Micro- and Nanotexture and Genesis of Ball Clays in the Lower Cretaceous (SE Iberian Range, NE Spain)
Abstract
:1. Introduction
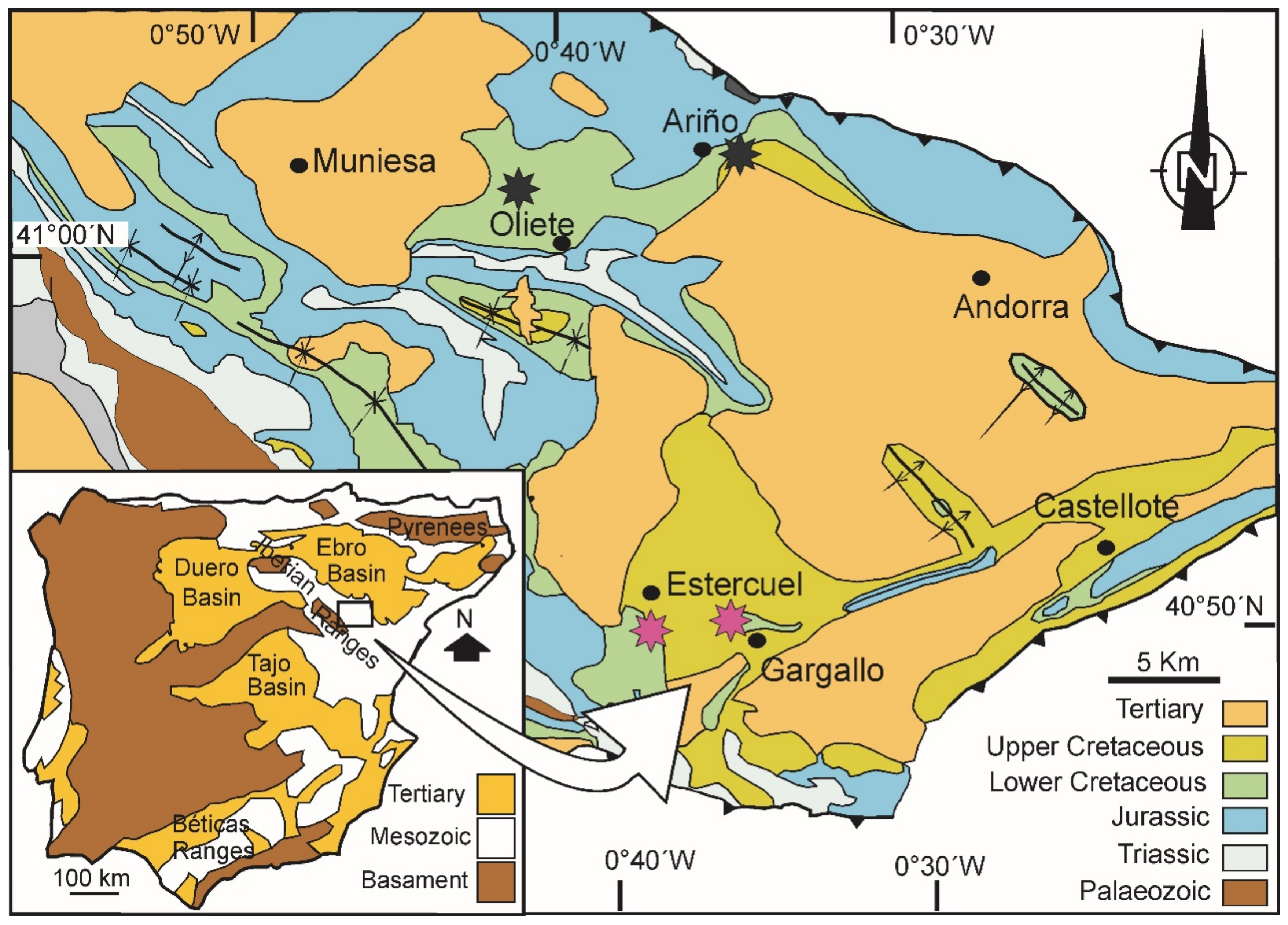
2. Geological Information
3. Materials and Methods
3.1. Impregnation with London Resin White (LRW) Resin and Ion Thinning
3.2. Particle Dispersion
4. Results
4.1. Mineralogy (XRD Studies)
4.2. Morphology of the Clays (FESEM/SE and TEM Images)
4.3. Microtexture and Composition of the Clays (FESEM/BSE Images)
4.4. Nanotextures of the Clays (TEM Images)
4.5. Chemical Composition of the Studied Ball Clays
5. Discussion
5.1. Mineralogy of the Ball Clay Deposits
5.2. Genesis of the Clay Minerals
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, I.R. The constitution, evaluation and ceramic properties of ball clays. Ceramica 1998, 44, 287–288. [Google Scholar] [CrossRef]
- Dondi, M.; Guarini, G.; Raimondo, M.; Salucci, F. Influence of mineralogy and particle size on the technological properties of ball clays for porcelain stoneware tiles. Tile Brick Int. 2003, 20, 2–11. [Google Scholar]
- Andreola, F.; Siligardi, C.; Manfredini, T.; Carbonchi, C. Rheological behaviour and mechanical properties of porcelain stoneware bodies containing Italian clay added with bentonites. Ceram. Int. 2009, 36, 1159–1164. [Google Scholar] [CrossRef]
- De Noni, A., Jr.; Hotza, D.; Cantavella Soler, V.; Sanchez Vilches, E. Analysis of the development of microscopic residual stresses on quartz particles in porcelain tile. J. Eur. Ceram. Soc. 2008, 28, 2629–2637. [Google Scholar] [CrossRef]
- Ferrari, S.; Gualtieri, A.F. The use of illitic clays in the production of stoneware tile ceramics. Appl. Clay Sci. 2006, 32, 73–81. [Google Scholar] [CrossRef]
- Leonelli, C.; Bondioli, F.; Veronesi, P.; Romagnoli, M.; Manfredini, T.; Pellacani, G.C.; Canillo, V. Enhancing the mechanical properties of porcelain stoneware tiles: A microstructural approach. J. Eur. Ceram. Soc. 2001, 21, 785–793. [Google Scholar] [CrossRef]
- Baioumy, H.M.; Ismael, I.S. Composition, origin and industrial suitability of the Aswan ball clays, Egypt. Appl. Clay Sci. 2014, 102, 202–212. [Google Scholar] [CrossRef]
- Dondi, M.; Raimondo, M.; Zanelli, C. Clays and bodies for ceramic tiles: Reappraisal and technological classification. Appl. Clay Sci. 2014, 96, 91–109. [Google Scholar] [CrossRef]
- Galos, K. Composition and ceramic properties of ball clays for porcelain stoneware tiles manufacture in Poland. Appl. Clay Sci. 2011, 51, 74–85. [Google Scholar] [CrossRef]
- Galos, K. Influence of mineralogical composition of applied ball clays on properties of porcelain tiles. Ceram. Int. 2011, 37, 851–861. [Google Scholar] [CrossRef]
- Jordán, M.M.; Meseguer, S.; Pardo, F.; Montero, M.A. Properties and possible ceramic uses of clays from lignite mine spoils of NW Spain. Appl. Clay Sci. 2015, 118, 158–161. [Google Scholar] [CrossRef] [Green Version]
- Bauluz, B.; Mayayo, M.J.; Yuste, A.; González López, J.M. Genesis of kaolinite from Albian sedimentary deposits of the Iberian Range (NE Spain): Analysis by XRD, SEM and TEM. Clay Miner. 2008, 43, 459–475. [Google Scholar] [CrossRef]
