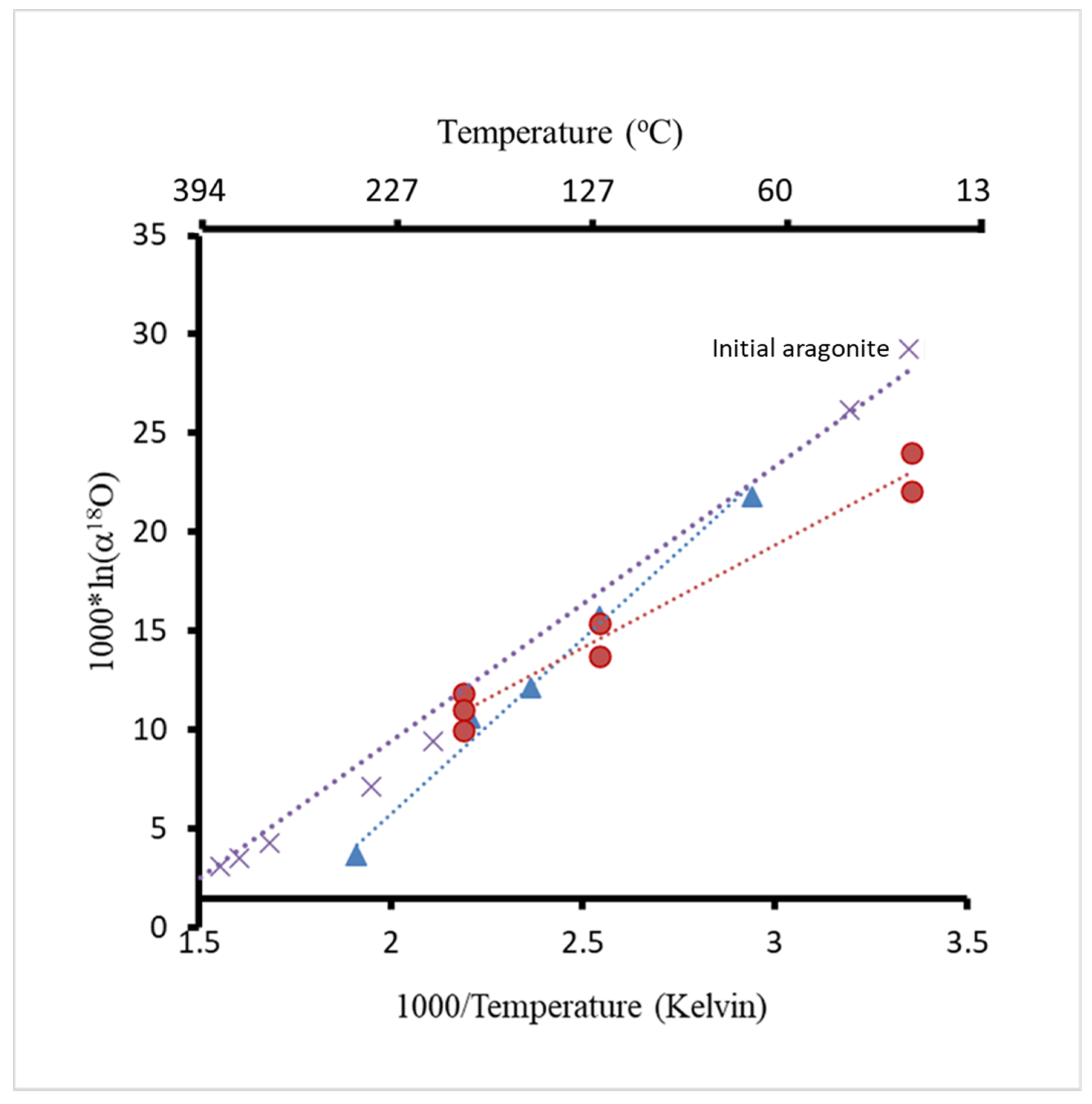
The values of 103⋅ln(α18O) in both Mg- and Sr-free aragonite experiments (blue triangles) and Mg–Sr aragonite experiments (red circles) are plotted against their precipitate temperature in Figure 7 (see also Table 2). The temperature used for calculations was in Kelvin. Mass spectrometry analyses showed that all the δ18O values of calcite were lower than the δ18O of the initial aragonite (Table 2, Figure 7). Initial isotopic data of the aragonite is plotted at 25 and 57 °C, which were the precipitation temperatures of the Mg- and Sr-bearing and Mg- and Sr-free aragonites, respectively. The fractionation of oxygen isotopes during the transformation of aragonite to calcite is controlled by the temperature of the experiments (Figure 7).
