Bentonite Powder XRD Quantitative Analysis Using Rietveld Refinement: Revisiting and Updating Bulk Semiquantitative Mineralogical Compositions
Abstract
:1. Introduction
- -
- 80 ± 10 wt.% smectite, mostly natural sodium exchanged forms. The sodium form has lower hydraulic conductivity and excellent swelling properties, in relation to other forms, such as calcium [11].
- -
- <30 wt.% accessory minerals.
- -
- -
- Highly variable structures, which generate different types of interstratification and defects in three-dimensional space lattice, cause greater complexity in the X-ray diffraction patterns, compared with phases with a high degree of crystallinity [15].
- -
- The preferential orientation of the smectite particles affects the signal intensities in pulverized samples. Phyllosilicates tend to be oriented in sheets, modifying the intensity of reflection compared to a random orientation and making analysis difficult. Preparation methods such as front loading tend to generate this preferential orientation [16].
- -
- The mixture of different accessory minerals imposes other difficulties. The presence of small crystal size or amorphous silica polymorphs produce very broad signals in XRD that do not allow good quantification. On the other hand, potassium feldspars and plagioclases generate numerous reflections with very little reproducible intensities, due to the difference of grinding size and effects of preferred orientation when mixed with soft clay [17,18].
2. Materials and Methods
2.1. Experiment
2.2. FEBEX Bentonite
2.3. XRD Quantification
2.3.1. Use of BGMN© and the Profex Interface
2.3.2. XRD Equipment Parameters
3. Results
3.1. FEBEX Bentonite
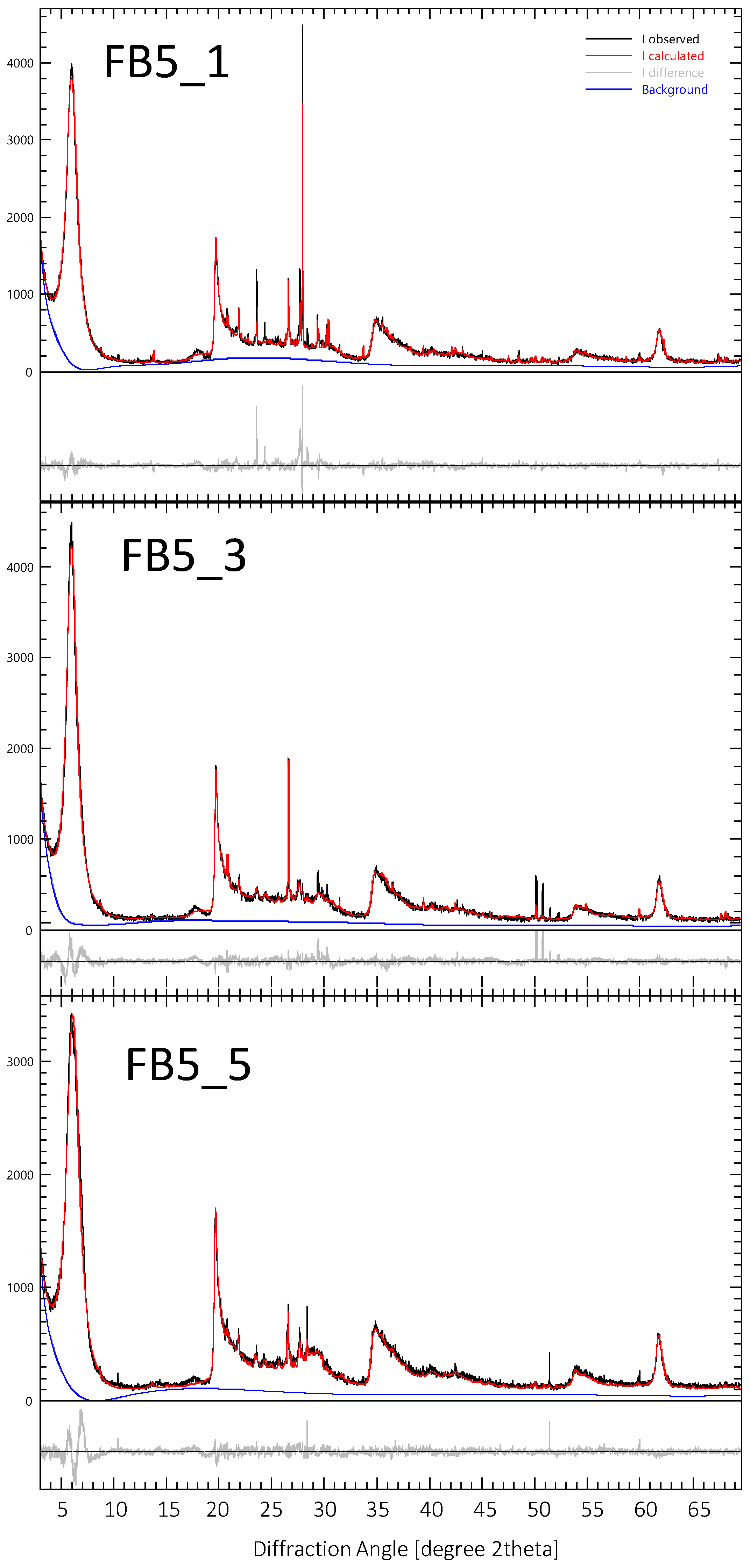
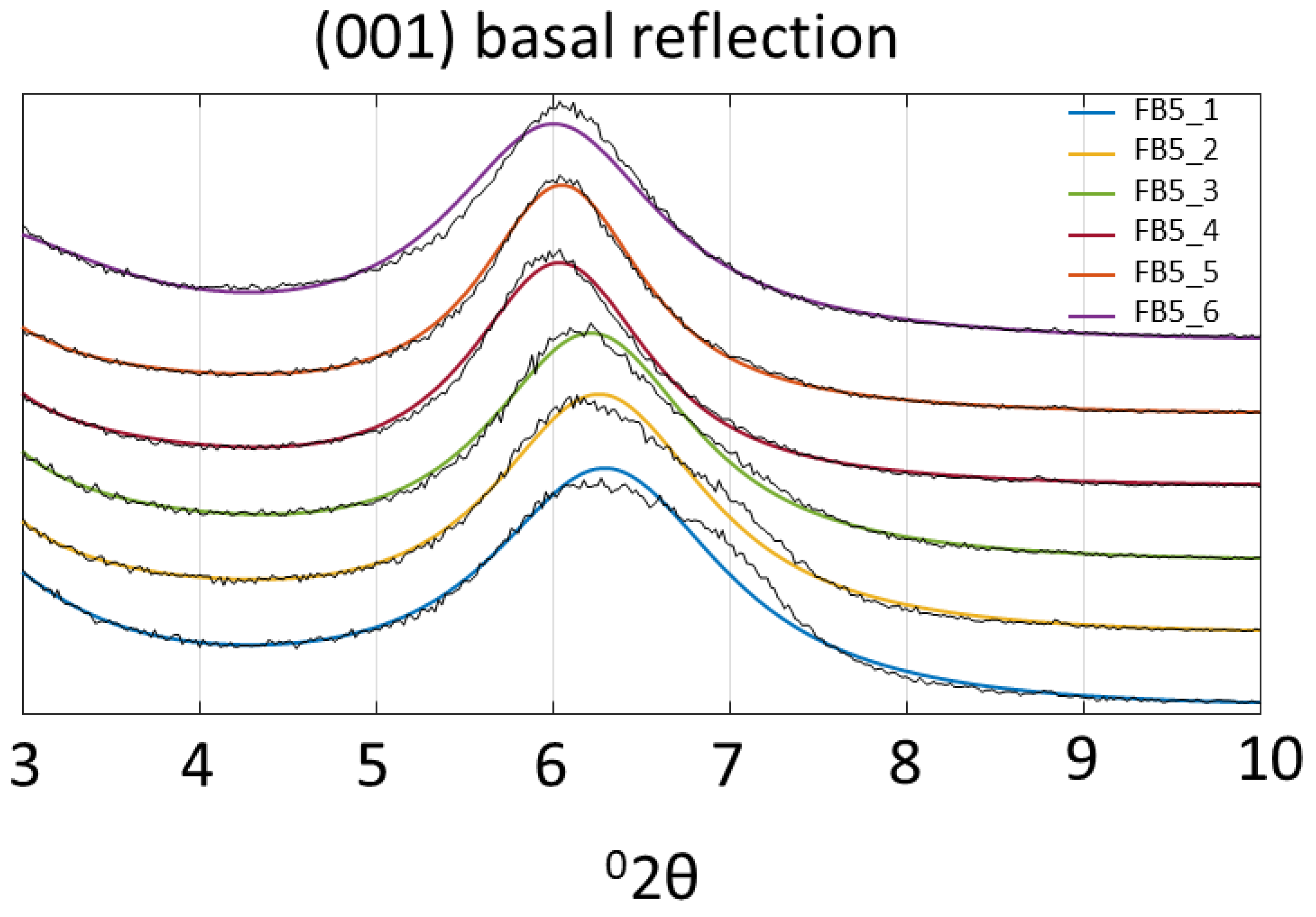
3.2. FB 5 Hydrothermal Experiment
4. Discussion of Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caballero, E.; de Cisneros, C.J.; Huertas, F.J.; Pozzuoli, A.; Linares, J. Bentonites from Cabo de Gata, Almería, Spain: A mineralogical and geochemical overview. Clay Miner. 2005, 40, 463–480. [Google Scholar] [CrossRef]
- Christidis, G.; Huff, W.D. Geological Aspects and Genesis of Bentonites. Elements 2009, 5, 93–98. [Google Scholar] [CrossRef]
- Gilg, H.A.; Kaufhold, S.; Ufer, K. Smectite and Bentonite Terminology, Classification, and Genesis. In Bentonites. Characterization, Geology, Mineralogy, Analysis, Mining, Processing and Uses; Kaufhold, S., Ed.; Publisher Geologisches Jahrbuch Reihe B: Hannover, Germany, 2002; pp. 1–18. [Google Scholar] [CrossRef]
- Güven, N. Molecular Aspects of Clay-Water Interactions. In Clay-Water Interface and Its Rheological Implications (CMS Workshop lectures, Vol 4); Güven, N., Pollastro, R.M., Eds.; The Clay Minerals Society: Boulder, CO, USA, 1992. [Google Scholar]