- Querol, X.; Salas, R.; Pardo, G.; Ardevol, L. Albian coal-bearing deposits of the Iberian Range in north eastern Spain. In Controls on the Distribution and Quality of Cretaceous Coals; McCabe, P.J., Totman, J.P., Eds.; Geological Special Paper 267; Geological Society of America: Boulder, CO, USA, 1992; pp. 193–208. [Google Scholar]
- Schultz, L.G. Quantitative interpretation of mineralogical composition from X-ray and chemical data for the Pierre shale. Prof. Pap. Geol. Surv. 1964, 391-c, 31. [Google Scholar]
- Biscaye, P.E. Mineralogy and sedimentation of recent deep-sea clay in the Atlantic Ocean and adjacent seas and ocean. Geol. Soc. Am. Bull. 1965, 76, 803–832. [Google Scholar] [CrossRef]
- Martin, J.D. A software package for powder x-ray diffraction analysis. Qual. Quant. Microtexture 2017, 5, 121. [Google Scholar]
- Churchman, G.J.; Theng, B.K.G. Interactions of halloysites with amides: Mineralogical factors affecting complex formation. Clay Miner. 1984, 19, 161–175. [Google Scholar] [CrossRef]
- Kim, J.W.; Peacor, D.R.; Tessier, D.; Elsass, F. A technique for maintaining texture and permanent expansion of smectite interlayers for TEM observations. Clays Clay Miner. 1995, 43, 51–57. [Google Scholar] [CrossRef]
- Sánchez-Roa, S.; Bauluz, B.; Nieto, F.; Abad, I.; Jimenéz-Millán, J.; Faulkner, D. Micro- and nano-scale study of deformation induced mineral transformations in Mg-phyllosilicate-rich fault gouges from the Galera Fault Zone (Betic Cordillera, SE Spain). Am. Mineral. J. Earth Planet. Mater. 2018, 103, 1604–1631. [Google Scholar] [CrossRef]
- Guthrie, G.D.; Veblen, D.R. High-resolution transmission electron microscopy of mixed-layer illite/Smectite: Computer simulation. Clays Clay Miner. 1989, 37, 1–11. [Google Scholar] [CrossRef]
- Guthrie, G.D.; Veblen, D.R. High-resolution Transmission electron microscopy applied to clay minerals. In Spectroscopic Characterization of Minerals and Their Surfaces; Coyne, L.M., Mckeever, S.W.W., Blake, D.F., Eds.; Symposia Series 415; American Chemical Society: Washington, DC, USA, 1989; pp. 75–93. [Google Scholar]
- Guthrie, G.D.; Veblen, D.R. Interpreting one-dimensional High-resolution transmission electron micrographs of sheet silicates by computer simulation. Am. Mineral. 1990, 75, 276–288. [Google Scholar]
- Bauluz, B.; Peacor, D.R.; Gonzalez-Lopez, J.M. Transmission electron microscopy study of illitization in pelites from the Iberian Range, Spain: Layer-by-layer replacement? Clays Clay Miner. 2000, 48, 374–384. [Google Scholar] [CrossRef]
- Djangang, C.N.; Elimbi, A.; Melo, U.C.; Lecomte, G.L.; Nkoumbou, C.; Soro, J.; Yvon, J.; Blanchart, P.; Njopwouo, D. Refractory ceramics from clays of Mayouom and Mvan in Cameroon. Appl. Clay Sci. 2008, 39, 10–18. [Google Scholar] [CrossRef]
