Hydrophobicity and Charge Distribution Effects in the Formation of Bioorganoclays
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
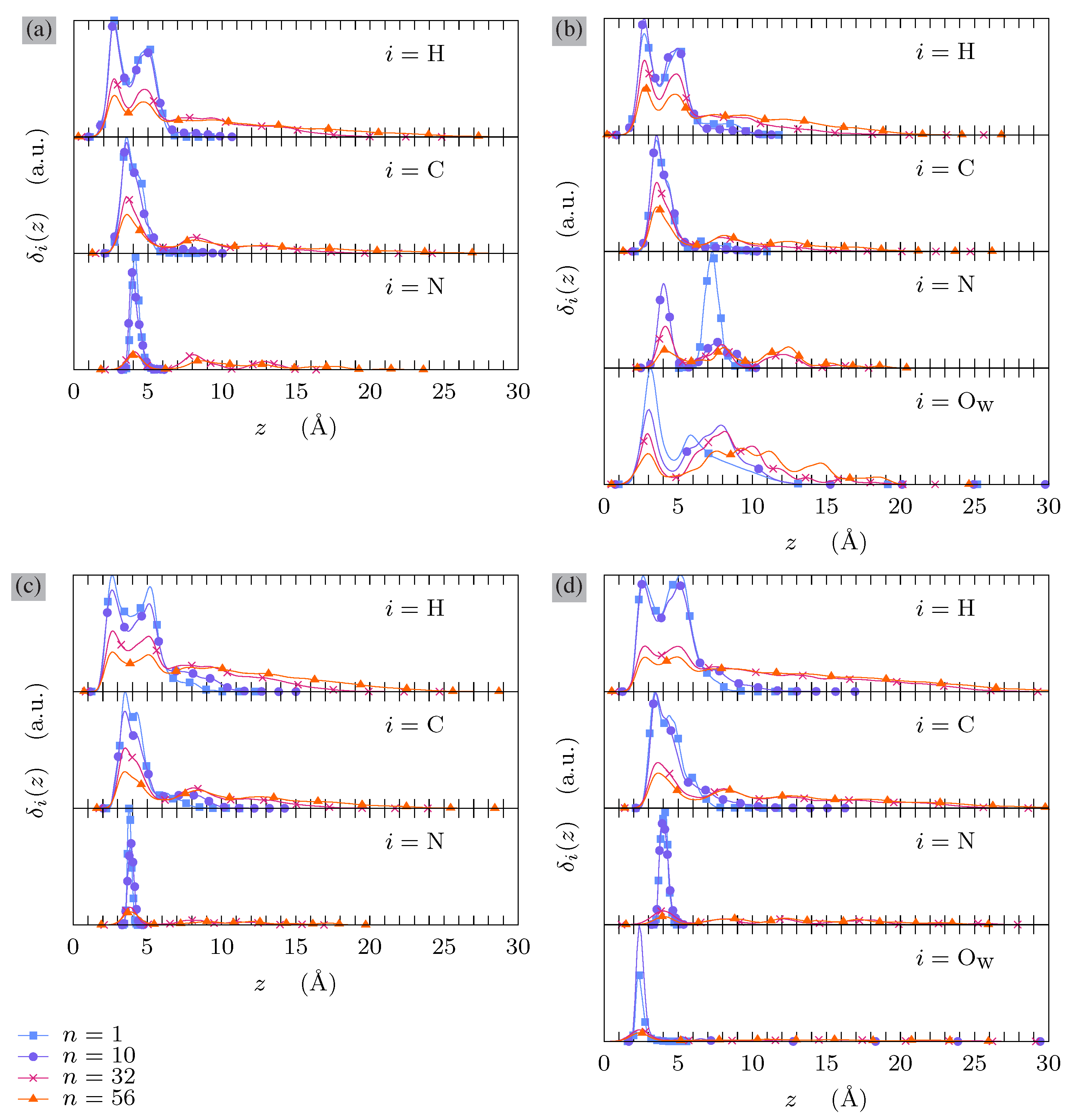
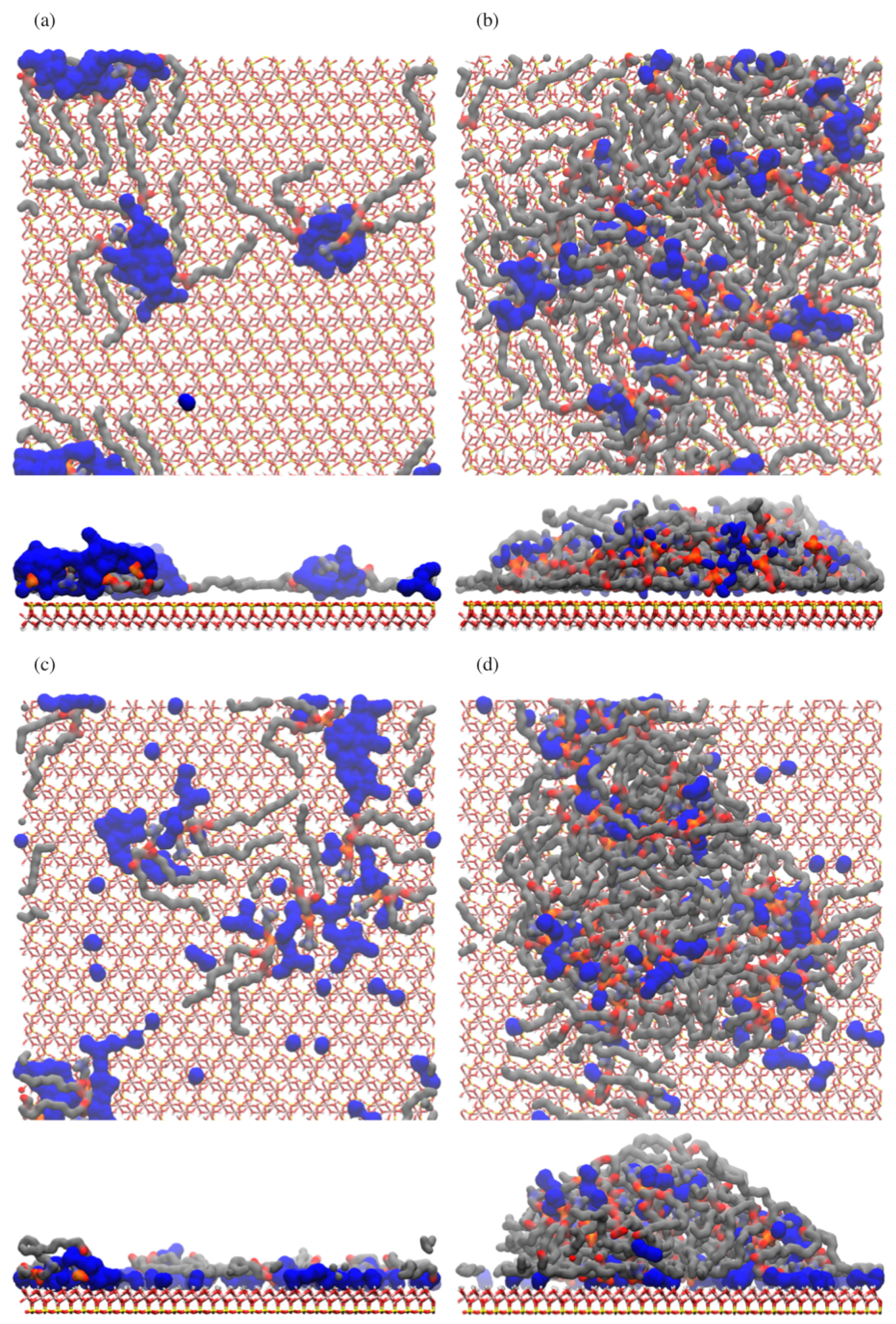
3.1. Atomic Density Profiles
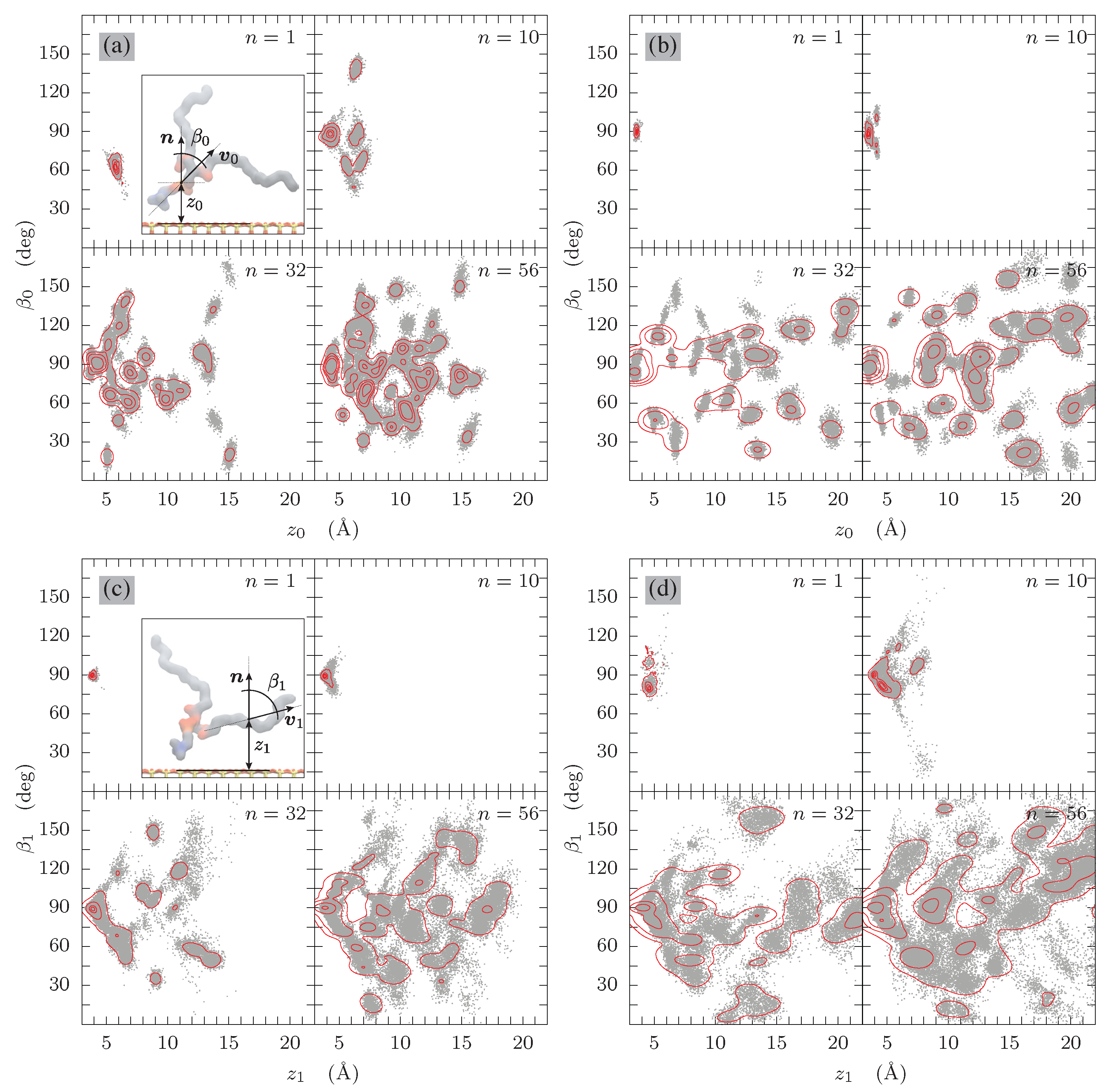
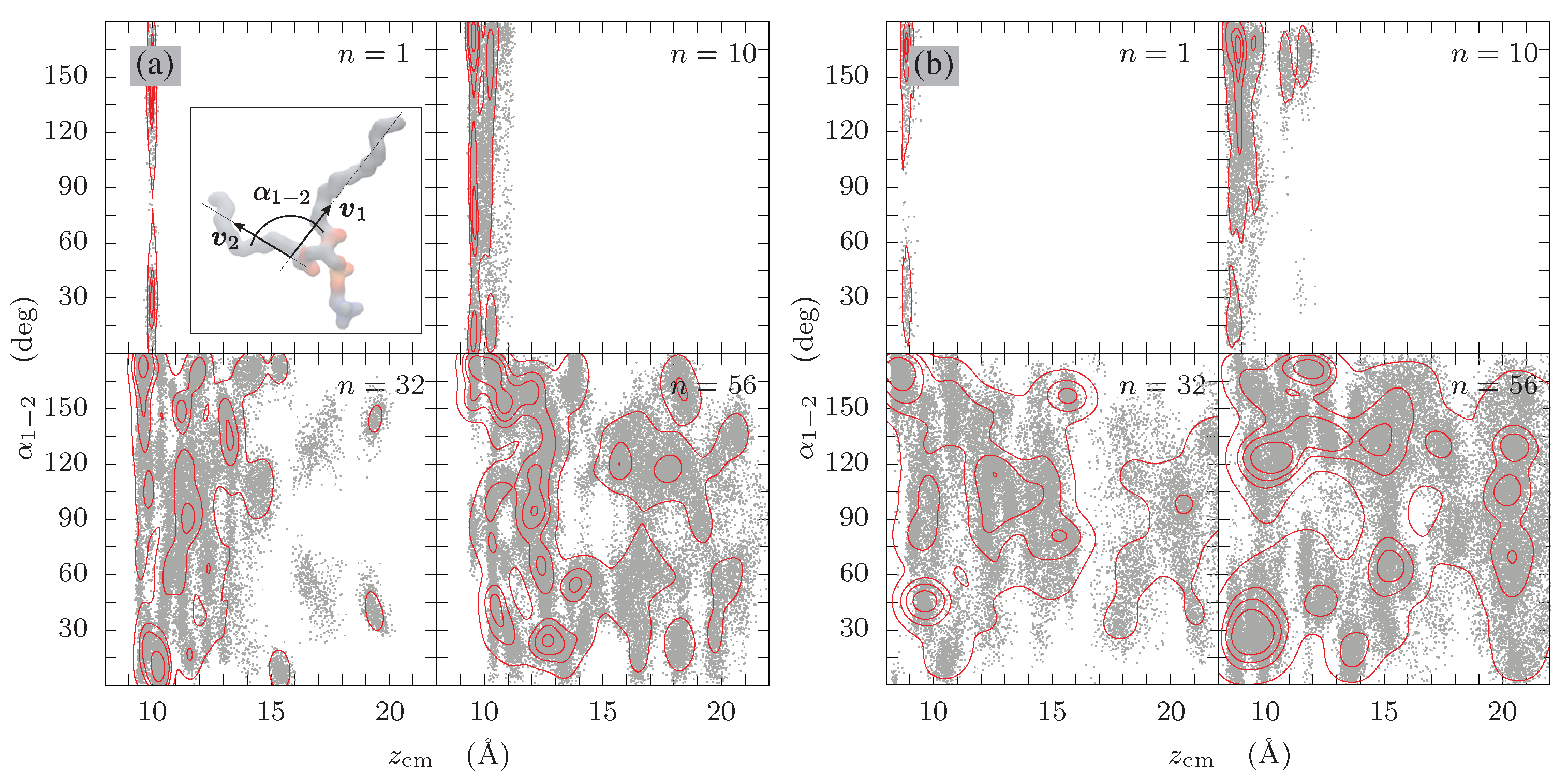
3.2. Tilt Angles
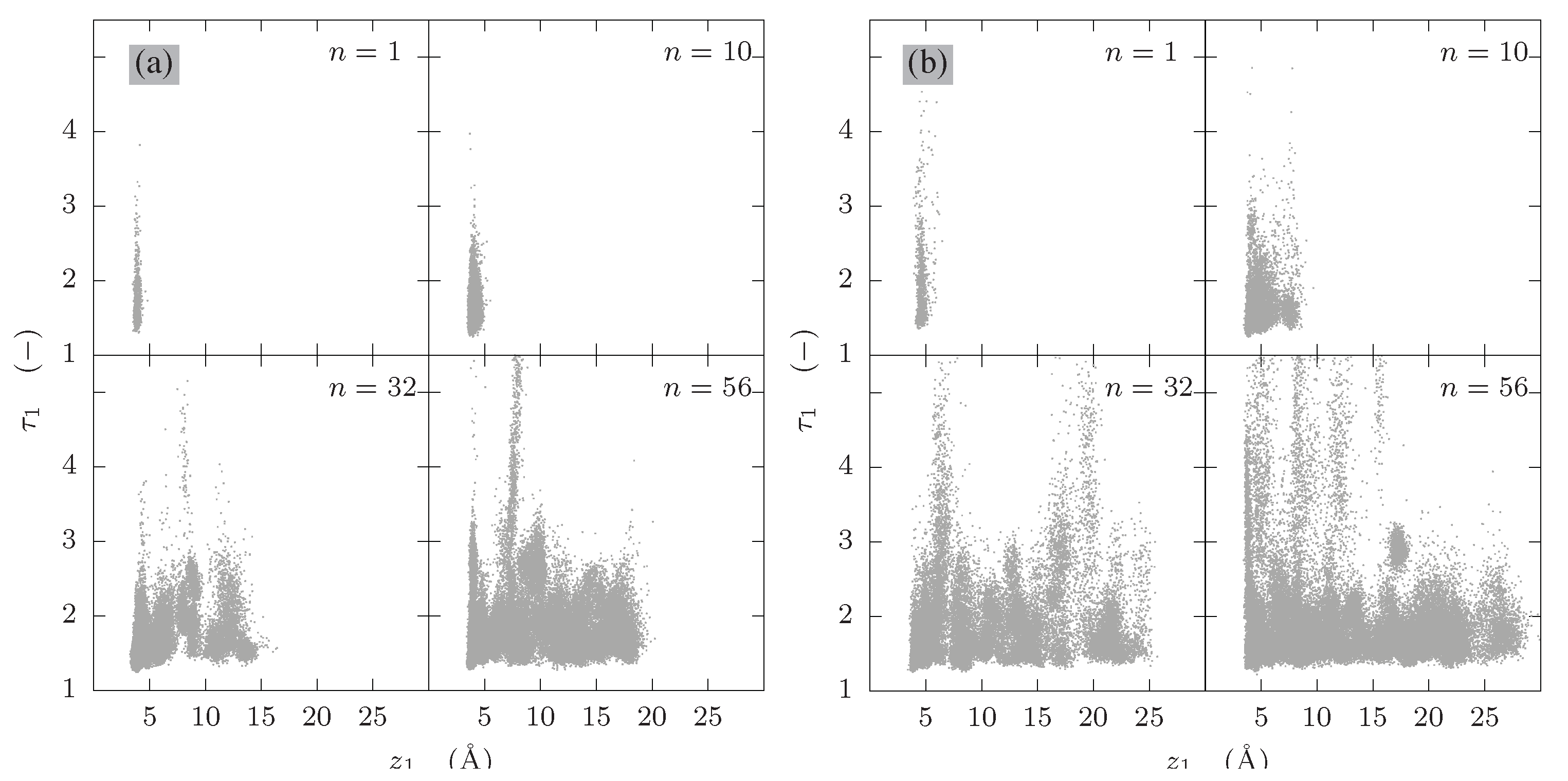
3.3. Internal Order
3.4. Residual Water Distribution
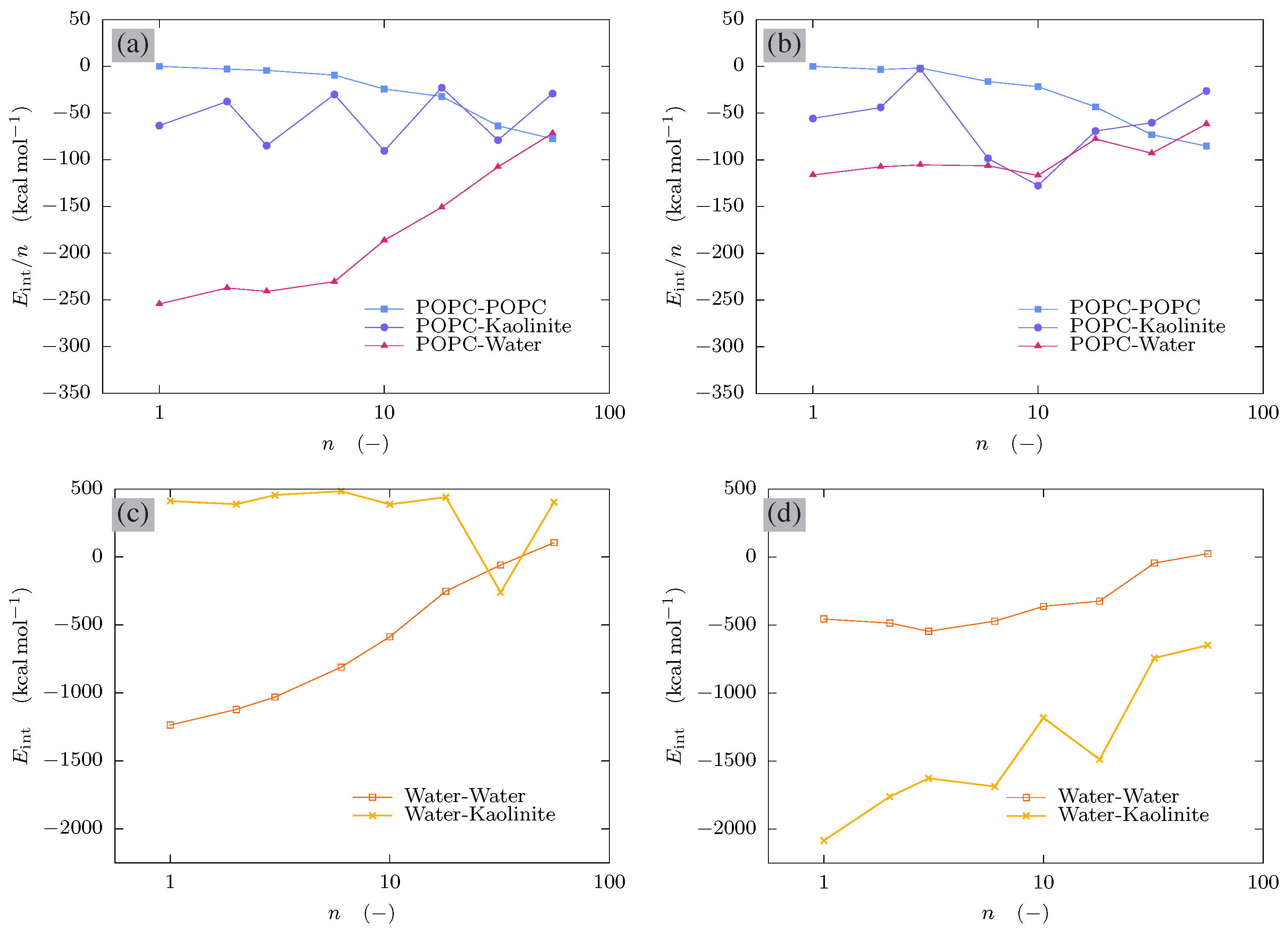
3.5. Interaction Energies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nafees, M.; Waseem, A. Organoclays as Sorbent Material for Phenolic Compounds: A Review. CLEAN—Soil Air Water 2014, 42, 1500–1508. [Google Scholar] [CrossRef]
- Lee, S.M.; Tiwari, D. Organo and Inorgano-Organo-Modified Clays in the Remediation of Aqueous Solutions: An Overview. Appl. Clay Sci. 2012, 59–60, 84–102. [Google Scholar] [CrossRef]
- Fornes, T.D.; Yoon, P.J.; Keskkula, H.; Paul, D.R. Nylon 6 Nanocomposites: The Effect of Matrix Molecular Weight. Polymer 2001, 42, 09929–09940. [Google Scholar] [CrossRef]
- Hedley, C.B.; Yuan, G.; Theng, B.K.G. Thermal Analysis of Montmorillonites Modified with Quaternary Phosphonium and Ammonium Surfactants. Appl. Clay Sci. 2007, 35, 180–188. [Google Scholar] [CrossRef]
