EXAFS Determination of Clay Minerals in Martian Meteorite Allan Hills 84001 and Its Implication for the Noachian Aqueous Environment
Abstract
:1. Introduction
2. Sample and Method
2.1. Sample
2.2. Iron K-Edge EXAFS
2.2.1. Reference Materials
2.2.2. ALH Sample
3. Results
4. Discussion
4.1. Carbonate Species
4.2. Other Fe-Bearing Minerals
4.3. Aqueous Environment of Ancient Mars
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, D.H.; Dohm, J.M.; Rice, J.W., Jr. Map of Mars showing channels and possible paleolake basins. In U.S. Geological Survey Miscellaneous Investigations Series Map I-2461; Geological Survey (U.S.): Denver, CO, USA, 1995. [Google Scholar]
- Carr, M.H.; Chuang, F.C. Martian drainage densities. J. Geophys. Res. Planets 1997, 102, 9145–9152. [Google Scholar] [CrossRef]
- Cabrol, N.A.; Grin, E.A. Distribution, classification, and ages of martian impact crater lakes. Icarus 1999, 142, 160–172. [Google Scholar] [CrossRef]
- Ori, G.G.; Marinangeli, L.; Baliva, A. Terraces and Gilbert-type deltas in crater lakes in Ismenius Lacus and Memnonia (Mars). J. Geophys. Res. Planets 2000, 105, 17629–17641. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Anderson, F.S.; Andrews-Hanna, J.; Catling, D.C.; Christensen, P.R.; Cohen, B.A.; Dressing, C.D.; Edwards, C.S.; Elkins-Tanton, L.T.; Farley, K.A.; et al. The sustainability of habitability on terrestrial planets: Insights, questions, and needed measurements from Mars for understanding the evolution of Earth-like worlds. J. Geophys. Res. Planets 2016, 121, 1927–1961. [Google Scholar] [CrossRef]
- Fialips, C.I.; Carey, J.W.; Vaniman, D.T.; Bish, D.L.; Feldman, W.C.; Mellon, M.T. Hydration state of zeolites, clays, and hydrated salts under present-day martian surface conditions: Can hydrous minerals account for Mars Odyssey observations of near-equatorial water-equivalent hydrogen? Icarus 2005, 178, 74–83. [Google Scholar] [CrossRef]
- Bibring, J.P.; Langevin, Y.; Mustard, J.; Poulet, F.; Arvidson, R.; Gendrin, A.; Gondet, B.; Mangold, N.; Pinet, P.; Forget, F.; et al. Global mineralogical and aqueous Mars history derived from OMEGA/Mars Express data. Science 2006, 312, 400–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustard, J.F.; Murchie, S.L.; Pelkey, S.M.; Ehlmann, B.L.; Milliken, R.E.; Grant, J.A.; Bibring, J.P.; Poulet, F.; Bishop, J.; Dobrea, E.N.; et al. Hydrated silicate minerals on Mars observed by the Mars Reconnaissance Orbiter CRISM instrument. Nature 2008, 454, 305–309. [Google Scholar] [CrossRef]
- Osterloo, M.M.; Hamilton, V.E.; Bandfield, J.L.; Glotch, T.D.; Baldridge, A.M.; Christensen, P.R.; Tornabene, L.L.; Anderson, F.S. Chloride-bearing materials in the Southern Highlands of Mars. Science 2008, 319, 1651–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulet, F.; Bibring, J.-P.; Mustard, J.F.; Gendrin, A.; Mangold, N.; Langevin, Y.; Arvidson, R.E.; Gondet, B.; Gomez, C. Phyllosilicates on Mars and implications for early Martian climate. Nature 2005, 438, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Ehlmann, B.L.; Mustard, J.F.; Murchie, S.L.; Bibring, J.P.; Meunier, A.; Fraeman, A.A.; Langevin, Y. Subsurface water and clay mineral formation during the early history of mars. Nature 2011, 479, 53–60. [Google Scholar] [CrossRef]
