The Effects of High-Grade Metamorphism on Cr-Spinel from the Archean Sittampundi Complex, South India
Abstract
:1. Introduction
2. Geology and Petrography
3. Materials and Methods
3.1. X-ray Single Crystal Diffraction
3.2. Electron Microprobe Analysis
3.3. Cation Distribution
3.4. Mössbauer Spectroscopic Analysis
4. Results
5. Discussion
6. Conclusions
- (i)
- Oxygen parameters of the studied Cr-spinels are close to the Amsaga chromites.
- (ii)
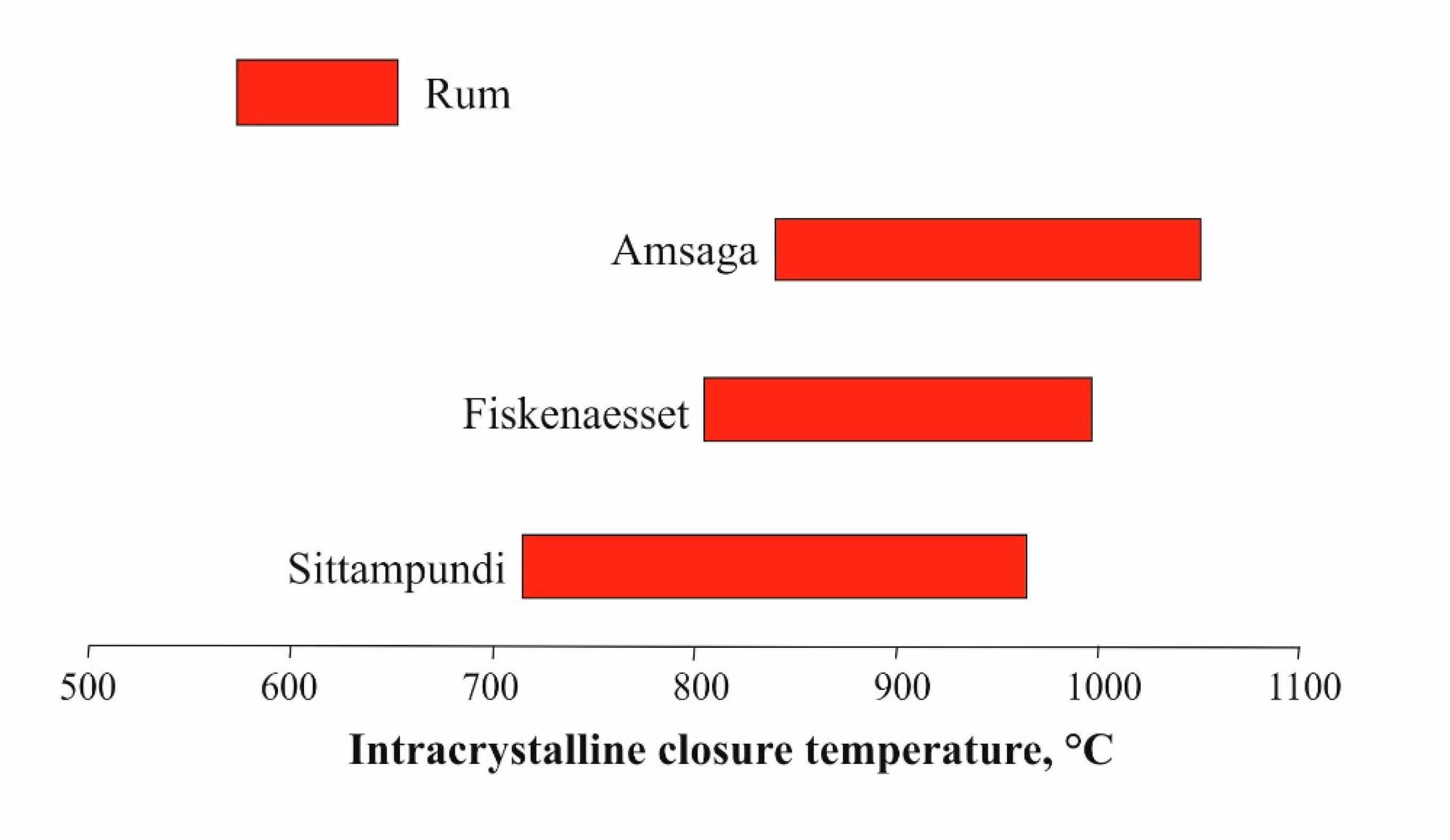
- The computed intracrystalline closure temperature of the Cr-spinels is comparable to the reported temperatures obtained by topological constraints and quantitative geothermometry.
- (iii)
- Estimated values of Fe3+/ΣFe ratios obtained by using different techniques are similar and establish that the studied Sittampundi Cr-spinels are stoichiometric.
