Distribution and Mode of Occurrence of Co, Ni, Cu, Zn, As, Ag, Cd, Sb, Pb in the Feed Coal, Fly Ash, Slag, in the Topsoil and in the Roots of Trees and Undergrowth Downwind of Three Power Stations in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Area and Materials
- Area A; high density of pine forests adjoining Kozienice Landscape Park [87]. In this area there are few towns, with a maximum population of 17,500 people. In area A, except PS-a, there are no other industrial emitters of particulates. The power station started operating in 1972. Currently, it generates energy of approximately 4000 MW. The soil samples were collected between 7.7 km and 13.1 km NE, E, and S of the PS-a.
- Area B is burdened with many years of industrial activities (coking plants, hard coal mines, zinc and lead ore mines, steel and zinc works, small PS, chemical plants) and 19 neighboring cities, with a total population of 2.013 million people, forming the Upper Silesian conurbation [88]. PS-b is located in this area, which has been combusting feed coals since 1954; and, currently, it generates energy of approximately 220 MW. In area B, oak and pine trees prevail.The soil samples were collected between 9.9 km and 12.9 km NNE and NE of PS-b.
- Area C includes mainly pine forests adjoining landscape conservation areas (Stobrawa—Turawa Forests, Niemodlin Forests). It includes a few towns and villages with a total population of 9800 people and a few small industry plants [89]. In area C, there is a coal-fired power station (PS-c), operating since 1993, which generates energy of approximately 1500 MW. Opole is located approximately 12 km south of area C (118,300 people). In the area, there were no and there are no metal works and no other power stations. The soil samples were collected at a distance between 5.2 km and 11.1 km NNE and E of PS-c.
