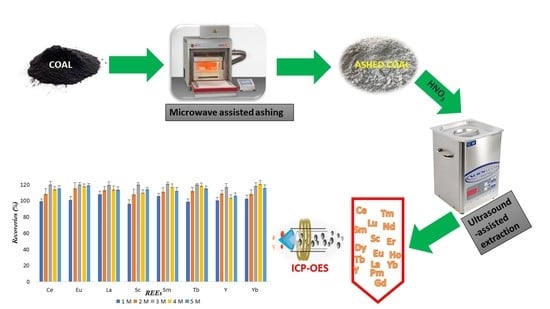
Simultaneous Determination of REEs in Coal Samples Using the Combination of Microwave-Assisted Ashing and Ultrasound-Assisted Extraction Methods Followed by ICP-OES Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Samples, Reagents, and Standards
2.3. Microwave-Assisted Ashing (MAA) Procedure
2.4. Ultrasound-Assisted Extraction Procedure
3. Results and Discussion
3.1. Effect of the HNO3 Acid Concentration during UAE of REEs in Coal
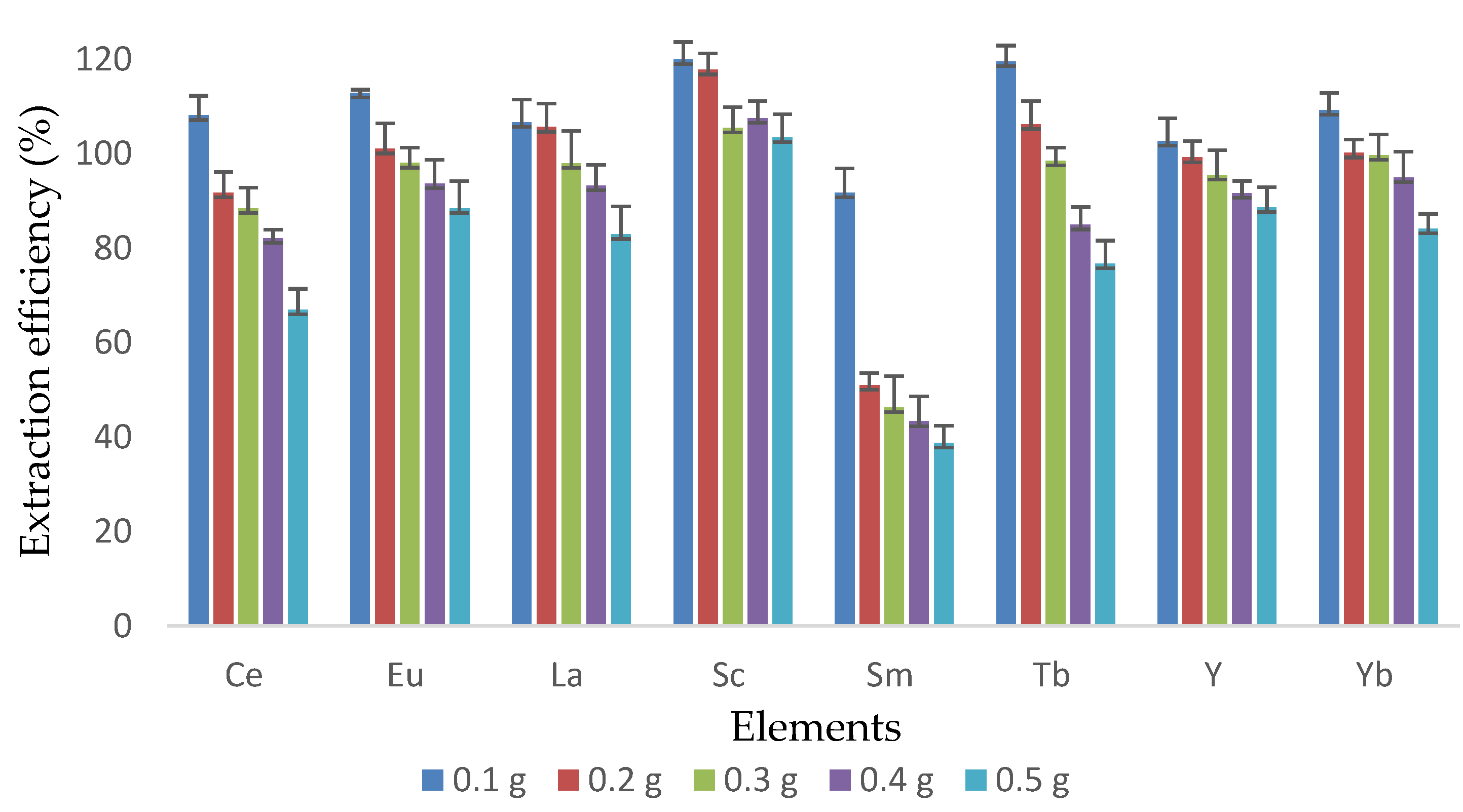
3.2. Effect of Sample Weight during UAE of REEs in Coal
3.3. Effect of the Time during UAE of REEs in Coal
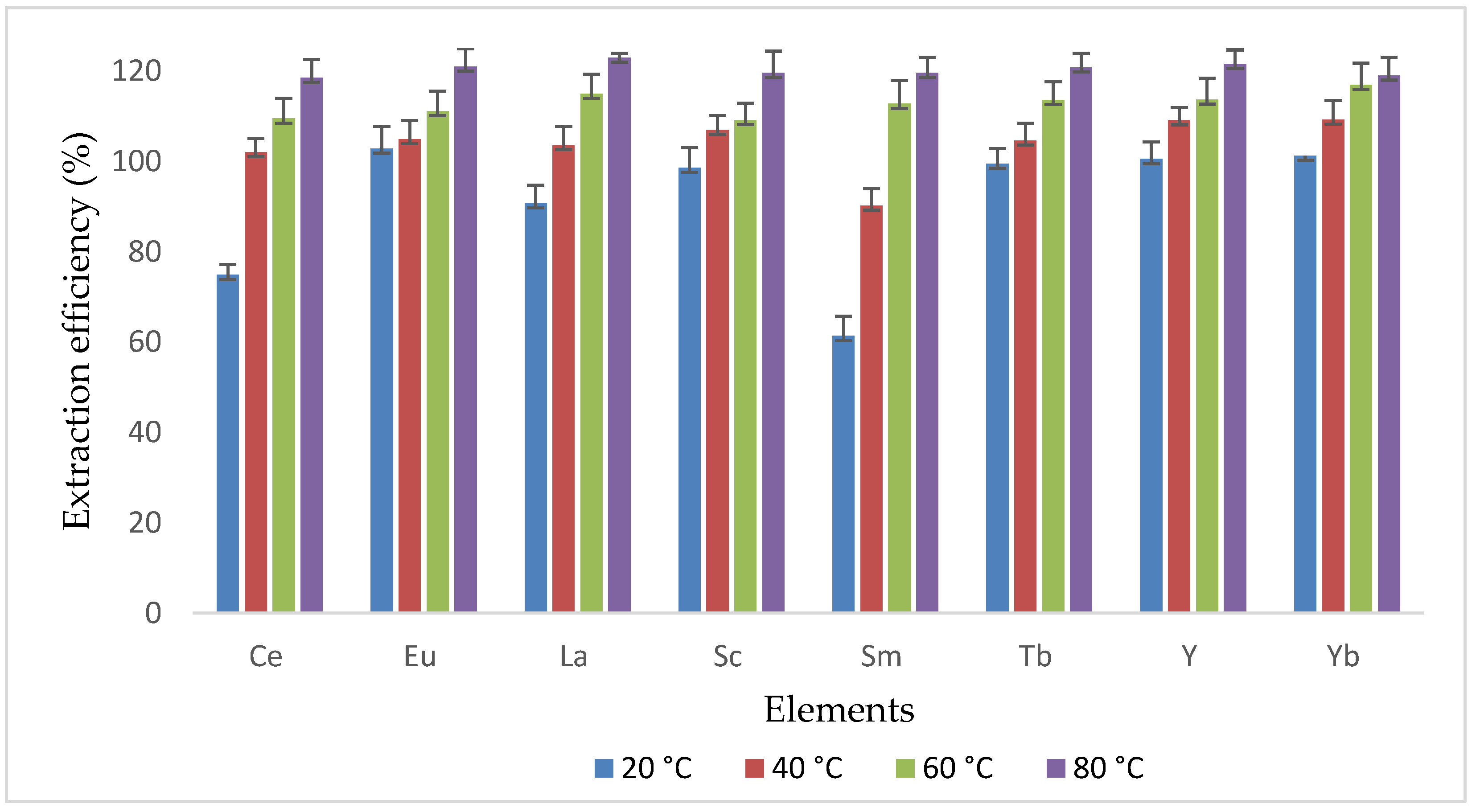
3.4. Effect of Temperature during UAE of REEs in Coal
3.5. Effect of Ultrasound Frequency during UAE of REEs in Coal
3.6. Validation of the MAA-UAE Method
3.6.1. Accuracy
3.6.2. Analytical Figures of Merits
3.7. Application of MAD-UAE Method in Real Coal Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, S.; Zhou, C.; Pan, J.-H.; Liu, C.; Tang, M.; Ji, W.; Hu, T.; Zhang, N. Study on influence factors of leaching of rare earth elements from coal fly ash. Energy Fuels 2018, 32, 8000–8005. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. A Comprehensive review of rare earth elements recovery from coal-related materials. Minerals 2020, 10, 451. [Google Scholar] [CrossRef]
- Phuoc, T.X.; Wang, P.; McIntyre, D. Detection of rare earth elements in Powder River Basin sub-bituminous coal ash using laser-induced breakdown spectroscopy (LIBS). Fuel 2016, 163, 129–132. [Google Scholar] [CrossRef]
- Kolker, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar] [CrossRef]
- Wu, S.; Hong, W.; Zhang, B.; Yang, C.; Wang, J.; Gao, J.; Mi, F.; Zhang, H.; Zhao, X.; Li, Q. Study on the determination of rare earth elements in coal ash by ICP-MS. Integr. Ferroelectr. 2019, 198, 116–121. [Google Scholar] [CrossRef]
- Krishna, M.V.B.; Venkateswarlu, G.; Karunasagar, D. Development of a simple and robust microwave-assisted decomposition method for the determination of rare earth elements in coal fly ash by ICP-OES. Anal. Methods 2017, 9, 2031–2040. [Google Scholar] [CrossRef]
- Ardini, F.; Soggia, F.; Rugi, F.; Udisti, R.; Grotti, M. Comparison of inductively coupled plasma spectrometry techniques for the direct determination of rare earth elements in digests from geological samples. Anal. Chim. Acta 2010, 678, 18–25. [Google Scholar] [CrossRef]