- Farley, K.A.; Malespin, C.; Mahaffy, P.; Grotzinger, J.P.; Vasconcelos, P.M.; Milliken, R.E.; Malin, M.; Edgett, K.S.; Pavlov, A.A.; Hurowitz, J.A.; et al. In situ radiometric and exposure age dating of the Martian surface. Science 2014, 343, 1247166. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Bish, D.L.; Ming, D.W.; Bristow, T.F.; Morris, R.V.; Blake, D.F.; Chipera, S.J.; Morrison, S.M.; Treiman, A.H.; Rampe, E.B.; et al. Mineralogy of a mudstone at Yellowknife Bay, Gale crater, Mars. Science 2014, 343, 1243480. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Keprta, K.L.; Wentworth, S.J.; McKay, D.S.; Gibson, J.E.K. Field emission gun scanning electron microscopy (FEGSEM) and transmission electron (TEM) microscopy of phyllosilicates in Martian meteorites ALH84001, Nakhla, and Shergotty. In Lunar and Planetary Science XXXI; Lunar and Planetary Institute: Houston, TX, USA, 2000; #1690. [Google Scholar]
- Lapen, T.J.; Righter, M.; Brandon, A.D.; Debaille, V.; Beard, B.L.; Shafer, J.T.; Peslier, A.H. A younger age for ALH 84001 and its geochemical link to shergottite sources in Mars. Science 2010, 328, 347–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittlefehldt, D.W. ALH 84001, a cumulate orthopyroxenite member of the Martian meteorite clan. Meteoritics 1994, 29, 214–221. [Google Scholar] [CrossRef]
- Treiman, A.H. A petrographic history of Martian meteorite ALH 84001: Two shocks and an ancient age. Meteoritics 1995, 30, 294. [Google Scholar] [CrossRef] [Green Version]
- Treiman, A.H. The history of Allan Hills 84001 revised: Multiple shock events. Meteorit. Planet. Sci. 1998, 33, 753–764. [Google Scholar] [CrossRef]
- Eiler, J.M.; Valley, J.W.; Graham, C.M.; Fournelle, J. Two populations of carbonate in ALH84001: Geochemical evidence for discrimination and genesis. Geochim. Cosmochim. Acta 2002, 66, 1285–1303. [Google Scholar] [CrossRef]
- Corrigan, C.M.; Harvey, R.P. Multi-generational carbonate assemblages in Martian meteorite Allan Hills 84001: Implications for nucleation, growth, and alteration. Meteorit. Planet. Sci. 2004, 39, 17–30. [Google Scholar] [CrossRef]
- Borg, L.E.; Connelly, J.N.; Nyquist, L.E.; Shih, C.-Y.; Wiesmann, H.; Reese, Y. The age of the carbonates in Martian meteorite ALH84001. Science 1999, 286, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Halevy, I.; Fischer, W.W.; Eiler, J.M. Carbonates in the Martian meteorite Allan Hills 84001 formed at 18 ± 4 °C in a near-surface aqueous environment. Proc. Natl. Acad. Sci. USA 2011, 108, 16895–16899. [Google Scholar] [CrossRef] [Green Version]
- McKay, D.S.; Gibson, E.K.; Thomas-Keprta, K.L.; Vali, H.; Romanek, C.S.; Clemett, S.J.; Chillier, X.D.F.; Maechling, C.R.; Zare, R.N. Search for past life on Mars: Possible relic biogenic activity in Martian meteorite ALH 84001. Science 1996, 273, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Thomas-Keprta, K.L.; Bazylinski, D.A.; Kirschvink, J.L.; Clemett, S.J.; McKay, D.S.; Wentworth, S.J.; Vali, H.; Gibson, E.K., Jr.; Romanek, C.S. Elongated prismatic magnetite crystals in ALH 84001 carbonate globules: Potential Martian magnetofossils. Geochim. Cosmochim. Acta 2000, 64, 4049–4081. [Google Scholar] [CrossRef]
- Thomas-Keprta, K.L.; Clemett, S.J.; McKay, D.S.; Gibson, E.K.; Wentworth, S.J. Origins of magnetite nanocrystals in Martian meteorite ALH 84001. Geochim.Cosmochim. Acta 2009, 73, 6631–6677. [Google Scholar] [CrossRef] [Green Version]
- Boctor, N.; Alexander, C.M.O.; Wang, J.; Hauri, E. The sources of water in Martian meteorites: Clues from hydrogen isotopes. Geochim. Cosmochim. Acta 2003, 67, 3971–3989. [Google Scholar] [CrossRef]
- Leshin, L.A.; Epstein, S.; Stolper, E.M. Hydrogen isotope geochemistry of SNC meteorites. Geochim. Cosmochim. Acta 1996, 60, 2635–2650. [Google Scholar] [CrossRef]
- Kopp, R.E.; Humayun, M. Kinetic model of carbonate dissolution in Martian meteorite ALH84001. Geochim. Cosmochim. Acta 2003, 67, 3247–3256. [Google Scholar] [CrossRef] [Green Version]