2.2. Research Range, Methods, and Calculations
- -
- -
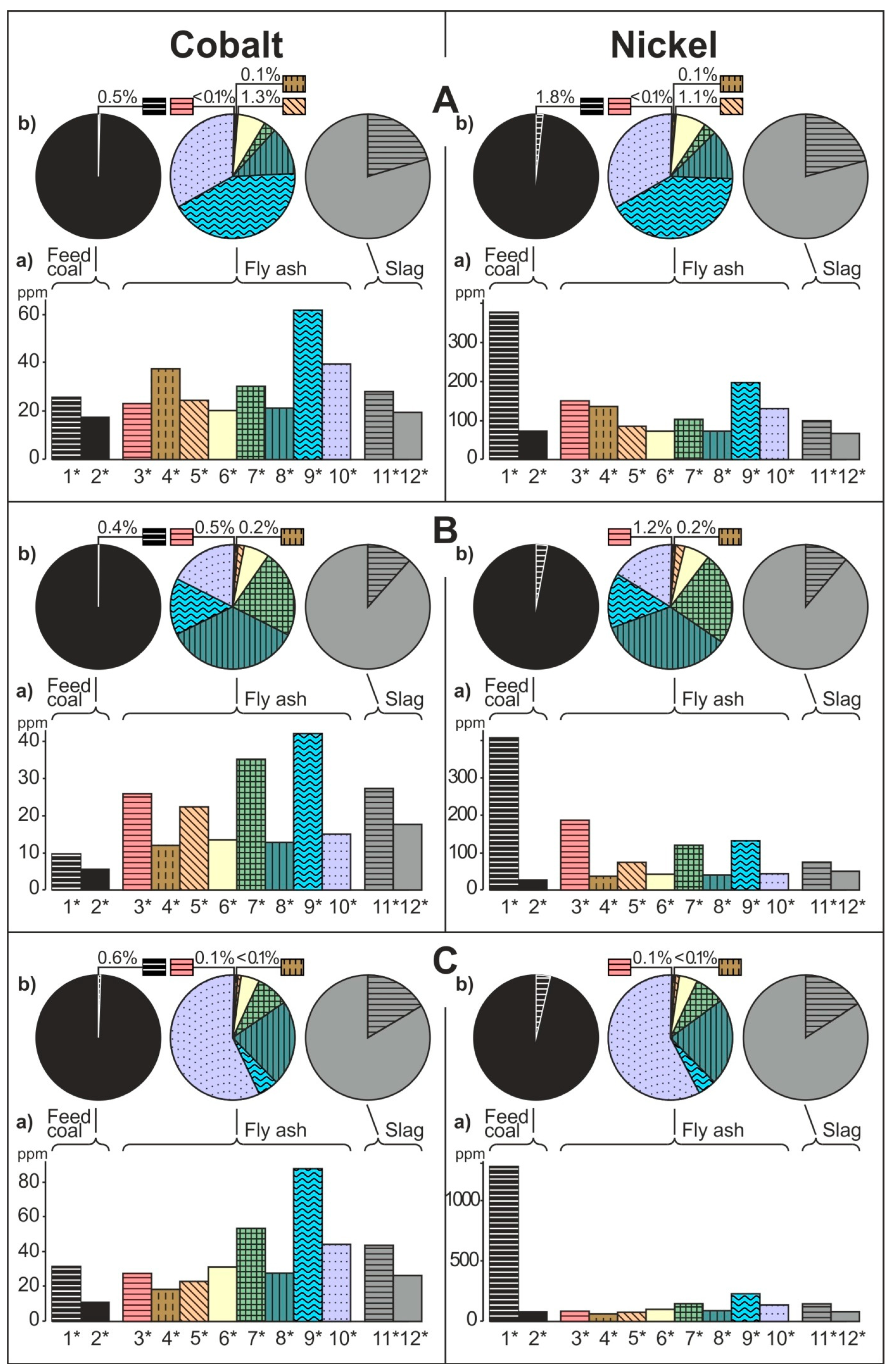
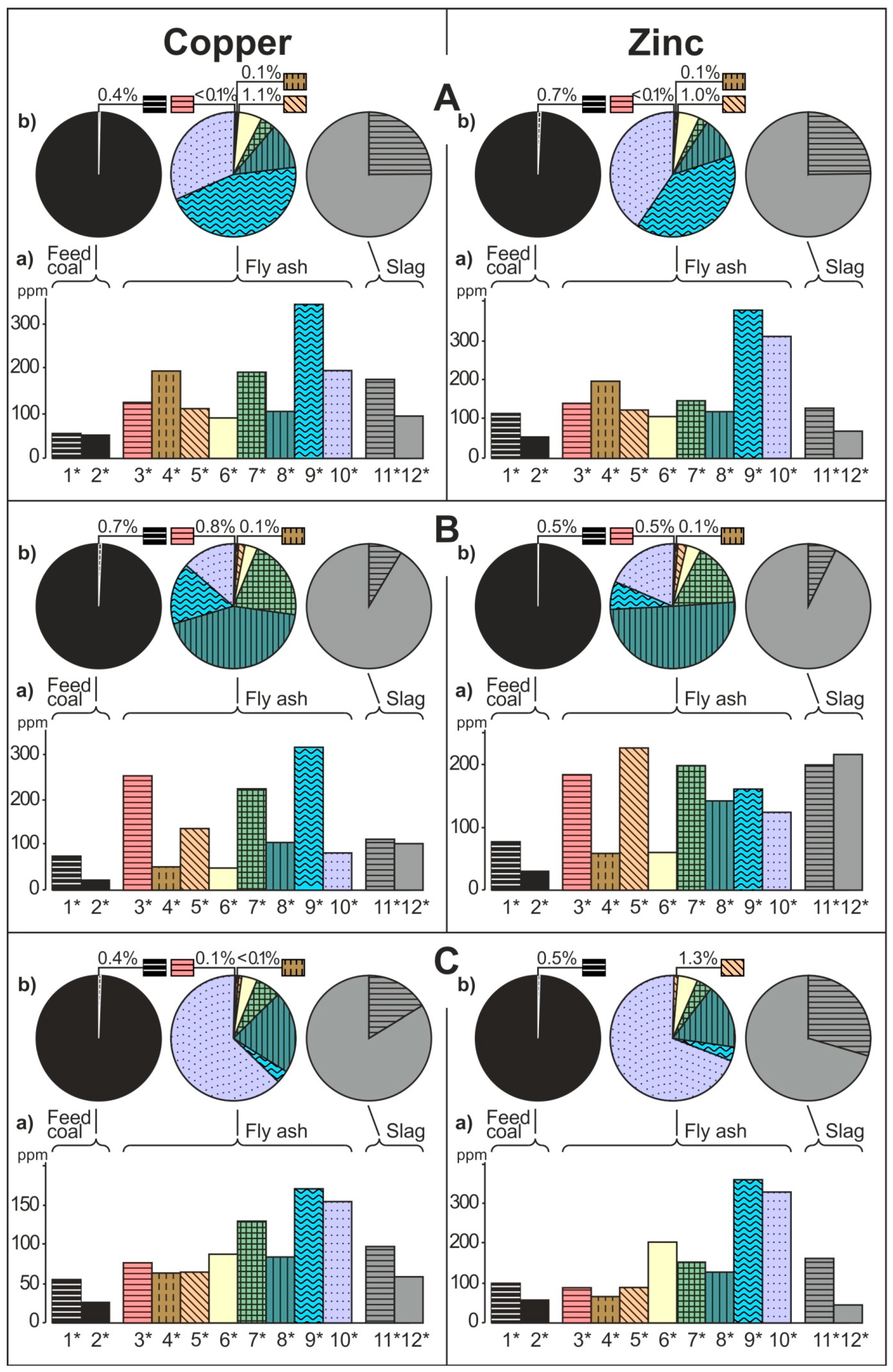
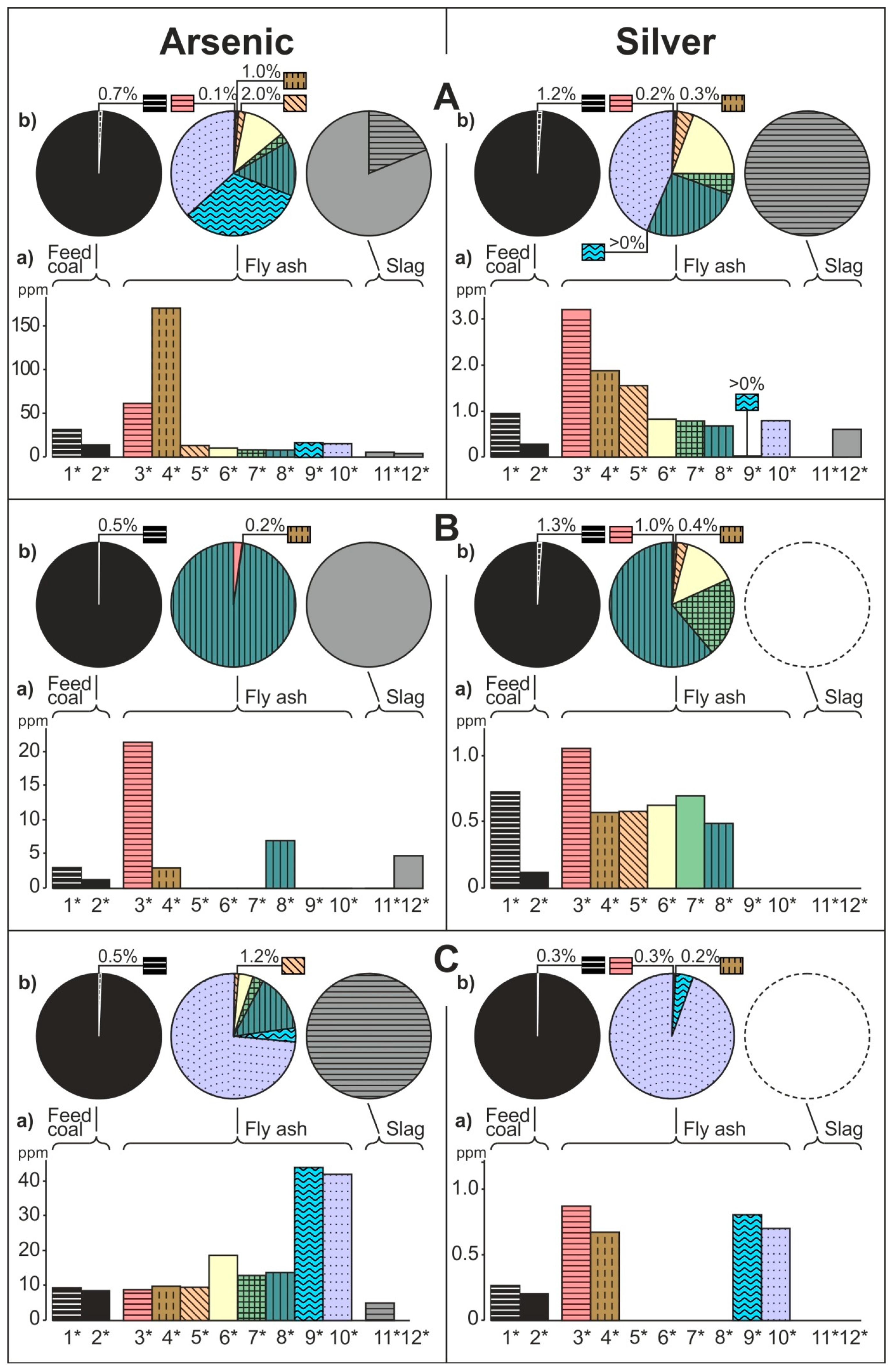
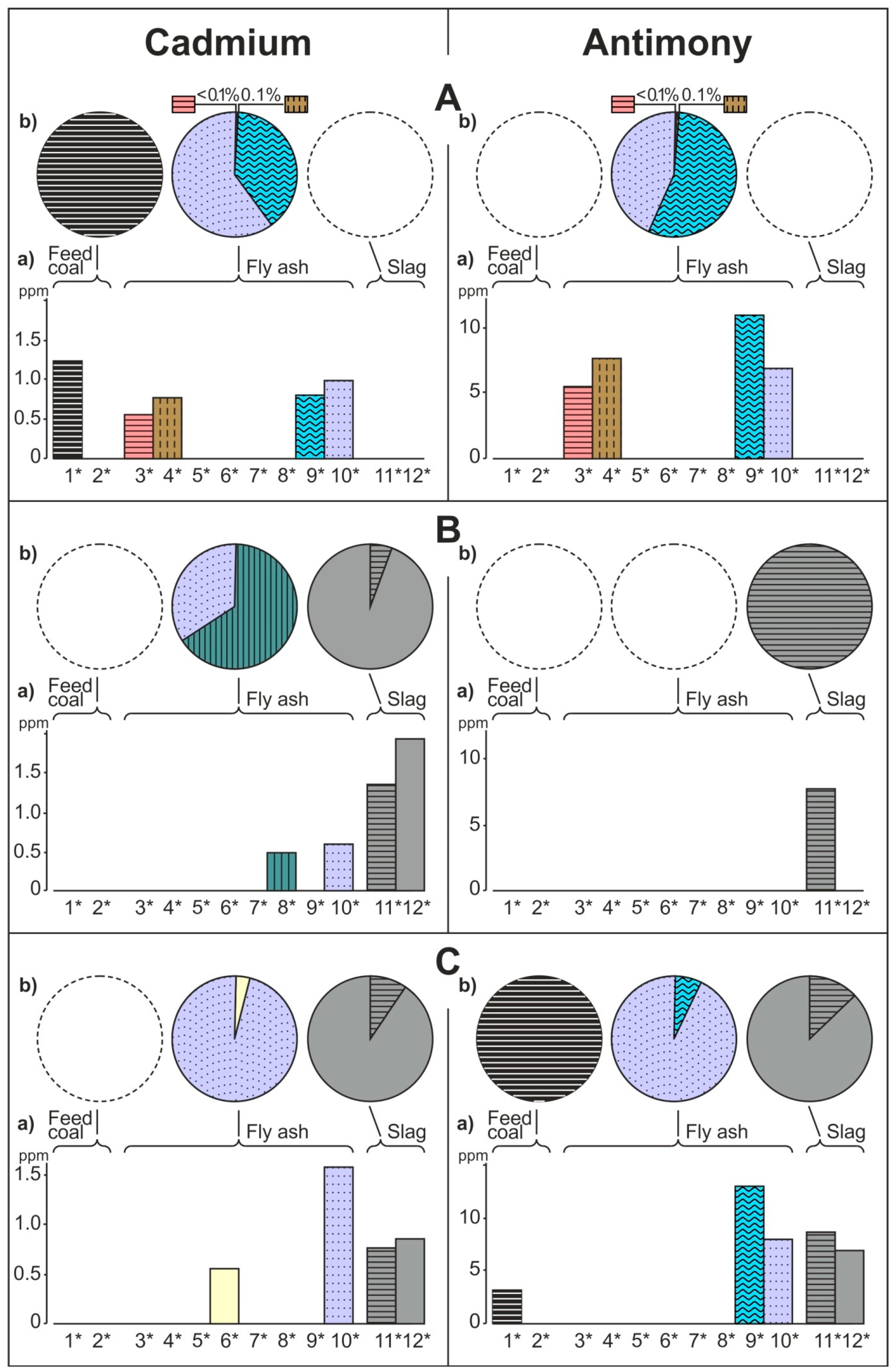
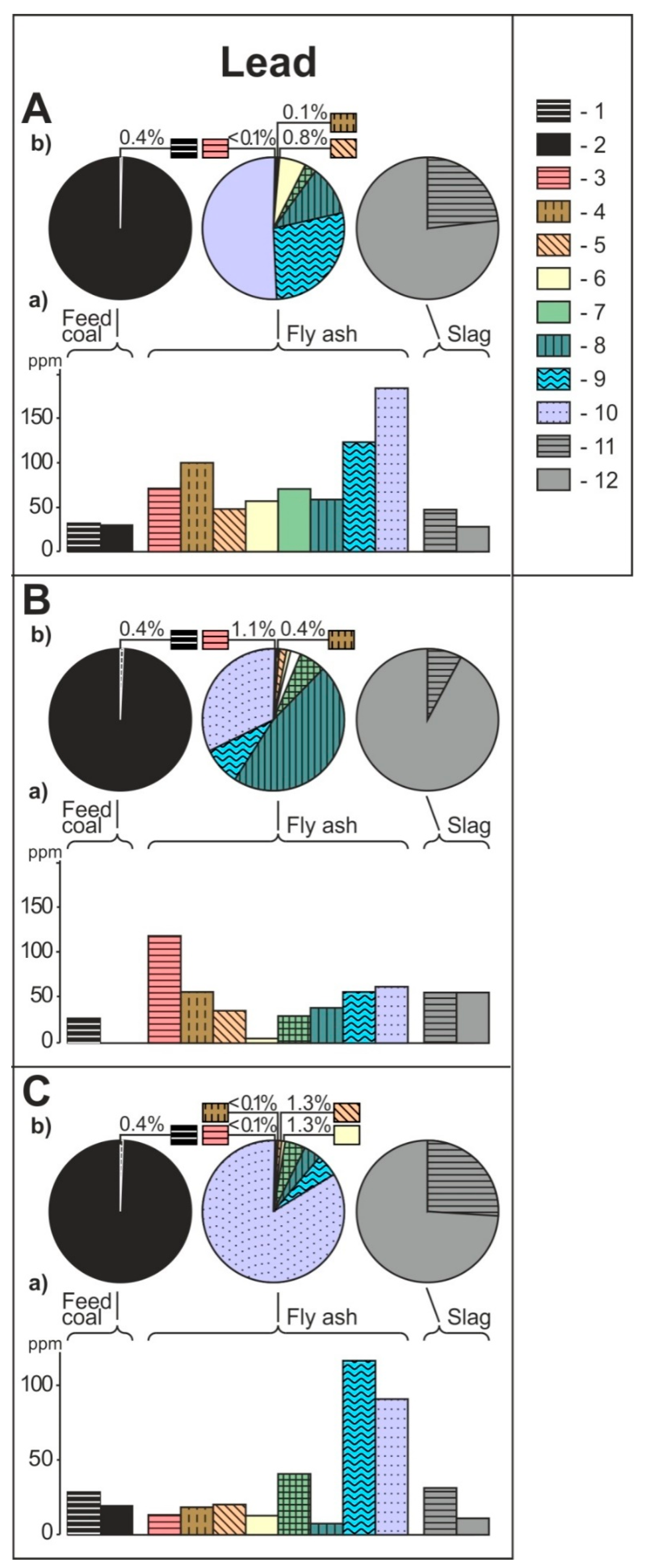
- The share of grain size classes and of magnetic and nonmagnetic fractions in the fly ash and the share of magnetic and nonmagnetic fraction in the feed coal and the slag in concentrating elements in the whole fly ash, whole feed coal, and whole slag. To do this, the percentage share of each of the components of the weighted average in the total value of the weighted average was calculated. The weighted average component is the product of the element content in each class of fly ash grains and in each slag fraction, as well as the percentage mass share of each class and grain fraction in the composition of whole fly ash and in the whole slag. The calculations were made according to the Equations (1) and (2) and their results are presented in Figure 2 (see Table S1);
- -
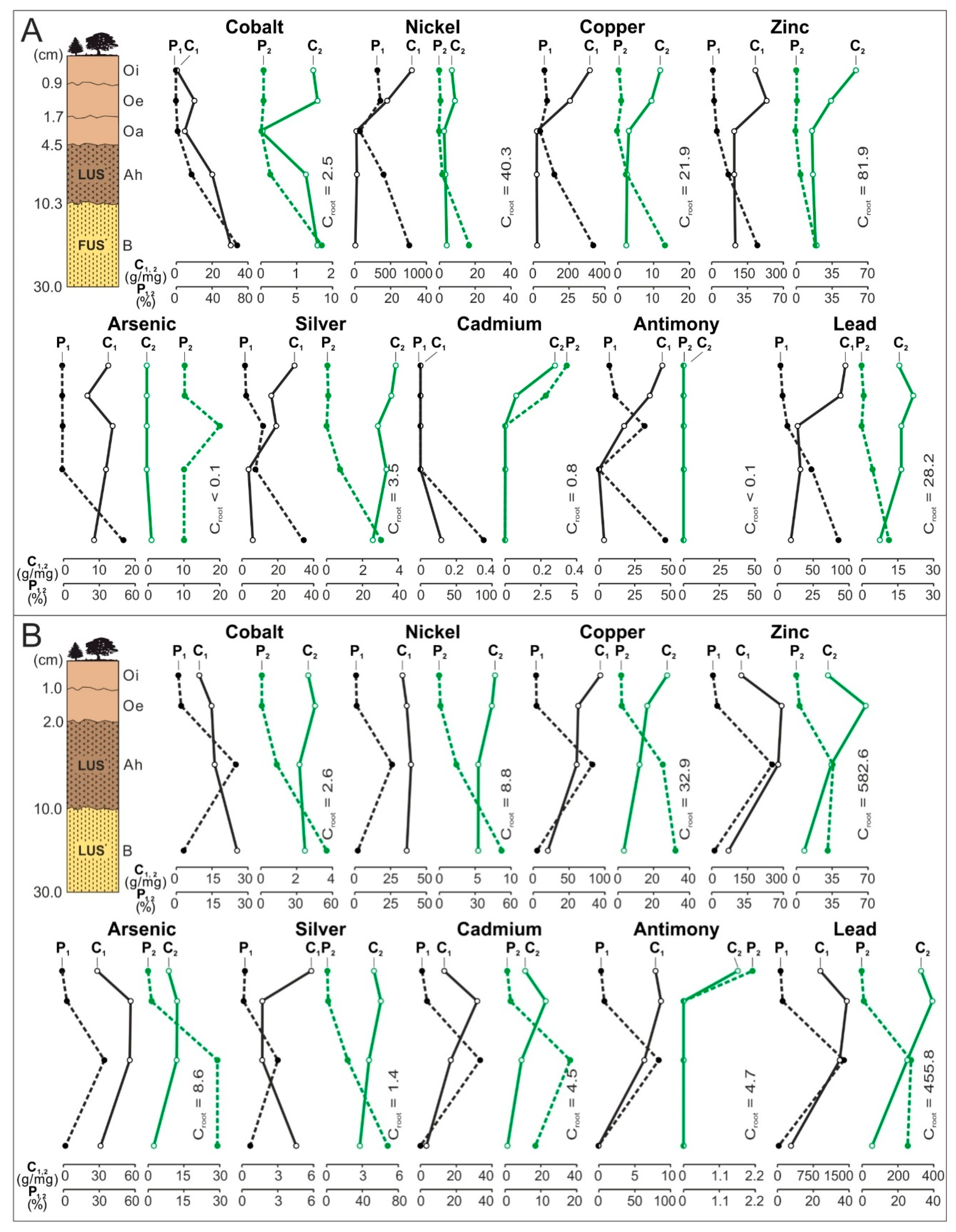
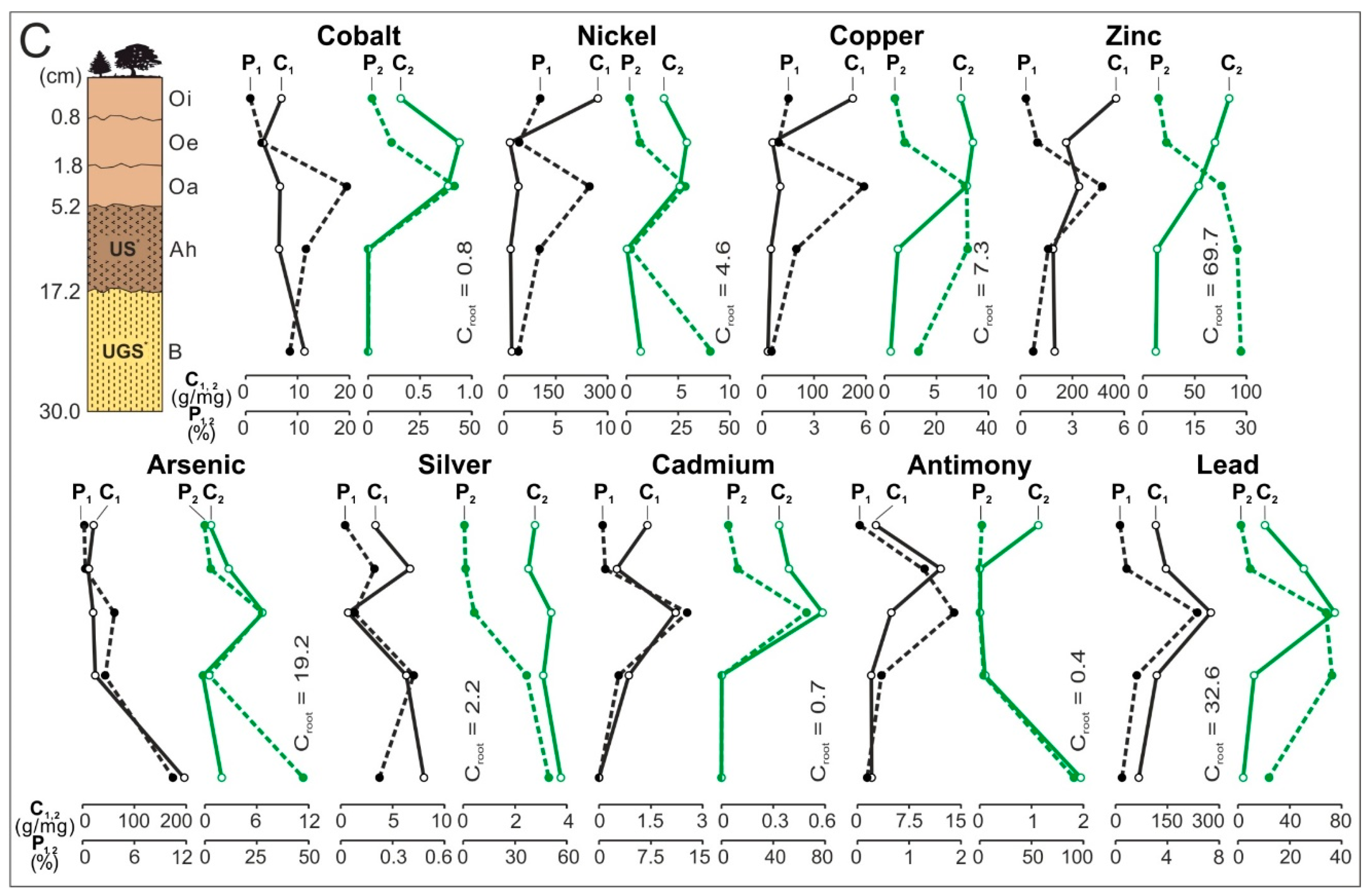
- The share of the horizon/subhorizon of soil (sorted into magnetic and nonmagnetic fractions) in concentrating elements in the whole topsoil of 30 cm thickness. To do this, the percentage share of each of the components of the weighted average was calculated. The component is the product of the content of a given element in each fraction of soil horizon/subhorizon and the percentage share of the weight of each fraction of soil horizon/subhorizon in the composition of the whole topsoil. The total of the components of the weighted average is 100%. The calculations were made according to the Equations (1) and (2) and their results are presented in Figure 3 (see Table S2).