- Żbik, M.S.; Martens, W.; Frost, R.L.; Song, Y.-F.; Chen, Y.-M.; Chen, J.-H. Transmission X-ray Microscopy (TXM) Reveals the Nanostructure of a Smectite Gel. Langmuir 2008, 24, 8954–8958. [Google Scholar] [CrossRef] [PubMed]
- Odom, I.E. Smectite clay minerals: Properties and uses. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1984, 311, 391–409. [Google Scholar] [CrossRef]
- Weber, W.; Navrotsky, A.; Stefanovsky, S.; Vance, E.R.; Vernaz, E. Materials Science of High-Level Nuclear Waste Immobilization. MRS Bull. 2009, 34, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Cohen, B.L. High-level radioactive waste from light-water reactors. Rev. Mod. Phys. 1977, 49, 1–20. [Google Scholar] [CrossRef]
- El-Showk, S. Final resting place. Science 2022, 375, 806–810. [Google Scholar] [CrossRef]
- Cuevas, J.; Fernández, R.; Ortega, A.; Ruiz, A.I. Comparison of Alternative Bentonites for Potential Use as Buffer and Sealing Materials in the Swiss Concept for Ra-Dioactive Waste Disposal. Part 2: Results; Nagra Arbeitsbericht Wettingen; NAB: Aarau, Switzerland, 2014; pp. 14–65. [Google Scholar]
- Gates, W.P.; Bouazza, A.; Churchman, G.J. Bentonite Clay Keeps Pollutants at Bay. Elements 2009, 5, 105–110. [Google Scholar] [CrossRef]
- Madsen, F.T.; Müller-Vonmoos, M. The swelling behaviour of clays. Appl. Clay Sci. 1989, 4, 143–156. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General Introduction: Clays, Clay Minerals, and Clay Science. In Handbook of Clay Science: Developments in Clay Science, Vol. 1; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, P.; Liu, H.; Cai, J.; Tan, D.; He, H.; Zhu, J.; Chen, T. Quantitative characterization of the solid acidity of montmorillonite using combined FTIR and TPD based on the NH3 adsorption system. Appl. Clay Sci. 2013, 80, 407–412. [Google Scholar] [CrossRef]
- Srodon, J. Quantitative mineralogy of sedimentary rocks with emphasis on clays and with applications to K-Ar dating. Miner. Mag. 2002, 66, 677–687. [Google Scholar] [CrossRef]
- Hillier, S. Accurate quantitative analysis of clay and other minerals in sandstones by XRD: Comparison of a Rietveld and a reference intensity ratio (RIR) method and the importance of sample preparation. Clay Miner. 2000, 35, 291–302. [Google Scholar] [CrossRef]
- Bish, D.L.; Reynolds, R.C., Jr. Sample preparation for X-ray Diffraction. In Reviews in Mineralogy, Vol. 20, Modern Powder Diffraction; Bish, D.L., Post, J.E., Eds.; The Mineralogical Society of America: Washington, WA, USA, 1989. [Google Scholar]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. II. Adiabatic principle of X-ray diffraction analysis of mixtures. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Fernández, R.; Torres, E.; Ruiz, A.I.; Cuevas, J.; Alonso, M.C.; Calvo, J.L.G.; Rodríguez, E.; Turrero, M.J. Interaction processes at the concrete-bentonite interface after 13 years of FEBEX-Plug operation. Part II: Bentonite contact. Phys. Chem. Earth Parts A B C 2017, 99, 49–63. [Google Scholar] [CrossRef]