- Mello, P.; Pereira, J.S.F.; Mesko, M.F.; Barin, J.S.; Flores, E.M. Sample preparation methods for subsequent determination of metals and non-metals in crude oil—A review. Anal. Chim. Acta 2012, 746, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Wilschefski, S.C.; Baxter, M.R. Inductively coupled plasma mass spectrometry: Introduction to analytical aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Mketo, N.; Nomngongo, P.N.; Ngila, J. An innovative microwave-assisted digestion method with diluted hydrogen peroxide for rapid extraction of trace elements in coal samples followed by inductively coupled plasma-mass spectrometry. Microchem. J. 2016, 124, 201–208. [Google Scholar] [CrossRef]
- Gholami, M.; Behkami, S.; Zain, M.G.S.B.S.M.; Bakirdere, S. A simple design for microwave assisted digestion vessel with low reagent consumption suitable for food and environmental samples. Sci. Rep. 2016, 6, 37186. [Google Scholar] [CrossRef]
- Szymczycha-Madeja, A.; Welna, M.; Pohl, P. Simple and fast sample preparation procedure prior to multi-element analysis of slim teas by ICP OES. Food Anal. Methods 2014, 7, 2051–2063. [Google Scholar] [CrossRef] [Green Version]
- Janski, R.; Neouze, M.-A.; Limbeck, A. Determination of rare earth elements in saline matrices using dispersed particle extraction and inductively coupled plasma mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Mketo, N.; Nomngongo, P.N.; Ngila, J.C. An overview on analytical methods for quantitative determination of multi-element in coal samples. TrAC Trends Anal. Chem. 2016, 85, 107–116. [Google Scholar] [CrossRef]
- Mketo, N.; Nomngongo, P.N.; Ngila, J.C. A rapid microwave-assisted acid extraction method based on the use of diluted HNO3-H2O2followed by ICP-MS analysis for simultaneous determination of trace elements in coal samples. Int. J. Environ. Anal. Chem. 2015, 95, 453–465. [Google Scholar] [CrossRef]
- Zhou, G.; Fu, L.; Li, X. Optimisation of ultrasound-assisted extraction conditions for maximal recovery of active monacolins and removal of toxic citrinin from red yeast rice by a full factorial design coupled with response surface methodology. Food Chem. 2015, 170, 186–192. [Google Scholar] [CrossRef]
- Ferrarini, S.F.; Maia, S.M. Application of ultrasound-assisted extraction and heating combined with icp-oes for coal sample analysis. At. Spectrosc. 2013, 25, 103–156. [Google Scholar] [CrossRef]
