Extraction of Potassium from Feldspar by Roasting with CaCl2 Obtained from the Acidic Leaching of Wollastonite-Calcite Ore
Abstract
:1. Introduction
2. Materials and Methods
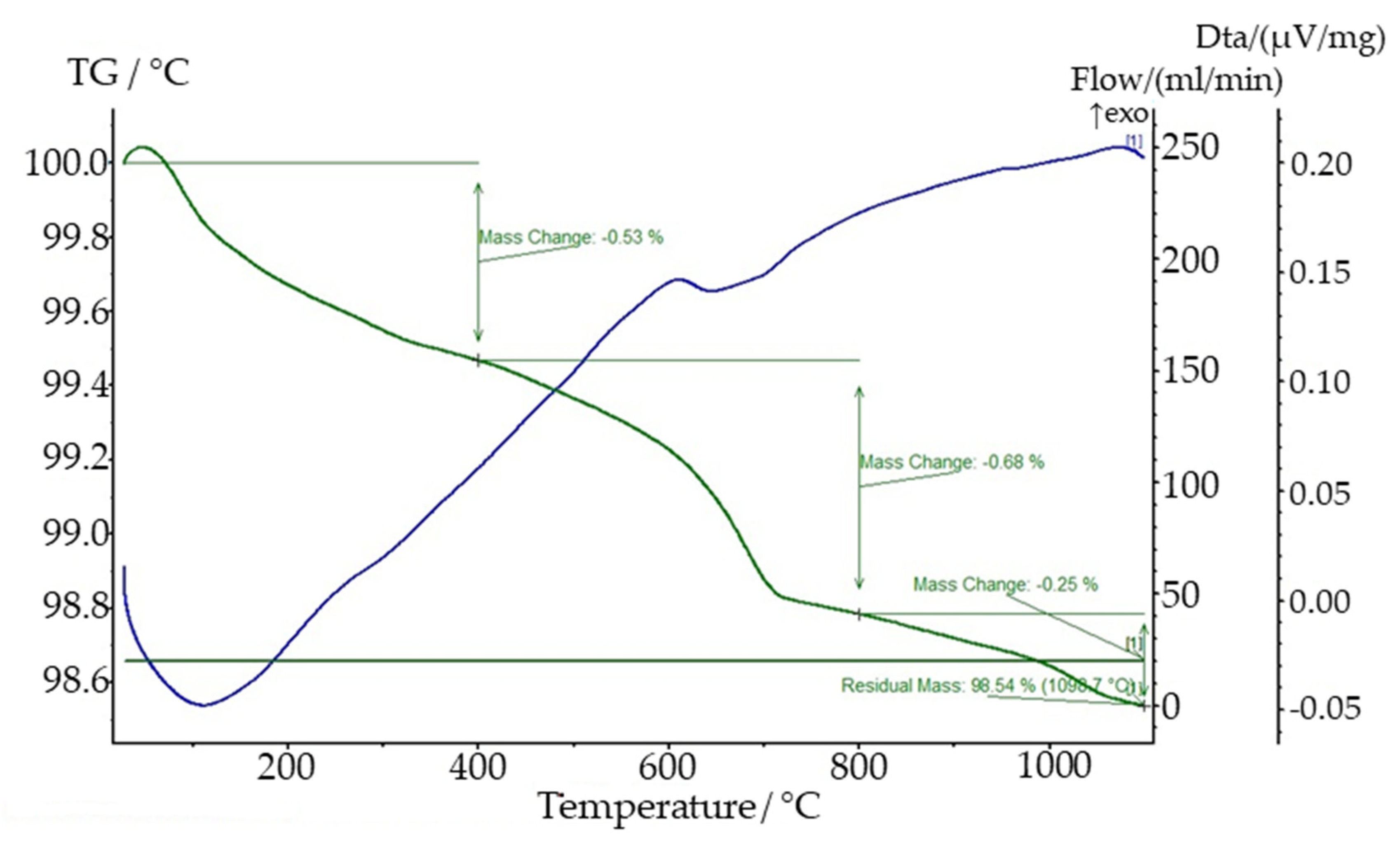
2.1. Material and Characterization
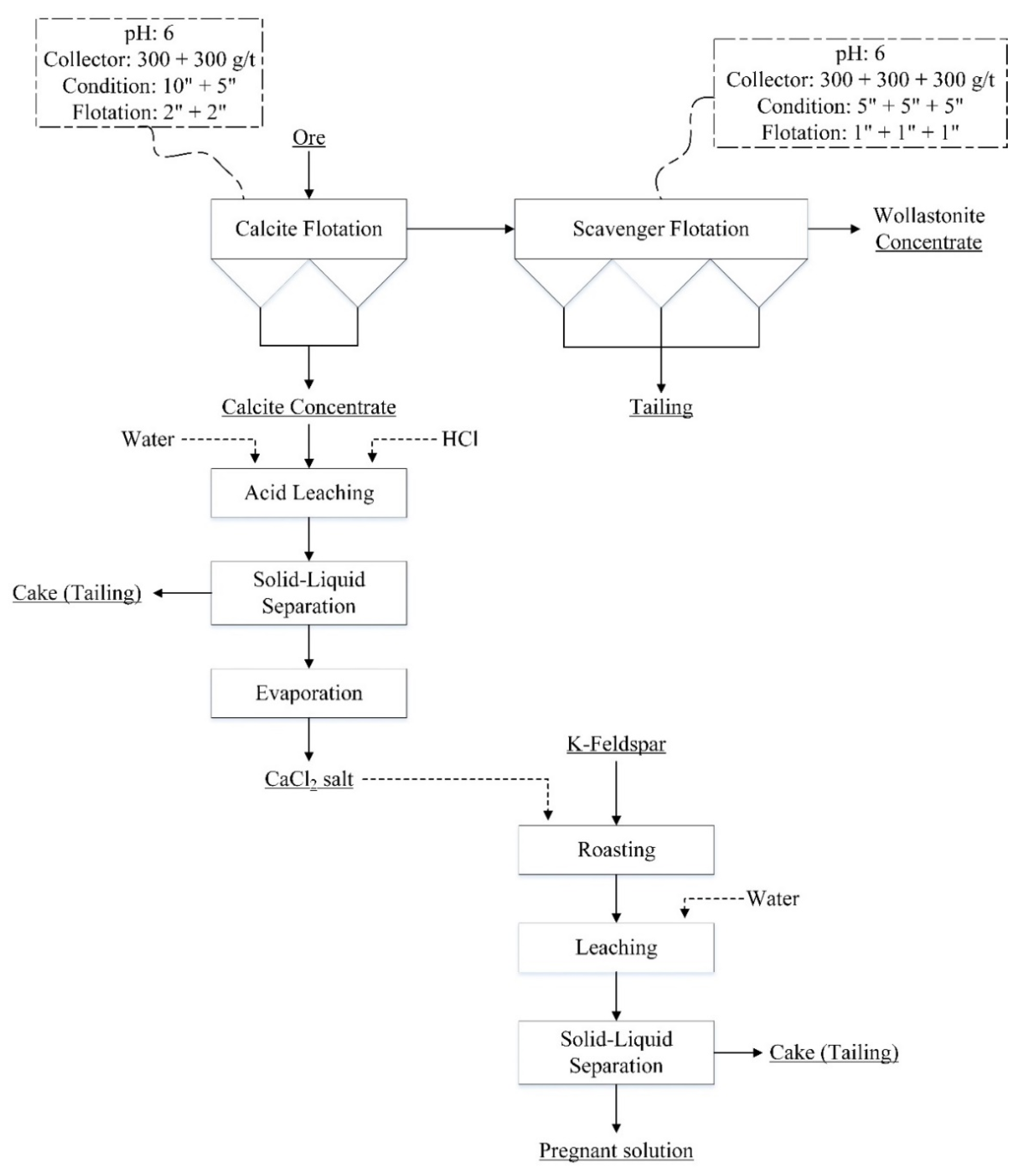
2.2. Methods
3. Results and Discussion
3.1. Flotation of Wollastonite-Calcite Ore
3.2. CaCl2 Production by Acid Leaching
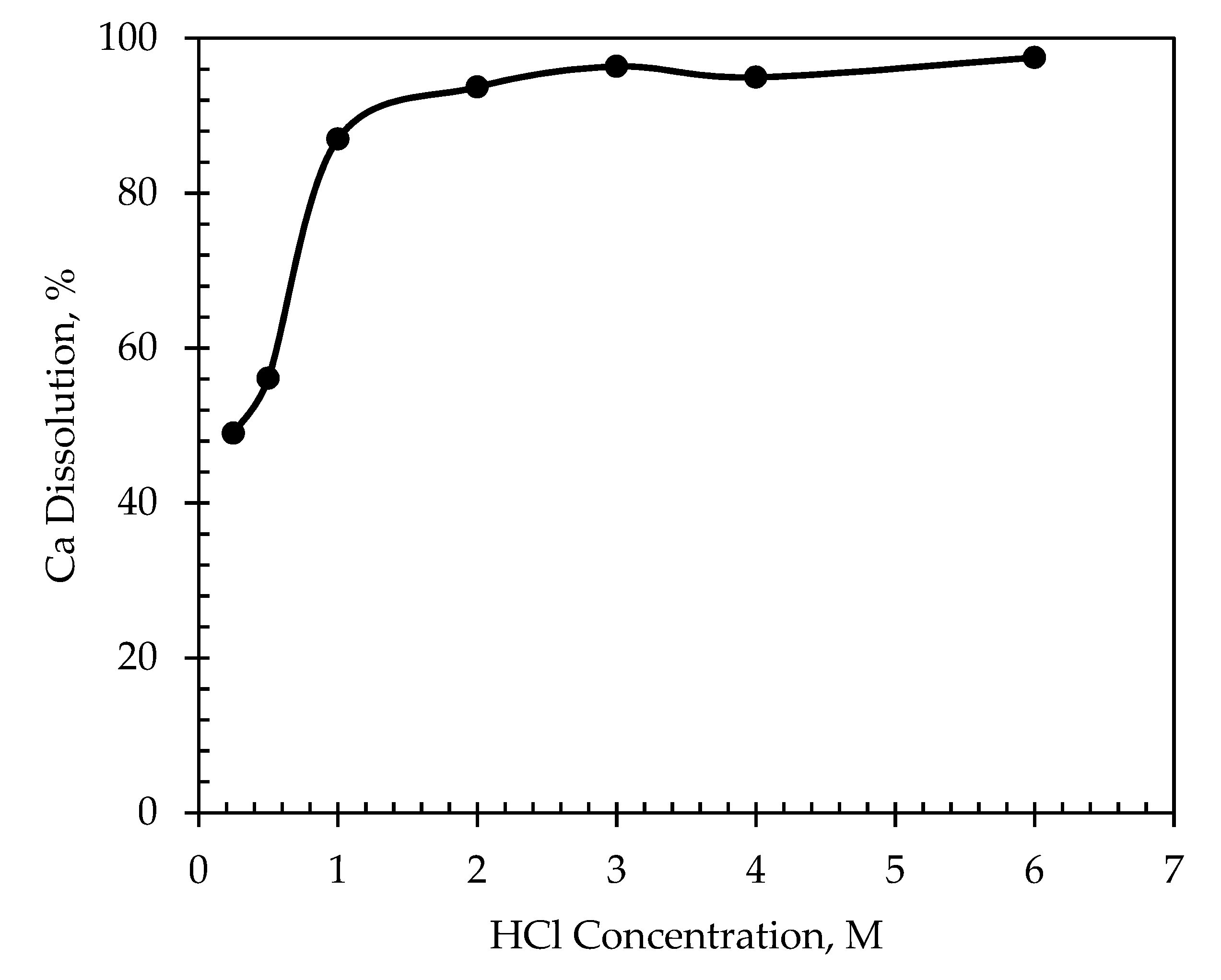
3.2.1. Effect of HCl Concentration
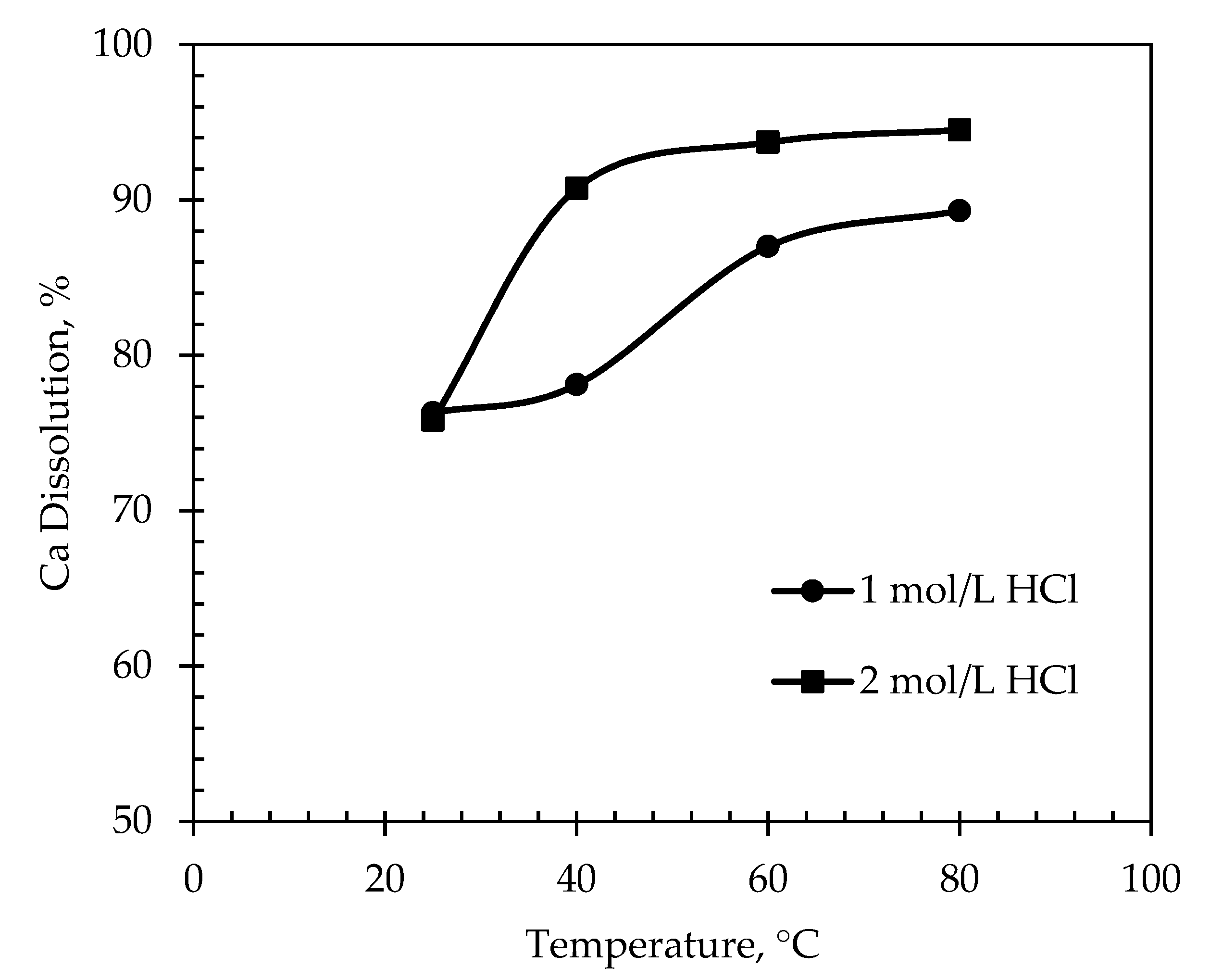
3.2.2. Effect of Temperature
3.2.3. Effect of Leaching Time
3.3. The Roasting Followed by Leaching
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherer, H.W.; Mengel, K.; Dittmar, H.; Drach, M.; Vosskamp, R.; Trenkel, M.E.; Gutser, R.; Steffens, G.; Czikkely, V.; Niedermaier, T.; et al. Fertilizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002. [Google Scholar]