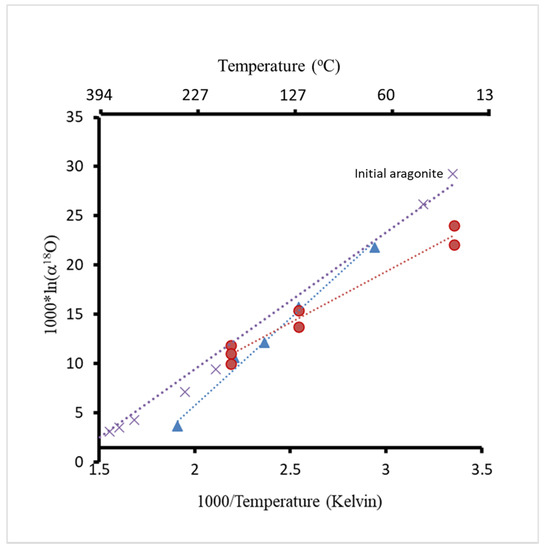
Figure 7.
Oxygen isotope fractionation from multiple experiments at different temperatures. Red circles present data from experiments using Mg and Sr carrying aragonite; blue triangles present data from Mg- and Sr-free aragonite experiments; purple cross symbols present data from [30,31].

Table 2.
δ18O, Sr/Ca, and Mg/Ca values in the initial aragonite and experimental samples at different temperatures.
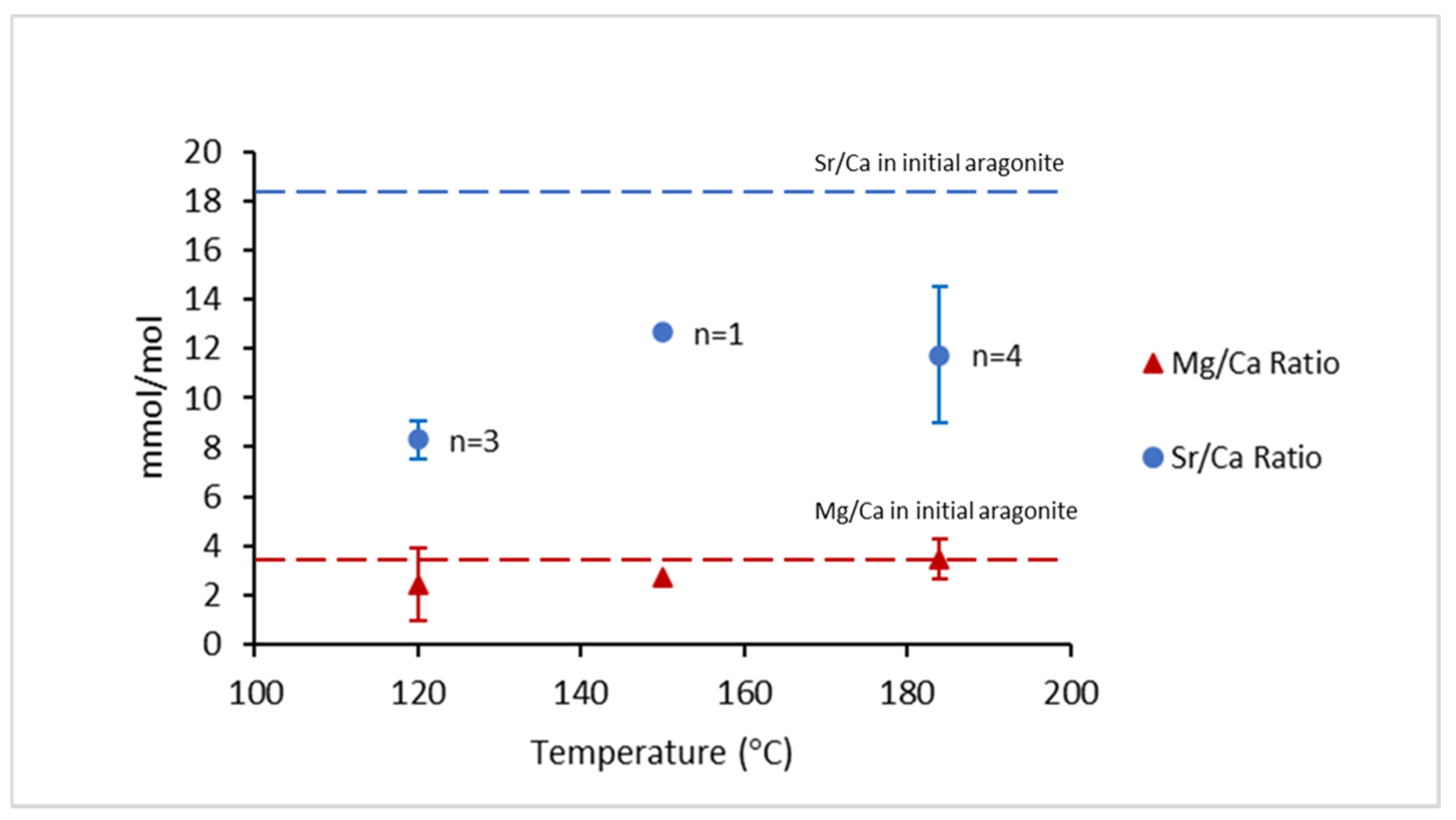
Elemental analyses showed that Sr/Ca (blue circles) and Mg/Ca ratios (red triangles) in calcite (transformation product) were lower than the ratios of the initial aragonite (Figure 8 and Table 2). The Sr/Ca ratio in the initial aragonite decreased approximately 52% at 120 °C, 27% at 150 °C, and 32% at 184 °C. Meanwhile, the Mg/Ca ratio decreased approximately 32% at 120 °C, 24% at 150 °C, and 3% at 184 °C (Figure 8).
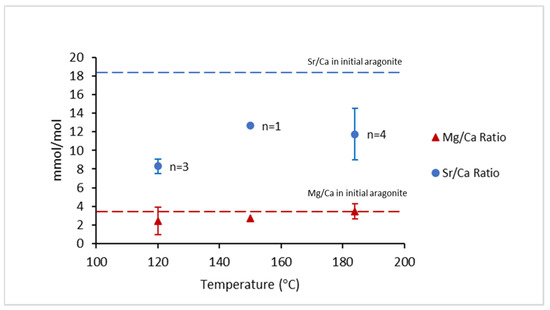
Figure 8.
Average ratios of Sr/Ca and Mg/Ca of the multiple (n) experiments where calcite was placed at the bottom of the autoclaves. Red triangles represent the Mg/Ca ratio and blue circles represent the Mg/Ca ratio. The red and blue dashed lines represent the Sr/Ca and Mg/Ca ratios of the initial aragonite, respectively.
4. Discussion
The Mg/Ca ratio signatures were fully retained during phase transformation at 184 °C (Figure 8). This observation can be explained by the high transformation rate, which positively correlated with temperature, i.e., Mg escape into the bulk solution from the crystal/fluid boundary layer was restricted during transformation. The transformation rate of the large aragonite grains into calcite was estimated as 0.69 ± 0.16 μm/day at 162 °C and as 26.28 ± 0.92 μm/day at 250 °C. At lower temperatures (i.e., 150 and 120 °C), the Mg/Ca ratio of aragonite was not completely retained by calcite. This observation is consistent with a decrease in the transformation rate with the temperature decrease, i.e., there was more time for Mg to escape. Molecular simulations showed that Mg diffusion in solution was slower by 13–18% in comparison to the diffusion of Sr and Ca, respectively, at 25 °C [32]. Therefore, if the difference in diffusion rates of Mg and Sr remained significant at high temperatures, then this can explain our data, i.e., the slower transport of Mg from the mineral transformation site resulted in better retention of Mg/Ca compared to Sr/Ca.