- Lagaly, G.; Dékany, I. Adsorption on Hydrophobized Surfaces: Clusters and Self-Organization. Adv. Colloid Interface Sci. 2005, 114–115, 189–204. [Google Scholar] [CrossRef]
- Koh, S.M.; Song, M.; Takagi, T. Mineralogy, Chemical Characteristics and Stabilities of Cetylpyridinium-Exchanged Smectite. Clay Miner. 2005, 40, 213–222. [Google Scholar] [CrossRef]
- He, H.; Zhou, Q.; Martens, W.N.; Kloprogge, T.J.; Yuan, P.; Xi, Y.; Zhu, J.; Frost, R.L. Microstructure of HDTMA+-Modified Montmorillonite and its Influence on Sorption Characteristics. Clays Clay Miner. 2006, 54, 689–696. [Google Scholar] [CrossRef]
- Dultz, S.; Riebe, B.; Bunnenberg, C. Temperature Effects on Iodine Adsorption on Organo-Clay Minerals. II. Structural effects. Appl. Clay Sci. 2005, 28, 17–30. [Google Scholar] [CrossRef]
- Park, Y.; Ayoko, G.A.; Horvath, E.; Kurdi, R.; Kristof, J.; Frost, R.L. Structural Characterisation and Environmental Application of Organoclays for the Removal of Phenolic Compounds. J. Colloid Interface Sci. 2013, 393, 319–334. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J. Expansion Characteristics of Organoclay as a Precursor to Nanocomposites. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 211, 19–26. [Google Scholar] [CrossRef]
