Effect of Sodium Metabisulfite on Selective Flotation of Chalcopyrite and Molybdenite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microflotation Test
2.3. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
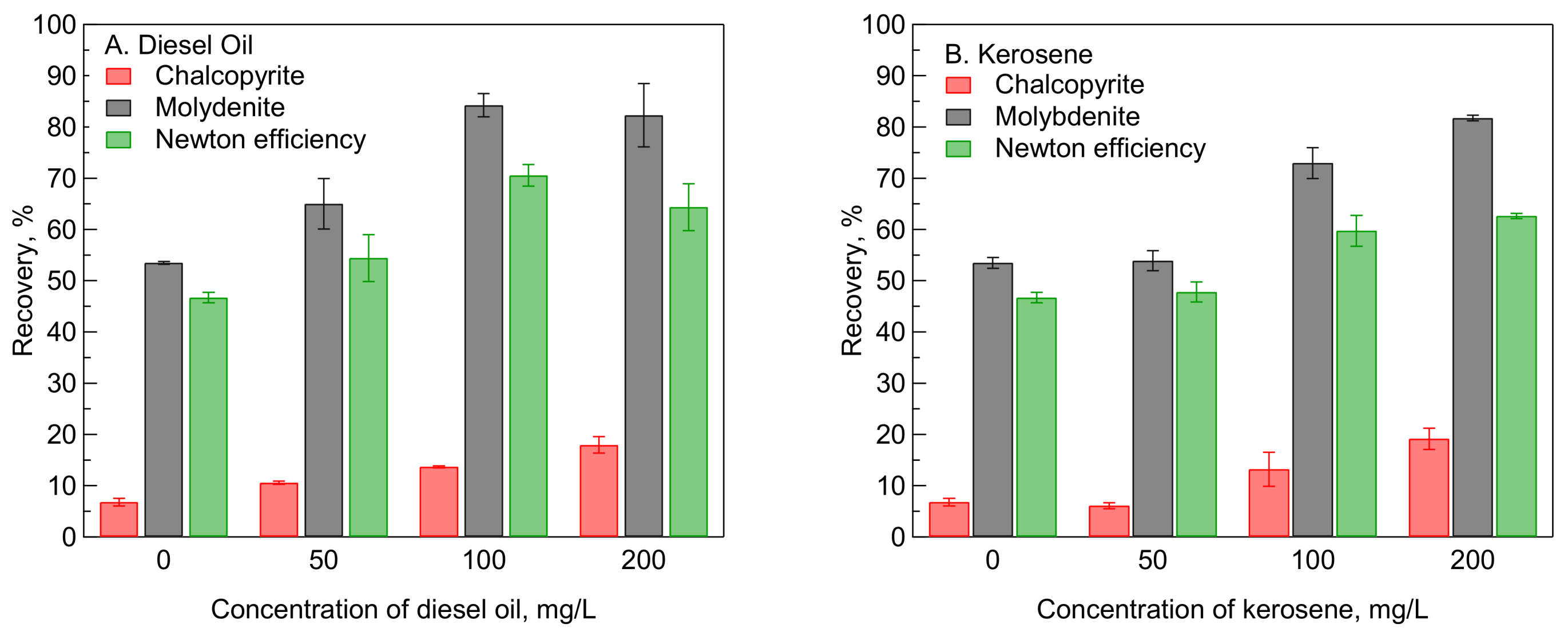
3.1. Microflotation of Single Mineral
3.2. XPS Analysis
3.3. Proposed Mechanism
3.4. Microflotation of Mixed Minerals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wills, B.A.; Napier-Munn, T.J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th ed.; Elsevier Science & Technology Books: Burlington, VT, USA, 2006. [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice: Volume 1: Flotation of Sulfide Ores; Elsevier Science: Amsterdam, The Netherlands, 2007; ISBN 978-0-08-047137-2. [Google Scholar]
- Liu, G.; Lu, Y.; Zhong, H.; Cao, Z.; Xu, Z. A Novel Approach for Preferential Flotation Recovery of Molybdenite from a Porphyry Copper–Molybdenum Ore. Miner. Eng. 2012, 36–38, 37–44. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Castro, S.; Ramos, O. Effect of Seawater Main Components on Frothability in the Flotation of Cu-Mo Sulfide Ore. Physicochem. Probl. Miner. Process. 2013, 50, 17–29. [Google Scholar]
- Ansari, A.; Pawlik, M. Floatability of Chalcopyrite and Molybdenite in the Presence of Lignosulfonates. Part I. Adsorption Studies. Miner. Eng. 2007, 20, 600–608. [Google Scholar] [CrossRef]
- Moreno, P.A.; Aral, H.; Cuevas, J.; Monardes, A.; Adaro, M.; Norgate, T.; Bruckard, W. The Use of Seawater as Process Water at Las Luces Copper–Molybdenum Beneficiation Plant in Taltal (Chile). Miner. Eng. 2011, 24, 852–858. [Google Scholar] [CrossRef]
- Pearse, M.J. An Overview of the Use of Chemical Reagents in Mineral Processing. Miner. Eng. 2005, 18, 139–149. [Google Scholar] [CrossRef]
- Prasad, M.S. Reagents in the Mineral Industry—Recent Trends and Applications. Miner. Eng. 1992, 5, 279–294. [Google Scholar] [CrossRef]
- Somasundaran, P. Reagents in Mineral Technology; CRC Press: Boca Raton, FL, USA, 1987; ISBN 978-0-8247-7715-9. [Google Scholar]
- Yin, W.; Zhang, L.; Xie, F. Flotation of Xinhua Molybdenite Using Sodium Sulfide as Modifier. Trans. Nonferrous Met. Soc. China 2010, 20, 702–706. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, Q.; Liu, W.; Zheng, J.; Sun, H. Flotation Separation of Copper–Molybdenum Sulfides Using Chitosan as a Selective Depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Kelebek, S.; Yoruk, S.; Smith, G.W. Wetting Behavior of Molybdenite and Talc in Lignosulphonate/Mibc Solutions and Their Separation by Flotation. Sep. Sci. Technol. 2001, 36, 145–157. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.; Liu, J.; Zhu, Y.; Han, Y. Dithiouracil, a Highly Efficient Depressant for the Selective Separation of Molybdenite from Chalcopyrite by Flotation: Applications and Mechanism. Miner. Eng. 2022, 175, 107287. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Wu, M.; Xu, Z.; Tian, M.; Yin, Z.; Sun, W.; Zhang, C. Flotation Separation of Molybdenite from Chalcopyrite Using Rhodanine-3-Acetic Acid as a Novel and Effective Depressant. Miner. Eng. 2021, 162, 106747. [Google Scholar] [CrossRef]