- (iv)
- Structural refinement study of Cr-spinels could be important in discriminating the various tectonic domains.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, Y.B.; Chetty, T.R.K.; Janardhan, A.S.; Gopalan, K. Sm-Nd and Rb-Sr ages and PT history of the Archean Sittampundi and Bhavani layered meta-anorthosite complexes in Cauvery shear zone, South India: Evidence for Neoproterozoic reworking of Archean crust. Contrib. Mineral. Petrol. 1996, 125, 237–250. [Google Scholar] [CrossRef]
- Subramaniam, A.P. Mineralogy and petrology of the Sittampundi Complex, Salem district, Madras state, India. Geol. Soc. Am. Bull. 1956, 67, 317–390. [Google Scholar] [CrossRef]
- Janardhanan, A.S.; Leake, B.E. The origin of the meta-anorthositic gabbros and garnetiferous granulites of the Sittampundi complex, Madras, India. J. Geol. Soc. India 1975, 16, 391–408. [Google Scholar]
- Windley, B.F.; Selvan, T.A. Anorthosites and associated rocks of Tamil Nadu, southern India. J. Geol. Soc. India 1975, 16, 209–215. [Google Scholar]
- Windley, B.F.; Bishop, F.C.; Smith, J.V. Metamorphosed layered igneous complexes in Archean granulite-gneiss belts. Ann. Rev. Earth Planet. Sci. 1981, 9, 175–198. [Google Scholar] [CrossRef]
- Sajeev, K.; Windley, B.F.; Connolly, J.A.D.; Kon, Y. Retrogressed eclogite (20 kbar, 1020 °C) from the Neoproterozoic Palghat–Cauvery suture zone, southern India. Precambrian Res. 2009, 171, 23–36. [Google Scholar] [CrossRef]
- Ghosh, B.; Konar, R. Chromites from meta-anorthosites, Sittampundi layered igneous complex, Tamil Nadu, southern India. J. Asian Earth Sci. 2011, 42, 1394–1402. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Arai, S.; Abdel-Aziz, Y.M.; Rahimi, A. Spinel composition as a petrogenetic indicator of the mantle section in the Neoproterozoic Bou Azzer ophiolite, Anti-Atlas, Morocco. Precambrian Res. 2005, 138, 225–234. [Google Scholar] [CrossRef]
- González Jiménez, J.M.; Kerestedjian, T.; Proenza, F.J.A.; Gervilla, F. Metamorphism on chromite ores from the Dobromirtsi ultramafic massif, Rhodope Mountains (SE Bulgaria). Geol. Acta 2009, 7, 413–429. [Google Scholar]
- Colas, V.; González-Jiménez, J.M.; Griffin, W.L.; Fanlo, I.; Gervilla, F.; O’Reilly, S.Y.; Pearson, N.J.; Kerestedjian, T.; Proenza, J.A. Fingerprints of metamorphism in chromite: New insights from minor and trace elements. Chem. Geol. 2014, 389, 137–152. [Google Scholar] [CrossRef]
- Evans, B.W.; Frost, B.R. Chrome–spinel in progressive metamorphism—A preliminary analysis. Geochim. Cosmochim. Acta 1975, 39, 959–972. [Google Scholar] [CrossRef]
- Kimball, K.L. Effects of hydrothermal alteration on the composition of chromian spinels. Contrib. Mineral. Petrol. 1990, 105, 337–346. [Google Scholar] [CrossRef]
- Burkhard, D.J.M. Accessory chromium spinels: Their coexistence and alteration in serpentinites. Geochim. Cosmochim. Acta 1993, 57, 1297–1306. [Google Scholar] [CrossRef]
- Barnes, S.J. Chromite in komatiites, II. Modification during greenschist to mid amphibolite facies metamorphism. J. Petrol. 2000, 41, 387. [Google Scholar] [CrossRef] [Green Version]
- Della Giusta, A.; Princivalle, F.; Carbonin, S. Crystal chemistry of a suite of natural Cr-bearing spinels with 0.15 < Cr < 1.07. Neues Jahrb. Mineralogie. Abh. 1986, 155, 319–330. [Google Scholar]
