EMPA, XRD, and Raman Characterization of Ag-Bearing Djurleite from the Lubin Mine, Lower Silesia, Poland
Abstract
:1. Introduction
2. Geological Setting
Mineralogy and Material
3. Analytical Techniques
3.1. Microscopy
3.2. Electron Probe Micro-Analyses (EPMA)
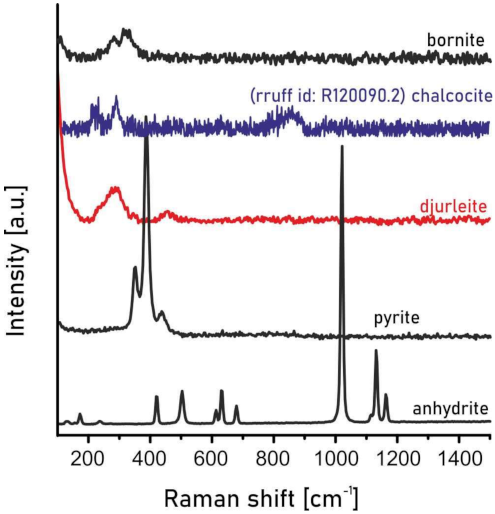
3.3. Raman Spectroscopy
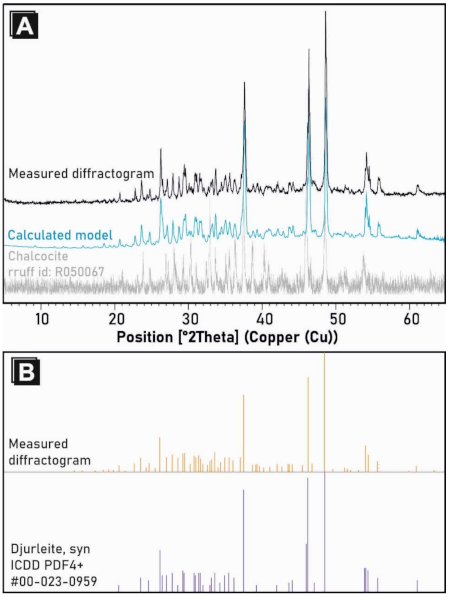
3.4. X-ray Diffraction (XRD)
4. Results and Discussion
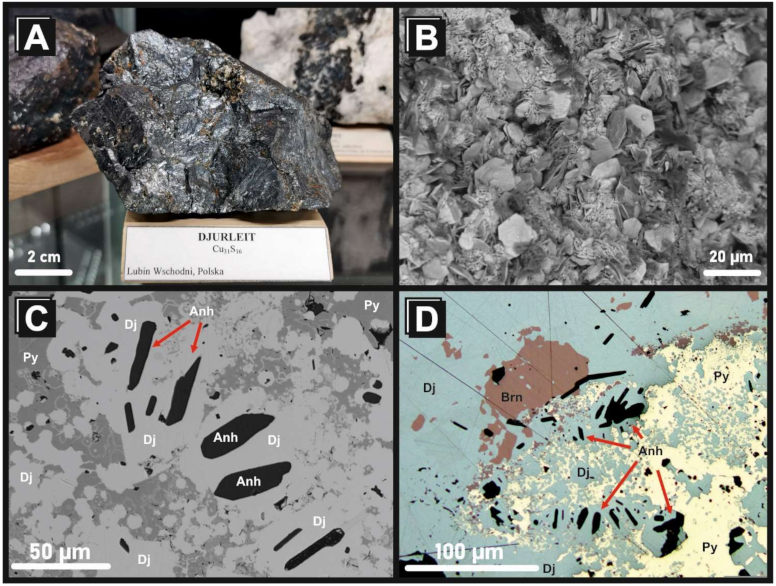
4.1. Mineralogy of Ag-Rich Djurleite from the Kupferschiefer Deposit, Lower Silesia, Poland
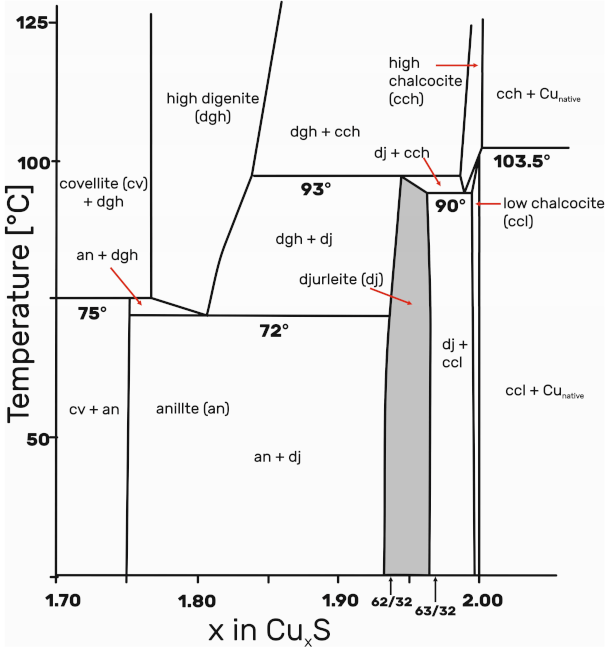
4.2. Formation Conditions of Ag-Djurleite
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morimoto, N. Djurleite, a new copper sulfide mineral. Mineral. J. 1962, 3, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Oszczepalski, S.; Speczik, S.; Małecka, K.; Chmielewski, A. Prospective copper resources in Poland. Gospod. Surowcami Miner. 2016, 32, 5–30. [Google Scholar] [CrossRef] [Green Version]
- Banaś, M.; Salamon, W.; Piestrzyński, A.; Mayer, W. Replacement phenomena of terrigenous minerals by sulphides in coppe-bearing Permian sandstones in Poland. In Ore Genesis—The State of the Art; Amstutz, G.C., Ed.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 3–9. [Google Scholar]
- Harańczyk, C.; Jarosz, J. Ore minerals from copper deposit in Fore-Sudetic Monocline. Rudy I Met. Niżel 1973, 18, 493–502. (In Polish) [Google Scholar]
- Kucha, H. Mineralogy and geochemistry of the Lubin-Sieroszowice orebody. Biuletyn Państwowego Inst. Geol. 2007, 423, 77–94, (In Polish with English Summary). [Google Scholar]
- Oszczypalski, S.; Chmielewski, A.; Speczik, S. Variability of ore mineralization in the north-west-trending extension of the Lubin–Sieroszowice deposit. Biuletyn Państwowego Inst. Geol. 2017, 468, 109–142, (In Polish with English Summary). [Google Scholar] [CrossRef]