- Torres, E.; Escribano, A.; Baldonedo, J.L.; Turrero, M.J.; Martin, P.L.; Peña, J.; Villar, M.V. Evolution of the Geochemical Conditions in the Bentonite Barrier and Its Influence on the Corrosion of the Carbon Steel Canister. In Proceedings of the Materials Research Society Symposium Fall Meeting, Boston, MA, USA, 1–5 December 2008. [Google Scholar]
- Torres, E. Geochemical Processes at the C-Steel/Bentonite Interface in a Deep Geological Repository: Experimental Approach and Modeling. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2011. [Google Scholar]
- Cuadros, J.; Linares, J. Experimental kinetic study of the smectite-to-illite transformation. Geochim. Cosmochim. Acta 1996, 60, 439–453. [Google Scholar] [CrossRef]
- Huertas, F.; Fariñas, P.; Farias, J.; García-Siñeriz, J.L.; Villar, M.V.; Fernández, A.M.; Martín, P.L.; Elorza, F.J.; Gens, A.; Sánchez, M.; et al. Full-Scale Engineered Barriers Experiment. Updated Final Report 1994–2004; Enresa Technical Report 05-0/2006; ENRESA: Madrid, Spain, 2006. [Google Scholar]
- Villar, M.; Fernández, A.; Rivas, P.; Lloret, A.; Daucausse, D.; Montarges-Pelletier, E.; Devineau, K.; Villieras, F.; Hynkova, E.; Cechova, Z.; et al. (Eds.) FEBEX Project Final Report. Post-Mortem Bentonite Analysis; Enresa Technical Report 05-1/2006; ENRESA: Madrid, Spain, 2006. [Google Scholar]
- Martín Pozas, I.M.; Martín Vivaldi, J.L.; Rodríguez Gallego, M. Análisis cuantitativo de filosilicatos de la arcilla por difracción de rayos X. An. Real Soc. Esp. Fis. Y Quim. Serie B 1969, 55, 109–112. [Google Scholar]
- Poppe, L.J.; Eliason, A.E. A basic program to calculate gravitational and centrifugal settling parameters. Geol. Soc. Am. 2009, 41, 21. [Google Scholar]
- Ramírez, S. Hydrothermal Alteration of “La Serrata” Bentonite (Almería, Spain) by Alkaline Solutions. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2002. [Google Scholar]
- Inoue, A.; Bouchet, A.; Velde, B.; Meunier, A. Convenient technique for estimating smectite layer percentage in randomly interstratified illite/smectite minerals. Clays Clay Miner. 1989, 37, 227–234. [Google Scholar] [CrossRef]
- Bergmann, J.; Kleeberg, R.; Taut, T.; Haase, A. Quantitative Phase Analysis Using a New Rietveld Algorithm—Assisted by Improved Stability and Convergence Behavior. In Proceedings of the 45th annual X-ray Conference, Denver, CO, USA, 3–8 August 1996. Advances in X-ray Analysis, 40 (1998), 425 (published on CD-ROM). [Google Scholar]
- Ufer, K.; Roth, G.; Kleeberg, R.; Stanjek, H.; Dohrmann, R.; Bergmann, J. Description of X-ray powder pattern of turbostratically disordered layer structures with a Rietveld compatible approach. Z. Für Krist.-Cryst. Mater. 2004, 219, 519–527. [Google Scholar] [CrossRef]
