Understanding of Blast Furnace Performance with Biomass Introduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass
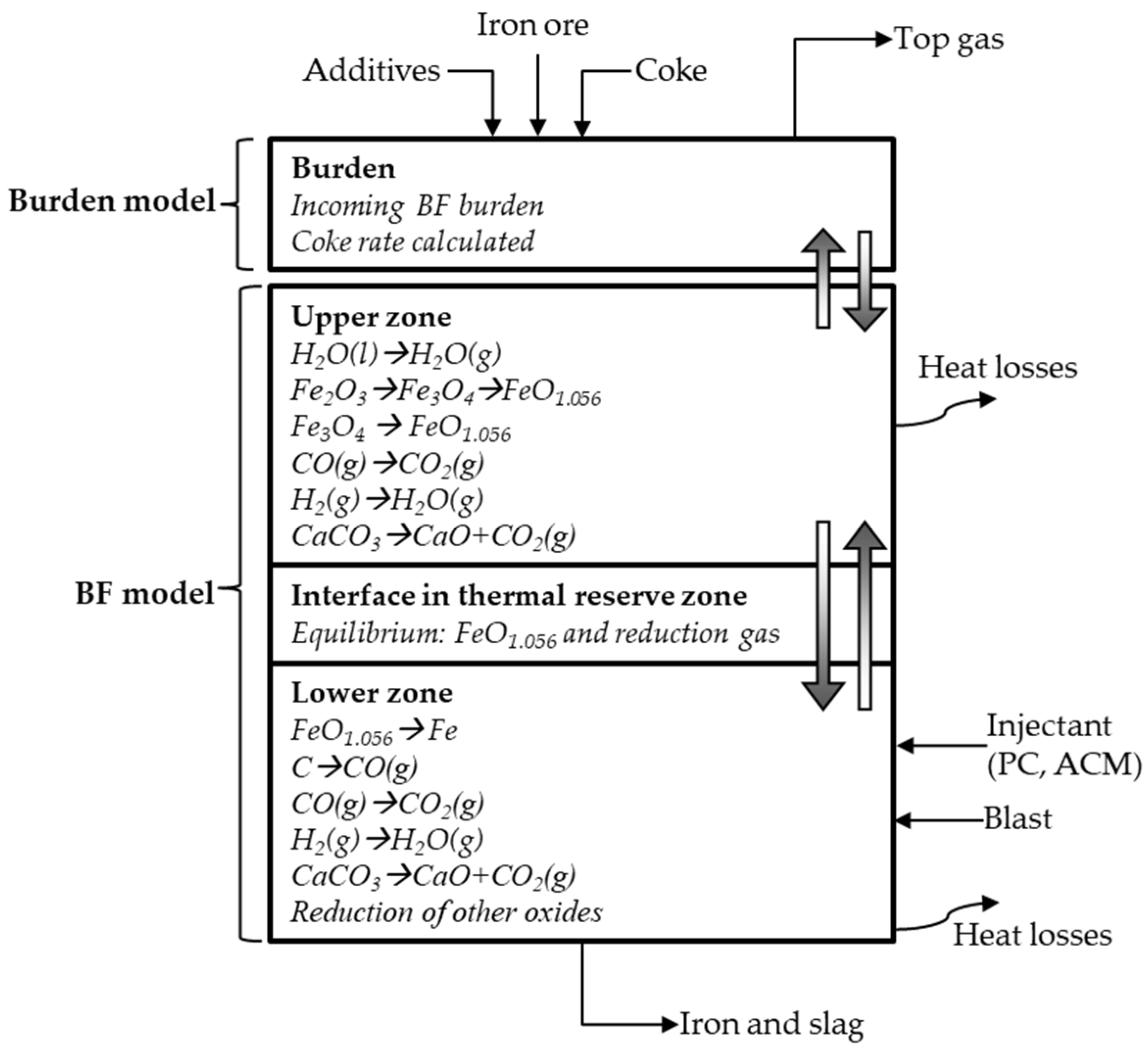
2.2. MASMOD