- Princivalle, F.; Della Giusta, A.; Carbonin, S. Comparative crystal chemistry of spinels from some suits of ultramafic rocks. Mineral. Petrol. 1989, 40, 117–126. [Google Scholar] [CrossRef]
- Carraro, A. Crystal chemistry of Cr-spinels from a suite of spinel peridotite mantle xenoliths from the Predazzo Area (Dolomites, Northern Italy). Eur. J. Mineral. 2003, 15, 681–688. [Google Scholar] [CrossRef]
- Bosi, F.; Andreozzi, G.B.; Ferrini, V.; Lucchesi, S. Behavior of cation vacancy in kenotetrahedral Cr-spinels from Albanian eastern belt ophiolites. Am. Mineral. 2004, 89, 1367–1373. [Google Scholar] [CrossRef]
- Uchida, H.; Lavina, B.; Downs, R.T.; Chesley, J. Single-crystal X-ray diffraction of spinels from the San Carlos Volcanic Field, Arizona: Spinel as a geothermometer. Am. Mineral. 2005, 90, 1900–1908. [Google Scholar] [CrossRef]
- Lenaz, D.; Logvinova, A.M.; Princivalle, F.; Sobolev, N.V. Structural parameters of chromite included in diamond and kimberlites from Siberia: A new tool for discriminating ultramafic source. Am. Mineral. 2009, 94, 1067–1070. [Google Scholar] [CrossRef]
- Lenaz, D.; De Min, A.; Garuti, G.; Zaccarini, F.; Princivalle, F. Crystal chemistry of Cr-spinels from the lherzolite mantle peridotite of Ronda (Spain). Am. Mineral. 2010, 95, 1323–1328. [Google Scholar] [CrossRef]
- Lenaz, D.; Youbi, N.; De Min, A.; Boumehdi, M.A.; Ben Abbou, M. Low intra-crystalline closure temperatures of Cr-bearing spinels from the mantle xenoliths of the Middle Atlas Neogene-Quaternary Volcanic Field (Morocco): A mineralogical evidence of a cooler mantle beneath the West African Craton. Am. Mineral. 2014, 99, 267–275. [Google Scholar] [CrossRef]
- Lenaz, D.; Musco, M.E.; Petrelli, M.; Caldeira, R.; De Min, A.; Marzoli, A.; Mata, J.; Perugini, D.; Princivalle, F.; Boumehdi, M.A.; et al. Restitic or not? Insights from trace element content and crystal—Structure of spinels in African mantle xenoliths. Lithos 2017, 278–281, 464–476. [Google Scholar] [CrossRef]
- Lavina, B.; Cesare, B.; Álvarez-Valero, A.M.; Uchida, H.; Downs, R.T.; Koneva, A.; Dera, P. Closure temperatures of intracrystalline ordering in anatectic and metamorphic hercynite, Fe2+Al2O4. Am. Mineral. 2009, 94, 657–665. [Google Scholar] [CrossRef]
- Ganguly, J. Mg-Fe order-disorder in ferromagnesian silicates. II Thermodynamics, kinetics and geological applications. In Advances in Physical Geochemistry; Saxena, S.K., Ed.; Springer: New York, NY, USA, 1982; Volume 2, pp. 58–59. [Google Scholar]
- Princivalle, F.; Della Giusta, A.; De Min, A.; Piccirillo, E.M. Crystal chemistry and significance of cation ordering in Mg–Al rich spinels from high-grade hornfels (Predazzo-Monzoni, NE Italy). Mineral. Mag. 1999, 63, 257–262. [Google Scholar] [CrossRef]
- Bosi, F.; Andreozzi, G.B. Chromium influence on Mg-Al intracrystalline exchange in spinels and geothermometric implications. Am. Mineral. 2017, 102, 333–340. [Google Scholar] [CrossRef]
- Mattioli, G.S.; Wood, B.J. Magnetite activities across the MgAl2O4−Fe3O4 spinel join, with application to thermobarometric estimates of upper mantle oxygen fugacity. Contrib. Mineral. Petrol. 1988, 98, 148–162. [Google Scholar] [CrossRef]