- Ufer, K.; Stanjek, H.; Roth, G.; Dohrmann, R.; Kleeberg, R.; Kaufhold, S. Quantitative phase analysis of bentonites by the Rietveld method. Clays Clay Miner. 2008, 56, 272–282. [Google Scholar] [CrossRef]
- Kleeberg, R.; Monecke, T.; Hillier, S. Preferred orientation of mineral grains in sample mounts for quantitative XRD measurements: How random are powder samples? Clays Clay Miner. 2008, 56, 404–415. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viani, A.; Gualtieri, A.F.; Artioli, G. The nature of disorder in montmorillonite by simulation of X-ray powder patterns. Am. Mineral. 2002, 87, 966–975. [Google Scholar] [CrossRef]
- Prewitt, C.T.; Sueno, S.; Papike, J.J. The crystal structures of high albite and monalbite at high temperatures. Am. Mineral. 1976, 61, 1213–1225. [Google Scholar]
- Wainwright, J.E.; Starkey, J. A refinement of the structure of anorthite. Zeitschrift für Kristallographie-Crystalline Materials 1971, 133, 75–84. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, A.; Sun, D. Three-dimensional thermal analysis of the repository for high-level radioactive nuclear waste. Int. J. Energy Res. 2020, 44, 8208–8220. [Google Scholar] [CrossRef]
- Gómez-Espina, R.; Villar, M.V. Effects of heat and humidity gradients on MX-80 bentonite geochemistry and mineralogy. Appl. Clay Sci. 2015, 109, 39–48. [Google Scholar] [CrossRef]
- Villar, M.V.; Martín, P.L.; Bárcena, I.; García-Siñeriz, J.L.; Gómez-Espina, R.; Lloret, A. Long-term experimental evidences of saturation of compacted bentonite under repository conditions. Eng. Geol. 2012, 149, 57–69. [Google Scholar] [CrossRef]
- Sellin, P.; Leupin, O.X. The use of clay as an engineered barrier in radioactive-waste management a Review. Clays Clay Miner. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Plançon, A. Consistent Modeling of the XRD Patterns of Mixed-layer Phyllosilicates. Clays Clay Miner. 2004, 52, 47–54. [Google Scholar] [CrossRef]
- Xie, M.; Miehe, R.; Kasbohm, J.; Herbert, H.-J.; Meyer, K.; Ziesche, U. Bentonite Barriers—New Experiments and State of the Art. Bentonite as Barrier Material for the Sealing of Underground Disposal Sites. Final Report; GRS–300: Koeln, Germany, 2012; ISBN 978-3-939355-79-3. [Google Scholar]
- Turrero, M.J.; Villar, M.V.; Torres, E.; Escribano, A.; Cuevas, J.; Fernández, R.; Ruiz, A.I.; Vigil de la Villa, R.; de Soto, I. Long-term Performance of Engineered Barrier Systems PEBS Contract (Grant Agreement) Number: FP7 249681, DELIVERABLE (D-N°:D2.3-3-1). Laboratory Tests at the Interfaces (First Results on the Dismantling of Tests FB3 and HB4), 31 October 2011. Available online: https://igdtp.eu/wp-content/uploads/2018/04/D2_3_6_2.pdf (accessed on 13 June 2022).