Based on our results, an effort was made to calculate apparent partitioning coefficients for Sr and Mg (KSr and KMg, respectively). Considering that initial aragonite was the only source for Sr and Mg, the Sr/Ca and Mg/Ca ratios in solution were assumed to be equal to the Sr/Ca and Mg/Ca ratios in aragonite, which is consistent with the ICP-OES analysis of the final fluid sample. These calculations yielded KSr = 0.64 ± 0.11 and KMg = 0.77 ± 0.11, respectively. In both cases, the K values are excessive compared to previous experimental data at low and high temperatures [33,34,35,36,37,38,39,40,41,42,43,44]. Based on this observation, we conclude that these K values were overestimated and were a result of complete or partial retention of Sr/Ca and Mg/Ca ratios during aragonite transformation into calcite.
Oxygen isotope fractionation is controlled by temperature and isotope composition of the growth media because the δ18O of calcite is very different from the δ18O of aragonite. The isotopic data are consistent with the calibrations in the following studies [39,40,41]. The calculated fractionation factors (1000lnα18O) are consistent with previously reported temperature calibrations [30,31,45] (Figure 7). There was a difference in the calibration slopes between experiments where Sr- and Mg-bearing aragonite was used compared to Sr- and Mg-free experiments, which indicates an effect of Mg and Sr on oxygen isotope fractionation [46]. The slope value derived from the previous studies is in between the slopes obtained in our study [30,31].
Our δ18O data suggested that the transformation process at 120–180 °C revealed conditions of alteration and eliminated protolith isotopic signatures. Data on elemental and isotopic retention suggest that the transformation process occurred simultaneously with the dissolution of carbonate phases in the bulk fluid. The transformation process itself likely occurred by the coupled dissolution of aragonite and the precipitation of calcite in the nearest proximity to carbonate grains, probably at the fluid boundary layer. We do not know the exact concentration of Mg and Sr in this thin layer; however, it was clearly different than the concentration of Mg and Sr in the bulk solution. Equilibrium between calcite and bulk solution can be achieved only if dissolution of the initial material (aragonite) is fast and precipitation of the product (calcite) is relatively slow. If more material is dissolved than precipitated, then the solutes can be transported elsewhere, which would cause calcite precipitation at a distance from the aragonite grains. This did not happen in our experiments, as calcite nucleated and grew on the surface of aragonite grains or on the aragonite powder. Oxygen isotopes, however, demonstrated an expected temperature dependence. Therefore, we can conclude that calcite–fluid isotope fractionation occurred at conditions closer to equilibrium, compared to the Mg and Sr incorporation into calcite observed in our study. This is likely because oxygen is a primary component of an aqueous carbonate species, and δ18O in the boundary layer is not very different from δ18O in the bulk solution. In addition, the difference in diffusion between oxygen isotopes is much smaller than the difference in diffusion between Mg, Sr, and Ca, which yielded smaller isotope fractionation between the boundary layer and bulk solution during transformation.
The formation of amorphous calcium carbonate (ACC) as an intermediate phase during aragonite–calcite transformation was observed as a lack of an electron backscatter diffraction signal (Figure 6). The unexpectedly high Sr/Ca and especially Mg/Ca ratios in our calcites support the formation of ACC as an intermediate phase during transformation of aragonite to calcite (Figure 8). The calculation of apparent partition coefficients between calcite and bulk fluid yielded very high values (i.e., K > 0.6). Therefore, a measured Sr/Ca and Mg/Ca in calcite may be a result of ACC formation as an intermediate phase. Lacking a crystal structure, ACC can entrap the composition (i.e., Sr/Ca and Mg/Ca) of the fluid boundary layer, where transformation takes place. The mechanism of formation of the amorphous phase in our experiment is not necessarily the same as the process of ACC growth at near-room temperatures [47], which was extensively studied in benchtop experiments, where the occurrence of ACC was reported as a precursor for crystalline CaCO3.