- Hirajima, T.; Mori, M.; Ichikawa, O.; Sasaki, K.; Miki, H.; Farahat, M.; Sawada, M. Selective Flotation of Chalcopyrite and Molybdenite with Plasma Pre-Treatment. Miner. Eng. 2014, 66–68, 102–111. [Google Scholar] [CrossRef]
- Miki, H.; Matsuoka, H.; Hirajima, T.; Suyantara, G.P.W.; Sasaki, K. Electrolysis Oxidation of Chalcopyrite and Molybdenite for Selective Flotation. Mater. Trans. 2017, 58, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Hirajima, T.; Miki, H.; Suyantara, G.P.W.; Matsuoka, H.; Elmahdy, A.M.; Sasaki, K.; Imaizumi, Y.; Kuroiwa, S. Selective Flotation of Chalcopyrite and Molybdenite with H2O2 Oxidation. Miner. Eng. 2017, 100, 83–92. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Yamane, M.; Takida, E.; Kuroiwa, S.; Imaizumi, Y. Selective Flotation of Chalcopyrite and Molybdenite Using H2O2 Oxidation Method with the Addition of Ferrous Sulfate. Miner. Eng. 2018, 122, 312–326. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Yamane, M.; Takida, E.; Kuroiwa, S.; Imaizumi, Y. Effect of Fenton-like Oxidation Reagent on Hydrophobicity and Floatability of Chalcopyrite and Molybdenite. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 34–48. [Google Scholar] [CrossRef]
- Grano, S.R.; Johnson, N.W.; Ralston, J. Control of the Solution Interaction of Metabisulphite and Ethyl Xanthate in the Flotation of the Hilton Ore of Mount Isa Mines Limited, Australia. Miner. Eng. 1997, 10, 17–39. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Hasanzadeh, M. Chalcopyrite and Pyrite Floatabilities in the Presence of Sodium Sulfide and Sodium Metabisulfite in a High Pyritic Copper Complex Ore. J. Dispers. Sci. Technol. 2017, 38, 782–788. [Google Scholar] [CrossRef]
- Houot, R.; Duhamet, D. The Use of Sodium Sulphite to Improve the Flotation Selectivity between Chalcopyrite and Galena in a Complex Sulphide Ore. Miner. Eng. 1992, 5, 343–355. [Google Scholar] [CrossRef]
- Khmeleva, T.; Skinner, W.; Beattie, D.A.; Georgiev, T.V. The Effect of Sulphite on the Xanthate-Induced Flotation of Copper-Activated Pyrite. Physicochem. Probl. Miner. Process. 2002, 36, 185–195. [Google Scholar]
- Khmeleva, T.N.; Chapelet, J.K.; Skinner, W.M.; Beattie, D.A. Depression Mechanisms of Sodium Bisulphite in the Xanthate-Induced Flotation of Copper Activated Sphalerite. Int. J. Miner. Process. 2006, 79, 61–75. [Google Scholar] [CrossRef]
- Miki, H.; Hirajima, T.; Muta, Y.; Suyantara, G.P.W.; Sasaki, K. Effect of Sodium Sulfite on Floatability of Chalcopyrite and Molybdenite. Minerals 2018, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Peng, Y. The Role of Sodium Metabisulphite in Depressing Pyrite in Chalcopyrite Flotation Using Saline Water. Miner. Eng. 2019, 142, 105921. [Google Scholar] [CrossRef]
- Petrus, H.T.B.M.; Hirajima, T.; Sasaki, K.; Okamoto, H. Effects of Sodium Thiosulphate on Chalcopyrite and Tennantite: An Insight for Alternative Separation Technique. Int. J. Miner. Process. 2012, 102–103, 116–123. [Google Scholar] [CrossRef]
- Haga, K.; Tongamp, W.; Shibayama, A. Investigation of Flotation Parameters for Copper Recovery from Enargite and Chalcopyrite Mixed Ore. Mater. Trans. 2012, 53, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, A.; Karakaş, F. The Kinetics Modeling of Chalcopyrite and Pyrite, and the Contribution of Particle Size and Sodium Metabisulfite to the Flotation of Copper Complex Ores. Part. Sci. Technol. 2017, 35, 455–461. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Kuroiwa, S.; Aoki, Y. Effect of Na2SO3 on the Floatability of Chalcopyrite and Enargite. Miner. Eng. 2021, 173, 107222. [Google Scholar] [CrossRef]