- Bibi, I.; Singh, B.; Silvester, E. Dissolution of illite in saline-acidic solutions at 25 °C. Geochim. Cosmochim. Acta 2011, 75, 33237–33249. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Schott, J.; Gauthier, J.M.; Roncal-Herrero, J. An experimental study of the dissolution mechanism and rates of muscovite. Geochim. Cosmochim. Acta 2008, 72, 4948–4961. [Google Scholar] [CrossRef]
- Rossel, N.C. Clay mineral diagenesis in Roetliegend aeolian sandstones of the southern North Sea. Clay Miner. 1992, 17, 69–77. [Google Scholar] [CrossRef]
- Blackbourn, G.A. Diagenetic history and reservoir quality of a Brent sand sequence. Clay Miner. 1984, 19, 377–389. [Google Scholar] [CrossRef]
- Goodchild, M.W.; Whitaker, J.C.M. A graphic study of the Rotliegendes sandstone reservoir North Sea. Clay Miner. 1986, 21, 459–477. [Google Scholar] [CrossRef]
- Pye, K.; Krinsley, D.H. Diagenetic carbonate and evaporite minerals in Rotliegend aeolian sandstones of the southern North Sea: Their nature and relationship to secondary porosity development. Clay Miner. 1986, 21, 443–457. [Google Scholar] [CrossRef]
- Ehrenberg, S.N. Kaolinized, potassium-leached zones at the contacts of the Garn formation, Haltenbanken, mid-Norwegian continental shelf. Mar. Pet. Geol. 1991, 8, 250–269. [Google Scholar] [CrossRef]
- Gaupp, R.; Matter, A.; Platt, J.; Ramseyer, K.; Walzebuck, J. Diagenesis and fluid evolution of a deeply buried Permian (Rotliegende) gas reservoir, Northwest Germany. AAPG Bull. 1993, 77, 1111–1128. [Google Scholar]
- Platt, J.D. Controls on clay mineral distribution and chemistry in the early Permian Rotliegend of Germany. Clay Miner. 1993, 28, 393–416. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Dolansky, L.; Piper, D.J.W. Sedimentary environment and diagenesis of the Lower Cretaceous Chaswood Formation, southeastern Canada: The origin of kaolin-rich mudstones. Sediment. Geol. 2005, 178, 75–97. [Google Scholar] [CrossRef]
- Piper, D.J.W.; Hundert, T.; Pe-Piper, G.; Okwese, A.C. The roles of pedogenesis and diagenesis in clay mineral assemblages: Lower Cretaceous fluvial mudrocks, Nova Scotia, Canada. Sediment. Geol. 2009, 213, 51–63. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauluz, B.; Mayayo, M.J.; Laita, E.; Yuste, A. Micro- and Nanotexture and Genesis of Ball Clays in the Lower Cretaceous (SE Iberian Range, NE Spain). Minerals 2021, 11, 1339. https://doi.org/10.3390/min11121339
Bauluz B, Mayayo MJ, Laita E, Yuste A. Micro- and Nanotexture and Genesis of Ball Clays in the Lower Cretaceous (SE Iberian Range, NE Spain). Minerals. 2021; 11(12):1339. https://doi.org/10.3390/min11121339
Chicago/Turabian StyleBauluz, Blanca, María José Mayayo, Elisa Laita, and Alfonso Yuste. 2021. "Micro- and Nanotexture and Genesis of Ball Clays in the Lower Cretaceous (SE Iberian Range, NE Spain)" Minerals 11, no. 12: 1339. https://doi.org/10.3390/min11121339
APA StyleBauluz, B., Mayayo, M. J., Laita, E., & Yuste, A. (2021). Micro- and Nanotexture and Genesis of Ball Clays in the Lower Cretaceous (SE Iberian Range, NE Spain). Minerals, 11(12), 1339. https://doi.org/10.3390/min11121339