5. Conclusions
In this study, we quantified the transformation rate of aragonite to calcite at the rim of aragonite grains. Our data confirmed an increase in the transformation rate with an increase in temperature. At 162 °C, the rate was about 0.69 μm/day. At 250 °C, the rate was 25.28 μm/day. Hydrothermal transformation of aragonite into calcite occurred via a coupled dissolution and precipitation mechanism in proximity to the crystal/fluid boundary with limited exchange with the bulk solution reservoir. We also showed that the transformation product (calcite) retained a significant amount of Sr and Mg from the protolith (aragonite) when transformation occurred in a Mg- and Sr-free solution. This effect, however, was not observed for oxygen isotopes, as oxygen is a primary component of carbonate aqueous species. The oxygen isotope composition of the protolith was not retained because fractionation was controlled by temperature and the δ18O of the bulk solution.
Author Contributions
Conceptualization, R.G. and A.N.; methodology, R.G., A.N., A.J., A.M., A.P.-H., V.P., B.K., P.D., A.D., and J.V.; writing—original draft preparation, A.N. and R.G.; writing—review and editing, A.N., R.G., and B.K.; supervision, R.G.; funding acquisition, R.G., A.M., and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
College of Arts and Sciences and Department of Geosciences (MSU) funding for research. Department of Energy National Energy Technology Laboratory under the Southern States Energy Board’s Cooperative Agreement Award Number DE-FE0029465. Los Alamos National Laboratory (LANL) under project number 20180007DR.
Data Availability Statement
Not applicable.
Acknowledgments
We are thankful to those who helped with this project, including Rooban Venkatesh K.G. Thirumalai and Iwei-Chu of the MSU Institute for Imaging and Analytical Technologies for helping with XRD analyses, the College of Arts and Sciences and Department of Geosciences (MSU) for supporting the experiments conducted at MSU, and the Environmental Isotope Laboratory, College of Science, Geosciences, The University of Arizona. Two experiments were conducted at Los Alamos National Laboratory (LANL) under project number 20180007DR. LANL, an affirmative action/equal opportunity employer, is managed by Triad National Security, LLC, for the National Nuclear Security Administration of the U.S. Department of Energy under contract 89233218CNA000001. Autoclave experimentation was conducted along with the shale experiments under funding from the Department of Energy National Energy Technology Laboratory under the Southern States Energy Board’s Cooperative Agreement Award Number DE-FE0029465.
Conflicts of Interest
The authors declare no conflict of interest in any personal circumstances that may be perceived as inappropriately influencing the representation or interpretation of the reported research results. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Solid preparation for ICP_OES.
Table A1.
Solid preparation for ICP_OES.
| Sample ID | Sample Weight for ICP (mg) in 10 mL of 3% HNO3 |
|---|---|
| Raw aragonite 30 July 2018 | 9.812 |
| A120-T2 | 9.989 |
| A120-T4 | 10.193 |
| A120-T6 | 9.967 |
| A120-B2 | 10.29 |
| A120-B4 | 10.404 |
| A120-B6 | 10.261 |
| A184-T2 | 9.846 |
| A184-T4 | 4.945 |
| A184-T6 | 9.771 |
| A184-B2 | 4.944 |
| A184-B4 | 9.926 |
| A184-B6 | 10.192 |
| A184-B10 | 10.644 |
| A150_B1 | 2.261 |

Table A2.
The widths of the calcite rim of sample A1 at selected locations.
Table A2.