- Burat, F.; Kökkiliç., O.; Kangal, O.; Gürkan, V.; Çelik, M.S. Quartz-feldspar separation for glass and ceramic industry. Miner. Metall. Process. 2007, 24, 75–80. [Google Scholar] [CrossRef]
- Kalyoncu, E.; Burat, F. Selective separation of coloring ımpurities from feldspar ore by ınnovative single-stage flotation. Miner. Process. Extr. Metall. Rev. 2021, 42, 1–6. [Google Scholar] [CrossRef]
- Rao, J.R.; Nayak, R.; Suryanarayana, A. Feldspar for potassium, fertilisers, catalysts and cement. Asian J. Chem. 1998, 10, 690–706. [Google Scholar]
- Kangal, M.O.; Bulut, G.; Yeşilyurt, Z.; Güven, O.; Burat, F. An alternative source for ceramics and glass augen-gneiss. Minerals 2017, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Kangal, M.O.; Bulut, G.; Yeşilyurt, Z.; Baştürkcü, H.; Burat, F. Characterization and production of Turkish nepheline syenites for industrial applications. Physicochem. Probl. Miner. Process. 2019, 55, 605–616. [Google Scholar]
- Kangal, M.O.; Bulut, G.; Guven, O. Physicochemical characterization of natural wollastonite and calcite. Minerals 2020, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Çinku, K.; Ipekoğlu, B.; Tulgarlar, A. Enrichment of Karaköy wollastonite ore. Ind. Miner. Symp. 1997, 86–91. [Google Scholar]
- Ravi, B.P.; Krishna, S.J.G.; Patil, M.R.; Kumar, P.S.; Rudrappa, C.; Naganoor, P.C. Beneficiation of a wollastonite from Sirohi, Rajasthan. Int. J. Innov. Sci. Eng. Technol. 2014, 1, 450–453. [Google Scholar]
- Bulut, G.; Akçin, E.S.; Kangal, M.O. Value added industrial minerals production from calcite-rich wollastonite. Gospod. Surowcami Miner. 2019, 35, 43–58. [Google Scholar]
- Zhang, J.; Zhang, R.; Geerlings, H.; Bi, J. A novel indirect wollastonite carbonation route for CO2 sequestration. Chem. Eng. Technol. 2010, 33, 1177–1183. [Google Scholar] [CrossRef]