3. Results
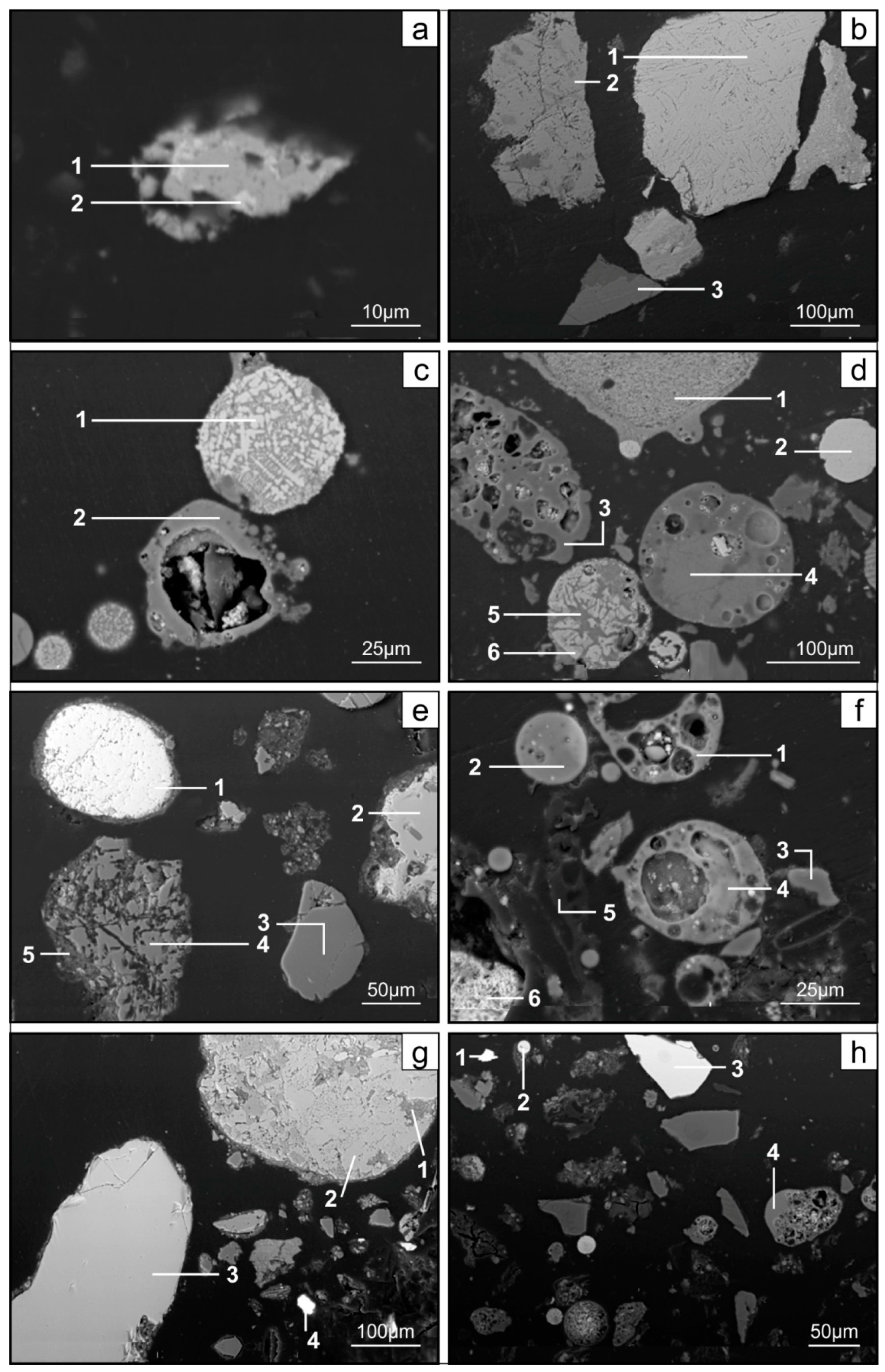
3.1. General Petrographic and Geochemical Characteristics of the Subject of the Research
3.2. Distribution and Mode of Occurrence of the Elements in Coal and Combustion Residues
3.3. Distribution and Mode of Occurrence of the Elements in Topsoil and Roots
4. Conclusions
- The hosts of the elements in the feed coal are most often: magnetite, fusinite (mainly with ingrowths or agglomerate with pyrite, carbonates, and clay minerals) together with pyrite and chalcopyrite, followed by siderite, vitrinite (often fused with minerals) and dolomite, present in the magnetic fractions of the feed coal. The highest point of content of the elements was observed in magnetite and cassiterite (Co, Ni, Cu, As, Ag, Cd, Pb), in macerals with siderite (Co, Zn, As, Ag, Cd, Sb, Pb) as well as in pyrite and chalcopyrite (Cu, Zn, and As). The nonmagnetic fraction has the greatest influence on the average content of the elements in the whole feed coal.
- The hosts of the highest content of the elements in the fly ash are microspheres and ferrospheres incrusted with crystals, dendrites and/or iron oxides (mainly of magnetite), and single grains of magnetite (concerns Co, Ni, Cu, Ag, Cd, Sb, and Pb), as well as tenuispheres and crassispheres, cenospheres, and subhedral grains of magnetite (Zn and As), being the main components of the magnetic fraction of the fly ash and the slag. The hosts of the highest content of theelements in the slag are iron oxide on the surface of cenospheres and the crassinetwork, ferrospheres, and iron oxide grains. More rarely the host is is apatite. The biggest influence onthe average content of the elements in the whole fly ash and the whole slag has a group of <0.05 mm nonmagnetic particles of the fly ash and the nonmagnetic fraction of the slag. If the finest solid particles found in the <0.05 mm fraction of the analyzed fly ash are emitted by the power stations, then they were the particles most enriched with Co and Cu (in areas A–C), Ni, Zn and Pb (A, C), As and Sb (C), and Cd (A), in comparison with the feed coal, which reached the analyzed topsoil, whereas the >0.5 mm fly ash particles, enriched mainly with As, Ag, and Cd, were probably separated by the electrostatic precipitators.
- In subhorizon Oi, and secondarily in Oe, the content of theelements is the highest. Most often their hosts are various morphotypes of microspheres and char, emitted by power stations located in large wooded areas (area A and C). The content of Zn, As, Cd, and Pb in each subhorizon/horizon of the topsoil in the area exposed to many years of industrial activities (area B) is greater than in the potentially emitted<0.05 mm particles of the fly ash. It is also greater than in the soil in areas A and C. Together with the power station(PS-b) the main emitters of the elements on the surface of the soil in area B were probably zinc, lead, and ironworks (operating in the past). The values of the enrichment factor for the magnetic fraction of the topsoil are always higher than the for the nonmagnetic fraction. The particles of the topsoil show lower enrichment with Co, Cu, Cd, and Sb and greater enrichment with Ni, Zn, As, Ag, and Pb, than the <0.05 mm particles of the fly ash. The elution of elements accumulated in the several-micron surface layer of sedimenting particles (mainly <0.05 mm), with the use of rainwater or flowing water, can still enrich topsoil with ecotoxic elements.
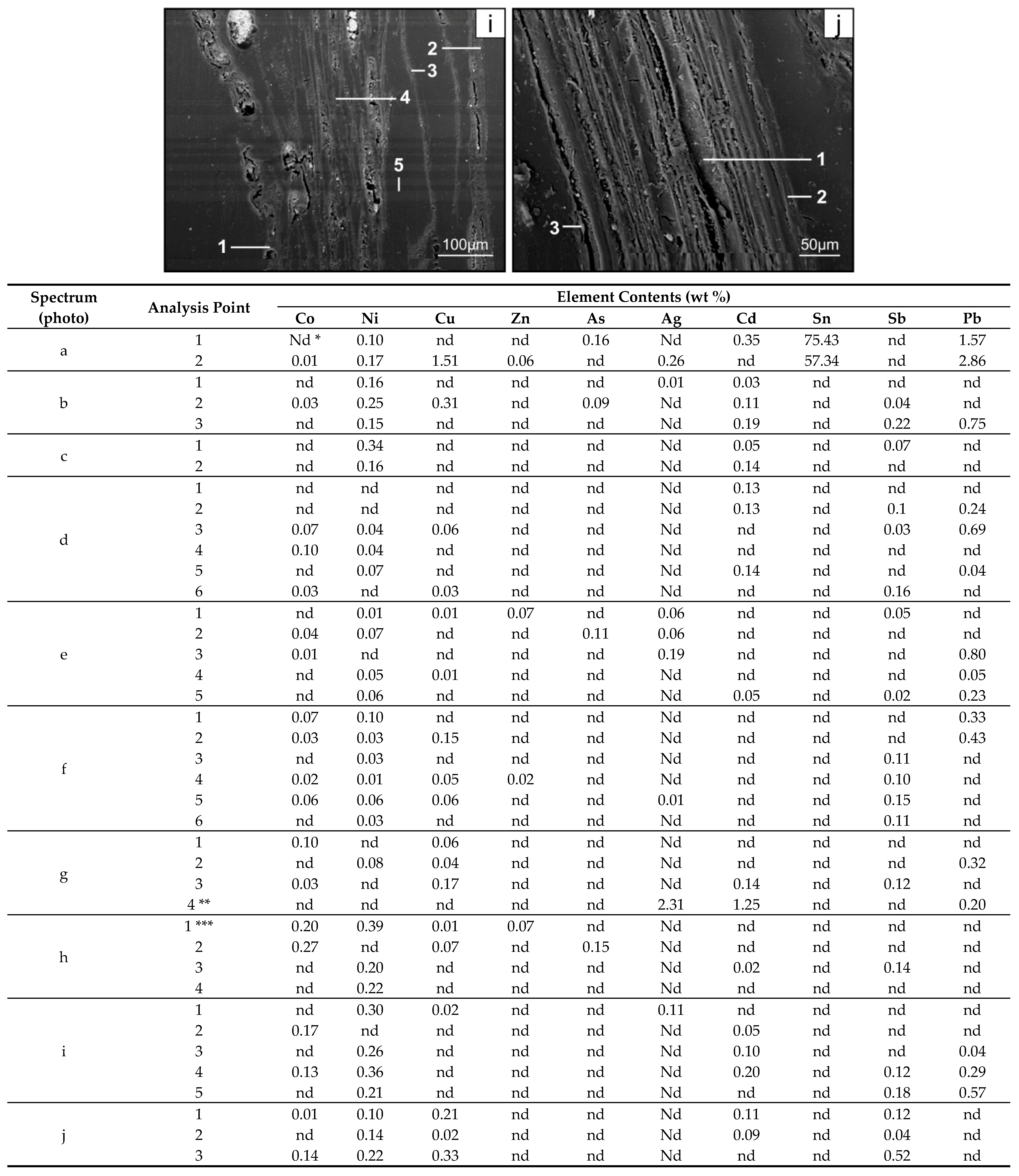
- The significant enrichment of the topsoil with the elements resulted in an increase in the content of the elements in the roots of trees and undergrowth. In the soil exposed to the long-term emission of the technogenic particles by thermo-emitters (area B), the roots have the highest content of Cu, Zn, Cd, Sb, and Pb, and in the topsoil in the wooded areas with the power station as the only pollution emitter, the roots had the highest content of As (area C) and Ag (area A). The enrichment of the roots with Ci, Ni, Zn, As, Ag, Sb, and Pb is between 0.2 for Co and 41.0 for Cd. It is usually proportional to the increase in the content of the elements in the whole topsoil and the value of the enrichment factor for the topsoil. The highest content of the elements occurs in the tiny roots, especially in the rhizodermis and the primary cortex, more seldom in the axle roller and cortex cells.