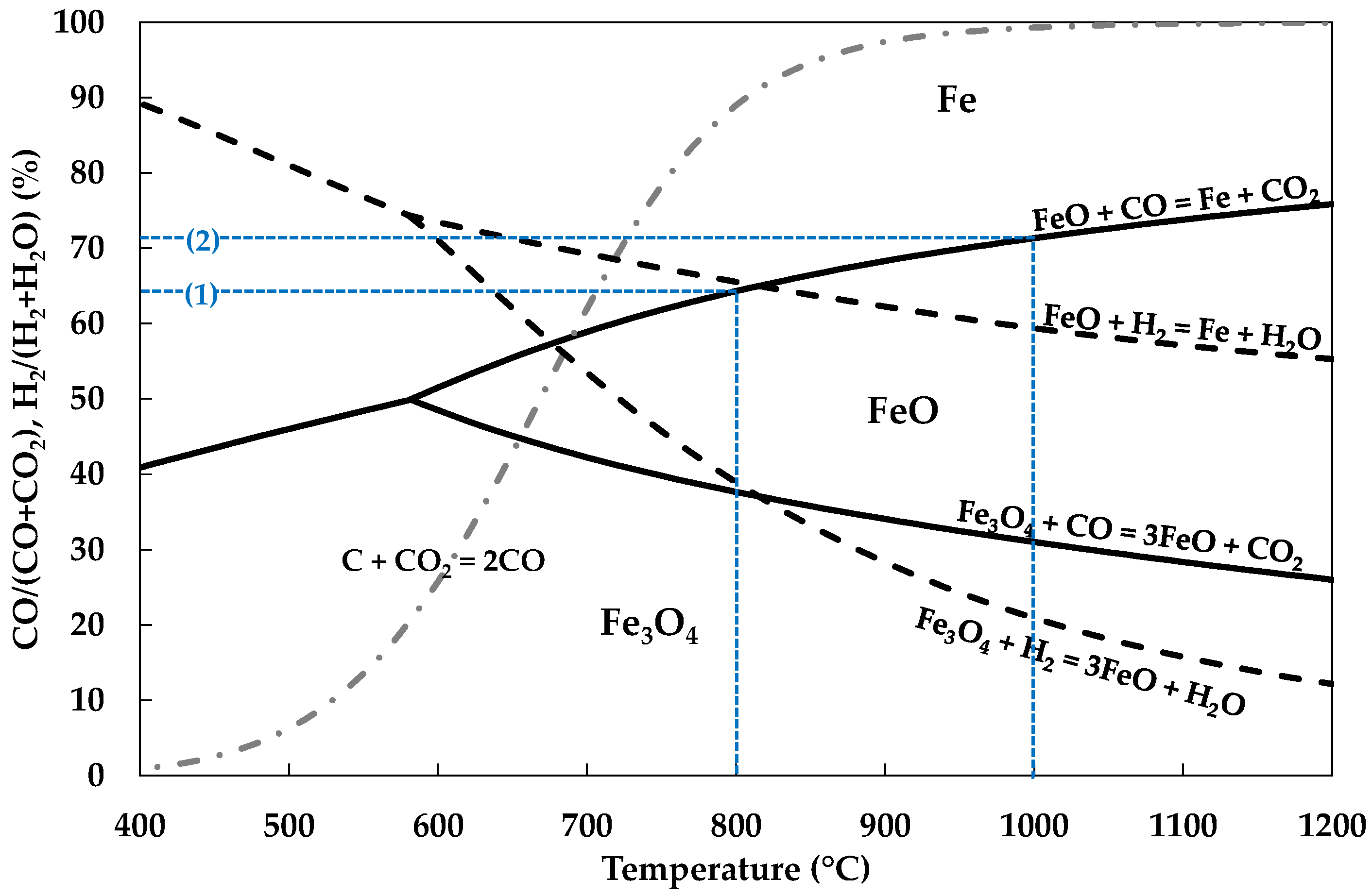
Interface between Upper and Lower Zones-TRZ-eq
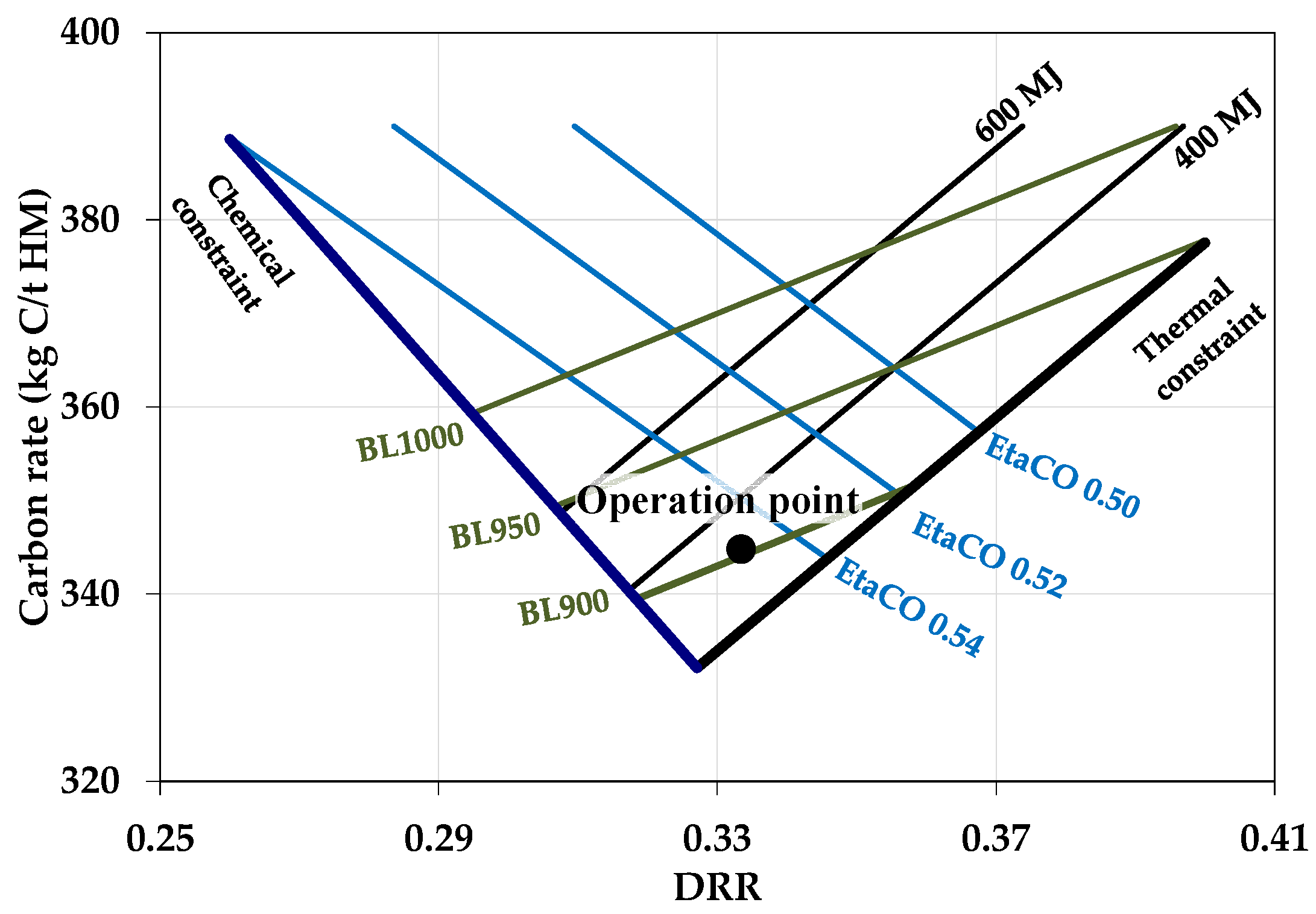
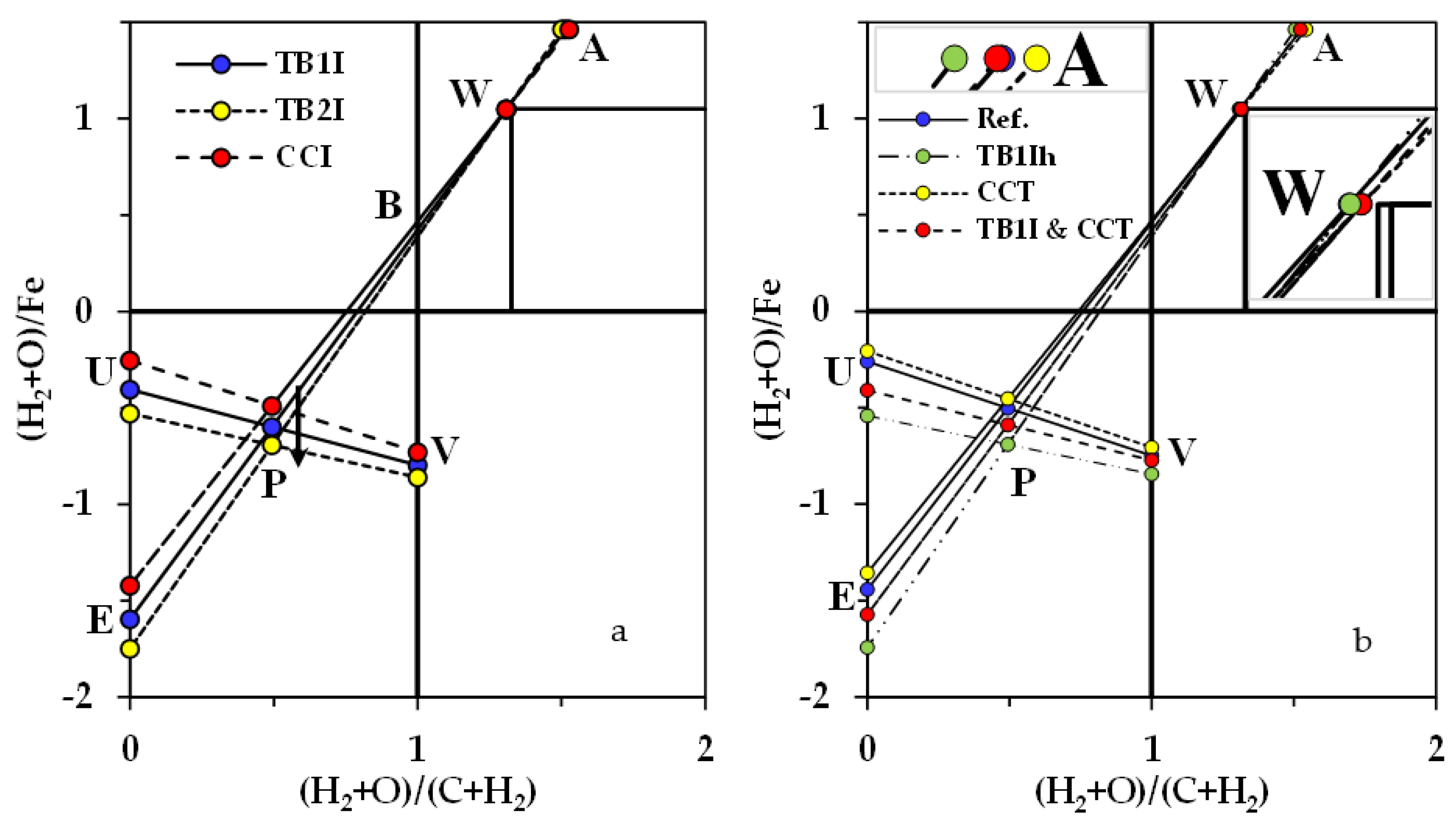