The widths of the calcite rim of sample A1 at selected locations.
| Location | Width (μm) |
|---|---|
| W1 | 23.12 |
| W2 | 31.12 |
| W3 | 43.37 |
| W4 | 23.69 |
| W5 | 20.45 |
| W6 | 32.29 |
| W7 | 40.03 |
| W8 | 31.39 |
| W9 | 39.11 |
| W10 | 51.26 |
| Average | 33.583 |
References
- Coleman, R.G.; Lee, D.E. Metamorphic aragonite in the glaucophane schists of Cazadero, California. Am. J. Sci. 1962, 260, 577–595. [Google Scholar] [CrossRef]
- Kunzler, R.H.; Goodell, H.G. The aragonite-calcite transformation; a problem in the kinetics of a solid-solid reaction. Am. J. Sci. 1970, 269, 360–391. [Google Scholar] [CrossRef]
- Folk, R.L. Some aspects of recrystallization in ancient limestones. Spec. Publ. Soc. Econ. Paleont. Mineral. 1965, 13, 14–48. [Google Scholar]
- Putnis, A.; Putnis, C.V. The mechanism of reequilibration of solids in the presence of a fluid phase. J. Solid State Chem. 2007, 180, 1783–1786. [Google Scholar] [CrossRef]
- Perdikouri, C.; Piazolo, S.; Kasioptas, A.; Schmidt, B.C.; Putnis, A. Hydrothermal replacement of aragonite by calcite: Interplay between replacement, fracturing and growth. Eur. J. Miner. 2013, 25, 123–136. [Google Scholar] [CrossRef]
- Epstein, S.; Mayeda, T. Variation of O18 content of waters from natural sources. Geochim. Cosmochim. Acta 1953, 4, 213–224. [Google Scholar] [CrossRef]
- Broecker, W.S. Oxygen isotope constraints on surface ocean temperatures. Quat. Res. 1986, 26, 121–134. [Google Scholar] [CrossRef]
- Labeyrie, L.D.; Duplessy, J.-C.; Blanc, P.L. Variations in mode of formation and temperature of oceanic deep waters over the past 125,000 years. Nat. Cell Biol. 1987, 327, 477–482. [Google Scholar] [CrossRef]
- Lea, D.W.; Mashiotta, T.A.; Spero, H. Controls on magnesium and strontium uptake in planktonic foraminifera determined by live culturing. Geochim. Cosmochim. Acta 1999, 63, 2369–2379. [Google Scholar] [CrossRef]
- Li, N.; Cao, Y.; Zhang, Z.; Du, Y.; Guo, C. Oxygen-Isotope-Based Modeling of the Hydrothermal Fluid Processes of the Taochong Skarn Iron Deposit, Anhui Province, China. Minerals 2021, 11, 375. [Google Scholar] [CrossRef]
- Matthews, A. Oxygen isotope geothermometers for metamorphic rocks. J. Metamorph. Geol. 1994, 12, 211–219. [Google Scholar] [CrossRef]
- Matera, P.F.; Ventruti, G.; Zucchi, M.; Brogi, A.; Capezzuoli, E.; Liotta, D.; Yu, T.-L.; Shen, C.-C.; Huntington, K.W.; Rinyu, L.; et al. Geothermal Fluid Variation Recorded by Banded Ca-Carbonate Veins in a Fault-Related, Fissure Ridge-Type Travertine Depositional System (Iano, southern Tuscany, Italy). Geofluids 2021, 2021, 8817487. [Google Scholar] [CrossRef]
- Bemis, B.E.; Spero, H.; Bijma, J.; Lea, D.W. Reevaluation of the oxygen isotopic composition of planktonic foraminifera: Experimental results and revised paleotemperature equations. Paleoceanography 1998, 13, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Eggins, S.; De Deckker, P.; Marshall, J. Mg/Ca variation in planktonic foraminifera tests: Implications for reconstructing palaeo-seawater temperature and habitat migration. Earth Planet. Sci. Lett. 2003, 212, 291–306. [Google Scholar] [CrossRef]
- Elderfield, H.; Ganssen, G. Past temperature and δ18O of surface ocean waters inferred from foraminiferal Mg/Ca ratios. Nat. Cell Biol. 2000, 405, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Dekens, P.S.; Lea, D.W.; Pak, D.K.; Spero, H.J. Core top calibration of Mg/Ca in tropical foraminifera: Refining paleotemperature estimation. Geochem. Geophys. Geosyst. 2002, 3, 1–29. [Google Scholar] [CrossRef]
