Cation Disorder Caused by Olivine-Ringwoodite Phase Transition Mechanism, Possible Explanation for Blue Olivine Inclusion in a Diamond
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synchrotron Radiation and the Diamond Anvil Cell (DAC)
2.2. Shear-Stress Experiments
2.3. X-ray Diffraction Experiments with Intense Synchrotron White X-ray Beam
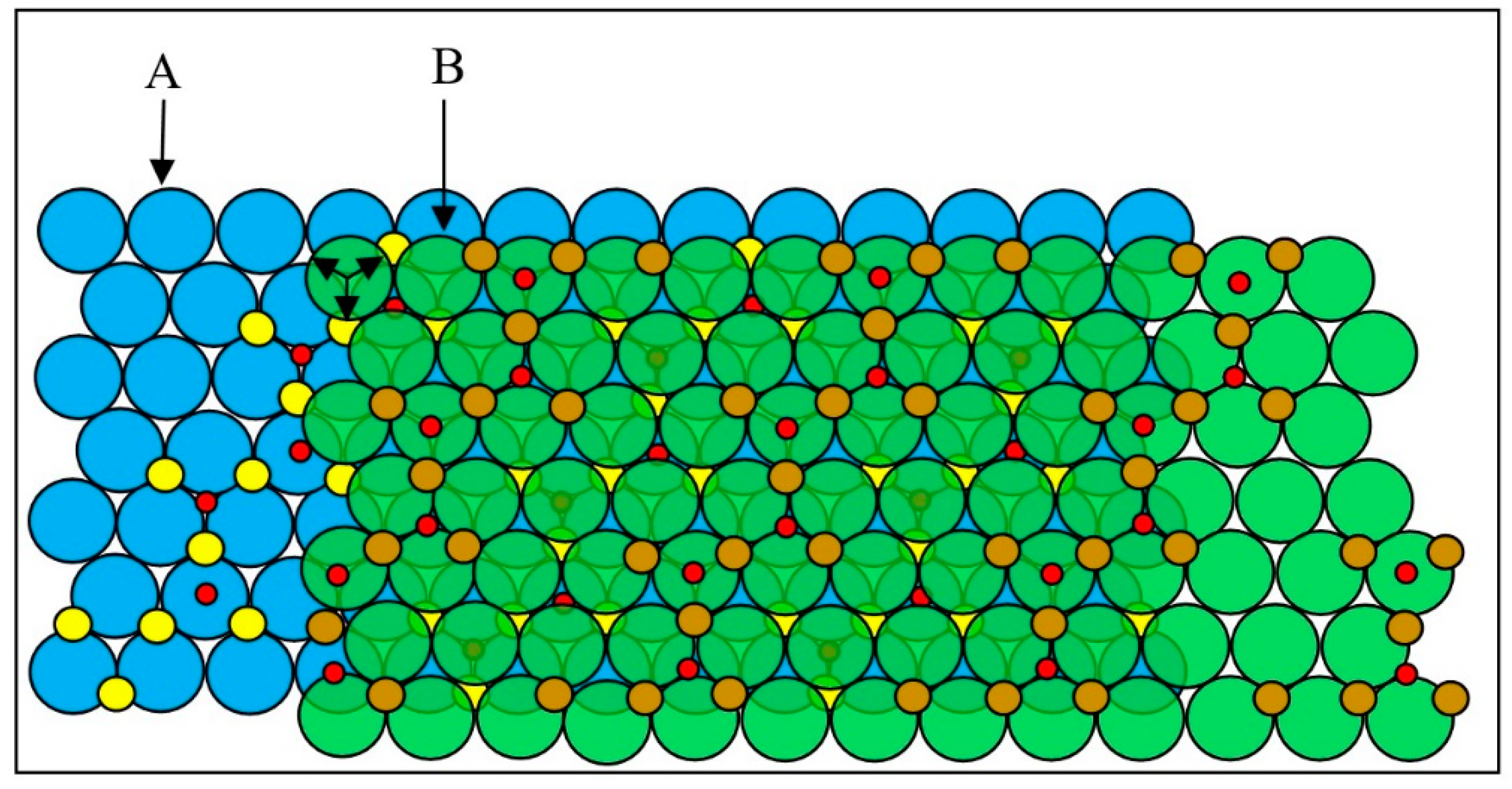
3. Results
3.1. X-ray Diffraction
3.2. Crystal Structures
3.2.1. The α and γ Structures Viewed Normal to the Oxygen Close-Packed Layers
3.2.2. View Parallel to Gliding Oxygen Layers
3.3. Visual Observations in the DAC
3.3.1. Fayalite
3.3.2. Other Samples with Olivine Structure
4. Wadsleyite, Ahrensite, and Asimowite
5. Sequence of Step 1 and Step 2 Transition
6. Implications of the Shear Induced Phase Transition
6.1. Blue Olivine Inclusion in Diamond
6.1.1. Proposed Pressure-Temperature History of the Blue Inclusion
6.1.2. Depth of Formation
6.1.3. Synthesized Ringwoodite
6.1.4. Ringwoodite in Meteorites
6.1.5. Blue Olivine in a Pallasitic Meteorite?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Poirier, J.P. Martensitic. Olivine-spinel transformation and plasticity of the mantle transition zone. In Anelastic Properties and Related Processes in the Earth’s Mantle; Stacey, F.D., Paterson, S.M., Nicolas, A., Eds.; American Geophysical Union Monograph, AGU: Washington, DC, USA, 1981; pp. 113–117. [Google Scholar]
- Furnish, M.D.; Bassett, W.A. Investigation of the mechanism of the olivine-spinel transition in fayalite by synchrotron radiation. J. Geophys. Res. 1983, 88, 10333–10341. [Google Scholar] [CrossRef]
- Bassett, W.A.; Shen, A.H.; Bucknum, M.; Chou, I.-M. A new diamond anvil cell for hydrothermal studies to 2.5 GPa and from –190 to 1200 °C. Rev. Sci. Instrum. 1993, 64, 2340–2345. [Google Scholar] [CrossRef] [Green Version]
- Bassett, W.A.; Ming, L.C. Disproportionation of Fe2SiO4 to FeO + SiO2 at pressures up to 250 kilobars and temperatures up to 3000 °C. Phys. Earth Planet. Int. 1972, 6, 154–160. [Google Scholar] [CrossRef]