- Ballhaus, C.; Berry, R.F.; Green, D.H. High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen geobarometer: Implications for the oxidation state of the upper mantle. Contrib. Mineral. Petrol. 1991, 107, 27–40. [Google Scholar] [CrossRef]
- Ozawa, K. Evaluation of olivine-spinel geothermometry as an indicator of thermal history for peridotites. Contrib. Mineral. Petrol. 1983, 82, 52–65. [Google Scholar] [CrossRef]
- Lenaz, D.; Braidotti, R.; Princivalle, F.; Garuti, G.; Zaccarini, F. Crystal chemistry and structural refinement of chromites from different chromitite layers and xenoliths of the Bushveld Complex. Eur. J. Mineral. 2007, 19, 599–609. [Google Scholar] [CrossRef]
- Lenaz, D.; O’Driscoll, B.; Princivalle, F. Petrogenesis of the anorthosite—Chromitite association: Crystal-chemical and petrological insights from the Rum Layered Intrusion, NW Scotland. Contrib. Mineral. Petrol. 2011, 162, 1201–1213. [Google Scholar] [CrossRef]
- Lenaz, D.; Garuti, G.; Zaccarini, F.; Cooper, R.W.; Princivalle, F. The Stillwater Complex: The response of chromite crystal chemistry to magma injection. Geol. Acta 2012, 10, 33–41. [Google Scholar]
- Lenaz, D.; Rigonat, N.; Skogby, H.; Berger, J. Following the amphibolite to greenschist metamorphic path through the structural parameters of spinels from Amsaga (Mauritania). Minerals 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Rollinson, H.; Adetunji, J.; Lenaz, D.; Szilas, K. Archaean chromitites show constant Fe3+/ΣFe in Earth’s asthenospheric mantle since 3.8 Ga. Lithos 2017, 282–283, 316–325. [Google Scholar] [CrossRef]
- Dharma Rao, C.V.; Santosh, M.; Sajeev, K.; Windley, B.F. Chromite–silicate chemistry of the Neoarchean Sittampundi Complex, southern India: Implications for subduction-related arc magmatism. Precambrian Res. 2013, 227, 259–275. [Google Scholar] [CrossRef]
- Chowdhury, P.; Talukdar, M.; Sengupta, P.; Sanyal, S.; Mukhopadhyay, D. Controls of P-T path and element mobility on the formation of corundum pseudomorphs in Paleoproterozoic high-pressure anorthosite from Sittampundi, Tamil Nadu, India. Am. Mineral. 2013, 98, 1725–1737. [Google Scholar] [CrossRef]
- Mohan, M.R.; Satyanarayanan, M.; Santosh, M. Neoarchean suprasubduction zone arc magmatism in southern India: Geochemistry, zircon U–Pb geochronology and Hf isotopes of the Sittampundi Anorthosite Complex. Gondwana Res. 2013, 23, 539–557. [Google Scholar] [CrossRef]
- Talukdar, M.; Sanyal, S.; Sengupta, P. Metasomatic alteration of chromite from parts of the late Archaean Sittampundi Layered Magmatic Complex (SLC), Tamil Nadu, India. Ore Geol. Rev. 2017, 90, 148–165. [Google Scholar] [CrossRef]
- Dutta, U.; Bhui, U.K.; Sengupta, P.; Sanyal, S.; Mukhopadhyay, D. Magmatic and metamorphic imprints in 2.9 Ga chromitites from the Sittampundi layered complex, Tamil Nadu, India. Ore Geol. Rev. 2011, 40, 90–107. [Google Scholar] [CrossRef]
- Karmakar, S.; Mukherjee, S.; Sanyal, S.; Sengupta, P. Origin of peraluminous minerals (corundum, spinel, and sapphirine) in a highly calcic anorthosite from the Sittampundi Layered Complex, Tamil Nadu, India. Contrib. Mineral. Petrol. 2017, 172, 67. [Google Scholar] [CrossRef]