- Schampera, B.; Dultz, S. Determination of Diffusive Transport in HDPy-Montmorillonite by H2O-D2O Exchange Using in situ ATR-FTIR Spectroscopy. Clay Miner. 2009, 44, 249–266. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Li, L.; Xu, J.; Sun, D. Surface Modification of Natural Na-Montmorillonite in Alkane Solvents Using a Quaternary Ammonium Surfactant. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 426, 26–32. [Google Scholar] [CrossRef]
- Schampera, B.; Šolc, R.; Tunega, D.; Dultz, S. Experimental and Molecular Dynamics Study on Anion Diffusion in Organically Modified Bentonite. Appl. Clay Sci. 2016, 120, 91–100. [Google Scholar] [CrossRef]
- Schampera, B.; Šolc, R.; Woche, S.K.; Mikutta, R.; Dultz, S.; Guggenberger, G.; Tunega, D. Surface Structure of Organoclays as Examined by X-ray Photoelectron Spectroscopy and Molecular Dynamics Simulations. Clay Miner. 2015, 50, 353–367. [Google Scholar] [CrossRef]
- Heinz, H.; Suter, U.W. Surface Structure of Organoclays. Angew. Chem. Int. Ed. 2004, 43, 2239–2243. [Google Scholar] [CrossRef]
- He, H.; Ding, Z.; Zhu, J.; Yan, P.; Xi, Y.; Yang, D.; Frost, R.L. Thermal Characteristics of Surfactant Modified Montmorillonites. Clays Clay Miner. 2005, 53, 287–293. [Google Scholar] [CrossRef]