- Pontes, F.V.M.; Mendes, B.A.D.O.; De Souza, E.M.; Ferreira, F.N.; Da Silva, L.I.; Carneiro, M.C.; Monteiro, M.I.; De Almeida, M.D.; Neto, A.A.; Vaitsman, D.S. Determination of metals in coal fly ashes using ultrasound-assisted digestion followed by inductively coupled plasma optical emission spectrometry. Anal. Chim. Acta 2010, 659, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Nascentes, C.C.; Korn, M.; Arruda, M.A. A fast ultrasound-assisted extraction of Ca, Mg, Mn and Zn from vegetables. Microchem. J. 2001, 69, 37–43. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Bhat, R.; Min-Tze, L.; Karim, A.A.; Mansor, S.M. Influence of sonication treatments and extraction solvents on the phenolics and antioxidants in star fruits. J. Food Sci. Technol. 2012, 49, 510–514. [Google Scholar] [CrossRef]
- Kenari, R.E.; Dehghan, B. Optimization of ultrasound-assisted solvent extraction of hemp (Cannabis sativa L.) seed oil using RSM: Evaluation of oxidative stability and physicochemical properties of oil. Food Sci. Nutr. 2020, 8, 4976–4986. [Google Scholar] [CrossRef]
- Ni, Z.; Tang, F.; Liu, Y.; Shen, D.; Mo, R. Multielemental analysis of camellia oil by microwave dry ashing and inductively coupled plasma mass spectrometry. Anal. Lett. 2015, 48, 1777–1786. [Google Scholar] [CrossRef]
- Mohammed, E.; Mohammed, T.; Mohammed, A. Optimization of an acid digestion procedure for the determination of Hg, As, Sb, Pb and Cd in fish muscle tissue. MethodsX 2017, 4, 513–523. [Google Scholar] [CrossRef]
- Uddin, A.H.; Khalid, R.S.; Alaama, M.; Abdualkader, A.M.; Kasmuri, A.; Abbas, S.A. Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J. Anal. Sci. Technol. 2016, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Sastre, J.; Sahuquillo, A.; Vidal, M.; Rauret, G. Determination of Cd, Cu, Pb and Zn in environmental samples: Microwave-assisted total digestion versus aqua regia and nitric acid extraction. Anal. Chim. Acta 2002, 462, 59–72. [Google Scholar] [CrossRef]
- Mketo, N.; Ngila, J.C. Development of Microwave Based Sample Preparation Methods for Extraction of Multi-Element for Coal Clean-Up and Spectrometric Determination; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2016. [Google Scholar]
- Druzian, G.T.; Pereira, L.S.F.; Mello, P.; Mesko, M.F.; Duarte, F.; Flores, E.M.M. Rare earth element determination in heavy crude oil by USN-ICP-MS after digestion using a microwave-assisted single reaction chamber. J. Anal. At. Spectrom. 2016, 31, 1185–1191. [Google Scholar] [CrossRef]
- Fariñas, J.C.; Rucandio, I.; Pomares-Alfonso, M.S.; Villanueva-Tagle, M.E.; Larrea, M.T. Determination of rare earth and concomitant elements in magnesium alloys by inductively coupled plasma optical emission spectrometry. Talanta 2016, 154, 53–62. [Google Scholar] [CrossRef]