- Rosenthal, Y.; Lohmann, G.; Lohmann, K.; Sherrell, R. Incorporation and preservation of Mg in Globigerinoides sacculifer: Implications for reconstructing the temperature and 18O/16O of seawater. Paleoceanography 2000, 15, 135–145. [Google Scholar] [CrossRef]
- Klein, R.T.; Lohmann, K.C.; Thayer, C.W. SrCa and 13C12C ratios in skeletal calcite of Mytilus trossulus: Covariation with metabolic rate, salinity, and carbon isotopic composition of seawater. Geochim. Cosmochim. Acta 1996, 60, 4207–4221. [Google Scholar] [CrossRef]
- Smith, S.V.; Buddemeier, R.W.; Redalje, R.C.; Houck, J.E. Strontium-calcium thermometry in coral skeletons. Science 1979, 204, 404–407. [Google Scholar] [CrossRef]
- Beck, J.W.; Edwards, R.L.; Ito, E.; Taylor, F.W.; Recy, J.; Rougerie, F.; Joannot, P.; Henin, C. Sea-surface temperature from coral skeletal strontium/calcium ratios. Science 1992, 257, 644–647. [Google Scholar] [CrossRef]
- Marshall, J.F.; McCulloch, M. An assessment of the Sr/Ca ratio in shallow water hermatypic corals as a proxy for sea surface temperature. Geochim. Cosmochim. Acta 2002, 66, 3263–3280. [Google Scholar] [CrossRef]
- Elfil, H.; Roques, H. Role of hydrate phases of calcium carbonate on the scaling phenomenon. Desalination 2001, 137, 177–186. [Google Scholar] [CrossRef]
- Clarkson, J.R.; Price, T.J.; Adams, C.J. Role of metastable phases in the spontaneous precipitation of calcium carbonate. J. Chem. Soc. Faraday Trans. 1992, 88, 243–249. [Google Scholar] [CrossRef]
- Brečević, L.; Nielsen, A.E. Solubility of amorphous calcium carbonate. J. Cryst. Growth 1989, 98, 504–510. [Google Scholar] [CrossRef]
- Loste, E.; Wilson, R.M.; Seshadri, R.; Meldrum, F.C. The role of magnesium in stabilising amorphous calcium carbonate and controlling calcite morphologies. J. Cryst. Growth 2003, 254, 206–218. [Google Scholar] [CrossRef]
- Purgstaller, B.; Mavromatis, V.; Immenhauser, A.; Dietzel, M. Transformation of Mg-bearing amorphous calcium carbonate to Mg-calcite—In situ monitoring. Geochim. Cosmochim. Acta 2016, 174, 180–195. [Google Scholar] [CrossRef]
- Littlewood, J.L.; Shaw, S.; Peacock, C.L.; Bots, P.; Trivedi, D.; Burke, I.T. Mechanism of enhanced strontium uptake into calcite via an amorphous calcium carbonate crystallization pathway. Cryst. Growth Des. 2017, 17, 1214–1223. [Google Scholar] [CrossRef]
- Gabitov, R.; Migdisov, A.; Nguyen, A.; Van Hartesveldt, N.; Perez-Huerta, A.; Sadekov, A.; Sauer, K.B.; Baker, J.; Paul, V.; Caporuscio, F.; et al. Uptake of uranium by carbonate crystallization from reduced and oxidized hydrothermal fluids. Chem. Geol. 2021, 564, 120054. [Google Scholar] [CrossRef]
- Casella, L.A.; Griesshaber, E.; Yin, X.; Ziegler, A.; Mavromatis, V.; Müller, D.; Ritter, A.-C.; Hippler, D.; Harper, E.M.; Dietzel, M.; et al. Experimental diagenesis: Insights into aragonite to calcite transformation of Arctica islandica shells by hydrothermal treatment. Biogeosciences 2017, 14, 1461–1492. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, J.R.; Clayton, R.N.; Mayeda, T.K. Oxygen isotope fractionation in divalent metal carbonates. J. Chem. Phys. 1969, 51, 5547–5558. [Google Scholar] [CrossRef]
- Kim, S.-T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Kerisit, S.; Liu, C. Molecular simulation of the diffusion of uranyl carbonate species in aqueous solution. Geochim. Cosmochim. Acta 2010, 74, 4937–4952. [Google Scholar] [CrossRef]