- Heinz, H.; Vaia, R.A.; Krishnamoorti, R.; Farmer, B.L. Self-Assembly of Alkylammonium Chains on Montmorillonite: Effect of Chain Length, Head Group Structure, and Cation Exchange Capacity. Chem. Mater. 2007, 19, 59–68. [Google Scholar] [CrossRef]
- Heinz, H.; Lin, T.J.; Kishore Mishra, R.; Emami, F.S. Thermodynamically Consistent Force Fields for the Assembly of Inorganic, Organic, and Biological Nanostructures: The INTERFACE Force Field. Langmuir 2013, 29, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Castelijns, H.J.; Suter, U.W. Structure and Phase Transitions of Alkyl Chains on Mica. J. Am. Chem. Soc. 2003, 125, 9500–9510. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.H.; Yu, A.B.; Lu, G.Q. Molecular Dynamics Simulation of the Structural and Dynamic Properties of Dioctadecyldimethylammonium in Organoclays. J. Phys. Chem. B 2004, 108, 10025–10033. [Google Scholar] [CrossRef]
- Fu, Y.T.; Heinz, H. Cleavage Energy of Alkylammonium-Modified Montmorillonite and Relation to Exfoliation in Nanocomposites: Influence of Cation Density, Head Group Structure, and Chain Length. Chem. Mater. 2010, 22, 1595–1605. [Google Scholar] [CrossRef]