- Guimarães-Silva, A.K.; De Lena, J.C.; Froes, R.; Costa, L.M.; Nascentes, C.C. Evaluation of signal-to-background and Mg II/Mg I ratios as response for the optimization of rare earth elements determination by inductively coupled plasma optical emission spectrometry. J. Braz. Chem. Soc. 2012, 23, 753–762. [Google Scholar] [CrossRef]
- Mketo, N.; Nomngongo, P.N.; Ngila, J.C. Development of a novel and green microwave-assisted hydrogen peroxide digestion method for total sulphur quantitative extraction in coal samples prior to inductively coupled plasma-optical emission spectroscopy and ion-chromatography determination. RSC Adv. 2015, 5, 38931–38938. [Google Scholar] [CrossRef]
- Masanabo, N.M.; Zinyemba, O.; Mketo, N. A greener microwave-assisted extraction method for rapid spectroscopic determination of selected metals in river and freshwater sediment certified reference materials. Int. J. Environ. Anal. Chem. 2019, 99, 33–46. [Google Scholar] [CrossRef]
- Maran, J.P.; Mekala, V.; Manikandan, S. Modeling and optimization of ultrasound-assisted extraction of polysaccharide from Cucurbita moschata. Carbohydr. Polym. 2013, 92, 2018–2026. [Google Scholar] [CrossRef]
- Ghitescu, R.-E.; Volf, I.; Carausu, C.; Bühlmann, A.-M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, N.; Qu, B. An improved ultrasonic-assisted extraction method by optimizing the ultrasonic frequency for enhancing the extraction efficiency of lycopene from tomatoes. Food Anal. Methods 2016, 9, 2288–2298. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Fu, M.-L.; Liu, J.; Zhang, H.-F.; He, G.-Q.; Ruan, H. Optimization of ultrasonic-assisted extraction (UAE) of betulin from white birch bark using response surface methodology. Ultrason. Sonochem. 2009, 16, 599–604. [Google Scholar] [CrossRef]
- Wang, H.-J.; Pan, M.-C.; Chang, C.-K.; Chang, S.-W.; Hsieh, C. Optimization of ultrasonic-assisted extraction of cordycepin from cordyceps militaris using orthogonal experimental design. Molecules 2014, 19, 20808–20820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mketo, N.; Nomngongo, P.N. An improved microwave assisted sequential extraction method followed by spectrometric analysis for metal distribution determination in South African coal samples. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Pandey, S.P.; Kumar, S.D. Determination of rare earth elements in Indian kimberlite using inductively coupled plasma mass spectrometer (ICP-MS). J. Radioanal. Nucl. Chem. 2012, 294, 419–424. [Google Scholar] [CrossRef]