- Kangal, M.O.; Bulut, G.; Burat, F.; Üçerler, Z.; Türk, T. Developing a Process for Obtaining High Value Added KCl with Calcium Chloride to be Produced from Kirşehir High Calcite Content Wollastonite; R&D Project, Final Report; ITU, Mineral Processing Engineering Department: İstanbul, Turkey, 2019; 39p. (In Turkish) [Google Scholar]
- Kangal, M.O.; Burat, F.; Güney, A.; Türk, T.; Şavran, C. Development of Processes for Obtaining High Value Added Potassium Salts from Turkish Potassium Feldspar Ore; Final Report; The Scientific and Technological Research Council of Turkey (Project No: 218M107): İstanbul, Turkey, 2019; 53p. (In Turkish) [Google Scholar]
- Yuan, B.; Li, C.; Liang, B.; Lü, L.; Yue, H.; Sheng, H.; Xie, H. Extraction of potassium from K-feldspar via the CaCl2 calcination route. Chin. J. Chem. Eng. 2015, 23, 1557–1564. [Google Scholar] [CrossRef]
- Serdengeçti, M.T.; Baştürkcü, H.; Burat, F.; Kangal, M.O. The correlation of roasting conditions in selective potassium extraction from K-feldspar ore. Minerals 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Sustainable process for the extraction of potassium from feldspar using eggshell powder. ACS Omega 2020, 5, 14990–14998. [Google Scholar] [CrossRef] [PubMed]
- Tanvar, H.; Dhawan, N. Recovery of potash values from feldspar. Sep. Sci. Technol. 2020, 55, 1398–1406. [Google Scholar] [CrossRef]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Production of potassium chloride from K-feldspar through roast–leach–solvent extraction route. Trans. Indian Inst. Met. 2019, 72, 2613–2622. [Google Scholar] [CrossRef]
- Lund, K.; Fogler, H.S.; McCune, C.C.; Ault, J.W. Acidization-II. The dissolution of calcite in hydrochloric acid. Chem. Eng. Sci. 1975, 30, 825–835. [Google Scholar] [CrossRef] [Green Version]