- Along the way from the feed coal to the roots, the highest enrichment with elements was observed in the <0.05 mm magnetic (for Co, Ni, Cu, Zn, Cd, Sb, and Pb) and nonmagnetic (Cd, Sb, and Pb)particles of the fly ash; in the >0.5 mm nonmagnetic particles of the fly ash (As and Ag); in the magnetic particles of the slag (Sb), and in the magnetic particles of the soil in subhorizon/horizon Oi (Ni, Cu, and Ag), Oe (Zn, Cd, Sb, and Pb), Ah (Co), and in horizon B (As). A high content of elements in particles of falling dust from power plants, which is potentially dangerous for topsoil and plants, can be significantly reduced by preparing coal which is free from extremely high content of ecotoxic elements. The choice of methods for obtaining such coal will be facilitated by studies of the occurrence and distribution of elements in the feed coal.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hower, J.C.; Trimble, A.S.; Eble, C.F. Temporal and spatial variations in fly ash quality. Fuel Proc. Technol. 2001, 73, 37–58. [Google Scholar] [CrossRef]
- Zhang, J.; Han, C.-L.; Xu, Y.-Q. The release of the hazardous elements from coal in the initial stage of combustion process. Fuel Proc. Technol. 2003, 84, 121–133. [Google Scholar] [CrossRef]
- Hower, J.C.; Fu, B.; Dai, S. Geochemical partitioning from pulverized coal to fly ash and bottom ash. Fuel 2020, 279, 118542. [Google Scholar] [CrossRef]
- Dzikuć, M.; Kuryło, P.; Dudziak, R.; Szufa, S.; Dzikuć, M.; Godzisz, K. Selected Aspects of Combustion Optimization of Coal in Power Plants. Energies 2020, 13, 2208. [Google Scholar] [CrossRef]
- Yudovich, Y.E.; Ketris, M.P. Toxic trace Elements in Coals; Russian Academy of Sciences: Ekaterinburg, Russia, 2005. (In Russian) [Google Scholar]
- Ketris, M.P.; Yudivich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World avarages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Palmer, C.A.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Dai, S.; French, D. The importance of minerals in coal as the hosts of chemical elements: A review. Int. J. Coal Geol. 2019, 212, 103251. [Google Scholar] [CrossRef]
- Dai, S.; Bechtel, A.; Eble, C.F.; Flores, R.M.; French, D.; Graham, I.T.; Hood, M.M.; Hower, J.C.; Korasidis, V.A.; Moore, T.A.; et al. Recognition of peat depositional environments in coal: A review. Int. J. Coal Geol. 2020, 219, 103383. [Google Scholar] [CrossRef]
- Dai, S.; Hower, J.C.; Finkelman, R.B.; Graham, I.T.; French, D.; Ward, C.R.; Eskenazy, G.; Wei, Q.; Zhao, L. Organic associations of non-mineral elements in coal: A review. Int. J. Coal Geol. 2020, 218, 103347. [Google Scholar] [CrossRef]
- Linak, W.P.; Wendt, J.O.L. Toxic metal emissions from incineration: Mechanisms and control. Prog. Energy Combust. Sci. 1993, 19, 145–185. [Google Scholar] [CrossRef]
- Querol, X.; Fernández-Turiel, J.L.; López-Soler, A. Trace elements in coal and their behavior during combustion in a large power station. Fuel 1995, 74, 331–343. [Google Scholar] [CrossRef]
- Huang, Y.; Jin, B.; Zhong, Z.; Xiao, R.; Tang, Z.; Ren, H. Trace elements (Mn, Cr, Pb, Se, Zn, Cd and Hg) in emissions from a pulverized coal boiler. Fuel Proc. Technol. 2004, 286, 23–32. [Google Scholar] [CrossRef]
- Sekine, Y.; Sakajin, K.; Kikuchi, E. Release behavior of trace elements from coal during high-temperature processing. Powder Technol. 2008, 180, 210–215. [Google Scholar] [CrossRef]
- Bhangare, R.C.; Ajmal, P.Y.; Sahu, S.K.; Pandit, G.G.; Puranik, V.D. Distribution of trace elements in coal and combustion residues from five thermal power plants in India. Int. J. Coal Geol. 2011, 86, 349–356. [Google Scholar] [CrossRef]
- Sia, S.G.; Abdullah, W.A. Enrichment of arsenic, lead, and antimony in Balingian coal from Sarawak, Malaysia: Modes of occurrence, origin, and partitioning behaviour during coal combustion. Int. J. Coal Geol. 2012, 101, 1–15. [Google Scholar] [CrossRef]
- Hower, J.C.; Dai, S.; Eskenazy, G. Distribution of uranium and other radionuclides in coal and coal combustion products, with discussion of occurrences of combustion products in Kentucky Power Plants. Coal Comb. Gasif. Prod. 2016, 8, 44–53. [Google Scholar]
- Zhao, S.; Duan, Y.; Li, Y.; Liu, M.; Lu, J.; Ding, Y.; Gu, X.; Tao, J.; Du, M. Emission characteristic and transformation mechanism of hazardous trace elements in a coal-fired power plant. Fuel 2018, 2014, 597–606. [Google Scholar] [CrossRef]
- Parzentny, H.R.; Róg, L. Distribution of some ecotoxic elements in fuel and solid combustion residues in Poland. Energies 2020, 13, 1131. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Karayigit, A.I.; Bulut, Y.; Alastuey, A.; Querol, X. Phase-mineral and chemical composition of composite samples from feed coals, bottom ashes and fly ashes at the Soma power station, Turkey. Int. J. Coal Geol. 2005, 61, 35–63. [Google Scholar] [CrossRef]
- Vejahati, F.; Xu, Z.; Gupta, R. Trace elements in coal: Associations with coal and mineralsmand their behaviour during coal utilization - A review. Int. J. Coal Geol. 2010, 14, 904–911. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Peng, S.; Chou, C.-L.; Wang, X.; Zhang, Y.; Li, D.; Sun, Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Konieczyński, J.; Zajusz-Zubek, E. Distribution of selected trace elements in dust containment and flue gas desulphurisation products from coal-fired Power plants. Arch. Environ. Prot. 2011, 37, 3–14. [Google Scholar]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Jiang, J.; Hower, J.C.; Song, X.; Jiang, Y.; Wang, X.; Gornostaeva, T.; Li, X.; et al. Composition and modes of occurrence of minerals and elements in coal combustion products derived from high-Ge coals. Int. J. Coal Geol. 2014, 121, 79–97. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Lewandowska, M. The high temperature ashes (HTA) from bituminous coal combustion as a potential resource of rare carth elements. Gospod. Sur. Miner. Miner. Resour. Manag. 2018, 34, 135–150. [Google Scholar]
- Oetari, P.S.; Hadi, S.P.; Huboyo, H.S. Trace elements in fine and coarse particles emitted from coal-fired power plants with different air pollution control systems. J. Environ. Manag. 2019, 250, 109497. [Google Scholar] [CrossRef]
- Wilczyńska-Michalik, W.; Dańko, J.; Michalik, M. Characteristics of particulate matter emitted from a coal-fired power plant. Pol. J. Environ. Sud. 2020, 29, 1411–1420. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching behaviour of elements from coal combustion fly ash: An overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef]
- Singh, R.K.; Gupta, N.C.; Guha, B.K. pH dependence leaching characteristics of selected metals from coal ash and its impact on ground water quality. Int. J. Chem. Environ. Eng. 2014, 5, 218–222. [Google Scholar]
- Silva, E.B.; Li, S.; Oliveira, L.M.; Gress, J.; Dong, X.; Wilkie, A.C.; Townsend, T.; Ma, L.Q. Metal leachability from coal combustion residues under different pHs and liquid/solid ratios. J. Hazard. Mater. 2018, 341, 66–74. [Google Scholar] [CrossRef]
- Tian, Q.; Guo, B.; Nakama, S.; Sasaki, K. Distributions and Leaching Behaviors of toxic elements in fly ash. ACS Omega 2018, 3, 13055–13064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dai, S.; Finkelman, R.B.; French, D.; Graham, I.T.; Yang, Y.; Li, J.; Yang, P. Leaching behavior of trace elements from fly ashes of five Chinese coal power plants. Int. J. Coal Geol. 2020, 219, 103381. [Google Scholar] [CrossRef]
- Querol, X.; Juan, R.; Lopez-Soler, A.; Fernandez-Turiel, J.L.; Ruiz, C.R. Mobility of trace elements from coal and combustion wastes. Fuel 1996, 75, 821–838. [Google Scholar] [CrossRef]
- Leyval, C.; Turnau, K.; Haselwandter, K. Effect of heavy metal pollution on mycorrhizal colonization and function: Physiological, ecological aspects. Mycorrhiza 1997, 7, 139–153. [Google Scholar] [CrossRef]
- Jamnická, G.; Bučinová, K.; Havranová, I.; Urban, A. Current state of mineral nutrition and risk elements in a beech ecosystem situated near the aluminium smelter in ŽiarnadHronom, Central Slovakia. For. Ecol. Manag. 2007, 248, 26–35. [Google Scholar] [CrossRef]
- Siwek, M. Plants in postindustrial sites, contaminated with heavy metals. Part, I. Uptake, transport and toxicity of heavy (trace) metals. Wiad. Botan. 2008, 52, 7–22. (In Polish) [Google Scholar]
- Kabata-Pendias, A. Trace Elements of Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2011; p. 534. [Google Scholar]
- Luo, X.; Bing, H.; Luo, Z.; Wang, Y.; Jin, L. Impacts of atmospheric particulate matter pollution on environmental biogeochemistry of trace metals in soil-plant system: A review. Environ. Pollut. 2019, 255, 113138. [Google Scholar] [CrossRef]
- Saikia, B.K.; Hower, J.C.; Hood, M.M.; Baruach, R.; Dekaboruah, H.P.; Boruah, R.; Sharma, A.; Baruah, B.P. Petrological and biological studies on some fly and bottom ashes collected at different times from an Indian coal-based captive power plant. Fuel 2015, 158, 572–581. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic Behaviour in Soil-Plant System: Biogeochemical Reactions and Chemical Speciation Influences. In Enhancing Cleanup of Environmental Pollutants; Anjum, N., Gill, S., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 97–140. [Google Scholar]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E. Essential and Beneficial Trace Elements in Plants, and Their Transport in Roots: A Review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef]
- Taylor, M.P.; Mold, S.A.; Kristensen, L.J.; Rouillon, M. Environmental arsenic, cadmium and lead dust emissions from metal mine operations: Implications for environmental management, monitoring and human health. Environ. Res. 2014, 135, 296–303. [Google Scholar] [CrossRef]
- Yang, J.S.; Yang, F.L.; Yang, Y.; Xing, G.L.; Deng, C.P.; Shen, Y.T.; Luo, L.Q.; Li, B.Z.; Yuan, H.L. A proposal of “core enzyme” bioindicator in long-term Pb-Zn ore pollution areas based on topsoil property analysis. Environ. Pollut. 2016, 213, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Shi, L.; Wu, C.; Wu, H.; Qin, Y.; Pan, W.; Hartkeya, W.; Ciu, M. Cadmium, lead, and arsenic contamination in paddy soils of a mining area and their exposure effects on human HEPG2 and keratinocyte cell-lines. Environ. Res. 2017, 156, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Jasminka, A.; Robert, Š. Distribution of chemical elements in an old metallurgical area, Zenica (Bosnia and Herzegovina). Geoderma 2011, 162, 71–85. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Liu, Z.; Wang, S.; Yao, J.; Borthwick, A.G.L. Vanadium contamination and associated health risk of farmland soil near smelters throughout China. Environ. Pollut. 2020, 263, 114540. [Google Scholar] [CrossRef] [PubMed]