- Melwani Daswani, M.; Schwenzer, S.P.; Reed, M.H.; Wright, I.P.; Grady, M.G. Alteration minerals, fluids and gases on early Mars: Predictions from 1D flow geochemical modelling of mineral assemblages in meteorite ALH 84001. Meteorit. Planet. Sci. 2016, 51, 2154–2174. [Google Scholar] [CrossRef] [Green Version]
- Nakada, R.; Ogawa, K.; Suzuki, N.; Takahashi, S.; Takahashi, Y. Late Triassic compositional changes of aeolian dusts in the pelagic Panthalassa: Response to the continental climatic change. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 393, 61–75. [Google Scholar] [CrossRef]
- Ikeda, M.; Hori, R.S.; Okada, Y.; Nakada, R. Volcanism and deep-ocean acidification across the end-Triassic extinction event. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 440, 725–733. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakada, R.; Watanabe, Y.; Takahashi, Y. Iron-depleted pelagic water at the end-Permian mass extinction inferred from chemical species of iron and molybdenum in deep-sea sedimentary rocks. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 516, 384–399. [Google Scholar] [CrossRef]
- Treiman, A.H.; Dyar, M.D.; McCanta, M.; Noble, S.K.; Pieters, C.M. Martian dunite NWA 2737: Petrographic constraints on geological history, shock events, and olivine color. J. Geophys. Res. Planets 2007, 112, E04002. [Google Scholar] [CrossRef] [Green Version]
- Hicks, L.J.; Bridges, J.C.; Gurman, S.J. Ferric saponite and serpentine in the nakhlite martian meteorites. Geochim. Cosmochim. Acta 2014, 136, 194–210. [Google Scholar] [CrossRef] [Green Version]
- Walton, E.L.; Jugo, P.J.; Herd, C.D.K.; Wilke, M. Martian regolith in Elephant Moraine 79001 shock melts? Evidence from major element composition and sulfur speciation. Geochim. Cosmochim. Acta 2010, 74, 4829–4843. [Google Scholar] [CrossRef]
- Shidare, M.; Nakada, R.; Usui, T.; Tobita, M.; Yokoyama, T. Sulfur K-Edge XANES Analyses of Shergottites: Implication for Aqueous Alteration Processes on Mars. In Lunar and Planetary Science XXXXVIIII; Lunar and Planetary Institute: Houston, TX, USA, 2018; #2083. [Google Scholar]
- Nakada, R.; Usui, T.; Ushioda, M.; Takahashi, Y. Vanadium micro-XANES determination of oxygen fugacity in olivine-hosted glass inclusion and groundmass glasses of martian primitive shergottite Yamato 980459. Am. Mineral. 2020, 105, 1695–1703. [Google Scholar] [CrossRef]
- Koike, M.; Nakada, R.; Kajitani, I.; Usui, T.; Tamenori, Y.; Sugahara, H.; Kobayashi, A. In-situ preservation of nitrogen-bearing organics in Noachian Martian carbonates. Nat. Commun. 2020, 11, 1988. [Google Scholar] [CrossRef] [Green Version]
- Schwandt, C.S.; McKay, G.A.; Lofgren, G.E. FESEM imaging reveals previously unseen detail and enhances interpretations of ALH 84001 carbonate petrogenesis. In Lunar and Planetary Science XXX; Lunar and Planetary Institute: Houston, TX, USA, 1999; #1346. [Google Scholar]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laborator, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2000; p. 103. [Google Scholar]
- Hirauchi, K.-I.; Katayama, I.; Uehara, S.; Miyahara, M.; Takai, Y. Inhibition of subduction thrust earthquakes by low-temperature plastic flow in serpentine. Earth Planet. Sci. Lett. 2010, 295, 349–357. [Google Scholar] [CrossRef]
- Sawai, M.; Katayama, I.; Hamada, A.; Maeda, M.; Nakashima, S. Dehydration kinetics of antigorite using in situ high-temperature infrared microspectroscopy. Phys. Chem. Miner. 2013, 40, 319–330. [Google Scholar] [CrossRef]
- Physical and Chemical Data of Source Clays. Available online: https://www.clays.org/sourceclays_data/ (accessed on 8 February 2021).