- Mitra, S.; Bidyananda, M.; Samanta, A.K. Cation distribution in Cr-spinels from the Sittampundi layered complex and their intracrystalline thermodynamics. Curr. Sci. 2006, 90, 435–439. [Google Scholar]
- Carbonin, S.; Menegazzo, G.; Lenaz, D.; Princivalle, F. Crystal chemistry of two detrital Cr -spinels with unusually low values of oxygen positional parameter: Oxidation mechanism and possible origin. Neues Jahrb. Mineral. Mon. 1999, 359–371. [Google Scholar]
- Thesniya, P.M.; Saranya, R.; Rajesh, V.J. Compositional and spectrochemical analyses of Cr-spinels in the Sittampundi Anorthosite Complex, Southern India: Implications for remote observations of spinels on the Moon. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119677. [Google Scholar] [CrossRef]
- D’Ippolito, V.; Andreozzi, G.B.; Bersani, D.; Lottici, P.P. Raman fingerprint of chromate, aluminate and ferrite spinels. J. Raman Spectrosc. 2015, 46, 1255–1264. [Google Scholar] [CrossRef]
- Lenaz, D.; Lughi, V. Raman study of MgCr2O4–Fe2+Cr2O4 and MgCr2O4–MgFe3+2O4 synthetic series: The effects of Fe2+ and Fe3+ on Raman shifts. Phys. Chem. Miner. 2013, 40, 491–498. [Google Scholar] [CrossRef]
- Lenaz, D.; Lughi, V. Raman spectroscopy and the inversion degree of natural Cr-bearing spinels. Am. Mineral. 2017, 102, 327–332. [Google Scholar] [CrossRef]
- Della Giusta, A.; Carbonin, S.; Ottonello, G. Temperature-dependent disorder in a natural Mg—Al—Fe2+—Fe3+—spinel. Mineral. Mag. 1996, 60, 603–616. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Prince, E. International Tables for X-ray Crystallography. Volume C: Mathematical, Physical and Chemical Tables, 3rd ed.; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Tokonami, M. Atomic scattering factor for O-2. Acta Crystallogr. 1965, 19, 486. [Google Scholar] [CrossRef]
- Carbonin, S.; Russo, U.; Della Giusta, A. Cation distribution in some natural spinels from X-ray diffraction and Mössbauer spectroscopy. Mineral. Mag. 1996, 60, 355–368. [Google Scholar] [CrossRef]
- Lavina, B.; Salviulo, G.; Della Giusta, A. Cation distribution and structure modeling of spinel solid solutions. Phys. Chem. Miner. 2002, 29, 10–18. [Google Scholar] [CrossRef]
- Lenaz, D.; Schmitz, B. Crystal structure refinement of chromites from two achondrites, their T-f(O2) conditions and implications. Meteor. Planet. Sci. 2017, 52, 1763–1775. [Google Scholar] [CrossRef]
- Lagarec, K.; Rancourt, G.K. Extended Voigt-based analytic line-shape method for determining N -dimensional correlated hyperfine parameter distributions in Mossbauer Spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1997, 129, 266–280. [Google Scholar] [CrossRef]
- Lagarec, K.; Rancourt, G.K. Recoil Spectral Analysis Software for Windows; Version 1.0; Mössbauer Group, Physics Department, University of Ottawa: Ottawa, ON, Canada, 1998. [Google Scholar]
- Prescher, C.; McCammon, C.; Dubrovinsky, L. MossA 1.01: A program for analyzing energy-domain Mössbauer spectra from Conventional and Synchrotron sources. J. Appl. Crystallogr. 2012, 45, 329–331. [Google Scholar] [CrossRef]