- Zhao, Q.; Burns, S.E. Modelling Sorption and Diffusion of Organic Sorbate in Hexadecyltrimethylammonium-Modified Clay Nanopores—A Molecular Dynamics Simulation Study. Environ. Sci. Technol. 2013, 47, 2769–2776. [Google Scholar] [CrossRef]
- Schampera, B.; Tunega, D.; Šolc, R.; Woche, S.K.; Mikutta, R.; Wirth, R.; Dultz, S.; Guggenberger, G. External Surface Structure of Organoclays Analyzed by Transmission Electron Microscopy and X-ray Photoelectron Spectroscopy in Combination with Molecular Dynamics Simulations. J. Colloid Interface Sci. 2016, 478, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Scholtzová, E.; Tunega, D. Density Functional Theory Study of the Stability of the Tetrabutylphosphonium and Tetrabutylammonium. Clay Miner. 2019, 54, 41–48. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Dairi, N. Elaboration of Cellulose Acetate Nanobiocomposites Using Acidified Gelatin-Montmorillonite as Nanofiller: Morphology, Properties, and Biodegradation Studies. Polym. Compos. 2013, 34, 1515–1524. [Google Scholar] [CrossRef]
- Wicklein, B.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bio-Organoclays Based on Phospholipids as Immobilization Hosts for Biological Species. Langmuir 2010, 26, 5217–5225. [Google Scholar] [CrossRef]