- Ravel, B.; Newville, M. Athena, Artemis, Hephaestus: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, R.P.; McSween, J.H.Y. A possible hightemperature origin for the carbonates in the Martian meteorite ALH84001. Nature 1996, 382, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Valley, J.W.; Eiler, J.M.; Graham, C.M.; Gibson, E.K.; Romanek, C.S.; Stolper, E.M. Low-temperature carbonate concretions in the Martian meteorite ALH 84001: Evidence from stable isotopes and mineralogy. Science 1997, 275, 1633–1637. [Google Scholar] [CrossRef]
- Scott, E.R.D.; Krot, A.N.; Yamagouchi, A. Carbonates in fractures of Martian meteorite ALH 84001: Petrologic evidence for impact origin. Meteorit. Planet. Sci. 1998, 33, 709–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyano-Cambero, C.E.; Trigo-Rodríguez, J.M.; Benito, M.I.; Alonso-Azcárate, J.; Lee, M.R.; Mestres, N.; Martínez-Jiménez, M.; Martín-Torres, F.J.; Fraxedas, J. Petrographic and geochemical evidence for multiphase formation of carbonates in the Martian orthopyroxenite Allan Hills 84001. Meteorit. Planet. Sci. 2017, 52, 1030–1047. [Google Scholar] [CrossRef] [Green Version]
- Kajitani, I.; Tanabe, G.; Nakada, R.; Usui, T.; Koike, M.; Matsuura, F.; Yokoyama, T. Finding of oxidized sulfur species in carbonates from a martian meteorite Allan Hills 84001 using μ-XANES. In Proceedings of the Ninth International Conference on Mars, Pasadena, CA, USA, 22–25 July 2019. [Google Scholar]
- Cooney, T.F.; Scott, E.R.D.; Krot, A.N.; Sharma, S.K.; Yamaguchi, A. Vibrational spectroscopic study of minerals in the Martian meteorite ALH 84001. Am. Mineral. 1999, 84, 1569–1576. [Google Scholar] [CrossRef]
- Steele, A.; Fries, M.D.; Amundsen, H.E.F.; Mysen, B.O.; Fogel, M.L.; Schweizer, M.; Boctor, N.Z. Comprehensive Imaging and Raman Spectroscopy of Carbonate Globules from Martian Meteorite ALH 84001 and a Terrestrial Analogue from Svalbard. Meteorit. Planet. Sci. 2007, 42, 1549–1566. [Google Scholar] [CrossRef] [Green Version]
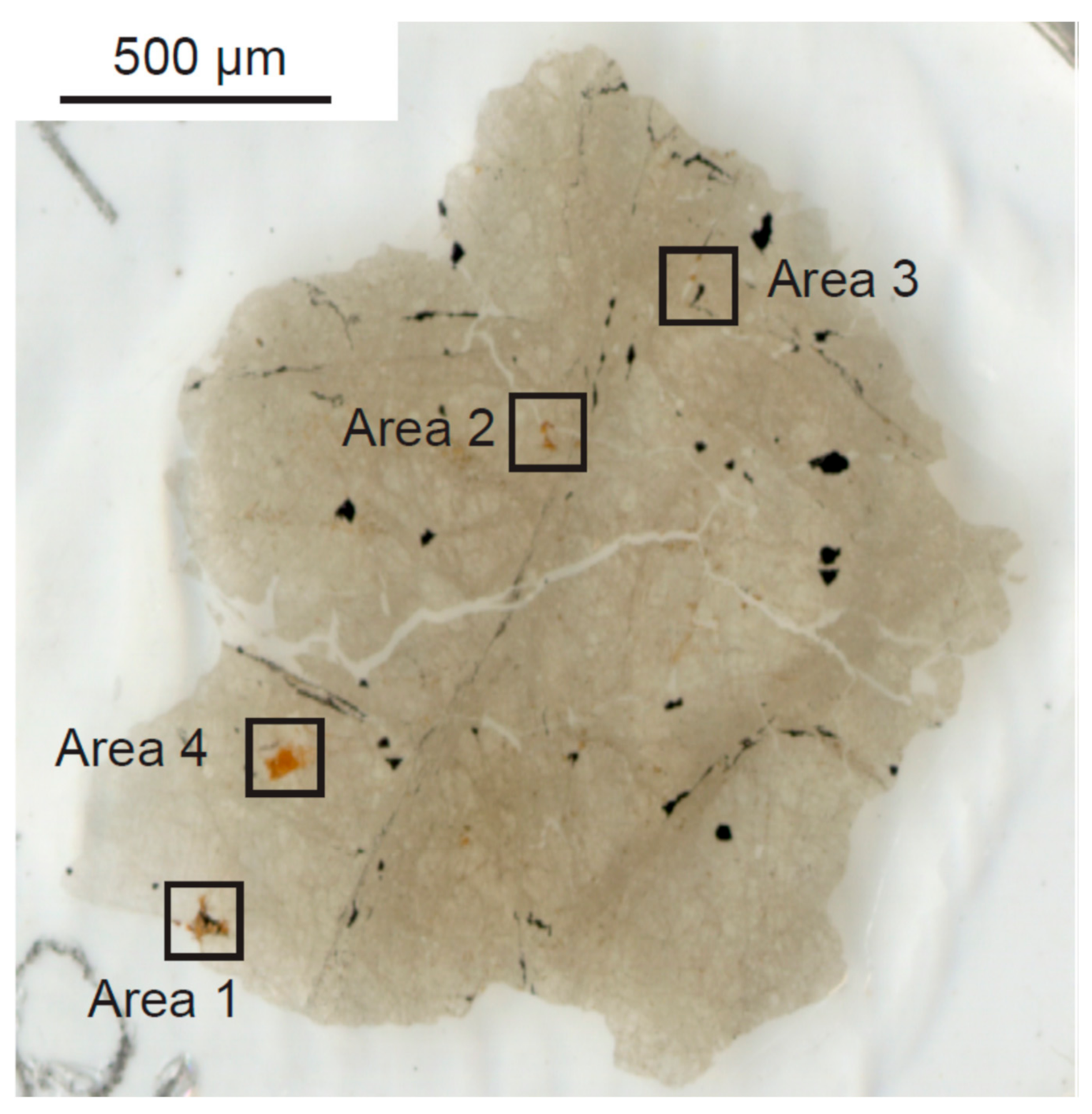







| Analytical Spots | Description | |
|---|---|---|
| Area 1 | spot 1 | Rim of a Mg-rich rosette carbonate |
| spot 2 | Between Mg-rich rim and Fe-rich core of the rosette carbonate | |
| spot 3 | Fe-rich core of the rosette carbonate | |
| spot 4 | Fe-rich core of the rosette carbonate | |
| spot 5 | Fe-rich core of the rosette carbonate | |