- Lenaz, D.; Skogby, H.; Logvinova, A.M.; Sobolev, N.V.; Princivalle, F. A micro-Mössbauer study of chromites included in diamond and other mantle related rocks. Phys. Chem. Miner. 2013, 40, 671–679. [Google Scholar] [CrossRef]
- De Grave, E.; Van Alboom, A. Evaluation of ferrous and ferric Mössbauer fractions. Phys. Chem. Miner. 1991, 18, 337–342. [Google Scholar] [CrossRef]
- Eeckhout, S.G.; De Grave, E. Evaluation of ferrous and ferric Mössbauer fractions. Part II. Phys. Chem. Miner. 2003, 30, 142–146. [Google Scholar] [CrossRef]
- Quintiliani, M. 57Fe Mössbauer spectroscopy analysis of spinels: Fe3+/ΣFe quantification accuracy and consequences on fO2 estimate. Period. Mineral. 2005, 74, 139–146. [Google Scholar]
- Rollinson, H.; Adetunji, J.; Yousif, A.A.; Gismelseed, A.M. New Mössbauer measurements of Fe3+/ΣFe in chromites from the mantle section of the Oman ophiolite: Evidence for the oxidation of the sub-oceanic mantle. Mineral. Mag. 2012, 76, 579–596. [Google Scholar] [CrossRef]
- Pal, T.; Moon, H.; Mitra, S. Distribution of iron cations in natural chromites at different stages of oxidation—A 57Fe Mössbauer investigation. J. Geol. Soc. India 1994, 44, 53–64. [Google Scholar]
- Rais, A.; Yousif, A.A.; Al-Shishi, M.H.; Al-Rawas, A.D.; Gismelseed, A.M.; El-Zain, M.E. Cation distribution and magnetic properties of natural chromites. Phys. Status Solidi (b) 2003, 739, 439–444. [Google Scholar] [CrossRef]
- Hill, R.J.; Craig, J.R.; Gibbs, G.V. Systematics of the spinel structure type. Phys. Chem. Miner. 1979, 4, 317–339. [Google Scholar] [CrossRef]
- Maibam, B.; Lenaz, D.; Foley, S.; Berndt, J.; Belousova, E.; Wangjam, M.; Goswami, J.N.; Kapsiotis, A. U-Pb and Hf isotope study of detrital zircon and Cr-spinel in Banavara quartzite and implications for the evolution of the Dharwar crato, South India. Geol. Mag. 2021, 158, 1671–1682. [Google Scholar] [CrossRef]
- Rollinson, H.R.; Reid, C.; Windley, B.F. Chromitites from the Fiskenæsset anorthositic complex, West Greenland: Clues to late Archaean mantle processes. Geol. Soc. Lond. Spec. Publ. 2010, 338, 197–212. [Google Scholar] [CrossRef]
- Polat, A.; Fryer, B.J.; Appel, P.W.U.; Kalvig, P.; Kerrich, R.; Dilek, Y.; Yang, Z. Geochemistry of anorthositic differentiated sills in the Archean (~2970 Ma) Fiskenaesset Complex, SW Greenland: Implications for parental magma compositions, geodynamic setting, and secular heat flow in arcs. Lithos 2011, 123, 50–72. [Google Scholar] [CrossRef]
- Lenaz, D.; Andreozzi, G.B.; Mitra, S.; Maibam, B.; Princivalle, F. Crystal chemical and 57Fe Mössbauer study of chromite from the Nuggihalli schist belt (India). Mineral. Petrol. 2004, 80, 45–57. [Google Scholar] [CrossRef]
- Sunder Raju, P.V. Textural and compositional relationships of rutile and chromite in Sittampundi anorthosite complex, Tamil Nadu. J. Geol. Soc. India 2013, 81, 709–712. [Google Scholar] [CrossRef]
- Lenaz, D.; Adetunji, J.; Rollinson, H. Determination of Fe3+/ΣFe ratios in chrome spinels using a combined Mössbauer and single-crystal X-ray approach: Application to chromitites from the mantle section of the Oman ophiolite. Contrib. Mineral. Petrol. 2014, 167, 958. [Google Scholar] [CrossRef]
- Lenaz, D.; Andreozzi, G.B.; Bidyananda, M.; Princivalle, F. Oxidation degree of chromite from Indian ophiolites: A crystal chemical and 57Fe Mössbauer study. Period. Mineral. 2014, 83, 241–255. [Google Scholar]