- Cabała, J.; Warchulski, R.; Rozmus, D.; Środek, D.; Szełęg, E. Pb-Rich Slags, Minerals, and Pollution Resulted from a Medieval Ag-Pb Smelting and Mining Operation in the Silesian-Cracovian Region (Southern Poland). Minerals 2020, 10, 28. [Google Scholar] [CrossRef]
- Kumar, S.; Zhao, M.; Zhang, H.; Rahman, M.A.; Luo, C.; Rahman, M.M. Distribution, contamination status and source of trace elements in the soil around brick kilns. Chemosphere 2021, 263, 127882. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.C.; Lin, C.; Gao, Y.; Sun, C.; Xu, L.; Zheng, L.; Zhang, Z. Health risk assessment of trace elements exposure through the soil-plant (maize)-human contamination pathway near a petrochemical industry complex, Northeast China. Environ. Pollut. 2020, 263, 114414. [Google Scholar] [CrossRef]
- Kowalska, J.; Mazurek, R.; Gąsiorek, M.; Setlak, M.; Zaleski, T.; Waroszewski, J. Soil pollution indices conditioned by medieval metallurgical activity—A case study from Krakow (Poland). Environ. Pollut. 2016, 218, 1023–1036. [Google Scholar] [CrossRef]
- Tume, P.; Barrueto, K.; Olguin, M.; Torres, J.; Cifuentes, J.; Ferraro, F.X.; Roca, N.; Bech, J.; Cornejo, O. The influence of the industrial area on the pollution outside its borders: A case study from Quintero and Puchuncavi district, Chile. Environ. Geochemi. Health 2019, 42, 2557–2572. [Google Scholar] [CrossRef]
- Askari, M.S.; Alamdari, P.; Chahardoli, S.; Afshari, A. Quantification of heavy metal pollution for environmental assessment of soil condition. Environ. Monit. Assess. 2020, 192, 162. [Google Scholar] [CrossRef]
- Bourliva, A.; Papadopoulou, L.; Aidona, E.; Giouri, K.; Simeonidis, K.; Vourlias, G. Characterization and geochemistry of technogenic magnetic particles (TMPs) in contaminated industrial soils: Assessing health risk via ingestion. Geoderma 2017, 295, 86–97. [Google Scholar] [CrossRef]
- Szuszkiewicz, M.M.; Łukasik, A.; Magiera, T.; Szuszkiewicz, M. Technogenic magnetic particles of topsoil from different sources of emission—A case study from upper silesian conurbation, Poland. MATEC Web Conf. 2018, 247, 00051. [Google Scholar] [CrossRef]
- Wilczyńska-Michalik, W.; Michalik, J.M.; Kapusta, C.; Michalik, M. Airborne magnetic technoparticles in soils as a record of anthropocene. Atmosphere 2020, 11, 44. [Google Scholar] [CrossRef]
- Kabala, C.; Galka, B.; Jezierski, P. Assessment and monitoring of soil and plant contamination with trace elements around Europe’s largest copper ore tailings impoundment. Sci. Total Environ. 2020, 738, 139918. [Google Scholar] [CrossRef]
- Vaněk, A.; Grösslová, Z.; Mihaljevič, M.; Ettler, V.; Trubač, J.; Chrastný, V.; Penížek, V.; Teper, L.; Cabała, J.; Voegelin, A.; et al. Thallium isotopes in metallurgical wastes/contaminated soils: A novel tool to trace metal source and behavior. J. Hazardous Mat. 2018, 343, 78–85. [Google Scholar] [CrossRef]
- Rahmonov, O.; Krzysztofik, R.; Środek, D.; Smolarek-Lach, J. Vegetation- and environmental changes on nonreclaimed spoil heaps in Southern Poland. Biology 2020, 9, 164. [Google Scholar] [CrossRef]
- Gür, F.; Yaprak, G. Natural radionuclide emission from coal-fired power plants in the southwestern of Turkey and the population exposure to external radiation in their vicinity. J. Environ. Sci. Health A 2010, 45, 1900–1908. [Google Scholar] [CrossRef]
- Vaněk, A.; Grösslová, Z.; Mihaljevič, M.; Trubač, J.; Ettler, V.; Teper, L.; Cabała, J.; Rohovec, J.; Zádorová, T.; Penižek, V.; et al. Isotopic Tracing of Thallium Contamination in Soils Affected by Emissions from Coal-Fired Power Plants. Environ. Sci. Techn. 2016, 50, 9864–9871. [Google Scholar] [CrossRef]
- Turhan, S.; Garad, A.M.K.; Hançerlioğulları, A.; Kurnaz, A.; Gőren, E.; Duran, C.; Karataşlı, M.; Altıkulaç, A.; Savacı, G.; Aydın, A. Ecological assessment of heavy metals in soil around a coal-fired thermal power plant in Turkey. Environ. Earth Sci. 2020, 79, 134. [Google Scholar] [CrossRef]
- Sengupta, S.; Chatterjee, T.; Ghosh, P.B.; Saha, T. Heavy metal accumulation in agricultural soils around a coal fired thermal power plant (Farakka) in India. J. Environ. Sci. Eng. 2010, 52, 299–306. [Google Scholar]
- Iruretagoiena, A.R.; Vallejuelo, S.F.O.; Gredilla, A.; Ramos, C.G.; Oliveira, M.L.S.; Arana, G.; Diego, A.; Madariaga, J.M.; Silva, L.F.O. Fate of hazardous elements in agricultural soils surrounding a coal power plant complex from Santa Catarina (Brasil). Sci. Total. Environ. 2015, 508, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hu, J.; Qin, F.; Quan, W.; Cao, R.; Fan, M.; Wu, X. Heavy metal pollution and ecological assessment around the Jinsha coal-fired power plant (China). Int. J. Environ. Res. Public Health 2017, 14, 1589. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, O.P.; Snitynskyy, V.V.; Shkumbatyuk, R.S. Monitoring of heavy metals content in soils of the areas surrounding Dobrotvir Thermal Power Plant. Sci. Bull. UNFU 2017, 27, 87–90. [Google Scholar] [CrossRef]
- Liu, D.; Quan, Y.; Ren, Z.; Wu, G. Assessment of heavy metal contamination in soil associated with Chinese coal-fired power plants: A case study in Xilingol, Inner Mongolia. Int. J. Sustain. Dev. World Ecol. 2017, 24, 439–443. [Google Scholar] [CrossRef]
- Jankiewicz, B.; Adamczyk, D. Assessing heavy metal content in soils surrounding a power plant. Pol. J. Environ. Stud. 2010, 19, 849–853. [Google Scholar]
- Clark, J.H.A.; Tredoux, M.; van Huyssteen, C. Heavy metals in the soils of Bloemfontein, South Africa: Concentration levels and possible sources. Environ. Monit. Assess. 2015, 187, 439–452. [Google Scholar] [CrossRef]
- Gune, M.M.; Harshavardhana, B.G.; Ma, W.-L.; Balakrishna, K.; Udayashankar, H.N.; Zhang, Z.; Li, Y.-F. Seasonal Variations of Heavy Metals in the Soil Around a Coal-Fired Thermal Power Plant, South-West Coast of India. Bull. Environ. Contam. Tox. 2020, 104, 602–608. [Google Scholar] [CrossRef]
- Zhai, M.; Totolo, O.; Modisi, M.P.; Finkelman, R.B.; Kelesitse, S.M.; Menyatso, M. Heavy metal distribution in soils near Palapye, Botswana: An evaluation of the environmental impact of coal mining and combustion on soils in a semi-arid region. Environ. Geoch. Health 2009, 31, 759–770. [Google Scholar] [CrossRef]
- Agrawal, P.; Mittal, A.; Prakash, R.; Kumar, M.; Singh, T.B.; Tripathi, S.K. Assessment of contamination of soil due to heavy metals around coal fired thermal power plants at Singrauli region of India. Bull. Environ. Contam. Toxicol. 2010, 85, 2019–2223. [Google Scholar] [CrossRef]
- Raja, R.; Nayak, A.K.; Shukla, A.K.; Rao, K.S.; Gautam, P.; Lal, B.; Tripathi, R.; Shahid, M.; Panda, B.B.; Kumar, A.; et al. Impairment of soil health due to fly ash-fugitive dust deposition from coal-fired thermal power plants. Environ. Monit. Assess. 2015, 187, 679. [Google Scholar] [CrossRef]
- Howladar, M.F.; Ahmed, T.; Deb, P.K.; Shine, F.M.M.; Rahman, M.A. Analysing the top soil chemistry for environments around the Barapukuria thermal power plant, Bangladesh. Int. J. Sci. Eng. Res. 2016, 7, 146–150. [Google Scholar]
- Linnik, W.G.; Minkina, T.M.; Bauer, T.V.; Saveliev, A.A.; Mandzhieva, S.S. Geochemical assessment and spatial analysis of heavy metals pollution around coal-fired power station. Environ. Geochem. Health 2019, 42, 4087–4100. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, W.; Zhao, C.; Chen, C. Environmental assessment of heavy metal and natural radioactivity in soil around a coal-fired power plant in China. J. Radioanal. Nucl. Chem. 2013, 295, 1845–1854. [Google Scholar] [CrossRef]
- Hajduk, E.; Kaniuczak, J.; Właśniewski, S. The Content of Heavy Metals in Arable Soils from the Vicinity of the Stalowa Wola Power Plant. Soil Sci. Annu. 2012, 63, 22–26. [Google Scholar] [CrossRef]
- Adeyi, A.A.; Torto, N. Profiling heavy metal distribution and contamination in soil of old power generation station in Lagos, Nigeria. Am. J. Sci. Technol. 2014, 1, 1–10. [Google Scholar]
- Pastrana-Corral, M.A.; Wakida, F.T.; Temores-Peña, J.; Rodriguez-Mendivil, D.D.; García-Flores, E.; Piñon-Colin, T.D.J.; Quiñonez-Plaza, A. Heavy metal pollution in the soil surrounding a thermal power plant in Playas de Rosarito, Mexico. Environ. Earth Sci. 2017, 76, 583. [Google Scholar] [CrossRef]
- Parzentny, H.R. Differences between the content of selected ecotoxic elements in feed coal, combustion residues, soils and common beech (Fagus sylvatica L.) in the surrounded of the power plant in Poland. Int. Multidiscip. Sci. GeoConference SGEM. 2019, 19, 271–293. [Google Scholar]
- Duffus, J.H. “Heavy metals”—A meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistryof trace elements in Chinese coals: A review of abundances, genetic types, impactson human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Ptak, B.; Różkowska, A. Geochemical Atlas of Coal Deposits Upper Silesian Coal Basin; Publishing of Polish Geological Institute: Warsaw, Poland, 1995; p. 53. [Google Scholar]
- Parzentny, H.R.; Róg, L. Evaluation the value of some petrographic, physico-chemical and geochemical indicatores of quality of coal in paralic series of the Upper Silesian Coal Basin and attempt to find a correlotion between thrm. Gospod. Miner. Resour. Manag. 2017, 33, 51–76. (In Polish) [Google Scholar]
- Parzentny, H.R.; Róg, L. Modes of occurrence of ecotoxic elements in coal from the Upper Silesian Coal Basin, Poland. Arabian J. Geosci. 2018, 11, 790. [Google Scholar] [CrossRef]
- Parzentny, H.R.; Róg, L. Dependences between certain petrographic, geochemical and technological indicators of coal quality in the Limnic Series of the Upper Silesian Coal Basin (USCB), Poland. Arch. Min. Sci. 2020, 65, 665–684. [Google Scholar]
- Masovian Voivodeship. Available online: https://pl.wikipedia.org/wiki/Wojew%C3%B3-dztwo_mazowieckie (accessed on 23 September 2020).
- Upper Silesian Conurbation. In Wikipedia, the Free Encyclopedia. Available online: https://pl.wikipedia.org/wiki/Konurbacja_górnośląska (accessed on 23 September 2020).
- Opole Voivodeship. Available online: https://pl.wikipedia.org/wiki/Wojew%C3%B3dztwo_opolskie (accessed on 23 September 2020).
- The Main Wind Directions in Poland. Available online: https://www.google.com/search?source=-univ&tbm=isch&q=kierunki+wiatru+w+polsce&sa=X&ved=2ahUKEwjU9OTUqonsAhVwxIsKHbKhDHAQjJkEegQICRAB&biw=1280&bih=891 (accessed on 23 September 2020).
- World Reference Base for soil resources. World Soil Resour. Rep. 2015, 106, 1–193.