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Transformation of two-line ferrihydrite to goethite and hematite as a function of pH and temperature. Environ. Sci. Technol. 2011, 45, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.V. FEBEX-DP Post-Mortem. THM/THG Analysis Report; Arbeitsbericht NAB; Nagra: Wettingen, Switzerland, 2017; pp. 16–17. [Google Scholar]
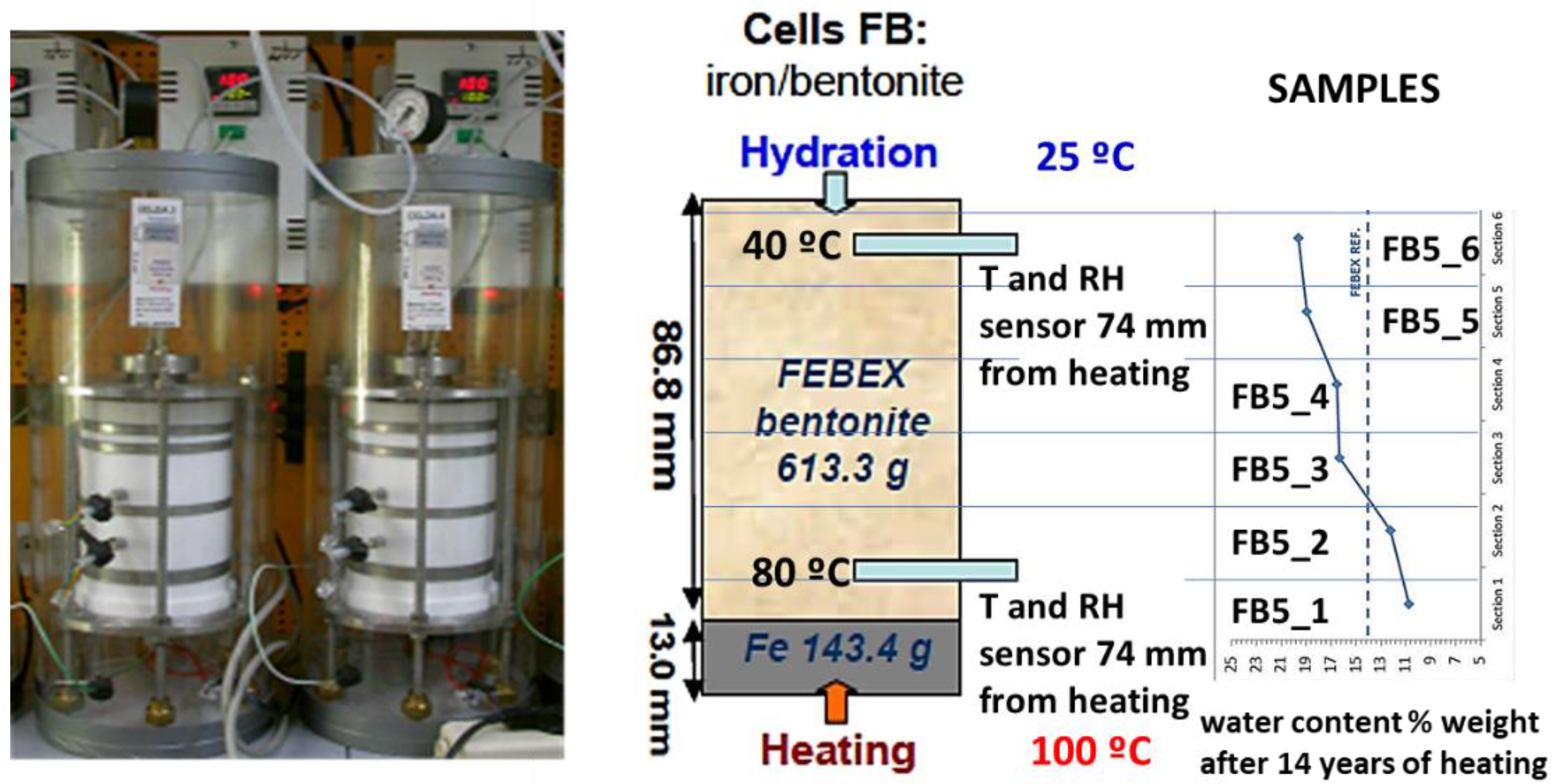
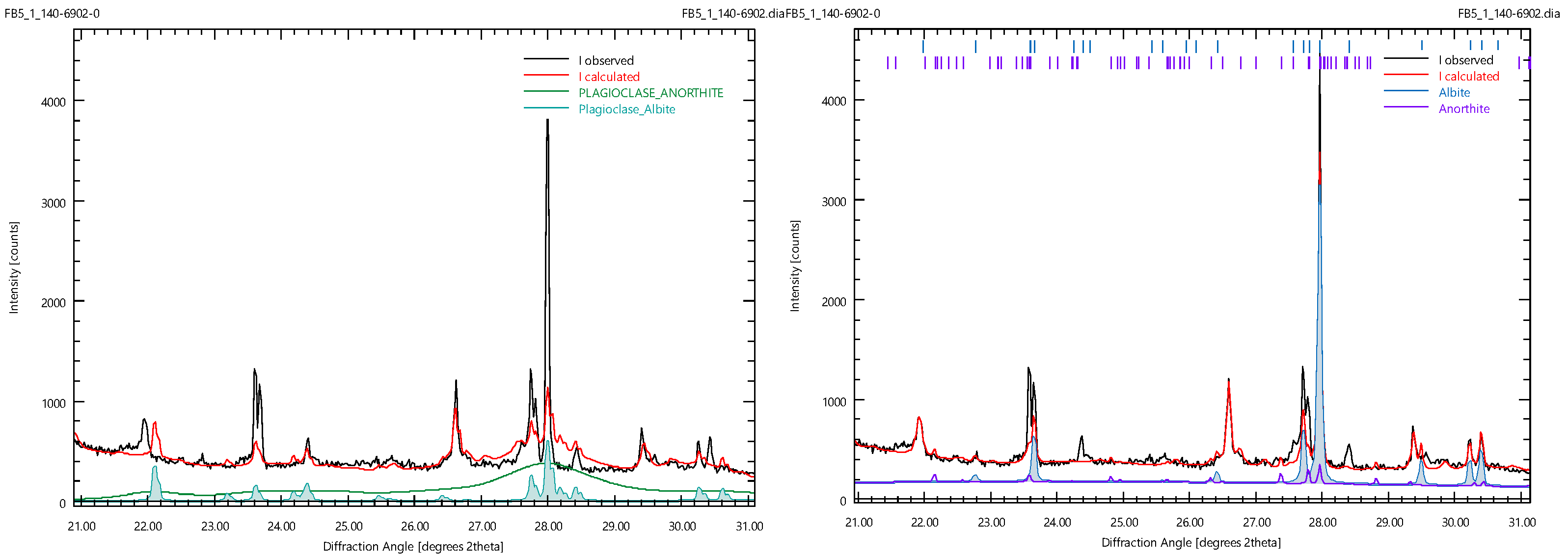
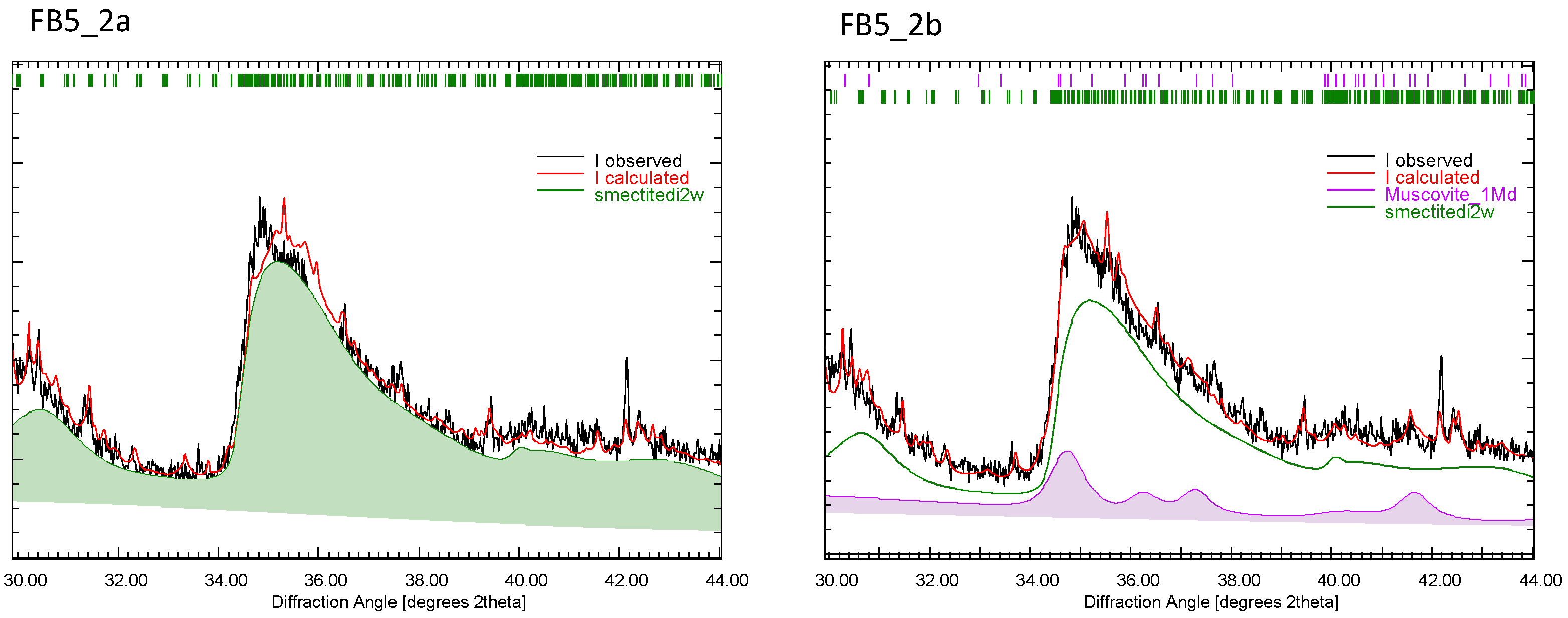
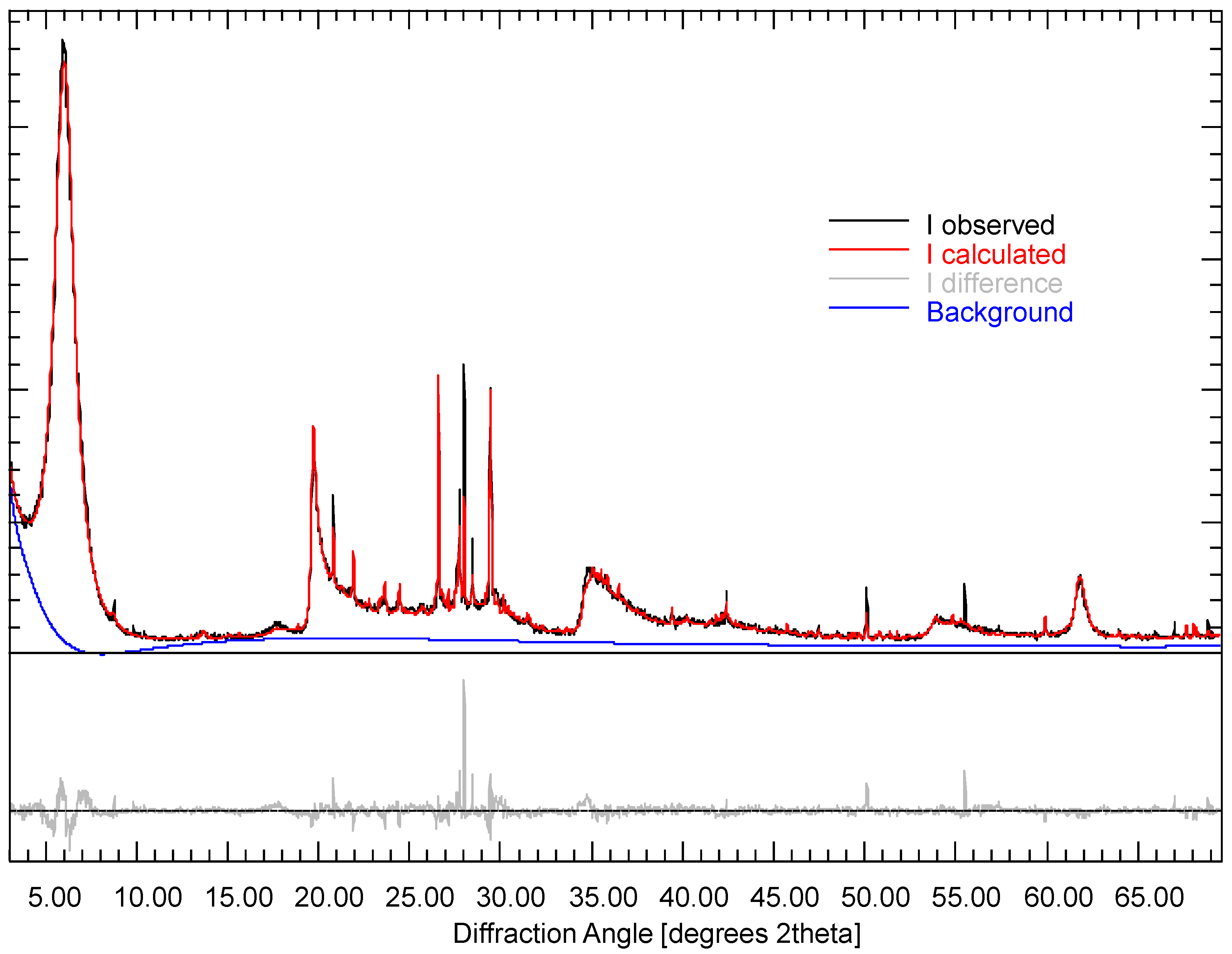
| Phase | [29] | [30] | [10] | (a) | (b) |
|---|---|---|---|---|---|
| Plagioclase | 2 ± 1 | 1 ± 0.5 | 0.8 ± 0.2 | 5 ± 1 | 11.2 |
| Cristobalite | 2 ± 1 | 2 ± 0.5 | 1.6 ± 0.5 | 4.5 ± 1 (**) | 1.2 |
| Calcite | <1 | 1 ± 0.5 | 0.9 ± 0.2 | 0.8 ± 0.1 (**) | 2.7 |
| Goethite | - | - | - | + | - |