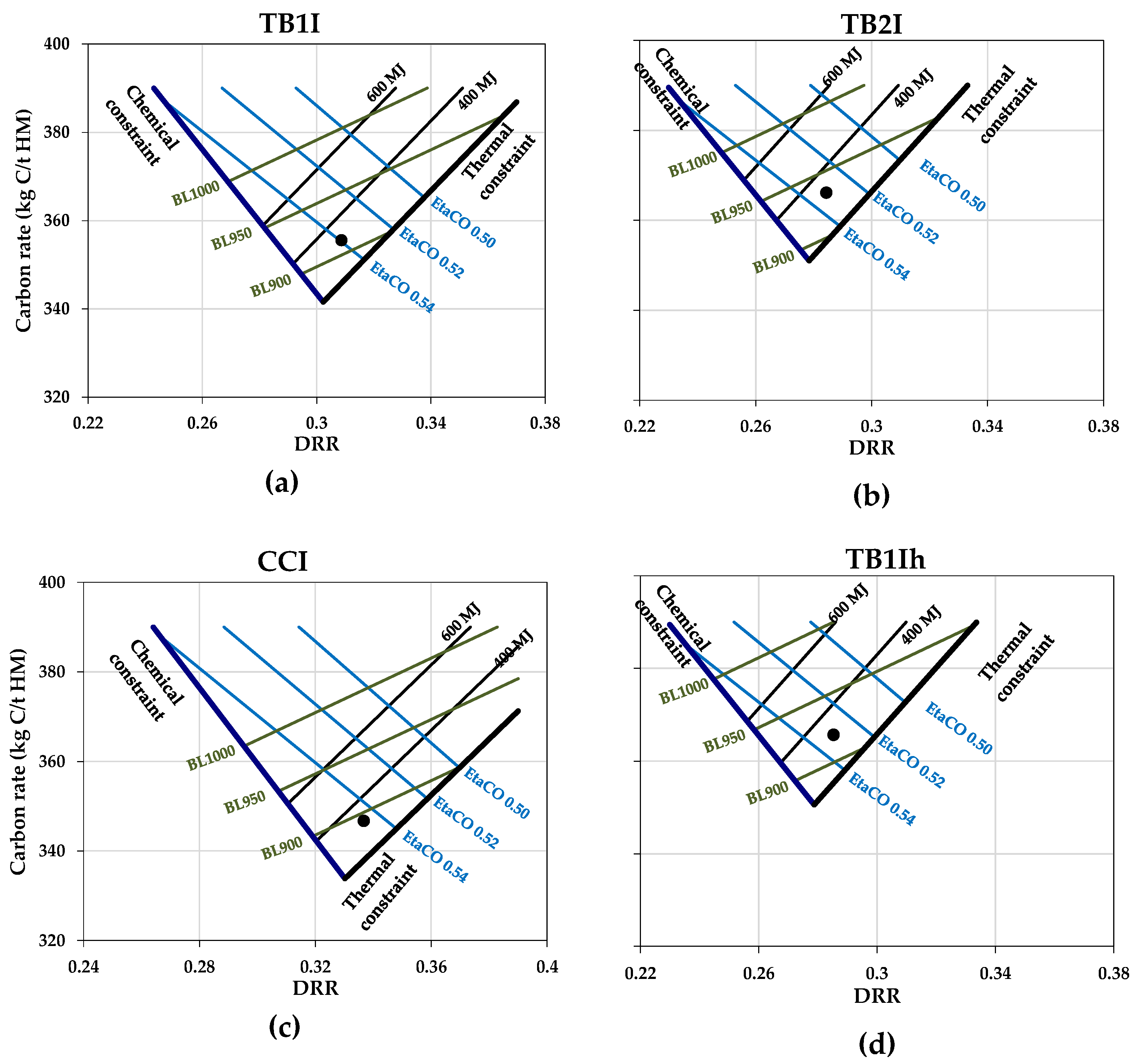
2.3. Visulisation of Effects of Biomass Introduction into the BF in RIST and CDRR Diagrams
2.4. Calculation Cases
3. Results
4. Discussion
5. Conclusions
- The model simulations indicate that the input of pre-treated bio-coal can theoretically enable lowering of fossil CO2-emissions by 9–34%.