- Adetunji, J.; Everitt, S.; Rollinson, H. New Mössbauer measurements of Fe3+/ΣFe ratios in chromites from the early Proterozoic Bushveld Complex, South Africa. Precambrian Res. 2013, 228, 194–205. [Google Scholar] [CrossRef]
- Perinelli, C.; Bosi, F.; Andreozzi, G.B.; Conte, A.M.; Armienti, P. Geothermometric study of Cr-spinels of peridotite mantle xenoliths from Northern Victoria Land (Antarctica). Am. Mineral. 2014, 99, 839–846. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; O’Driscoll, B.; Lenaz, D.; Gertisser, R.; Kronz, A. Compositionally heterogeneous podiform chromitite in the Shetland Ophiolite Complex (Scotland): Implications for chromitite petrogenesis and late-stage alteration in the upper mantle portion of a supra-subduction zone ophiolite. Lithos 2013, 162–163, 279–300. [Google Scholar] [CrossRef]
- O’Driscoll, B.; Leuthold, J.; Lenaz, D.; Skogby, H.; Day, J.M.D.; Adetunji, J. Melt Percolation, Melt-Rock Reaction and Oxygen Fugacity in Supra-Subduction Zone Mantle and Lower Crust from the Leka Ophiolite Complex, Norway. J. Petrol. 2021, 62, egab078. [Google Scholar] [CrossRef]
- Stowe, C.W. Compositions and tectonic settings of chromite deposits through time. Econ. Geol. 1994, 89, 528–546. [Google Scholar] [CrossRef]
- Suita, M.T.; Streider, A.J. Cr-spinels from Brazilian mafic-ultramafic complexes: Metamorphic modifications. Int. Geol. Rev. 1996, 38, 245–267. [Google Scholar] [CrossRef]
- Candia, M.A.F.; Gaspar, J.C. Chromian spinels in metamorphosed ultramafic rocks from Mangabal I and II complexes, Goias, Brazil. Mineral. Petrol. 1997, 60, 27–40. [Google Scholar] [CrossRef]
- Proenza, J.A.; Ortega-Gutierrez, F.; Camprubi, A.; Tritlla, J.; Elias-Herrera, M.; Reyes-Salas, M. Paleozoic serpentinite-enclosed chromitites from Tehuitzingo, (Acatlan complex, southern Mexico): A petrological and mineralogical study. J. S. Am. Earth Sci. 2004, 16, 649–666. [Google Scholar] [CrossRef]
- Wood, B.J.; Virgo, D. Upper mantle oxidation state: Ferric iron contents of lherzolite spinels by 57Fe Mössbauer spectroscopy and resultant oxygen fugacities. Geochim. Cosmochim. Acta 1989, 53, 1277–1291. [Google Scholar] [CrossRef]
- Quintiliani, M.; Andreozzi, G.B.; Graziani, G. Fe2+ and Fe3+ quantification by different approaches and fO2 estimation for Albanian Cr-spinels. Am. Mineral. 2006, 91, 907–916. [Google Scholar] [CrossRef]

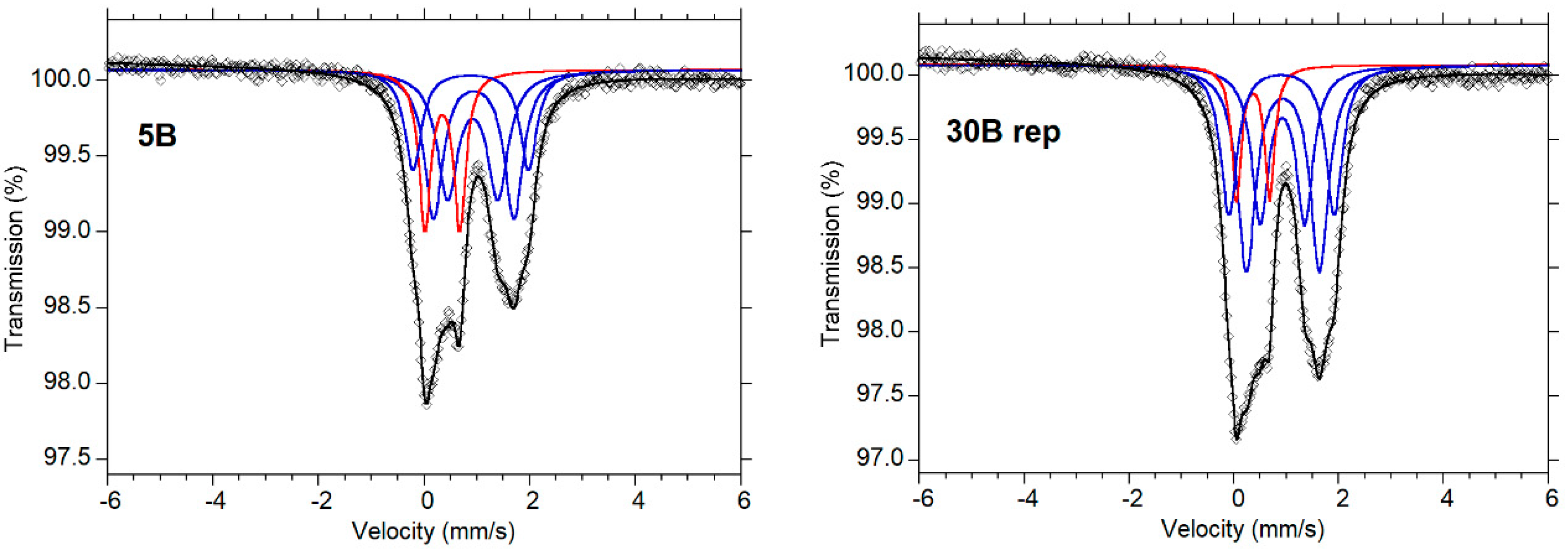

| Sample | 5B | 24B | 30A | 30B | |
|---|---|---|---|---|---|
| XRD | a | 8.2364 (4) | 8.2477 (3) | 8.2448 (4) | 8.2399 (4) |
| u | 0.26355 (5) | 0.26360 (6) | 0.2633 (1) | 0.2637 (1) | |
| R1 | 1.83 | 2.10 | 2.56 | 2.72 | |
| wR2 | 3.92 | 4.12 | 6.08 | 6.33 | |
| GooF | 1.186 | 1.311 | 1.244 | 1.198 | |
| EMPA | MgO | 8.2 (1) | 6.80 (9) | 9.2 (3) | 6.8 (1) |
| Al2O3 | 27.6 (2) | 27.3 (3) | 24.1 (7) | 26.7 (3) | |
| TiO2 | 0.22 (4) | 0.14 (3) | 0.23 (2) | 0.14 83) | |
| Cr2O3 | 35.8 (4) | 36.0 (5) | 38.5 (8) | 36.4 (3) | |
| MnO | 0.23 (3) | 0.26 (3) | 0.23 (3) | 0.26 (3) | |
| FeOtot | 29.3 (4) | 30.2 (2) | 28.5 (2) | 30.2 (3) | |
| NiO | 0.20 (3) | 0.12 (3) | 0.13 (3) | 0.13 (4) | |