- Mikulski, S.Z.; Oszczepalski, S.; Sadłowski, K.; Chmielewski, A.; Małek, R. Trace Element Distributions in the Zn-Pb (Mississippi Valley-Type) and Cu-Ag (Kupferschiefer) Sediment-Hosted Deposits in Poland. Minerals 2020, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Borg, G.; Piestrzyński, A.; Bachman, G.H.; Puttmann, W.; Walther, S.; Fidler, M. An overview of the European Kupferschiefer deposits. In Geology and Genesis of Major Copper Deposits and Districts of the World: A Tribute to Richard, H. Sillitoe, 1st ed.; Hedenquist, J.W., Camus, F., Eds.; Society of Econmic Geologists, Special Publication: Littleton, CO, USA, 2012; Volume 16, pp. 455–486. [Google Scholar]
- Jowett, E.C. Genesis of Kupferschiefer Cu-Ag deposits by convective flow of Rotliegendes brines during Triassic rifting. Econ. Geol. 1986, 81, 1823–1837. [Google Scholar] [CrossRef]
- Oszczepalski, S. Origin of the Kupferschiefer polymetallic mineralization in Poland. Miner. Depos. 1999, 34, 599–613. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Sweeney, M.; Diedel, G.F.R.; Haranczyk, C. The Kupferschiefer: An overview with an appraisal of the different types of mineralization. Econ. Geol. 1989, 84, 1003–1027. [Google Scholar] [CrossRef]
- Wierzchowska-Kicułowa, H. Charakterystyka geologiczna podłoża permu obszaru przedsudeckiego. Geol. Q. 1987, 31, 515–516. (In Polish) [Google Scholar]
- Juroszek, C.; Kłapciński, J.; Sachańbiński, M. Wulkanity dolnego permu południowej części Monokliny Przedsudeckiej i Perykliny Żar. Ann. Soc. Geol. Pol. 1981, 51, 517–546. (In Polish) [Google Scholar]
- Nemec, W.; Porębski, S.J. Sedimentary environment of the Weissliegendes sandstone in Fore-Sudetic monocline. In Proceedings of the SCEP-Proceedings, International Symposium on Central European Permian, Jabłonna, Poland, 27–29 April 1979; Pakulska, Z., Ed.; Geological Institute: Warsaw, Poland, 1981; pp. 273–293. [Google Scholar]
- Oszczepalski, S.; Rydzewski, A. Paleogeography and sedimentary model of the Kupferschiefer in Poland. Lect. Not. Earth Sci. 1987, 10, 189–205. [Google Scholar]
- Oszczepalski, S.; Speczik, S.; Zieliński, K.; Chmielewski, A. The Kupferschiefer Deposits and Prospects in SW Poland: Past, Present and Future. Minerals 2019, 9, 592. [Google Scholar] [CrossRef] [Green Version]
- Peryt, T.M.; Oszczepalski, S. Stratygrafia serii złożowej. In Monografia KGHM Polska Miedź S.A.; Piestrzyński, A., Ed.; KGHM Cuprum Sp. z o.o.: Lubin, Poland, 2007; pp. 108–111. (In Polish) [Google Scholar]