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 633. [Google Scholar]
- ISO 7404-3. Methods for the Petrographic Analysis of Bituminous Coal and Anthracite—Part. 3: Method of Determining Maceral Group Composition; International Organization for Standardization: Geneva, Switzerland, 2009; p. 7. [Google Scholar]
- ISO 7404-5. Methods for the Petrographic Analysis of Bituminous Coal and Anthracite—Part. 5: Method of Determining Microscopically the Reflectance of Vitrinite; International Organization for Standardization: Geneva, Switzerland, 2009; p. 14. [Google Scholar]
- Taylor, J.C. Computer programs for standardless quantitative analysis of minerals using the full powder diffraction profile. Powder Diffr. 1991, 6, 2–9. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Ruan, C.-D.; Ward, C.R. Quantitative X-ray powder diffraction analysis of clay minerals in Australian coals using Rietveld methods. Appl. Clay Sci. 2002, 21, 227–240. [Google Scholar] [CrossRef]
- Mahieux, P.Y.; Aubert, J.E.; Cyr, M.; Coutand, C.; Husson, B. Quantitative mineralogical composition of complex mineral wastes—Contribution of the Rietveld method. Waste Manag. 2010, 30, 378–388. [Google Scholar] [CrossRef]
- Guo, Z.X.; Geng, H.; Zhang, J.H.; Zhou, H.; Peng, Y.; Zhai, S.Y.; Li, J.L.; Chen, Y.S. Ecological and health risks of trace heavy metals in atmospheric PM2.5 collected in Wuxiang Town, Shanxi Province. Huan Jing Ke Xue 2018, 39, 1004–1013. (In Chinese) [Google Scholar] [CrossRef]
- Silva, L.F.O.; Oliveira, M.L.S.; da Boit, K.M.; Finkelman, R.B. Characterization of Santa Catarina (Brazil) coal with respect to human health and environmental concerns. Environ. Geochem Health 2009, 31, 475–485. [Google Scholar] [CrossRef]
- Bureau Veritas Mineral Laboratories Schedule of Services Bro. Vancouver, Canada. 2020. Available online: http://acmelab.com (accessed on 23 September 2020).
- The Regulation of the Minister of the Environment of 15 July 2011 on the criteria for the classification of extractive waste for inert waste. In Polish Journal of Laws 2011 Item 1048; The Regulation of the Minister of the Environment: Warsaw, Poland, 2011.
- Directive 2006/21/EC of the European Parliament and of the Council of 15 March 2006 on the Management of Waste from Extractive Industries and Amanding Directive 2004/35/EC. Available online: http://www.legislation.gov.uk/eudr/2006/21/introduction (accessed on 23 September 2020).
- The Regulation of the Minister of the Environment of 1 September 2016 on the assessment of the pollution of the earth’s surface. In Polish Journal of Laws 2016 Item 1395; The Regulation of the Minister of the Environment: Warsaw, Poland, 2016.
- Economic Commission for Europe; Committee on Sustainable Energy. International Classification of in-Seam Coals; United Nations Report Energy/1998/19; UN; UN: New York, NY, USA; Geneva, Switzerland, 1998. [Google Scholar]
- Ratajczak, T.; Gaweł, A.; Górniak, K.; Muszyński, M.; Szydłak, T.; Wyszomirski, P. Characteristics of fly ash from combustion of some hard and brown coals. Spec. Pap. Mineral. Soc. Pol. 1999, 15, 1–34. (In Polish) [Google Scholar]
- Wierońska, F.; Makowska, D.; Strugała, A.; Bytnar, K. Analysis of the content of nickel, chromium, lead and zinc in solid products of coal combustion (CCPs) coming from Polish power plants. IOP Publ. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 012029. [Google Scholar] [CrossRef]
- Llorens, J.F.; Fernandez, J.L.; Querol, X. The fate of trace elements in a large coal-fired power plant. Environ. Geol. 2000, 40, 409–416. [Google Scholar] [CrossRef]
- Karayiğit, A.I.; Yigitler, Ő.; İşerli, S.; Querol, X.; Mastalerz, M.; Oskay, G.; Hower, J.C. Mineralogy and Geochemistry of Feed Coals and Combustion Residues from Tunçbilek and Seyitömer Coal-Fired Power Plants in Western Turkey. Coal Combust. Gasif. Prod. 2019, 11, 18–31. [Google Scholar]
- Wei, Q.; Song, W. Mineralogical and chemical characteristics of coal ashes from two high-sulfur coal-fired power plants in Wuhai, Inner Mongolia, China. Minerals 2020, 10, 323. [Google Scholar] [CrossRef]
- Lin, W.; Wu, K.; Lao, Z.; Hu, W.; Lin, B.; Li, Y.; Fan, H.; Hu, J. Assessment of trace metal contamination and ecological risk in the forest ecosystem of dexing mining area in northeast Jianxi Province, China. Ecotoxicol. Environ. Saf. 2019, 167, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Sengupta, D. An assessment of soil contamination due to heavy metals around a coal-fired thermal power plant in India. Environ. Geol. 2006, 51, 409–420. [Google Scholar] [CrossRef]
- Parzentny, H. Differences in content and bonding pattern of certain elements in coal of the Upper Silesian Caol Basin throughout a single seam pfofile). PrzeglądGórniczy 1989, 45, 17–21. (In Polish) [Google Scholar]
- Parzentny, H. Lead distribution in coal and coaly shales in the Upper Silesian Coal Basin. Geol. Quat. 1994, 38, 43–58. [Google Scholar]
- Parzentny, H.R. Spatial macroscale variability of the role of mineral matter in concentrating some trace elements in bituminous coal in a coal basin—a case study from the Upper Silesian Coal Basin in Poland. Minerals 2020, 10, 422. [Google Scholar] [CrossRef]
- Hill, P.A. Vertical distribution of elements in Deposit No. 1, Hat Creek, British Columbia: A preliminary study. Int. J. Coal Geol. 1990, 15, 77–111. [Google Scholar] [CrossRef]
- Mohanty, M.K.; Honaker, R.Q.; Mondal, K.; Paul, B.C.; Ho, K. Trace element reductions in fine coal using advanced physical cleaning. Coal Prep. 1998, 19, 195–211. [Google Scholar] [CrossRef]
- Makowska, D.; Strugała, A.; Wierońska, F.; Włodek, A. Investigations of the effectiveness of lead disposal from hard coal trough the cleaning process. E2S Web Conf. 2016, 10, 00117. [Google Scholar] [CrossRef]
- Chen, J.; Chen, P.; Yao, D.; Huang, W.; Tang, S.; Wang, W.; Liu, W.; Hu, Y.; Zhang, B.; Sha, J. Abundance, distribution, and modes of occurrence of uranium in Chinese Coals. Minerals 2017, 7, 239. [Google Scholar] [CrossRef]
- Duan, P.; Wang, W.; Sang, S.; Tang, Y.; Ma, M.; Zhang, W.; Liang, B. Geochemistry of toxic elements and their removal via the preparation of high-uranium coal in Southwestern China. Minerals 2018, 8, 83. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, C.; Zhang, N.; Pan, J.; Cao, S.; Tang, M.; Ji, W.; Hu, T. Modes of occurrence and partitioning behavior of trace elements during coal preparation - A case study in Guizhou Province, China. Fuel 2019, 243, 79–87. [Google Scholar] [CrossRef]
- Parzentny, H.R.; Lewińska-Preis, L. The role of sulphide and carbonate minerals in the concentration of chalcophile elements in the bituminous coal seams of a paralic series (Upper Carboniferous) in the Upper Silesian Coal Basin (USCB), Poland. Chem. Erde Geochem. 2006, 66, 227–247. [Google Scholar] [CrossRef]
- Hower, J.C.; Campbell, J.L.; Teesdale, W.J.; Nejedly, Z.; Robertson, J.D. Scanning proton microprobe analysis of mercury and other trace elements in Fe-sulfides from a Kentucky coal. Int. J. Coal Geol. 2008, 75, 88–92. [Google Scholar] [CrossRef]
- Diehl, S.F.; Goldhaber, M.B.; Koenig, A.E.; Lowers, H.A.; Ruppert, L.F. Distribution of arsenic, selenium, and other trace elements in high pyrite Appalachian coals: Evidence for multiple episodes of pyrite formation. Int. J. Coal Geol. 2012, 94, 238–249. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Li, W. Mineralogy, distribution, occurrence and removability of trace elements during the coal preparation of No. 6 coal from Heidaigou mine. Int. J. Coal Sci. Technol. 2014, 1, 402–420. [Google Scholar] [CrossRef]
- Kokowska-Pawłowska, M. Petrographic and mineral variability of the rocks accompanying selected coal seams of the Poruba beds and their influenceof the trace elements content. Gospod. Surowcami Miner. Miner. Resour. Manag. 2015, 31, 73–92. [Google Scholar]
- Bielowicz, B.; Misiak, J. The forms of occurrence and geochemistry of sulfides in hard coal deposits of the Libiąż Beds in the Upper Silesian Coal Basin, Southern Poland. Geol. Geophys. Environ. 2017, 43, 109–125. [Google Scholar] [CrossRef]
- Kolker, A.; Finkelman, R.B. Potentially hazardous elements in coal: Modes of occurrence and summary of concentration data for coal components. Int. J. Coal Prep. Util. 1998, 19, 133–157. [Google Scholar] [CrossRef]
- Kolker, A. Minor element distribution in iron disulfides in coal: A geochemical review. Int. J. Coal Geol. 2012, 94, 32–43. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, H.; Zhou, G. Mineralogy and geochemistry of different morphological pyrite in Late Permian coals, South Chin. Arab. J. Geosci. 2016, 9, 590. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, R.; Walshe, J. Trace metal nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Nowińska, K. Chemical composition of pyrite in the feed mixture into zinc and lead pirometallurgical process. In Support Systems in Production Engineering Geochemistry and Environmental Geology of Industrial Areas; Pozzi, M., Ed.; “Panova” Publishing House: Gliwice, Poland, 2016; Volume 5, pp. 38–46. (In Polish) [Google Scholar]
- Reich, M.; Deditius, A.; Chryssoulius, S.; Li, J.-W.; Ma, C.-Q.; Parada, M.A.; Barra, F.; Mittemayr, F. Pyrite as a record of hydrothermal fluid evolution in a porphyry copper system: A SIMS/EMPA trace element study. Geoch. Cosmoch. Acta 2013, 104, 42–62. [Google Scholar] [CrossRef]
- Serranti, S.; Ferrini, V.; Masi, U.; Cabri, L.J. Trace-element distribution in cassiterite and sulfides from Rubané and massive ores of the Corvo deposit, Portugal. Can. Mineral. 2002, 40, 815–835. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Zhong, H.; Bai, Z.; Yao, J.; Xu, C. Using trace elements of magnetite to constrain the origin of the Pingchuan hydrothermal low-Ti magnetite deposit in the Panxi area, SW China. Acta Geochim. 2019, 38, 376–390. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Eskenazy, G.M.; Vassileva, C.G. Behaviour of elements and minerals during preparation and combustion of the Pernik coal, Bulgaria. Fuel Proc. Technol. 2001, 72, 103–129. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Quin, S.; Panchal, B.; Sun, Y.; Niu, H. Distribution characteristics and migration patterns of hazardous trace elements in coal combustion products of power plants. Fuel 2019, 258, 116062. [Google Scholar] [CrossRef]
- Parzentny, H.R.; Róg, L. Distribution of heavy metals in fly ash originating from burning coal of Upper Silesian Coal Basin. Przegląd Górniczy 2001, 57, 52–60. (In Polish) [Google Scholar]
- Misz, M.A. Comparasion of chars in slag and fly ash as formed in pf boilers from Będzin Power Station (Poland). Fuel 2002, 81, 1351–1358. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kalugin, V.M.; Nigmatulina, E.N.; Volkova, N.I.; Frenkel, A.E.; Maksimova, N.V. Ferrospheres from fly ashes of Chelyabinsk coals: Chemical composition, morphology and formation conditions. Fuel 2002, 81, 867–876. [Google Scholar] [CrossRef]
- Wilczyńska-Michalik, W.; Moryl, R.; Sobczyk, J.; Michalik, M. Composition of coal combustion by-products: The importance of combustion technology. FuelProc. Tech. 2014, 124, 35–43. [Google Scholar] [CrossRef]
- Valentim, B.; Shreya, N.; Paul, B.; Gomes, C.S.; Sant’Ovaia, H.; Guedes, A.; Ribeiro, J.; Flores, D.; Pinho, S.; Suárez-Ruiz, I.; et al. Characteristics of ferrospheres in fly ashes derived from Bokaro and Jharia (Jharkand, India) coals. Int. J. Coal Geol. 2016, 153, 52–74. [Google Scholar] [CrossRef]
- Hower, J.C.; Cantando, E.; Eble, C.F.; Copley, G.C. Characterization of stoker ash from the combustion of high-lanthanide coal at a Kentucky bourbon distillery. Int. J. Coal Geol. 2019, 213, 103260. [Google Scholar] [CrossRef]
- Hower, J.C.; Qian, D.; Briot, N.J.; Santillan-Jimenez, E.; Hood, M.M.; Taggart, R.K.; Hsu-Kim, H. Nano-scale rare earth distribution in fly ash derived from the combustion of the fire clay coal, kentucky. Minerals 2019, 9, 206. [Google Scholar] [CrossRef]
- Maity, R.; Venkateshwarlu, M.; Mondal, S.; Kapawar, M.R.; Gain, D.; Paul, P. Magnetic and microscopic characterization of anthropogenically produced magnetic particles: A proxy for environmental pollution. Int. J. Environ. Sci. Technol. 2020, 114. [Google Scholar] [CrossRef]
- Xu, M.; Yan, R.; Zheng, C.; Oiao, Y.; Han, J.; Sheng, C. Status of trace element emission in a coal combustion process: A review. Fuel Proc. Techn. 2003, 85, 215–237. [Google Scholar] [CrossRef]
- The Engineering ToolBox. Melting and Boiling Temperatures—Evaporation and Melting Heats of Common Materials. Available online: https://www.engineeringtoolbox.com/melting-boiling-temperatures-d_392.html (accessed on 23 September 2020).