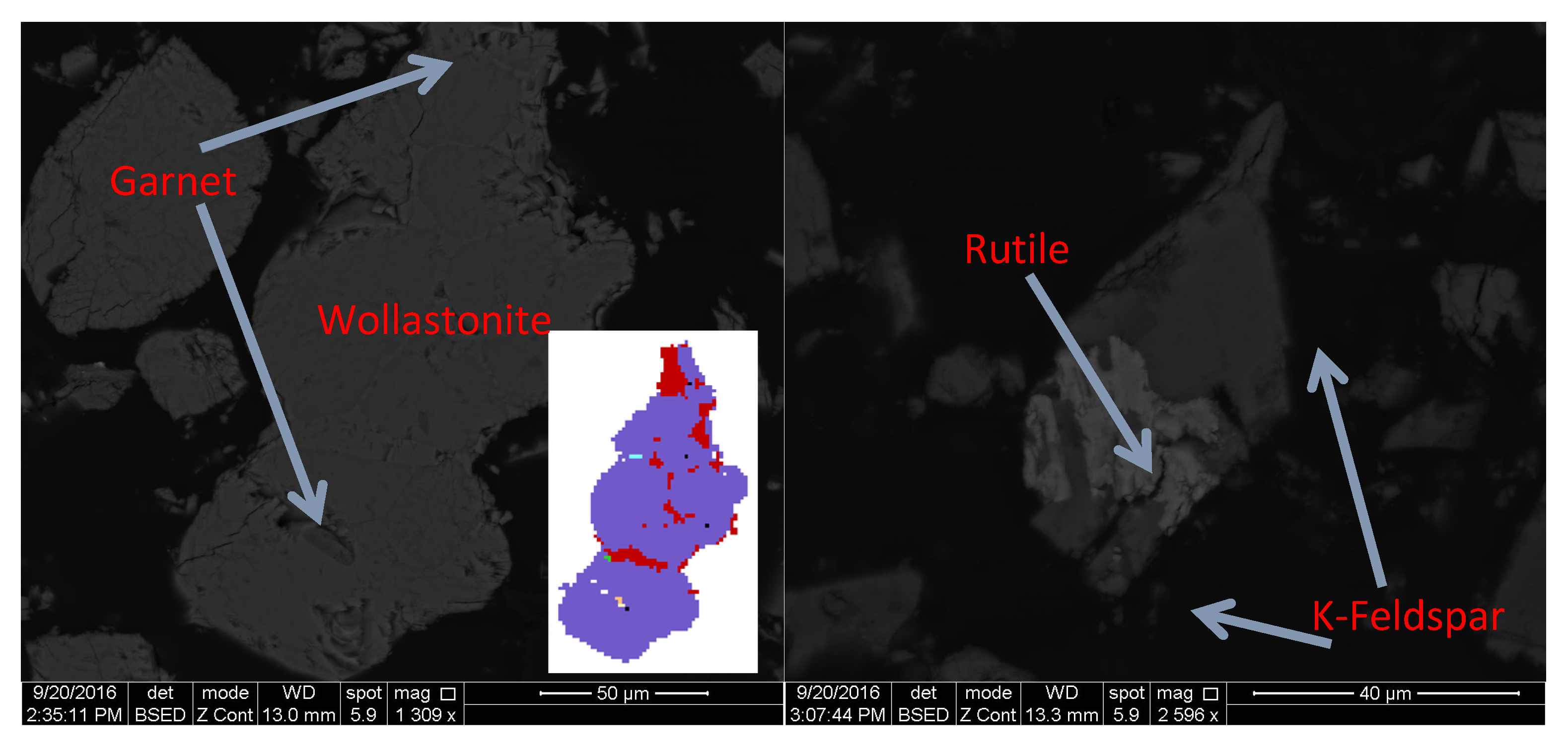
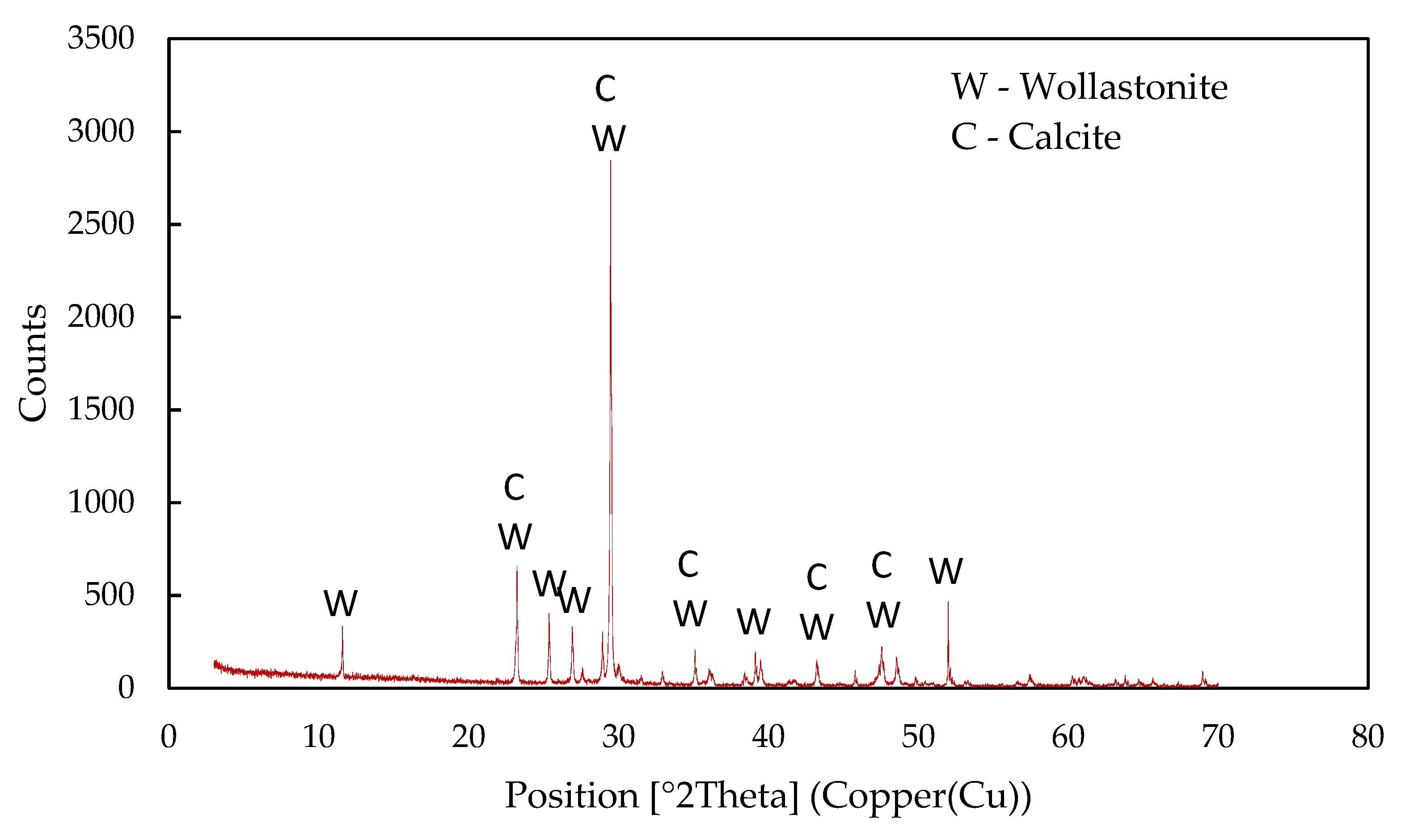
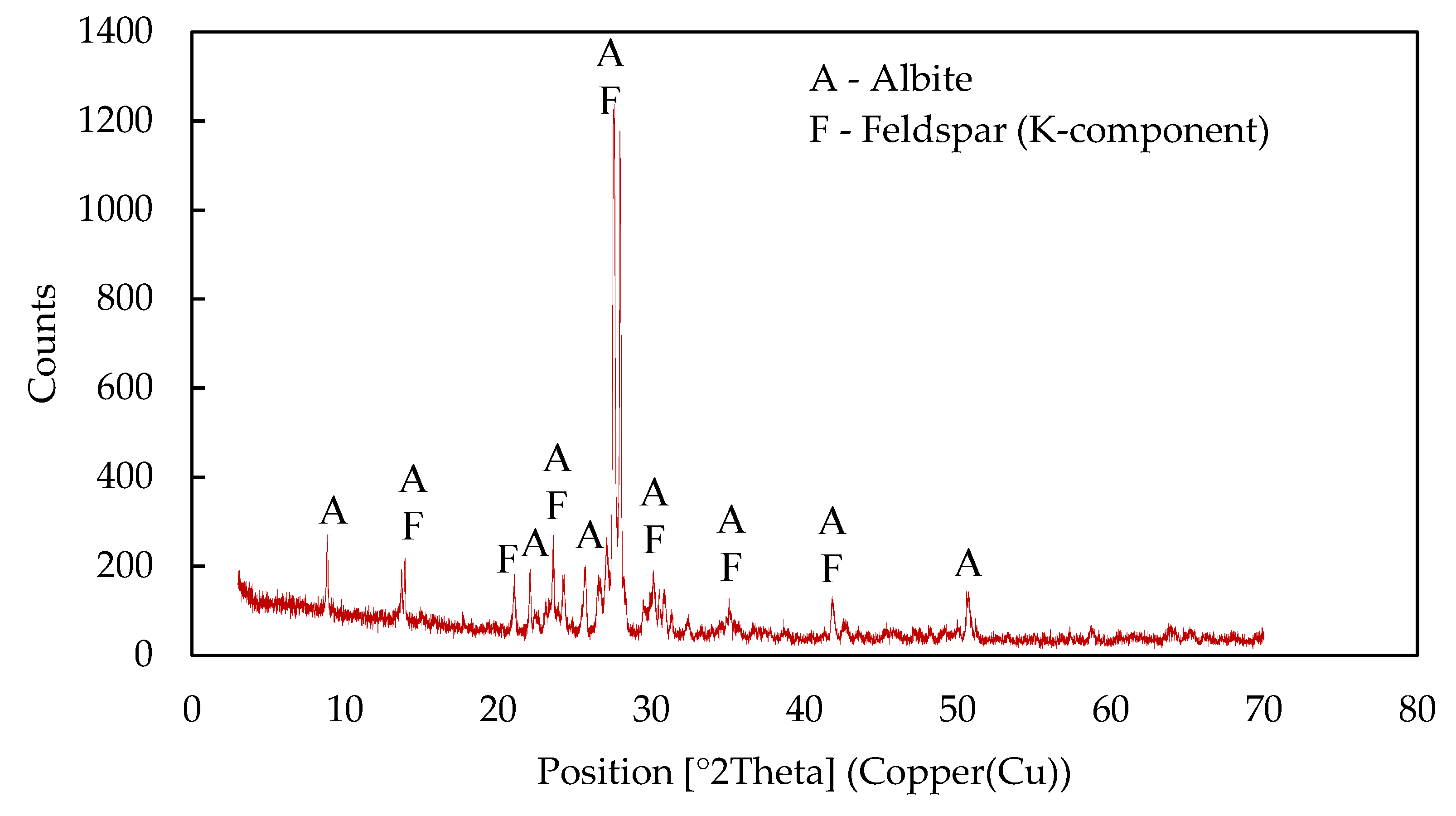
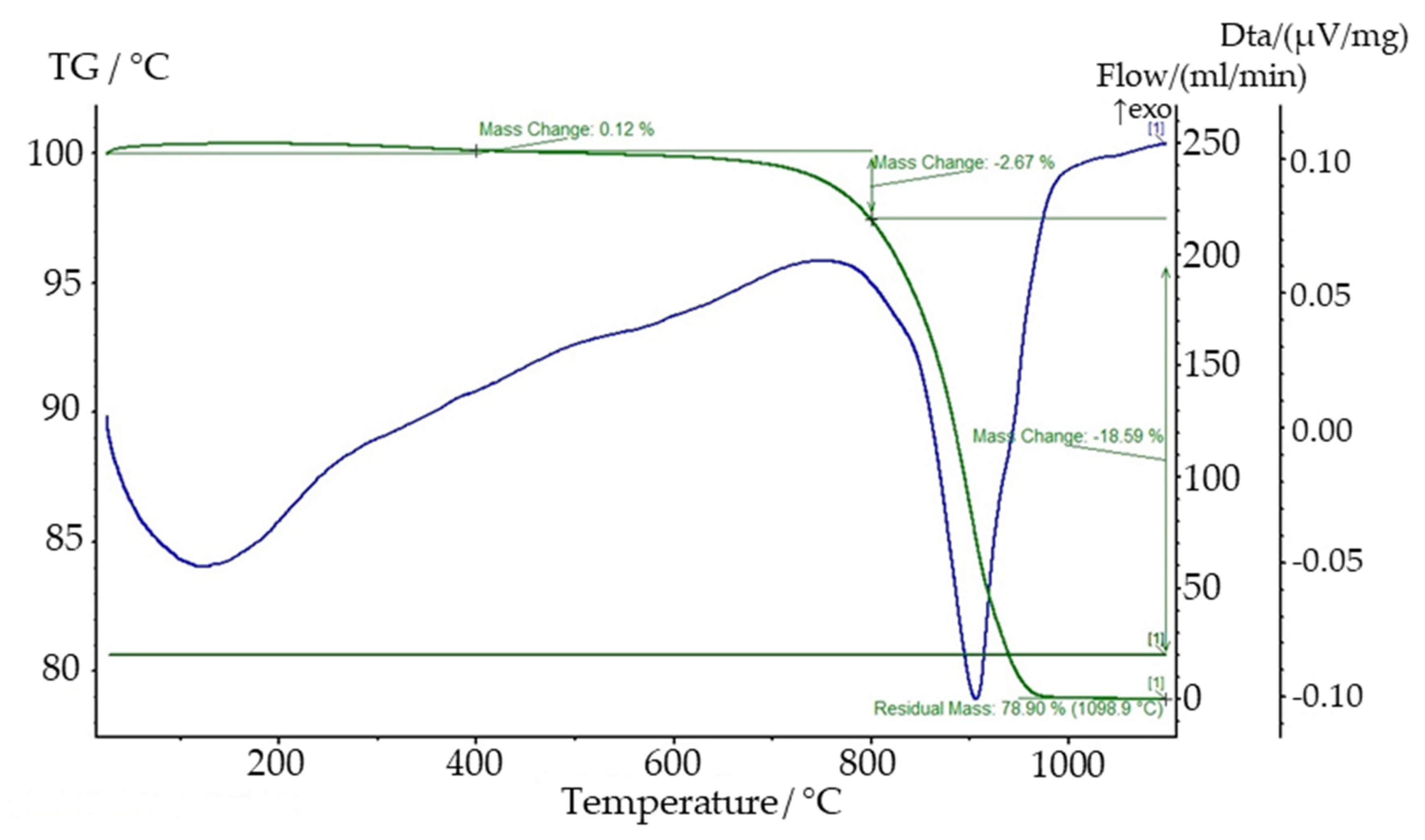


| Compound | Content, % | |
|---|---|---|
| Wollastonite-Calcite | K-Feldspar | |
| SiO2 | 28.00 | 62.30 |
| Al2O3 | 1.91 | 18.70 |
| Fe2O3 | 0.45 | 1.89 |
| TiO2 | 0.04 | 0.20 |
| Na2O | 0.47 | 4.32 |
| K2O | 0.40 | 8.40 |
| CaO | 48.20 | 2.16 |
| MgO | 1.15 | 0.26 |
| P2O5 | 0.03 | 0.09 |
| SrO | 0.04 | 0.14 |
| MnO | 0.03 | 0.06 |
| LOI | 20.80 | 1.83 |
| Particle Size | −74 µm |
|---|---|
| pH | 6.0 |
| KOl Dosage | 300 + 300 + 300 + 300 + 300 g/t |
| Conditioning Time | 10 + 5 + 5 + 5 + 5 min |
| Flotation Time | 2 + 2 + 1 + 1 + 1 min |
| Products | Weight (%) | Fe2O3 | SiO2 | Loss on Ignition | Calcite | Wollastonite | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | D | C | D | C | D | P | D | P | D | ||