- Amaral, C.D.B.; Machado, R.C.; Virgilio, A.; Schiavo, D.; Nogueira, A.R.A.; Nobrega, A. Determination of Rare Earth Elements in Geological Samples Using the Agilent SVDV ICP-OES; Agilent Technologies: Santa Clara, CA, USA, 2016; pp. 1–6. [Google Scholar]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Mello, P.A.; Pedrotti, M.F.; Cruz, S.M.; Muller, E.I.; Dressler, V.L.; Flores, E. Determination of rare earth elements in graphite by solid sampling electrothermal vaporization-inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2015, 30, 2048–2055. [Google Scholar] [CrossRef]
- Pereira, J.S.F.; Mello, P.; Guimarães, R.; Guarnieri, R.; Fonseca, T.; Flores, E. Microwave-induced combustion of crude oil for further rare earth elements determination by USN–ICP-MS. Anal. Chim. Acta 2014, 844, 8–14. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, S.; Wang, C.Y.; Xu, P. Determination of rare earth elements and thorium at nanogram levels in ultramafic samples by inductively coupled plasma-mass spectrometry combined with chemical separation and pre-concentration. Geostand. Geoanal. Res. 2013, 37, 65–76. [Google Scholar] [CrossRef]
- Yan, X.; Dai, S.; Graham, I.T.; He, X.; Shan, K.; Liu, X. Determination of Eu concentrations in coal, fly ash and sedimentary rocks using a cation exchange resin and inductively coupled plasma mass spectrometry (ICP-MS). Int. J. Coal Geol. 2018, 191, 152–156. [Google Scholar] [CrossRef]
- Silva, J.; Henn, A.S.; Dressler, V.L.; Mello, P.A.; Flores, E.M.M. Feasibility of rare earth element determination in low concentration in crude oil: Direct sampling electrothermal vaporization-inductively coupled plasma mass spectrometry. Anal. Chem. 2018, 90, 7064–7071. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Conditions |
|---|---|
| RF generator power (kW): | 1.20 |
| Plasma flow (L/min): | 15.0 |
| Nebulizer (L/min): | 0.75 |
| Replication reding time (s): | 1.0 |
| Auxiliary gas (L/min): | 1.50 |
| Instru stabilization delay (s): | 15 |
| Pump rate (rpm) | 15 |
| Sample uptake delay (s): | 30 |
| Replicates: | 3 |
| Flush time (s): | 10 |
| Wavelengths (nm): | Ce (418.659 nm), Eu (397.197 nm), Er (361.265 nm), Gd (358.496 nm), Ho (347.425 nm), La (333.749 nm), Lu (219.556 nm), Nd (401.224 nm), Pr (390.843) Sc (357.634 nm), Sm (442.434 nm), Tb (350.914 nm), Tm (349.919 nm) Y (320.027 nm), Yb (218.572 nm) |
| Step | Temperature (°C) | Ramping Time (hours) | Hold (min) |
|---|---|---|---|
| 1 | 380 | 2 | 30 |
| 2 | 550 | 1 | 50 |
| CRM | Average Weight before Ashing (AWBA) (g) | Average Weight after Ashing (AWAA) (g) | Weight Difference (AWBA) − (AWAA) (g) | Weight Loss (%) |
|---|---|---|---|---|
| SARM 18 | 2.011 (1.99) | 0.4991 (2.65) | 1.5119 | 75.52 |
| SARM 19 | 2.009 (1.86) | 0.7382 (2.22) | 1.2708 | 63.52 |