- Injection of the selected type of charcoal results in minor changes in the BF conditions and results in decreased slag volume, and therefore decreased need for limestone addition, because of low content of acid ash forming oxides.
- Injection of torrefied biomass significantly changes the conditions, resulting in lower raceway adiabatic flame temperature (RAFT), higher volumes of reducing gas and higher top gas temperatures, which limits the maximum amount possible to inject.
- Top charging of charcoal with lowering of thermal reserve zone temperature results in higher gas efficiency, lower top gas temperature and higher RAFT.
- By combining the two methods with opposite impact on the BF conditions theses impacts level out and an operational state quite similar to the reference conditions can be achieved. This is exemplified by combining injection of torrefied biomass with top charging of charcoal, where the contrary change in BF operating conditions evens out and resembles those of the reference case.
- The highest reduction of fossil CO2 emissions (34%) is estimated for injection of 143 kg per tonne of hot metal of torrefied biomass and top charging of 30 kg per tonne of hot metal charcoal.
- The slag rate and need for limestone to control the basicity at 1.07 is lower for all cases with input of pre-treated biomass.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Variable | Description |
| AO | Alloying oxides SiO2, P2O5, MnO, etc. |
| BL | Blast |
| BR | Boudouard reaction |
| CC | Charcoal |
| CCI | Charcoal injection |
| CCT | Charcoal top-charging |
| CDRR | Carbon Direct Reduction Rate |
| DRR | Direct reduction rate |
| Eta | Gas utilisation |
| EtaCO | EtaCO = %CO2/(%CO2 + %CO) |
| EtaH2 | EtaH2 = %H2O/(%H2O + %H2) |
| Eta(CO + H2) | Eta(CO + H2)= (%CO2 + %H2O /(%CO2 + %CO + %H2O + %H2) |
| HHV | Higher heating value |
| ΔH | Heat of reaction |
| MASMOD | Name of 1 dimensional static BF model used |
| n | Moles of element (nO, nC, nH) |
| PC | Pulverized coal |
| RAR | Reductant rate |
| SE | Shaft efficiency |
| TB | Torrefied biomass |
| TB1I, TB2I | TB injection of highly torrefied biomass 1 or lowly torrefied biomass 2 |
| TRZ | Thermal reserve zone |
| TRZ-eq | Interface in TRZ where reduction gas composition corresponds to equilibrium with wüstite and metallic iron |
| TRZT | TRZ temperature |
| VM | Volatile matter |
References
- Danloy, G.; Berthelemot, A.; Grant, M.; Borlée, J.; Sert, D.; van der Stel, J.; Jak, H.; Dimastromatteo, V.; Hallin, M.; Eklund, N.; et al. ULCOS-Pilot testing of the Low-CO2 Blast Furnace process at the experimental BF in Luleå. Revu De Métallurgie 2009, 106, 1–8. [Google Scholar] [CrossRef]
- Knop, K.; Hallin, M.; Burström, E. ULCORED SP 12 Concept for minimized CO2 emission. Revue De Métallurgie 2009, 106, 419–421. [Google Scholar] [CrossRef]
- Watakabe, S.; Miyagawa, K.; Matsuzaki, S.; Inada, T.; Tomita, Y.; Saito, K.; Osame, M.; Sikström, P.; Sundqvist Ökvist, L.; Wikström, J.-O. Operation Trial of Hydrogenous Gas Injection of COURSE50 Project at an Experimental Blast Furnace. ISIJ Int. 2013, 53, 2065–2071. [Google Scholar] [CrossRef]
- Okvist, L.S.; Hu, X.; From, L.-E.; Sandström, D.; Ölund, M.; Hirsch, A.; Mittelstädt, H.; Bolle, M.; Hensmann, M.; Möhring, S.; et al. Improved Coal Combustion under Variable BF Conditions; Final Report, EUR 29519 EN; Publications Office of the European Union: Luxembourg, 2018; pp. 111–116. ISBN 978-92-79-98296-5. [Google Scholar]
- Lingiardi, O.; Burrai, O.; Gomez Fuentealba, C.; Etchevarne, P.; Gonzalez, J.M. Natural gas injection at Siderar #2 Blast Furnace. Ironmak. Conf. Proc. 1999, 58, 135–142. [Google Scholar]
- Starshinov, B.N.; Korwlnikov, I.V.; Sinitskii, V.I.; Laverentev, M.L.; Sinitskii, V.D. Blast-Furnace operation with the addition of natural gas to the blast. Trans. Metall. 1961, 7, 4–8. [Google Scholar] [CrossRef]
- Agarwal, J.C.; Brown, F.C.; Chin, D.L.; Stevens, G.S. Results from ultra high rates of natural gas injection into the blast furnace at ACME steel company. In Proceedings of the ICSTI Ironmaking, Toronto, ON, Canada, 22–25 March 1998. [Google Scholar]