- Bartoňová, L.; Raclavská, H.; Čech, B.; Kucbel, M. Behavior of Pb during coal combustion: An overview. Sustainability 2019, 11, 6061. [Google Scholar] [CrossRef]
- Chen, G.; Sun, Y.; Wang, Q.; Yan, B.; Cheng, Z.; Ma, W. Partitioning of trace elements in coal combustion products: A comparative study of different applications in China. Fuel 2019, 240, 31–39. [Google Scholar] [CrossRef]
- Cui, W.; Meng, Q.; Feng, Q.; Zhou, L.; Cui, Y.; Li, W. Occurrence and release of cadmium, chromium, and lead from stone coal combustion. Int. J. Coal Sci Technol. 2019, 6, 586–594. [Google Scholar] [CrossRef]
- Vassileva, C.G.; Vassilev, S.V. Behaviour of inorganic matter during heting of Bulgarian coals. 2. Subbiltuminous and bituminous coals. Fuel Proc. Technol. 2006, 87, 1095–1116. [Google Scholar] [CrossRef]
- Magiera, T.; Parzentny, H.R.; Róg, L.; Chybiorz, R.; Wawer, M. Spatial variation of soil magnetic susceptibility in relation to different emission sources in southern Poland. Geoderma 2015, 255–256, 94–103. [Google Scholar] [CrossRef]
- Magiera, T.; Mendakiewicz, M.; Szuszkiewicz, M.; Jabłońska, M.; Chróst, L. Technogenic magnetic particles in soils as evidence of historical mining and smelting activity: A case of the Brynica River Valley, Poland. Sci. Total Environ. 2016, 566–567, 536–551. [Google Scholar] [CrossRef]
- Strzyszcz, Z.; Magiera, T. Heavy metal contamination and magnetic susceptibility in soils of southern Poland. Phys. Chem. Earth 1998, 23, 1127–1131. [Google Scholar] [CrossRef]
- Jordanova, N.; Jordanova, D.; Tsacheva, T. Application of magnetometry for delineation of anthropogenic pollution in areas covered by various soil types. Geoderma 2008, 144, 557–571. [Google Scholar] [CrossRef]
- Minkina, T.; Konstatinova, E.Y.; Bauer, T.V.; Mandzhieva, S.S.; Sushkova, S.N.; Chapligin, V.A.; Burachevskaya, M.V.; Nazarenko, O.; Kizilkaya, R.; Gülser, C.; et al. Environmental and human health risk assessment of potentially toxic elements in soils around the largest coal-fired power station in Southern Russia. Environ. Geoch. Health. 2020. [Google Scholar] [CrossRef]
- Kapper, K.L.; Bautista, F.; Goguitchaishvili, A.; Bógalo, M.F.; Cejudo-Ruíz, R.; Cervantes, S.M. The use and misuse of magnetic methods to monitor environmental pollution in urban areas. Boletín Soc. Geológica Mex. 2020, 72, 1–44. [Google Scholar] [CrossRef]
- Yean, S.; Cong, L.; Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Kan, A.T.; Colvin, V.L.; Tomson, M.B. Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate. J. Mater. Res. 2005, 20, 3255–3264. [Google Scholar] [CrossRef]
- Usman, M.; Byrne, M.; Chaundhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderalein, S.B. Magnetite and green rust: Synthesis, properties, and environmental applications of mixed-valent iron minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, Z.; Usman, M.; Anastopoulos, I.; Qadeer, A.; Zhu, R.; Wakeel, A.; Dong, R. Use of nano-/micro-magnetite for abatement of cadmium and lead contamination. J. Environ. Manag. 2020, 264, 110477. [Google Scholar] [CrossRef] [PubMed]
- Kukier, U.; Ishak, C.F.; Sumner, M.E.; Miller, W.P. Composition and element solubility of magnetic and non-magnetic fly ash fractions. Environ. Pollut. 2003, 123, 255–266. [Google Scholar] [CrossRef]
- Seferenioğlu, M.; Paul, M.; Sandström, Ǻ.; Köker, A.; Toprak, S.; Paul, J. Acid leaching of coal and coal-ashes. Fuel 2003, 82, 1721–1734. [Google Scholar] [CrossRef]
- Brownfield, M.E.; Cathcart, J.D.; Aolter, R.H.; Brownfield, I.K.; Rice, C.A.; O’Connor, J.T.; Zielinski, J.R.A.; Bullock, J.H.; Hower, J.C.; Meeker, G.P. Characterization and Modes of Occurrence of Elements in Feed Coal and Coal Combustion Products from a Power Plant. Utilizing Low-Sulfur Coal from the Powder River Basin, Wyoming; Scientific Investigations Report 2004-5271; U.S. Geological Survey: Reston, VA, USA, 2005; p. 36. Available online: http://pubs.usgs.gov/sir/2004/5271/ (accessed on 23 September 2019).
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Niemiec, M.; Chowaniak, M.; Paluch, Ł. Accumulation of chromium, aluminum, barium and arsenic in selected elements of a forest ecosystem in the Przedbabiogórskie Mountain Range in the Western Carpathians. J. Elem. 2017, 22, 1107–1116. [Google Scholar]
- Parzentny, H.; Róg, L. Importance of geological conditions for industry development on area between Bytom and Katowice. PrzeglądGórniczy 1999, 56, 26–34. (In Polish) [Google Scholar]
- Pająk, M.; Jasik, M. The level of Zn, Cd and Pb accumulation in top layer of forest soil in the neighbourhood of metallurgic complex „MiasteczkoSląskie”. Scientific Journals of the University of Zielona Góra No. 137. Environ. Eng. 2010, 17, 112–122. (In Polish) [Google Scholar]
- Adamczyk, Z.; Nowińska, K. Environmental mobility of trace elements present in dusts emitted from Zn–Pb metallurgical processes. Environ. Earth. Sci. 2016, 75, 956. [Google Scholar] [CrossRef]
- Walthert, L.; Pannatier, E.G.; Meier, E.S. Shortage of nutrients and excess of toxic elements in soils limit the distribution of soil-sensitive tree species in temperate forests. Forest Ecol. Manag. 2013, 297, 94–107. [Google Scholar] [CrossRef]
- Brunner, I.; Luster, J.; Günthardt-Goerg, M.S.; Frey, B. Heavy metal accumulation and phytostabilisation potential of tree fine roots in a contaminated soil. Environ. Pollut. 2008, 152, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Akburak, S. Variations of element concentrations in roots of different tree species. Cerne 2020, 26, 118–129. [Google Scholar] [CrossRef]
- Kidd, P.; Barceló, J.; Bernal, M.P.; Navari-Izzo, F.; Poschenrieder, C.; Shilev, S.; Clemente, R.; Monterroso, C. Trace element behaviour at the root–soil interface: Implications in phytoremediation. Environ. Exp. Bot. 2009, 67, 243–259. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]


| Object/Formula | A | B | C |
|---|---|---|---|
| (In vol. %) | |||
| Vitrinite | 66.0 | 62.8 | 52.0 |
| Liptinite | 2.1 | 5.0 | 6.0 |
| Inertinite | 11.1 | 22.9 | 23.0 |
| Pyrite/FeS2 | 5.8 | 2.0 | 3.8 |
| Magnetite/Fe2+Fe3+2O4 | 0.2 | 0.1 | 0.3 |
| Hematite/Fe2O3 | <0.1 | <0.1 | not found |
| Quartz/SiO2 | 0.1 | 0.1 | 1.4 |
| Feldspar/KAlSi3O8-NaAlSi3O8-CaAl2Si2O8 | <0.1 | <0.1 | <0.1 |
| Apatite/Ca5(PO4)3(F,Cl,OH | not found | not found | <0.1 |
| Chlorite/(X,Y)4–6(Si,Al)4O10(OH,O)8; X and Y = Fe+2, Fe+3, Mg+2, Mn+2, Ni+2, Zn+2, Al+3, Li+1, or Ti+4 | not found | not found | <0.1 |
| Kaolinite/Al2Si2O5(OH)4 | 8.8 | 4.0 | 4.1 |
| Illite+muscovite/K0.65Al2.0[Al0.65Si3.35O10](OH)2-KAl2(AlSi3O10)(OH)2 | 0.1 | 0.1 | 1.9 |
| Calcite/CaCO3/ | not found | not found | not found |
| Dolomite/CaMg[CO3]2 | not found | 2.7 | 7.0 |
| Ankerite/Ca(Fe2+, Mg, Mn)(CO3)2 | not found | 0.1 | not found |
| Siderite/Fe2+CO3 | 5.8 | 0.2 | 0.5 |
| Gypsum/CaSO4·2H2O) | not found | not found | not found |
| Reflectance (%) | 0.92 | 0.81 | 0.81 |
| Ash yield (wt %) | 43.92 | 11.38 | 24.57 |
| Element | Object | Sample from Studied Areas | Research Results of Other | PCS ** | ||
|---|---|---|---|---|---|---|
| A | B | C | Authors * | |||
| Co *** | 1 **** | 17.1 | 5.5 | 10.3 | 6.0 ± 0.2 1 | |
| 2 | 37.6 | 17.9 | 38.5 | 44.6 2, 14.6 3 | ||
| 3 | 20.4 | 18.3 | 27.5 | 12.0 3, 15 4 | ||
| 4 | 10.1 | 3.3 | 0.1 | World cambisols 10 5 | 50 | |
| Topsoil in the vicinity of the PS | 38.5 16, 4.8 7, 18.0 8, 1.4 9, 0.9 10 | |||||
| 5 | 2.5 | 2.6 | 0.8 | nd ***** | ||
| Ni | 1 | 72.3 | 28.2 | 72.7 | 17 ± 1 1 | |
| 2 | 123.7 | 58.2 | 111.5 | 138.8 2, 35.5 3, 97.3 11 | ||
| 3 | 70.5 | 52.6 | 77.4 | 33.2 3 | ||
| 4 | 15.0 | 7.7 | 1.4 | World cambisols 26 5 | 150 | |
| Topsoil in the vicinity of the PS | 52.4 16, 21.8 7, 30.3 8, 2.9 9, 2.4 10, 36.0 12, 3.5 13, 40.4 14, 73 15 | |||||
| 5 | 40.3 | 8.8 | 4.6 | 1.85 6 | ||
| Cu | 1 | 51.0 | 20.4 | 25.8 | 16 ± 1 1 | |
| 2 | 196.6 | 124.4 | 123.2 | 123.4 2, 72 17 | ||
| 3 | 106.1 | 101.3 | 63.1 | 35.7 3, 41.0 18 | ||
| 4 | 10.1 | 9.4 | 2.3 | World cambisols 23 5 | 200 | |
| Topsoil in the vicinity of the PS | 70.4 16, 12.3 7, 40.3 8, 1.7 10, 35.6 12, 33.9 14, 0.6 19, 28.0 20, 8.1 21 | |||||
| 5 | 21.9 | 32.9 | 7.3 | 1.2–3.5 5 | ||
| Zn | 1 | 53.9 | 29.6 | 58.9 | 28 ± 2 1 | |
| 2 | 245.8 | 139.6 | 242.4 | 199.2 2, 282 4,148 18 | ||
| 3 | 77.1 | 214.3 | 58.7 | 31.7 3, 317 4, 79.0 18 | ||
| 4 | 48.5 | 221.0 | 17.7 | World cambisols 605 | 500 | |
| Topsoil in the vicinity of the PS | 148.5 16, 56.1 7, 124.7 8, 715 9, 0.9 10,33.4 12, 83.0 14, 0.7 19, 34 21 | |||||
| 5 | 81.9 | 582.7 | 369.7 | nd | ||
| As | 1 | 14.4 | 1.1 | 8.3 | 9.0 ± 0.7 1 | |
| 2 | 13.6 | 3.5 | 28.5 | 28.22, 60.0 17, 24.8 22 | ||
| 3 | 4.8 | 4.3 | 0.5 | 0.173, 19.6 17, 7.0 22 | ||
| 4 | 5.0 | 8.4 | 1.8 | World cambisols 8.4 5 | 25 | |
| Topsoil in the vicinity of the PS | 3.5 16, 5.1 12, 17.8 13, 6.7 14, <0.1 19, 12.0 20 | |||||
| 5 | <0.1 | 8.6 | 19.2 | 1.96 6 | ||
| Ag | 1 | 0.3 | 0.1 | 0.2 | 0.090 ± 0.016 1 | |
| 2 | 0.6 | 0.4 | 0.4 | 0.7 2, 0.8 3 | ||
| 3 | 0.1 | <0.0 | <0.0 | 0.6 23 | ||