| Halite | - | - | - | - | - |
| Hematite | - | - | - | - | - |
| Maghemite | - | - | - | 1 | - |
| Magnetite | - | - | + | + | - |
| Illite | - | (10) * | 1 ± 0.5 | <1 | - |
| Orthoclase | <1 | 2 ± 1 | 0.8 ± 0.2 | + | - |
| Quartz | 3 ± 1 | 2 ± 1 | 0.7 ± 0.2 | 4 ± 1 | 3.1 |
| Smectite | >90 | 92 ± 3 | 94 ± 1 | 85 ± 2 | 81.5 |
| Phase | FEBEX | FB5_1 | FB5_2 | FB5_3 | FB5_4 | FB5_5 | FB5_6 |
|---|---|---|---|---|---|---|---|
| Plagioclase | 7.7 | 11.8 | 14.8 | 9.5 | 13.0 | 9.7 | 16.5 |
| Cristobalite | - | 0.5 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 |
| Calcite | 0.9 | 0.6 | 1.8 | 0.6 | 3.9 | 1.1 | 2.2 |
| Goethite | 0.5 | - | 0.6 | 0.5 | 1.4 | 2.2 | - |
| Halite | - | - | 0.1 | 0.1 | 0.1 | 0.1 | - |
| Hematite | - | - | - | - | - | - | - |
| Maghemite | - | - | - | 0.1 | - | 0.2 | - |
| Magnetite | 0.4 | - | - | - | - | 0.1 | - |