| spot 6 | Ca-rich core of the rosette carbonate | |
| spot 7 | Ca-rich core of the rosette carbonate | |
| spot 8 | Silica glass located in the rosette carbonate | |
| spot 9 | Silica glass adjacent to the rosette carbonate | |
| spot 10 | Fe-rich core of the rosette carbonate | |
| spot 11 | Silica glass located in the rosette carbonate | |
| spot 12 | Silica glass | |
| spot 13 | Fe-rich core of the rosette carbonate | |
| spot 14 | Fe-rich core of the rosette carbonate | |
| spot 15 | Between Mg-rich rim and Fe-rich core of the rosette carbonate | |
| spot 16 | Rim of a Mg-rich rosette carbonate | |
| spot 17 | Silica glass located in the rosette carbonate | |
| Area 2 | spot 1 | Silica glass located in the slab carbonate |
| spot 2 | Slab carbonate closely adjacent to the silica glass | |
| spot 3 | Fe-rich slab carbonate | |
| spot 4 | Ca-rich slab carbonate | |
| spot 5 | Slab carbonate with moderate Fe contents | |
| spot 6 | Slab carbonate with moderate Fe contents | |
| Area 3 | spot 1 | Silica glass |
| spot 2 | Core of the rosette carbonate rich in Ca | |
| spot 3 | Core of the rosette carbonate rich in Fe | |
| spot 4 | Between Mg-rich rim and Fe-rich core of the rosette carbonate | |
| Area 4 | spot 1 | Slab carbonate with moderate Fe |
| spot 2 | Slab carbonate with moderate Fe | |
| spot 3 | Fe-rich slab carbonate | |
| spot 4 | Slab carbonate with depleted Ca | |
| Name | Mineral | Locality | Reference |
|---|---|---|---|
| CCa-2 | Ripidolite (chlorite) | California, USA | [43] |
| IMt-2 | Illite | Montana, USA | [43] |
| ISCz-1 | Illite-smectite mixedlayer (70/30 ordered) | Slovakia | [43] |
| KGa-1b | Kaolin (low-defect) | Georgia, USA | [43] |
| NAu-1 | Nontronite (Al-enriched) | South Australia | [43] |
| NAu-2 | Nontronite (Al-poor) | South Australia | [43] |
| NG-1 | Nontronite | Hohen Hagen, Germany | [43] |
| STx-1b | Ca-montmorillonite | Texas, USA | [43] |
| SWy-3 | Na-rich Montmorillonite | Wyoming, USA | [43] |
| Serpentine (L) | Lizardite and chrysotile | Chiba, Japan | [41] |
| Serpentine (H) | Antigorite | Nagasaki, Japan | [42] |
| Analytical Spots | Mgs | Sd | STx | NAu | ISCz | Srp | Hem | Mag | Py | R (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area 1 | spot 1 | 59 | 24 | 17 | 0.122 | ||||||
| spot 2 | 48 | 36 | 16 | 0.195 | |||||||
| spot 3 | 48 | 39 | 13 | 0.116 | |||||||