- Castro, S. Physico-Chemical Factors in Flotation of Cu-Mo-Fe Ores with Seawater: A Critical Review. Physicochem. Probl. Miner. Process. 2018, 54, 1223–1236. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Peng, Y. The Depression of Molybdenite Flotation by Sodium Metabisulphite in Fresh Water and Seawater. Miner. Eng. 2021, 168, 106939. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Takatsugi, K.; Ishikura, K.; Hirajima, T. Spectroscopic Study on Oxidative Dissolution of Chalcopyrite, Enargite and Tennantite at Different pH Values. Hydrometallurgy 2010, 100, 144–151. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Cook, M.G. X-ray Photoelectron Studies on Some Oxides and Hydroxides of Cobalt, Nickel, and Copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS Analysis of Chalcopyrite (CuFeS2) Dissolution in Sulfuric Acid Solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Brion, D. Etude Par Spectroscopie de Photoelectrons de La Degradation Superficielle de FeS2, CuFeS2, ZnS et PbS a l’air et Dans l’eau. Appl. Surf. Sci. 1980, 5, 133–152. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. X-ray Photoelectron Spectroscopy Studies of Solvated Metal Atom Dispersed Catalysts. Monometallic Iron and Bimetallic Iron-Cobalt Particles on Alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar] [CrossRef]
- Shuxian, Z.; Hall, W.K.; Ertl, G.; Knözinger, H. X-ray Photoemission Study of Oxygen and Nitric Oxide Adsorption on MoS2. J. Catal. 1986, 100, 167–175. [Google Scholar] [CrossRef]
- Benoist, L.; Gonbeau, D.; Pfister-Guillouzo, G.; Schmidt, E.; Meunier, G.; Levasseur, A. XPS Analysis of Lithium Intercalation in Thin Films of Molybdenum Oxysulphides. Surf. Interface Anal. 1994, 22, 206–210. [Google Scholar] [CrossRef]
- Seifert, G.; Finster, J.; Müller, H. SW Xα Calculations and X-ray Photoelectron Spectra of Molybdenum(II) Chloride Cluster Compounds. Chem. Phys. Lett. 1980, 75, 373–377. [Google Scholar] [CrossRef]
- Kim, K.S.; Baitinger, W.E.; Amy, J.W.; Winograd, N. ESCA Studies of Metal-Oxygen Surfaces Using Argon and Oxygen Ion-Bombardment. J. Electron Spectrosc. Relat. Phenom. 1974, 5, 351–367. [Google Scholar] [CrossRef]
- Stevens, G.C.; Edmonds, T. Electron Spectroscopy for Chemical Analysis Spectra of Molybdenum Sulfides. J. Catal. 1975, 37, 544–547. [Google Scholar] [CrossRef]
- Zingg, D.S.; Makovsky, L.E.; Tischer, R.E.; Brown, F.R.; Hercules, D.M. A Surface Spectroscopic Study of Molybdenum-Alumina Catalysts Using X-ray Photoelectron, Ion-Scattering, and Raman Spectroscopies. J. Phys. Chem. 1980, 84, 2898–2906. [Google Scholar] [CrossRef]
- Patterson, T.A.; Carver, J.C.; Leyden, D.E.; Hercules, D.M. A Surface Study of Cobalt-Molybdena-Alumina Catalysts Using X-ray Photoelectron Spectroscopy. J. Phys. Chem. 1976, 80, 1700–1708. [Google Scholar] [CrossRef]