| Float-1 * | 31.1 | 0.09 | 6.2 | 1.55 | 1.8 | 40.28 | 59.3 | 91.55 | 59.3 | 3.10 | 1.8 |
| Float-2 * | 15.8 | 0.15 | 5.3 | 3.30 | 2.0 | 40.82 | 30.6 | 92.77 | 30.6 | 6.60 | 2.0 |
| Float-3 | 2.9 | 1.31 | 8.5 | 24.20 | 2.7 | 36.47 | 5.1 | 82.89 | 5.1 | 48.40 | 2.7 |
| Float-4 | 3.3 | 2.17 | 16.0 | 40.80 | 5.2 | 17.52 | 2.8 | 39.82 | 2.8 | 81.60 | 5.2 |
| Float-5 | 3.1 | 3.09 | 21.4 | 45.60 | 5.4 | 8.83 | 1.3 | 20.07 | 1.3 | 91.20 | 5.4 |
| Sink | 43.8 | 0.44 | 42.6 | 49.70 | 82.9 | 0.42 | 0.9 | 0.95 | 0.9 | 99.40 | 82.9 |
| Total | 100.0 | 0.45 | 100.0 | 26.24 | 100.0 | 21.08 | 100.0 | 47.90 | 100.0 | 52.47 | 100.0 |
| Roasting Temperature, °C | Potassium Recovery, % |
|---|---|
| 800 | 97.1 |
| 850 | 97.1 |
| 900 | 98.6 |
| 950 | 97.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türk, T.; Üçerler, Z.; Burat, F.; Bulut, G.; Kangal, M.O. Extraction of Potassium from Feldspar by Roasting with CaCl2 Obtained from the Acidic Leaching of Wollastonite-Calcite Ore. Minerals 2021, 11, 1369. https://doi.org/10.3390/min11121369
Türk T, Üçerler Z, Burat F, Bulut G, Kangal MO. Extraction of Potassium from Feldspar by Roasting with CaCl2 Obtained from the Acidic Leaching of Wollastonite-Calcite Ore. Minerals. 2021; 11(12):1369. https://doi.org/10.3390/min11121369
Chicago/Turabian StyleTürk, Tülay, Zeynep Üçerler, Fırat Burat, Gülay Bulut, and Murat Olgaç Kangal. 2021. "Extraction of Potassium from Feldspar by Roasting with CaCl2 Obtained from the Acidic Leaching of Wollastonite-Calcite Ore" Minerals 11, no. 12: 1369. https://doi.org/10.3390/min11121369
APA StyleTürk, T., Üçerler, Z., Burat, F., Bulut, G., & Kangal, M. O. (2021). Extraction of Potassium from Feldspar by Roasting with CaCl2 Obtained from the Acidic Leaching of Wollastonite-Calcite Ore. Minerals, 11(12), 1369. https://doi.org/10.3390/min11121369