| SARM 20 | 2.021 (1.56) | 0.9820 (2.95) | 1.0386 | 51.41 |
| CSA | 2.005 (0.006) | 0.6252 (0.018) | 1.3520 | 67.45 |
| CSB | 2.004 (0.005) | 0.5404 (0.047) | 1.3633 | 70.58 |
| CSC | 2.004 (0.002) | 0.3399 (0.012) | 1.6637 | 83.04 |
| Element | Sensitivity (L µg−1) | Correlation Coefficient (R2) | MLOD a (µg g−1) | MLOQ b (µg g−1) | Precision (Intraday) (%) |
|---|---|---|---|---|---|
| Cerium (Ce) | 22.430 | 0.9993 | 0.089 | 0.29 | 3.285 |
| Europium (Eu) | 183.392 | 0.9992 | 0.0075 | 0.025 | 2.96 |
| Lanthanum (La) | 75.943 | 0.9985 | 0.03 | 0.093 | 3.458 |
| Samarium (Sm) | 42.060 | 0.9992 | 0.14 | 0.46 | 2.509 |
| Scandium (Sc) | 565.996 | 0.9999 | 0.0039 | 0.013 | 3.053 |
| Yttrium (Y) | 47.692 | 0.9998 | 0.057 | 0.19 | 3.008 |
| Ytterbium (Yb) | 3.993 | 0.9985 | 0.59 | 1.96 | 4.805 |
| Terbium (Tb) | 44.597 | 0.9980 | 0.046 | 0.15 | 3.655 |
| Sample | Digestion Method | Sample Preparation Remarks | Detection Technique | Elements | MLOD (µg g−1) | Accuracy (%) | Precision (%) | Time (Hour) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Heavy crude oil | MAD-SRC | 500 mg, HNO3 (5 mL) + H2O2, (1 mL) and 220 °C | USN-ICP-MS | Ce, Dy, Er, Eu, Gd, Ho, La, Lu, Nd, Pr, Sm, Tb, Tm, Y, Yb | 0.00003–0.0006 | 94–110 | NR | 1 | [27] |
| Kimberlite Rock | 0.2 g, Na2O2 (1.5 g), and then dissolved in water and HCl | Y, La, Ce, Pr, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu | 0.12–1.54 | NR | <14 | NR | [38] | ||
| Crude oil | MAD-UV | 500 mg, (HNO3 + H2O2) (10 mL) and 220 °C | USN-ICP-MS | La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y | 0.00009–0.0006 | 97–102 | NR | 1 | [42] |
| Ultramafic rock | HPD | Step 1: 0.2 g, HF 0.5 (mL) + HNO3 (0.5 mL), and 150 °C Step 2: HF (0.5 mL), and heated to dryness, then, heated 190 °C in (HF (0.2 mL) + HNO3 (2 mL) and 190 °C | ICP-MS | Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Er, Tm, Yb, Lu | 0.00003–0.00051 | 93–102 | NR | 36 | [43] |
| Geological samples | MAD | Step 1: 50 mg, HNO3 (5 mL) + HF (2 mL), and 240 °C Step 2: HNO3, (5 mL) and 180 °C | ICP-MS | Eu | 0.000006 | 97.24 | 0.923 | 5.25 | [44] |
| Geological Rock | MAD | 100 mg, aqua regia, (9 mL) and 220 °C | SVDV-ICP-OE | La, Ce, Pr, Nd, Sm, Eu Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu | 0.02–2.6 | 90–107 | <5 | 0.66 | [39] |
| Geological sample | HPD | 250 mg, HCl + HNO3 + HF and 110 °C | ICP-OES | La, Nd, Eu, Gd, Dy, Er, Yb | 0.0003–0.0067 | 92–112 | <7 | 6 | [29] |
| Graphite mineral | MAD | HNO3 (5 mL) and H2O2 (1 mL) | USN-ICP-MS | Ce, Eu, Gd, La, Lu, Pr, Sm | 0.00006–0.018 | <100.2 | <24 | 1 | [41] |