- Nagy, K.; Bíró, G.; Berkesi, O.; Benczédi, D.; Ouali, L.; Dékány, I. Intercalation of Lecithins for Preparation of Layered Nanohybrid Materials and Adsorption of Limonene. Appl. Clay Sci. 2013, 72, 155–162. [Google Scholar] [CrossRef]
- Merino, D.; Ollier, R.; Lanfranconi, M.; Alvarez, V. Preparation and Characterization of Soy Lecithin-Modified Bentonites. Appl. Clay Sci. 2016, 127–128, 17–22. [Google Scholar] [CrossRef]
- Grančič, P.; Tunega, D. Geometry and Molecular Arrangement of Phosphatidylcholine-Montmorillonite Bioclays via Classical Molecular Dynamics Simulation. Appl. Clay Sci. 2020, 198, 105815. [Google Scholar] [CrossRef]
- Gaylor, M.O.; Miro, P.; Vlaisavljevich, B.; Kondage, A.A.S.; Barge, L.M.; Omran, A.; Videau, P.; Swenson, V.A.; Leinen, L.J.; Fitch, N.W.; et al. Plausible Emergence and Self Assembly of a Primitive Phospholipid from Reduced Phosphorus on the Primordial Earth. Orig. Life Evol. Biosph. 2021. [Google Scholar] [CrossRef]
- Schoonheydt, R.; Johnston, C. Surface and Interface Chemistry of Clay Minerals. In Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 139–172. [Google Scholar] [CrossRef]
- Ma, C.; Eggleton, R.A. Cation Exchange Capacity of Kaolinite. Clays Clay Miner. 1999, 47, 174–180. [Google Scholar] [CrossRef]