- Burnley, P.C.; Green, H.W. Stress dependence of the mechanism of the olivine-spinel transformation. Nature 1989, 338, 753–755. [Google Scholar] [CrossRef]
- Burnley, P.C.; Green, H.W.; Prior, D.J. Faulting associated with the olivine to spinel transformation in Mg2GeO4 and its implication for deep-focus earthquakes. J. Geophys. Res. 1991, 96, 425–443. [Google Scholar] [CrossRef]
- Wu, T.-C.; Bassett, W.A.; Burnley, P.C.; Weathers, M.S. Shear-promoted phase transitions in Fe2SiO4 and Mg2SiO4 and the mechanism of deep earthquakes. J. Geophys. Res. 1993, 98, 19767–19776. [Google Scholar] [CrossRef]
- Burnley, P.C.; Bassett, W.A.; Wu, T.-C. Diamond anvil cell study of the transformation mechanism from olivine to spinel phase in Co2SiO4, Ni2SiO4, and Mg2SiO4, J. Geophys. Res. 1995, 100, 17715–17723. [Google Scholar] [CrossRef]
- Suito, K. Phase relations of pure Mg2SiO4 up to 200 kilobars. In High-Pressure Research: Application in Geophysics; Manghnani, M.H., Akimoto, S., Eds.; Academic Press: New York, NY, USA, 1977; pp. 255–266. [Google Scholar]
- Ito, E.; Okayama University, Misasa, Japan. Personal communication, 1991.
- Burnley, P.C. The effect of non-hydrostatic stress on the olivine-spinel transformation in Mg2GeO4. Ph.D. Thesis, University of California at Davis, Davis, CA, USA, 1990. [Google Scholar]
- Rubie, D.C.; Bayerisches Geoinstitut, University of Bayreuth, Bayreuth, Germany. Personal communication, 1994.
- Ringwood, A.E. Olivine-spinel transformation in cobalt orthosilicate. Nature 1963, 198, 79–80. [Google Scholar] [CrossRef]
- Akimoto, S. High-pressure synthesis of a “modified” spinel and some geophysical implications Phys. Earth Planet Int. 1970, 3, 189–195. [Google Scholar] [CrossRef]
- Ringwood, A.E. The constitution of the mantle 1. Thermodynamics of the olivine-spinel transition. Geochim. Cosmochim. Acta 1958, 13, 303–321. [Google Scholar]
- Yagi, T.M.; University of Tokyo, Tokyo, Japan. Personal communication, 1991.