- Hooey, P.L.; Bodén, A.; Wang, C.; Grip, C.-E.; Jansson, B. Design and application of a spread sheet based model of the blast furnace factory. ISIJ Int. 2010, 50, 924–930. [Google Scholar] [CrossRef]
- Ueda, S.; Watanabe, K.; Yanagiya, K.; Inoue, R.; Ariyama, T. Improvement of reactivity of carbon iron ore composite with biomass char for blast furnace. ISIJ Int. 2009, 49, 1505–1512. [Google Scholar] [CrossRef]
- Kasai, A.; Matsui, Y. Lowering the Thermal Reserve Zone Temperature in Blast Furnace by Adjoining Carbonaceous Material and Iron Ore. ISIJ Int. 2004, 44, 2073–2078. [Google Scholar] [CrossRef]
- Kasai, A.; Toyota, H.; Nozawa, K.; Kitayama, S. Reduction of Reducing Agent in Blast Furnace Operation by Carbon Composite Iron Ore Hot Briquette. ISIJ Int. 2011, 51, 1333–1335. [Google Scholar] [CrossRef]
- Marques, M.B.; Assis, A.R.; Dias, S.M.B.; Araujo, F.H.; De Andrade, F.C.; Dos Santos, R.J. Co-Injection of Natural Gas, Charcoal Fines and Pulverized Coal in the Arcelormittal Monlevade Blast Furnace “A”. Technol. Metal. Mater. Min. 2011, 20110912, 544–554. [Google Scholar]
- Ökvist, L.S.; Brandell, C.; Lundgren, M. Impact of activated nut coke on energy efficiency in the blast furnace. In Proceedings of the Aistech 2014, Indianapolis, IN, USA, 3–8 May 2014. [Google Scholar]
- Lundgren, M.; Sundqvist Ökvist, L.; Brandell, C. Development of nut coke activation for energy efficient blast furnace operation. In Proceedings of the AisTech/ICSTI, Cleveland, OH, USA, 4–7 May 2015. [Google Scholar]
- Mousa, E.; Lundgren, M.; Sundqvist Ökvist, L.; From, L.E.; Robles, A.; Hällsten, S.; Sundelin, B.; Friberg, H.; El-Tawil, A. Reduced Carbon Consumption and CO2 Emission at the Blast Furnace by Use of Briquettes Containing Torrefied Sawdust. J. Sustain. Metall. 2019, 5, 391–401. [Google Scholar] [CrossRef]
- Nishioka, K.; Ujisawa, Y.; Inada, T. Effect of Large Quantity of Ferrocoke Charging on Reduction of Reducing Agent Rate of Blast Furnace. Tetsu-to-Hagané 2014, 100, 1347–1354. [Google Scholar] [CrossRef]
- Ökvist, L.S.; Jansson, B.; Hahlin, P. Effect of coal properties, injection rate and O2 addition on BF conditions. In Proceedings of the ICSTI, Osaka, Japan, 26–30 November 2006. [Google Scholar]
- Sundqvist Ökvist, L.; Lagerwall, P.; Sundelin, B.; Orre, J.; Brämming, M.; Lundgren, M. Low CO2 ironmaking in the blast furnace: Roheisenerzeugung im Hochofen mit niedrigen CO2 Emissionen. Stahl Und Eisen 2017, 137, 29–37. [Google Scholar]
- Ng Wing, K.; Giroux, L.; MacPhee, T.; Todoschuk, T. Combustibility of Charcoal for Direct Injection in Blast Furnace Ironmaking; Association for Iron & Steel Technology: Warrendale, PA, USA, 2012. [Google Scholar]
- Spanlang, A.; Wukovits, W.; Weiss, B. Development of a Blast Furnace Model with Thermodynamic Process Depiction by Means of the Rist Operating Diagram. Chem. Eng. Trans. 2016, 52, 973–978. [Google Scholar]
- Bhattacharya, A.; Muthusamy, S. Static Heat Energy Balance Mathematical Model for an Iron Blast Furnace. Int. J. Miner. Process. Extract. Metall. 2017, 2, 57–67. [Google Scholar] [CrossRef][Green Version]
- Ryman, C.; Grip, C.-E.; Franck, P.-Å.; Wikström, J.-O. Similarities between pinch analysis and classical blast furnace analysis methods—Possible improvement by synthesis. In Proceedings of the 1st International Green Energy Conference, Waterloo, ON, Canada, 12–16 June 2005. [Google Scholar]
- Ökvist, L.S.; Lundgren, M.; Hyllander, G.; Hensmann, M.; Olsson, E.; Antila, O.; Schuster, S. Injection of Alternative Carbon Containing Materials into the Blast Furnace. In Proceedings of the TMS 2012 141st Annual Meeting & Exhibition, Orlando, FL, USA, 11–15 March 2012. [Google Scholar]
- Ökvist, L.S.; Hyllander, G.; Olsson, E.T.; Wikström, J.-O. Injection of pulverized materials into the blast furnace raceway. In Proceedings of the 6th ICSTI, Rio de Janerio, Brazil, 14–17 October 2012. [Google Scholar]
- Flexible Production of Coke Using Alternative Coals, Effects on Coke Properties under Blast Furnace Conditions, Coordination of Application on Cooperation with Colleague; 2012: FLEXCOKE (RFSR-CT-2013-00001); Publications Office of the European Union: Luxembourg, 2012.