| Illite | 12.7 | 1.3 | 9.5 | 11.9 | 11.0 | 8.1 | 7.1 |
| Orthoclase | 2.4 | 2.5 | 1.8 | 1.8 | 1.8 | 1.3 | 2.55 |
| Quartz | 2.5 | 3 | 1.1 | 2.5 | 1.0 | 1.0 | 1.5 |
| Smectite | 73.0 | 82.0 | 70.0 | 72.6 | 67.5 | 75.9 | 69.2 |
| χ2 | 2.93 | 3.6 | 3.6 | 2.6 | 2.9 | 2.5 | 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, J.; Cabrera, M.Á.; Fernández, C.; Mota-Heredia, C.; Fernández, R.; Torres, E.; Turrero, M.J.; Ruiz, A.I. Bentonite Powder XRD Quantitative Analysis Using Rietveld Refinement: Revisiting and Updating Bulk Semiquantitative Mineralogical Compositions. Minerals 2022, 12, 772. https://doi.org/10.3390/min12060772
Cuevas J, Cabrera MÁ, Fernández C, Mota-Heredia C, Fernández R, Torres E, Turrero MJ, Ruiz AI. Bentonite Powder XRD Quantitative Analysis Using Rietveld Refinement: Revisiting and Updating Bulk Semiquantitative Mineralogical Compositions. Minerals. 2022; 12(6):772. https://doi.org/10.3390/min12060772
Chicago/Turabian StyleCuevas, Jaime, Miguel Ángel Cabrera, Carlos Fernández, Carlos Mota-Heredia, Raúl Fernández, Elena Torres, María Jesús Turrero, and Ana Isabel Ruiz. 2022. "Bentonite Powder XRD Quantitative Analysis Using Rietveld Refinement: Revisiting and Updating Bulk Semiquantitative Mineralogical Compositions" Minerals 12, no. 6: 772. https://doi.org/10.3390/min12060772
APA StyleCuevas, J., Cabrera, M. Á., Fernández, C., Mota-Heredia, C., Fernández, R., Torres, E., Turrero, M. J., & Ruiz, A. I. (2022). Bentonite Powder XRD Quantitative Analysis Using Rietveld Refinement: Revisiting and Updating Bulk Semiquantitative Mineralogical Compositions. Minerals, 12(6), 772. https://doi.org/10.3390/min12060772