| spot 4 | 47 | 43 | 10 | 0.130 | |||||||
| spot 5 | 42 | 49 | 10 | 0.117 | |||||||
| spot 6 | 44 | 45 | 11 | 0.093 | |||||||
| spot 7 | 63 | 26 | 11 | 0.122 | |||||||
| spot 8 | 45 | 41 | 13 | 0.114 | |||||||
| spot 9 | 45 | 39 | 15 | 0.089 | |||||||
| spot 10 | 44 | 44 | 12 | 0.078 | |||||||
| spot 11 | 46 | 37 | 17 | 0.155 | |||||||
| spot 12 | 49 | 38 | 14 | 0.239 | |||||||
| spot 13 | 41 | 36 | 23 | 0.142 | |||||||
| spot 14 | 41 | 42 | 16 | 0.191 | |||||||
| spot 15 | 49 | 33 | 18 | 0.107 | |||||||
| spot 16 | 50 | 28 | 22 | 0.104 | |||||||
| spot 17 | 45 | 26 | 29 | 0.217 | |||||||
| Area 2 | spot 1 | 51 | 23 | 27 | 0.189 | ||||||
| spot 2 | 56 | 31 | 14 | 0.078 | |||||||
| spot 3 | 47 | 46 | 7 | 0.068 | |||||||
| spot 4 | 46 | 41 | 13 | 0.064 | |||||||
| spot 5 | 49 | 43 | 8 | 0.074 | |||||||
| spot 6 | 52 | 40 | 8 | 0.046 | |||||||
| Area 3 | spot 1 | 36 | 33 | 31 | 0.196 | ||||||
| spot 2 | 43 | 49 | 8 | 0.065 | |||||||
| spot 3 | 47 | 47 | 6 | 0.047 | |||||||
| spot 4 | 52 | 39 | 10 | 0.060 | |||||||
| Area 4 | spot 1 | 58 | 31 | 11 | 0.096 | ||||||
| spot 2 | 43 | 42 | 15 | 0.077 | |||||||
| spot 3 | 45 | 48 | 7 | 0.045 | |||||||
| spot 4 | 37 | 52 | 11 | 0.127 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakada, R.; Tanabe, G.; Kajitani, I.; Usui, T.; Shidare, M.; Yokoyama, T. EXAFS Determination of Clay Minerals in Martian Meteorite Allan Hills 84001 and Its Implication for the Noachian Aqueous Environment. Minerals 2021, 11, 176. https://doi.org/10.3390/min11020176
Nakada R, Tanabe G, Kajitani I, Usui T, Shidare M, Yokoyama T. EXAFS Determination of Clay Minerals in Martian Meteorite Allan Hills 84001 and Its Implication for the Noachian Aqueous Environment. Minerals. 2021; 11(2):176. https://doi.org/10.3390/min11020176
Chicago/Turabian StyleNakada, Ryoichi, Gaku Tanabe, Iori Kajitani, Tomohiro Usui, Masashi Shidare, and Tetsuya Yokoyama. 2021. "EXAFS Determination of Clay Minerals in Martian Meteorite Allan Hills 84001 and Its Implication for the Noachian Aqueous Environment" Minerals 11, no. 2: 176. https://doi.org/10.3390/min11020176
APA StyleNakada, R., Tanabe, G., Kajitani, I., Usui, T., Shidare, M., & Yokoyama, T. (2021). EXAFS Determination of Clay Minerals in Martian Meteorite Allan Hills 84001 and Its Implication for the Noachian Aqueous Environment. Minerals, 11(2), 176. https://doi.org/10.3390/min11020176