| 4 | 4.4 | 3.0 | 3.5 | World cambisols 0.1 5 | nd | |
| Topsoil in the vicinity of the PS | nd | |||||
| 5 | 3.5 | 1.4 | 2.2 | nd | ||
| Cd | 1 | <0.01 | <0.01 | <0.01 | 0.20 ± 0.04 1 | |
| 2 | 0.52 | 0.37 | 0.83 | 0.9 2, 0.8 3, 0.7 4, 1.3 17 | ||
| 3 | <0.01 | 1.89 | 0.85 | 0.6 3, 0.5 4, 0.6 17 | ||
| 4 | 0.03 | 2.55 | 0.07 | World cambisols 0.45 5 | 2.0 | |
| Topsoil in the vicinity of the PS | 0.45 5, 1.08 16, 0.33 7, 2.94 9, 0.08 10, 0.69 13, 3.85 14, 0.01 15, 0.58 20, 0.10 21 | |||||
| 5 | 0.80 | 4.53 | 0.72 | 0.28 6 | ||
| Sb | 1 | <0.01 | <0.01 | 0.01 | 1.00 ± 0.09 1 | |
| 2 | 5.00 | <0.01 | 4.30 | 7.92, 5.8 4, 3.8 17 | ||
| 3 | <0.01 | 0.61 | 7.03 | 4.3 4, 2.0 17, 0.6 18 | ||
| 4 | 1.49 | 0.38 | 0.99 | World cambisols 0.62 5 | nd | |
| Topsoil in the vicinity of the PS | 1.14 21 | |||||
| 5 | <0.01 | 4.67 | 0.38 | nd | ||
| Pb | 1 | 34.8 | 12.2 | 18.8 | 9.0 ± 0.7 1 | |
| 2 | 114.3 | 40.3 | 54.2 | 196.8 2, 52.0 3, 48 4, 66.0 7 | ||
| 3 | 30.2 | 55.3 | 12.4 | 31.0 3, 61 4, 16.3 7, 23.0 10 | ||
| 4 | 35.2 | 178.2 | 16.5 | World cambisols 28 5 | 200 | |
| Topsoil in the vicinity of the PS | 26.6 16, 13.5 7, 39.7 8, 138 9, 1.3 10, 18.9 12, 13.7 13, 46.9 14, 33.7 20 | |||||
| 5 | 28.2 | 455.8 | 32.6 | 0.1–0.3 16, 11.64 8 | ||
| Element | Area | Feed Coal | Fly Ash | Slag | Topsoil | Root | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cn ** | Compound | Cn | Compound | Cn | Compound | Cn | Compound | Cn | Compound | ||
| Co | A * | 0.13 | Anhedral pyrite | 0.12 | Al-Si crassisphere with Fe | 0.22 | Apatite on microsphere | 0.34 | Fe-oxide in cenosphere | 0.17 | Primary cortex |
| B * | 0.22 | Hematite with siderite | 0.08 | Calcimagnesiaferrosphere | 0.14 | Cenosphere | 0.27 | Ferrosphere | 0.11 | Primary cortex | |
| C * | 0.45 | Fusinite with siderite | 0.07 | Fe-oxide on ferrosphere | 0.07 | Al-Si-Fe crassinetwork | 0.34 | Ca-Mg-Fe-Mn carbonate | 0.14 | Rhizodermis | |
| Ni | A | 0.17 | Cassiterite with maceral | 0.34 | Fe-oxide on microsphere | 0.27 | Fe-oxide on microsphere | 0.30 | Organic matter | 0.36 | Primary cortex |
| B | 1.63 | Hematite skeletal | 0.32 | Fe-oxide on microsphere | 0.16 | Magnetite? grain | 0.39 | Barite | 0.11 | Primary cortex | |
| C | 4.44 | iron oxide grain | 0.42 | Fe-oxide on cenosphere | 1.32 | Al-Si-Fe crassinetwork | 3.46 | Siderite after hematite | 0.60 | Primary cortex | |
| Cu | A | 1.51 | Cassiterite with maceral | 1.51 | Fe-oxide on cenosphere | 0.22 | Fe-oxide on cenosphere | 0.51 | Fe-oxide in cenosphere | 0.18 | Rhizodermis |
| B | 17.61 | Chalcopyrite in siderite | 0.21 | Magnetite? grain | 0.12 | Magnetite? grain | 0.24 | Fe-dendrite on ferrosphere | 0.81 | Axle roller | |
| C | 0.33 | Hematite grain | 2.44 | Fe-oxide on ferrosphere | 0.12 | Al-Si-Fe crassinetwork | 0.73 | Ca-Mg-Fe-Mn carbonate | 0.49 | Primary cortex | |
| Zn | A | 0.50 | Vitrinite with siderite | 0.11 | Teniusphere with Fe-oxide | 0.60 | Cenosphere | 0.16 | Magnetite in cenosphere | <0.01 | No data |
| B | 0.72 | Pyrite in siderite | 0.07 | Si microsphere | 0.01 | No data | 0.31 | Fusinoid | <0.01 | No data | |
| C | 0.84 | Siderite grain | 1.23 | Cenosphere | 0.17 | Al-Si-Fe crassinetwork | 0.46 | Hematite in organic | <0.01 | No data | |
| As | A | 0.41 | Fusinite with siderite | 0.17 | Crassisphere wit Fe-oxide | 0.29 | Fe-oxide on microsphere | 0.58 | Fe-oxide in cenosphere | <0.01 | No data |
| B | 0.12 | Chalcopyrite in siderite | 0.04 | Cenosphere | 0.22 | Calcimagnesiaferrosphere | 0.17 | Ferrosphere | 0.14 | Rhizodermis | |
| C | 0.19 | Magnetite massive grain | <0.01 | No data | 0.39 | Al-Si-Fe crassinetwork | 0.52 | Ca-Fe aluminosilicate | <0.01 | No data | |
| Ag | A | 0.26 | Cassiterite with maceral | 0.22 | Fe-oxide on Si-microsphere | 0.28 | Fe-oxide in cenosphere | 0.19 | Quartz with organic | 0.22 | Cortex cell |
| B | 0.19 | Hematite with siderite | 0.12 | Magnetite? on microsphere | 0.10 | Si-Ca microsphere | 0.28 | Ferrosphere | 0.11 | Axle roller | |
| C | 0.65 | Fusinite with siderite | 0.52 | Fe-oxide in cenosphere | 0.60 | Crassinetwork with Fe-oxide | 2.31 | Monazite | 0.04 | Primary cortex | |
| Cd | A | 0.35 | Cassiterite | 0.14 | Fe-oxide on cenosphere | 0.21 | Fe-oxide in cenosphere | 0.12 | Fe-oxide on Si-cenosphere | 0.42 | Rhizodermis |
| B | 0.29 | Hematite with siderite | 0.23 | Cenosphere | 0.18 | Crassinetwork | 0.24 | Barite | 0.21 | Primary cortex | |
| C | 0.61 | Siderite grain | 0.39 | Fe-oxide in cenosphere | 0.44 | Crassinetwork with Fe-oxide | 1.21 | Monazite | 0.37 | Primary cortex | |
| Sb | A | 0.53 | Vitrinite with siderite | 0.24 | Fe-oxide on microsphere | 0.36 | Fe-oxide in cenosphere | 0.78 | Organic matter | 0.62 | Primary cortex |
| B | 0.34 | Siderite | 0.43 | Magnetite? on microsphere | 0.29 | Ferrosphere | 0.22 | Cenosphere with Fe-oxide | 1.28 | Axle roller | |
| C | 0.37 | Fusinite with minerals? | 0.42 | Fe-oxide in cenosphere | 0.28 | Al-Si-Fe crassinetwork | 1.20 | Ca-Mg-Fe-Mn carbonate | 0.52 | Rhizodermis | |
| Pb | A | 2.86 | Cassiterite with maceral | 1.37 | Fe-oxide on cenosphere | 1.72 | Fe-oxide on microsphere | 1.47 | Cenosphere | 1.16 | Primary cortex |
| B | 1.62 | Hematite with siderite | 0.68 | Cenosphere | 0.69 | Cenosphere | 1.02 | Siderite after hematite | 0.63 | Axle roller | |
| C | 3.79 | Siderite grain | 1.07 | Fe-oxide on crassinetwork | 0.47 | Crassinetwork with Fe-oxide | 1.60 | Siderite after hematite | 0.80 | Primary cortex | |
| Object/PS/Element | Fly Ash | Slag | |||
|---|---|---|---|---|---|
| >0.5 mm | 0.5–0.2 mm | 0.2–0.05 mm | <0.05 mm | ||
| Yield of Magnetic Fraction (wt %) | |||||
| PS-a | 0.03 | 1.96 | 3.98 | 26.01 | 15.07 |
| PS-b | 0.37 | 1.85 | 11.67 | 6.30 | 7.84 |
| PS-c | 0.11 | 3.57 | 6.26 | 2.39 | 10.54 |
| Yield of Nonmagnetic Fraction (wt %) | |||||
| PS-a | 0.08 | 13.73 | 22.42 | 31.79 | 84.93 |
| PS-b | 0.28 | 8.88 | 49.58 | 21.07 | 92.16 |
| PS-c | 0.09 | 5.99 | 31.32 | 50.27 | 89.16 |
| Fe content in Magnetic Fraction (wt %) | |||||
| PS-a | 5.30 | 7.65 | 15.33 | 21.60 | 13.57 |
| PS-b | 39.30 | 12.72 | 33.47 | 33.01 | 7.60 |
| PS-c | 4.24 | 2.66 | 9.84 | 14.14 | 12.61 |
| Content of Unburned Organic Matter in Magnetic Fraction (vol %) | |||||
| PS-a | 5 | 4 | <0.5 | <0.5 | 1 |
| PS-b | 2 | 6 | <0.5 | <0.5 | <0.5 |
| PS-c | 8 | 3 | <0.5 | <0.5 | 4 |
| Element Content in Unburned Organic Matter in Magnetic Fraction (wt %) | |||||
| PS-a | |||||
| Co | 0.07 | no data | no data | 0.02 | 0.11 |
| Ni | <0.01 | no data | no data | <0.01 | <0.01 |
| Cu | <0.01 | no data | no data | <0.01 | <0.01 |
| Zn | 0.08 | no data | no data | <0.01 | 0.60 |
| As | 0.15 | no data | no data | <0.01 | 0.08 |
| Ag | <0.01 | no data | no data | <0.01 | <0.01 |
| Cd | <0.01 | no data | no data | <0.01 | <0.01 |
| Sb | <0.01 | no data | no data | 0.26 | 0.15 |
| Pb | <0.01 | no data | no data | 0.52 | <0.01 |
| PS-b | |||||
| Co | <0.01 | no data | <0.01 | <0.01 | 0.14 |
| Ni | <0.01 | no data | 0.10 | <0.01 | 0.08 |
| Cu | <0.01 | no data | 0.16 | <0.01 | <0.01 |
| Zn | <0.01 | no data | <0.01 | <0.01 | <0.01 |
| As | <0.01 | no data | <0.01 | <0.01 | <0.01 |
| Ag | <0.01 | no data | <0.01 | <0.01 | 0,01 |
| Cd | <0.01 | no data | <0.01 | <0.01 | 0.14 |
| Sb | <0.01 | no data | <0.01 | <0.01 | <0.01 |
| Pb | 0.54 | no data | <0.01 | <0.01 | 0.09 |
| PS-c | |||||
| Co | <0.01 | 0.10 | no data | 0.02 | <0.01 |
| Ni | 0.05 | 0.04 | no data | <0.01 | 0.07 |
| Cu | <0.01 | <0.01 | no data | <0.01 | <0.01 |
| Zn | <0.01 | <0.01 | no data | <0.01 | 0.13 |
| As | <0.01 | <0.01 | no data | <0.01 | 0.28 |
| Ag | <0.01 | <0.01 | no data | <0.01 | 0.60 |
| Cd | <0.01 | <0.01 | no data | <0.01 | 0.29 |
| Sb | <0.01 | <0.01 | no data | 0.26 | <0.01 |
| Pb | 0.12 | <0.01 | no data | 0.53 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parzentny, H.R.; Róg, L. Distribution and Mode of Occurrence of Co, Ni, Cu, Zn, As, Ag, Cd, Sb, Pb in the Feed Coal, Fly Ash, Slag, in the Topsoil and in the Roots of Trees and Undergrowth Downwind of Three Power Stations in Poland. Minerals 2021, 11, 133. https://doi.org/10.3390/min11020133
Parzentny HR, Róg L. Distribution and Mode of Occurrence of Co, Ni, Cu, Zn, As, Ag, Cd, Sb, Pb in the Feed Coal, Fly Ash, Slag, in the Topsoil and in the Roots of Trees and Undergrowth Downwind of Three Power Stations in Poland. Minerals. 2021; 11(2):133. https://doi.org/10.3390/min11020133
Chicago/Turabian StyleParzentny, Henryk R., and Leokadia Róg. 2021. "Distribution and Mode of Occurrence of Co, Ni, Cu, Zn, As, Ag, Cd, Sb, Pb in the Feed Coal, Fly Ash, Slag, in the Topsoil and in the Roots of Trees and Undergrowth Downwind of Three Power Stations in Poland" Minerals 11, no. 2: 133. https://doi.org/10.3390/min11020133
APA StyleParzentny, H. R., & Róg, L. (2021). Distribution and Mode of Occurrence of Co, Ni, Cu, Zn, As, Ag, Cd, Sb, Pb in the Feed Coal, Fly Ash, Slag, in the Topsoil and in the Roots of Trees and Undergrowth Downwind of Three Power Stations in Poland. Minerals, 11(2), 133. https://doi.org/10.3390/min11020133