| Crude oil | MIC | 250 mg and 1400 with 20 bar of oxygen and 50 mL of 6 M ammonium nitrate NH4NO3 as igniter | USN-ICP-MS | La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y | 0.00018–0.00153 | 97.8–102 | <5 | 5 | [45] |
| Coal | MAA-UAE | Ashed at 550 °C, extracted at 40 °C, in (1 mol L−1) HNO3, for 20 min at high frequency. | ICP-OES | Ce, Eu, La, Sc, Sm, Tb, Y, Yb | 0.0075–0.59 | 92–120 | <5 | 4.25 | Current study |
| Element | Coal Sample A (µg g−1) | Coal Sample B (µg g−1) | Coal Sample C (µg g−1) |
|---|---|---|---|
| Cerium (Ce) | <ND | <ND | 104.369 (1.22) |
| Europium (Eu) | <ND | <ND | 1.644 (4.46) |
| Erbium (Er) | 0.266 (3.78) | 0.272 (1.69) | 3.11 (1.87) |
| Gadolinium (Gd) | 0.382 (4.14) | 0.44 (2.62) | 7.13 (0.34) |
| Holmium (Ho) | 0.954 (4.40) | 0.979 (2.93) | 22.261 (2.67) |
| Lanthanum (La) | <ND | <ND | 54.989 (2.626) |
| Lutetium (Lu) | 1.109 (3.98) | 1.32 (4.75) | 1.330 (4.85) |
| Neodymium (Nd) | 1.24 (4.40) | 1.34 (4.687) | 43.63 (3.81) |
| Praseodymium (Pr) | <ND | <ND | 30.44 (3.98) |
| Samarium (Sm) | <ND | <ND | 7.163 (3.15) |
| Scandium (Sc) | <ND | <ND | 8.928 (2.42) |
| Yttrium (Y) | 0.111 (2.985) | 0.133 (4.988) | 31.463 (2.835) |
| Ytterbium (Yb) | 1.047 (3.018) | 1.609 (1.53) | 3.462 (1.658) |
| Terbium (Tb) | 0.0695 (2.147) | 0.0796 (3.31) | 1.459 (3.131) |
| Thulium (Tm) | <ND | <ND | 4.67 (4.46) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuma, M.C.; Nomngongo, P.N.; Mketo, N. Simultaneous Determination of REEs in Coal Samples Using the Combination of Microwave-Assisted Ashing and Ultrasound-Assisted Extraction Methods Followed by ICP-OES Analysis. Minerals 2021, 11, 1103. https://doi.org/10.3390/min11101103
Zuma MC, Nomngongo PN, Mketo N. Simultaneous Determination of REEs in Coal Samples Using the Combination of Microwave-Assisted Ashing and Ultrasound-Assisted Extraction Methods Followed by ICP-OES Analysis. Minerals. 2021; 11(10):1103. https://doi.org/10.3390/min11101103
Chicago/Turabian StyleZuma, Mceliseni C., Philiswa N. Nomngongo, and Nomvano Mketo. 2021. "Simultaneous Determination of REEs in Coal Samples Using the Combination of Microwave-Assisted Ashing and Ultrasound-Assisted Extraction Methods Followed by ICP-OES Analysis" Minerals 11, no. 10: 1103. https://doi.org/10.3390/min11101103
APA StyleZuma, M. C., Nomngongo, P. N., & Mketo, N. (2021). Simultaneous Determination of REEs in Coal Samples Using the Combination of Microwave-Assisted Ashing and Ultrasound-Assisted Extraction Methods Followed by ICP-OES Analysis. Minerals, 11(10), 1103. https://doi.org/10.3390/min11101103