- Kahr, G.; Madsen, F. Determination of the Cation Exchange Capacity and the Surface Area of Bentonite, Illite and Kaolinite by Methylene Blue Adsorption. Appl. Clay Sci. 1995, 9, 327–336. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short–Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Bish, D.L. Rietveld Refinement of the Kaolinite Structure at 1.5 K. Clays Clay Miner. 1993, 41, 738–744. [Google Scholar] [CrossRef]
- Hestenes, M.R.; Stiefel, E. Methods of Conjugate Gradients for Solving Linear Systems. J. Res. Natl. Bur. Stand. 1952, 49, 409–436. [Google Scholar] [CrossRef]
- Scholzová, E.; Tunega, D.; Madejová, J.; Pálková, H.; Komadel, P. Theoretical and Experimental Study of Montmorillonite Intercalated with Tetramethylammonium Cation. Vib. Spectrosc. 2013, 66, 123–131. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical Dynamics: Equilibrium Phase-Space Distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. A Unified Formulation of the Constant Temperature Molecular Dynamics Methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Verlet, L. Computer “Experiments” on Classical Fluids. I. Thermodynamical Properties of Lennard-Jones Molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Hockney, R.W.; Eastwood, J.W. Particle-Particle/Particle-Mesh Algorithms. In Computer Simulation Using Particles; CRC Press: Boca Raton, FL, USA, 1988; pp. 267–304. [Google Scholar]