- Sundqvist Ökvist, L.; From, L.-E.; Ölund, M.; Orre, J.; Sundelin, B.; Ahmed, H. Lowering of CO2 Emissions at the BF by Using Bio-coal—Theoretical and Practical Possibilities and Limitations. In Proceedings of the AISTech 2018, Philadephia, PA, USA, 10 May 2018. [Google Scholar]
- Orre, J.; Sundqvist, L.; Brämming, M.; Sundelin, B.; Lagerwall, P.; Björkman, B. Modelling of blast furnace process modification for lowering CO2 emissions from integrated steel plant. In Proceedings of the EMECR 2017, Kobe, Japan, 11–13 October 2017. [Google Scholar]
- IEAGHG Iron and Steel CCS Study (Techno-Economics Integrated Steel Mill). Available online: http://documents.ieaghg.org/index.php/s/P3rYI5vSh80SPM7 (accessed on 4 December 2020).
- Rist, A. Analogue Diagrams for Process Metallurgists. ISIJ Int. 1992, 32, 1034–1043. [Google Scholar] [CrossRef][Green Version]
- Nakatani, F.; Mukai, T.; Nakamura, F. Theoretical consideration on blast furnace coke ratio. Trans. ISIJ 1966, 6, 263–280. [Google Scholar] [CrossRef]
- Rist, A.; Meysson, N. A dual graphic representation of the blast-furnace mass and heat balances. J. Metals 1967, 50–59. [Google Scholar] [CrossRef]
- Kundrat, D.M. Development of an Analytical Equation for Calculation of the Blast Furnace Fuel Rate. Metall. Trans. B 1986, 17B, 705–724. [Google Scholar] [CrossRef]
- Kundrat, D.M.; Miwa, T.; Rist, A. Injections in the Iron Blast Furnace: A Graphics Study by Means of the Rist Operating Diagram. Metall. Trans. B 1991, 22B, 363–383. [Google Scholar]
- Roine, A. HSC Chemistry® [Software]; Outotec: Pori, Finland, 2018; Available online: www.outotec.com/HSC (accessed on 15 December 2020).