- Lorens, R.B. Sr, Cd, Mn and Co distribution coefficients in calcite as a function of calcite precipitation rate. Geochim. Cosmochim. Acta 1981, 45, 553–561. [Google Scholar] [CrossRef]
- Tesoriero, A.J.; Pankow, J.F. Solid solution partitioning of Sr2+, Ba2+, and Cd2+ to calcite. Geochim. Cosmochim. Acta 1996, 60, 1053–1063. [Google Scholar] [CrossRef]
- Fairchild, I.J.; Borsato, A.; Tooth, A.F.; Frisia, S.; Hawkesworth, C.J.; Huang, Y.; McDermott, F.; Spiro, B. Controls on trace element (Sr–Mg) compositions of carbonate cave waters: Implications for speleothem climatic records. Chem. Geol. 2000, 166, 255–269. [Google Scholar] [CrossRef]
- Gabitov, R.; Weremeichik, J.; Novak, A.; Sadekov, A.; Thirumalai, R. Effect of pH on the precipitation of synthetic CaCO3 polymorphs and determination of Mg/Ca ratios in synthetic low-magnesium calcite: An experimental investigation. Am. Geophys. Union 2016, 2016, AH14A-0026. [Google Scholar]
- Mavromatis, V.; Gautier, Q.; Bosc, O.; Schott, J. Kinetics of Mg partition and Mg stable isotope fractionation during its incorporation in calcite. Geochim. Cosmochim. Acta 2013, 114, 188–203. [Google Scholar] [CrossRef]
- Gabitov, R.; Sadekov, A.; Leinweber, A. Crystal growth rate effect on Mg/Ca and Sr/Ca partitioning between calcite and fluid: An in situ approach. Chem. Geol. 2014, 367, 70–82. [Google Scholar] [CrossRef]
- Gregory, R.T.; Taylor, H.P., Jr. An oxygen isotope profile in a section of Cretaceous oceanic crust, Samail Ophiolite, Oman: Evidence for δ18O buffering of the oceans by deep (>5 km) seawater-hydrothermal circulation at mid-ocean ridges. J. Geophys. Res. Solid Earth 1981, 86, 2737–2755. [Google Scholar] [CrossRef] [Green Version]
- Taylor, H.P. Oxygen and hydrogen isotope studies of hydrothermal interactions at submarine and subaerial spreading centers. In Hydrothermal Processes at Seafloor Spreading Centers; Springer: Berlin/Heidelberg, Germany, 1983; pp. 83–139. [Google Scholar]
- McKibbin, R.; Absar, A.; Blattner, P. The transport of oxygen isotopes in hydrothermal systems. In Proceedings of the 8th NZ Geothermal Workshop, Auckland, New Zealand, 5–7 November 1986; pp. 29–36. [Google Scholar]
- Clayton, R.N.; Epstein, S. The use of oxygen isotopes in high-temperature geological thermometry. J. Geol. 1961, 69, 447–452. [Google Scholar] [CrossRef]
- Gabitov, R.; Sadekov, A.; Yapaskurt, V.; Borrelli, C.; Bychkov, A.; Sabourin, K.; Perez-Huerta, A. Elemental uptake by calcite slowly grown from seawater solution: An in-situ study via depth profiling. Front. Earth Sci. 2019, 7, 51. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Watson, E.B. Partitioning of strontium between calcite and fluid. Geochem. Geophys. Geosyst. 2006, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Gabitov, R.I.; Watson, E.B.; Sadekov, A.Y. Oxygen isotope fractionation between calcite and fluid as a function of growth rate and temperature: An in situ study. Chem. Geol. 2012, 306–307, 92–102. [Google Scholar] [CrossRef]
- Mavromatis, V.; Schmidt, M.; Botz, R.; Comas-Bru, L.; Oelkers, E.H. Experimental quantification of the effect of Mg on calcite–aqueous fluid oxygen isotope fractionation. Chem. Geol. 2012, 310–311, 97–105. [Google Scholar] [CrossRef]
- Millero, F.J. Chemical Oceanography; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).