- Tomioka, N.; Okuchi, T. A new high-pressure form of Mg2SiO4 highlighting diffusionless phase transitions of olivine. Sci. Rep. 2017, 7, 17351. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Tchauner, O.; Beckett, J.R.; Liu, Y.; Rossman, G.R.; Sinogeikin, S.V.; Smith, J.S.; Taylor, L.A. Ahrensite, γ-Fe2SiO4, a new shock-metamorphic mineral from the Tissint meteorite: Implications for the Tissint shock event on Mars. Geochimica et Cosmochimica Acta 2016, 184, 240–256. [Google Scholar] [CrossRef] [Green Version]
- Bindi, L.; Brenker, F.E.; Nestola, F.; Koch, T.E.; Prior, D.J.; Lilly, K.; Krot, A.N.; Bizzarro, M.; Xie, X. Discovery of asimowite, the Fe-analog of wadsleyite, in shock-melted silicate droplets of the Suizhou L6 and the Quebrada Chimborazo 001 CB3.0 chondrites. Am. Mineral. 2019, 4, 775–778. [Google Scholar] [CrossRef]
- Poirier, J.P. Introduction to the Physics of the Earth’s Interior, 2nd ed.; Cambridge University Press (Virtual Publishing): New York, NY, USA, 2000; pp. 251–255. [Google Scholar]
- Kohlstedt, D.; Keppler, H.; Rubie, D. Solubility of water in the α, β and γ phases of (Mg,Fe)2SiO4. Contrib. Miner. Petrol. 1996, 123, 345–357. [Google Scholar] [CrossRef]
- Smyth, J.R.; Holl, C.M.; Frost, D.J.; Jacobsen, S.D.; Langenhorst, F.; McCammon, C.A. Structural systematics of hydrous ringwoodite and water in the Earth’s interior. Am. Mineral. 2003, 88, 1402–1407. [Google Scholar] [CrossRef]
- Jacobsen, S.; van Lee, S. (Eds.) Earth’s Deep Water Cycle; American Geophysical Union: Washington, DC, USA, 2006; 313p, ISBN 978-875-90433-7. [Google Scholar]
- Pearson, D.G.; Brenker, F.E.; Nestola, F.; McNeill, J.; Nasdala, L.; Hutchison, M.T.; Matveev, S.; Mather, K.; Silversmit, G.; Schmitz, S.; et al. Hydrous mantle transition zone indicated by ringwoodite included within diamond. Nature 2014, 507, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sui, Z.; Fei, H.; Yan, W.; Ma, W.Y.; Ye, Y. Cation Disorder IR Features of Hydrous Mg2SiO4-Ringwoodite, Unannealed and Annealed at 200–600 °C and 1 atm, with Implications to Hydrogen Defects and Water-Coupled Cation Disorder. Minerals 2020, 10, 499. [Google Scholar] [CrossRef]
- Koivula, J.I. The MicroWorld of Diamonds; GemWorld International, Inc.: Northbrook, IL, USA, 2000; p. 53. [Google Scholar]
- Jacobsen, S.D.; Bassett, W.A.; Skalwold, E.A.; Koivula, J.I. Message in a bottle: Secrets from the deep Earth in a diamond inclusion. In Proceedings of the 2012 Packard Fellows Meeting, David and Lucile Packard Foundation, Monterey, CA, USA, 5–8 September 2012. funding provided by the Fellowship for Science and Engineering by the David and Lucile Packard Foundation. [Google Scholar]
- Jacobsen, S.D.; Northwestern University, Evanston, IL, USA. Personal communication, 2020.
- Coleman, L.C. Ringwoodite and majorite in the Catherwood meteorite. Can. Mineral. 1977, 15, 97–101. [Google Scholar]
- Binns, R.A.; Davis, R.J.; Reed, S.J.B. Ringwoodite, Natural Mg2SiO4 spinel in the Tenham meteorite. Nature 1969, 221, 943–944. [Google Scholar] [CrossRef]
- Putnis, A.; Price, G.D. High-pressure Mg2SiO4 phases in the Tenham chondrite meteorite. Nature 1979, 280, 217–218. [Google Scholar] [CrossRef]
















| Sample | From Literature * “Hydrostatic”, °C | In DAC Experiments “Nonhydrostatic”, °C | Difference |
|---|---|---|---|
| Mg2SiO4 | 700 a,b | 575 c | >125 |
| Mg2GeO4 | 860 d | 600 e | >260 |
| Ni2SiO4 | 650 f | 525 e | >125 |
| Co2SiO4 | 800 g,h | 525 e | >275 |
| Fe2SiO4 | 600 i,j | 380 c | >220 |
| Analytical Techniques and Rationale |
|---|
|
|
|
|
|
|
|
|
|
| Element | Moles per Four Oxygen Atoms |
|---|---|
| Ca | 0.00 |
| Mn | 0.00 |
| Fe | 0.17 |
| Co | 0.00 |
| Ni | 0.01 |
| Si | 1.00 |
| Mg | 1.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassett, W.A.; Skalwold, E.A. Cation Disorder Caused by Olivine-Ringwoodite Phase Transition Mechanism, Possible Explanation for Blue Olivine Inclusion in a Diamond. Minerals 2021, 11, 202. https://doi.org/10.3390/min11020202
Bassett WA, Skalwold EA. Cation Disorder Caused by Olivine-Ringwoodite Phase Transition Mechanism, Possible Explanation for Blue Olivine Inclusion in a Diamond. Minerals. 2021; 11(2):202. https://doi.org/10.3390/min11020202
Chicago/Turabian StyleBassett, William A., and Elise A. Skalwold. 2021. "Cation Disorder Caused by Olivine-Ringwoodite Phase Transition Mechanism, Possible Explanation for Blue Olivine Inclusion in a Diamond" Minerals 11, no. 2: 202. https://doi.org/10.3390/min11020202
APA StyleBassett, W. A., & Skalwold, E. A. (2021). Cation Disorder Caused by Olivine-Ringwoodite Phase Transition Mechanism, Possible Explanation for Blue Olivine Inclusion in a Diamond. Minerals, 11(2), 202. https://doi.org/10.3390/min11020202