| Unit | PC | TB1 | TB2 | Charcoal | |
|---|---|---|---|---|---|
| C | % wt. | 82 | 70 | 57 | 87 |
| H | % wt. | 4.1 | 5 | 5.8 | 3.4 |
| N | % wt. | 2.2 | 0.1 | 0.1 | 0.25 |
| O | % wt. | 3.9 | 24 | 36.7 | 8.3 |
| S | % wt. | 0.28 | 0.01 | 0.01 | 0.01 |
| Ash | % wt. | 7.8 | 0.9 | 0.4 | 0.85 |
| Volatiles | % wt. | 21 | 50 | 74 | 12 |
| C fix | % wt. | 71 | 49 | 26 | 87 |
| H2O | % wt. | 0.5 | 1.2 | 1.2 | 1.2 |
| HHV | MJ/kg | 32 | 28 | 23 | 33 |
| Ash Composition | |||||
| SiO2 | % wt. | 49 | 8.6 | 7.0 | 20 |
| Fe2O3 | % wt. | 11 | 2.7 | 1.7 | 3.1 |
| Al2O3 | % wt. | 35 | 1.6 | 1.8 | 9.4 |
| CaO | % wt. | 12 | 27 | 56 | 29 |
| MgO | % wt. | 4.4 | 5.3 | 8.1 | 8.1 |
| Name | Description |
|---|---|
| Ref. | Reference case |
| TB1I | Injection of 100 kg/tHM highly torrefied biomass |
| TB2I | Injection of 100 kg/tHM lowly torrefied biomass |
| CCI | Injection of 100 kg/tHM charcoal |
| TB1Ih | Injection of TB1 to replace all PC |
| CCT | 30 kg/tHM of top-charged CC, 30 °C lower TRZ temperature |
| TB1I & CCT | 30 kg top-charged CC, injection of TB1 to replace all PC |
| Unit | Process Data | Ref. | TB1I | TB2I | CCI | TB1Ih | CCT | TB1I & CCT | |
|---|---|---|---|---|---|---|---|---|---|
| Pellets | kg/tHM | 1331 | 1332 | 1332 | 1332 | 1333 | 1333 | 1332 | 1333 |
| CCT | kg/tHM | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 30 |
| Coke | kg/tHM | 309 | 309 | 309 | 309 | 309 | 309 | 309 | 309 |
| PCI | kg/tHM | 143 | 143 | 70 | 99 | 39 | 0 | 104 | 0 |
| TB/CC injection | kg/tHM | 0 | 0 | 100 | 100 | 100 | 195 | 0 | 142 |
| RAR | kg/tHM | 452 | 452 | 479 | 508 | 448 | 505 | 443 | 481 |
| Slag rate | kg/tHM | 171 | 171 | 163 | 166 | 160 | 156 | 167 | 156 |
| Limestone | kg/tHM | 20.1 | 20.1 | 14.9 | 16.9 | 13.0 | 9.9 | 17.4 | 10.0 |
| Basicity (B2) | %CaO/%SiO2 | 1.03 | 1.07 | 1.07 | 1.07 | 1.07 | 1.07 | 1.07 | 1.07 |
| Blast volume | Nm3/tHM | 914 | 905 | 913 | 935 | 895 | 921 | 880 | 891 |
| Tuyere gas volume | Nm3/tHM | - | 1218 | 1282 | 1357 | 1202 | 1343 | 1166 | 1255 |
| O2 enrichment | % | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 |
| RAFT | °C | - | 2157 | 2039 | 1931 | 2170 | 1937 | 2242 | 2061 |
| Top gas temperature | °C | 124 | 124 | 153 | 179 | 125 | 180 | 81 | 124 |
| Top gas LHV | MJ/Nm3 | 3.0 | 3.0 | 3.2 | 3.3 | 3.0 | 3.3 | 2.9 | 3.1 |
| Top gas energy | GJ/tHM | 4.2 | 4.2 | 4.6 | 4.9 | 4.2 | 4.9 | 3.9 | 4.4 |
| EtaCO | % | 54.9 | 54.9 | 54.0 | 53.2 | 54.7 | 53.2 | 56.2 | 54.9 |
| SE | - | - | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| TRZT | °C | - | 850 | 850 | 850 | 850 | 850 | 820 | 820 |
| PC replacement ratio | kg PC/kg Bio | - | - | 0.73 | 0.44 | 1.04 | 0.73 | 1.30 | 0.83 |
| Unit | Ref. | TB1I | TB2I | CCI | TB1Ih | CCT | TB1I & CCT | |
|---|---|---|---|---|---|---|---|---|
| CO2 Bio | kg/tHM | 0 | 258 | 209 | 319 | 504 | 96 | 462 |
| CO2 fossil | kg/tHM | 1272 | 1051 | 1140 | 957 | 840 | 1154 | 840 |
| Total CO2 emission | kg/tHM | 1272 | 1309 | 1349 | 1276 | 1344 | 1250 | 1302 |
| Δ fossil CO2 | % | 0 | −17 | −10 | −25 | −34 | −9 | −34 |
| Δ fossilCO2 | kg/tHM | 0 | −221 | −132 | −315 | −432 | −118 | −432 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orre, J.; Ökvist, L.S.; Bodén, A.; Björkman, B. Understanding of Blast Furnace Performance with Biomass Introduction. Minerals 2021, 11, 157. https://doi.org/10.3390/min11020157
Orre J, Ökvist LS, Bodén A, Björkman B. Understanding of Blast Furnace Performance with Biomass Introduction. Minerals. 2021; 11(2):157. https://doi.org/10.3390/min11020157
Chicago/Turabian StyleOrre, Joel, Lena Sundqvist Ökvist, Axel Bodén, and Bo Björkman. 2021. "Understanding of Blast Furnace Performance with Biomass Introduction" Minerals 11, no. 2: 157. https://doi.org/10.3390/min11020157
APA StyleOrre, J., Ökvist, L. S., Bodén, A., & Björkman, B. (2021). Understanding of Blast Furnace Performance with Biomass Introduction. Minerals, 11(2), 157. https://doi.org/10.3390/min11020157