| Sum | 101.6 | 100.8 | 100.9 | 100.7 | |
| T-site | |||||
| Mg | 0.339 | 0.274 | 0.3644 | 0.2578 | |
| Al | 0.0033 | 0.0298 | 0.0101 | 0.0353 | |
| Fe2+ | 0.5622 | 0.6488 | 0.5357 | 0.6557 | |
| Fe3+ | 0.0866 | 0.0362 | 0.0817 | 0.0393 | |
| Mn | 0.006 | 0.0069 | 0.0061 | 0.0069 | |
| Zn | 0.0025 | 0.0041 | 0.0016 | 0.0046 | |
| Cation distribution | M-site | ||||
| Al | 1.0187 | 0.9804 | 0.9444 | 0.9715 | |
| Cr | 0.8679 | 0.8854 | 0.9122 | 0.8958 | |
| Mg | 0.0375 | 0.0425 | 0.0683 | 0.0609 | |
| Fe2+ | 0.0454 | 0.0219 | 0.0208 | 0.0061 | |
| Fe3+ | 0.0186 | 0.063 | 0.0443 | 0.057 | |
| Ni | 0.0049 | 0.003 | 0.0032 | 0.0033 | |
| Ti | 0.005 | 0.0033 | 0.0054 | 0.0033 | |
| Cr# | 0.46 | 0.48 | 0.49 | 0.47 | |
| Mg# | 0.38 | 0.32 | 0.44 | 0.33 | |
| Fe# | 0.05 | 0.05 | 0.06 | 0.05 | |
| Tc | 715 | 924 | 754 | 966 |
| Samples (a) | δ (mm/s) | ΔEQ mm/s | Γ (mm/s) | % Area | Oxidation State | χ2 (±0.126) | (Fe2+/ΣFe) | (Fe3+/ΣFe) | (Fe3+/Fe2+) | (Fe3+/ΣFe) Corrected |
|---|---|---|---|---|---|---|---|---|---|---|
| 5B | 0.927 0.920 0.878 0.347 | 1.454 0.883 2.155 0.657 | 0.246 0.221 0.201 0.142 | 40.000 21.200 18.600 20.490 | Fe2+ Fe2+ Fe2+ Fe3+ | 1.07 | 79.80 | 20.49 | 0.26 | 0.166 |
| 24B | 0.934 0.919 0.901 0.348 | 1.479 0.915 2.133 0.651 | 0.446 0.431 0.401 0.257 | 36.412 25.282 21.989 16.316 | Fe2+ Fe2+ Fe2+ Fe3+ | 1.04 | 83.68 | 16.32 | 0.20 | 0.131 |
| 30A | 0.935 0.926 0.917 0.364 | 1.402 2.042 0.853 0.616 | 0.396 0.38 0.392 0.256 | 31.303 25.614 22.005 21.078 | Fe2+ Fe2+ Fe2+ Fe3+ | 1.02 | 78.92 | 21.08 | 0.27 | 0.168 |
| 30B | 0.934 0.930 0.917 0.345 | 1.489 0.910 2.084 0.660 | 0.424 0.413 0.359 0.225 | 37.298 30.121 19.896 12.684 | Fe2+ Fe2+ Fe2+ Fe3+ | 1.12 | 87.32 | 12.68 | 0.15 | 0.101 |
| Samples (b) | ||||||||||
| 5B replica | 0.934 0.914 0.878 0.340 | 1.521 0.951 2.188 0.664 | 0.427 0.473 0.381 0.272 | 31.032 29.045 18.808 21.118 | Fe2+ Fe2+ Fe2+ Fe3 | 1.29 | 78.89 | 21.13 | 0.28 | 0.172 |
| 24B replica | 0.934 0.922 0.898 0.353 | 1.452 0.905 2.090 0.653 | 0.437 0.425 0.407 0.241 | 34.034 25.467 25.320 15.180 | Fe2+ Fe2+ Fe2+ Fe3+ | 1.33 | 84.82 | 15.18 | 0.18 | 0.126 |
| 30A replica | 0.939 0.936 0.920 0.359 | 1.461 2.075 0.895 0.621 | 0.397 0.369 0.405 0.262 | 31.041 23.495 24.758 20.707 | Fe2+ Fe2+ Fe2+ Fe3+ | 1.35 | 79.29 | 20.71 | 0.26 | 0.170 |
| 30B replica | 0.932 0.920 0.904 0.360 | 1.397 0.852 2.012 0.634 | 0.421 0.391 0.379 0.219 | 37.001 25.873 24.502 12.624 | Fe2+ Fe2+ Fe2+ Fe3+ | 1.73 | 87.39 | 12.62 | 0.14 | 0.101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenaz, D.; Maibam, B.; Adetunji, J.; Skogby, H. The Effects of High-Grade Metamorphism on Cr-Spinel from the Archean Sittampundi Complex, South India. Minerals 2021, 11, 1370. https://doi.org/10.3390/min11121370
Lenaz D, Maibam B, Adetunji J, Skogby H. The Effects of High-Grade Metamorphism on Cr-Spinel from the Archean Sittampundi Complex, South India. Minerals. 2021; 11(12):1370. https://doi.org/10.3390/min11121370
Chicago/Turabian StyleLenaz, Davide, Bidyananda Maibam, Jacob Adetunji, and Henrik Skogby. 2021. "The Effects of High-Grade Metamorphism on Cr-Spinel from the Archean Sittampundi Complex, South India" Minerals 11, no. 12: 1370. https://doi.org/10.3390/min11121370