- Castro, S.; López-Valdivieso, A.; Laskowski, J.S. Review of the Flotation of Molybdenite. Part I: Surface Properties and Floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Wada, M.; Majima, H. Flotation of Molybdenite. Flotation 1962, 1962, 9–18. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K. Bubble Interactions with Chalcopyrite and Molybdenite Surfaces in Seawater. Miner. Eng. 2020, 157, 106536. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Yang, B.; Lopez-Mendoza, A. Flotation of Molybdenite Fines as Hydrophobic Agglomerates. Sep. Purif. Technol. 2012, 98, 451–455. [Google Scholar] [CrossRef]
- Tanaka, Y.; Miki, H.; Suyantara, G.P.W.; Aoki, Y.; Hirajima, T. Mineralogical Prediction on the Flotation Behavior of Copper and Molybdenum Minerals from Blended Cu–Mo Ores in Seawater. Minerals 2021, 11, 869. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Elmahdy, A.M.; Miki, H.; Sasaki, K. Effect of Kerosene Emulsion in MgCl2 Solution on the Kinetics of Bubble Interactions with Molybdenite and Chalcopyrite. Colloids Surf. A Physicochem. Eng. Asp. 2016, 501, 98–113. [Google Scholar] [CrossRef]
- Lin, Q.; Gu, G.; Wang, H.; Liu, Y.; Fu, J.; Wang, C. Flotation Mechanisms of Molybdenite Fines by Neutral Oils. Int. J. Miner. Metall. Mater. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K. Floatability of Molybdenite and Chalcopyrite in Artificial Seawater. Miner. Eng. 2018, 115, 117–130. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration-Flotation of Finely Ground Chalcopyrite and Quartz: Effects of Agitation Strength during Agglomeration Using Emulsified Oil on Chalcopyrite. Minerals 2020, 10, 380. [Google Scholar] [CrossRef]






| Elements | Chalcopyrite | Molybdenite |
|---|---|---|
| Al | 0.12 | 0.07 |
| Si | 5.74 | 0.17 |
| S | 24.19 | 41.90 |
| Fe | 25.90 | 0.16 |
| Cu | 31.59 | - |
| Zn | - | 0.18 |
| Mo | - | 51.56 |
| No | Collector Condition | MBS Concentration | Recovery, % | η, % | |
|---|---|---|---|---|---|
| Cu | Mo | ||||
| 1 | Without collector | 0 mM MBS | 35.9 | 61.1 | 25.2 |
| 2 | 100 mg/L diesel | 0 mM MBS | 30.8 | 88.0 | 57.3 |
| 3 | 100 mg/L diesel | 1 mM MBS | 13.7 | 84.2 | 70.5 |
| 4 | 200 mg/L kerosene | 0 mM MBS | 43.3 | 89.5 | 46.2 |
| 5 | 200 mg/L kerosene | 1 mM MBS | 19.1 | 81.7 | 62.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semoto, Y.; Suyantara, G.P.W.; Miki, H.; Sasaki, K.; Hirajima, T.; Tanaka, Y.; Aoki, Y.; Ura, K. Effect of Sodium Metabisulfite on Selective Flotation of Chalcopyrite and Molybdenite. Minerals 2021, 11, 1377. https://doi.org/10.3390/min11121377
Semoto Y, Suyantara GPW, Miki H, Sasaki K, Hirajima T, Tanaka Y, Aoki Y, Ura K. Effect of Sodium Metabisulfite on Selective Flotation of Chalcopyrite and Molybdenite. Minerals. 2021; 11(12):1377. https://doi.org/10.3390/min11121377
Chicago/Turabian StyleSemoto, Yuki, Gde Pandhe Wisnu Suyantara, Hajime Miki, Keiko Sasaki, Tsuyoshi Hirajima, Yoshiyuki Tanaka, Yuji Aoki, and Kumika Ura. 2021. "Effect of Sodium Metabisulfite on Selective Flotation of Chalcopyrite and Molybdenite" Minerals 11, no. 12: 1377. https://doi.org/10.3390/min11121377
APA StyleSemoto, Y., Suyantara, G. P. W., Miki, H., Sasaki, K., Hirajima, T., Tanaka, Y., Aoki, Y., & Ura, K. (2021). Effect of Sodium Metabisulfite on Selective Flotation of Chalcopyrite and Molybdenite. Minerals, 11(12), 1377. https://doi.org/10.3390/min11121377