- Yeh, I.C.; Berkowitz, M.L. Ewald Summation for Systems with Slab Geometry. J. Chem. Phys. 1999, 111, 3155. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar] [CrossRef]
- Sánchez-Verdejo, T.; Undabeytia, T.; Nir, S.; Maqueda, C.; Morillo, E. Environmentally Friendly Slow Release Formulations of Alachlor Based on Clay-Phosphatidylcholine. Environ. Sci. Technol. 2008, 42, 5779–5784. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grančič, P.; Tunega, D. Hydrophobicity and Charge Distribution Effects in the Formation of Bioorganoclays. Minerals 2021, 11, 1102. https://doi.org/10.3390/min11101102
Grančič P, Tunega D. Hydrophobicity and Charge Distribution Effects in the Formation of Bioorganoclays. Minerals. 2021; 11(10):1102. https://doi.org/10.3390/min11101102
Chicago/Turabian StyleGrančič, Peter, and Daniel Tunega. 2021. "Hydrophobicity and Charge Distribution Effects in the Formation of Bioorganoclays" Minerals 11, no. 10: 1102. https://doi.org/10.3390/min11101102
APA StyleGrančič, P., & Tunega, D. (2021). Hydrophobicity and Charge Distribution Effects in the Formation of Bioorganoclays. Minerals, 11(10), 1102. https://doi.org/10.3390/min11101102