- Pieczonka, J.; Piestrzyński, A.; Sawlowicz, Z. The sediment hosted copper silver deposits in the Lubin-Głogów mining district (Poland). In Proceedings of the Digging Deeper, Joint 9th Biennial SGA-SEG Meeting, Dublin, Ireland, 20–23 August 2007; Sass-Gustkiewicz, M., Sawlowicz, Z., Eds.; Rafael: Kraków, Poland, 2007; pp. 7–23. [Google Scholar]
- Oszczepalski, S. Kupferschiefer in southwestern Poland. Sedimentary environments, metal zoning, and ore controls. In Sediment-Hosted Stratiform Copper Deposits; Boyle, R.W., Brown, A.C., Jowett, E.C., Kirkham, R.V., Eds.; Geological Association of Canada Special Paper; Geological Association of Canada: Waterloo, ON, Canada, 1989; pp. 571–600. [Google Scholar]
- Piestrzyński, A.; Sawlowicz, Z. Exploration for Au and PGE in the Polish Zechstein copper deposits (Kupferschiefer). J. Geochem. Explor. 1999, 66, 17–25. [Google Scholar] [CrossRef]
- Pieczonka, J.; Piestrzyński, A. Minerały kruszcowe złoża rud miedzi na monoklinie przedsudeckiej i ich znaczenie dla genezy. Gosp. Sur. Miner. 2006, 22, 187–202, (In Polish with English Abstract). [Google Scholar]
- Piestrzyński, A. Okruszcowanie. In Monografia KGHM Polska Miedź S.A.; Piestrzyński, A., Ed.; KGHM Cuprum Sp. z o.o.: Lubin, Poland, 2007; pp. 232–282. (In Polish) [Google Scholar]
- Pieczonka, J. Factors Controlling Distribution of Ore Minerals within Copper Deposit, Fore-Sudetic Monocline, SW Poland; Wyd. AGH: Kraków, Poland, 2011; pp. 1–195. (In Polish) [Google Scholar]
- Kozub-Budzyń, G.A.; Piestrzyński, A. The first occurrence of cupropearceite in the Kupferschiefer deposit, Lubin mine, SW Poland. Geol. Q. 2018, 62, 319–326. [Google Scholar] [CrossRef]
- Kucha, H. Geochemistry of the Kupferschiefer, Poland. Geol. Rundschau 1990, 79, 387–399. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. “PAP”, Procedure for Improved Quantitative Microanalysis. Microbeam Analysis Proc; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Evans, H.T. The crystal structures of low chalocite and djurleite. Z. Krist. Cryst. Mater. 1979, 150, 299–320. [Google Scholar] [CrossRef]
- De Caro, T.; Caschera, D.; Ingo, G.M.; Calandra, P. Micro-Raman innovative methodology to identify Ag–Cu mixed sulphides as tarnishing corrosion products. J. Raman Spectrosc. 2016, 47, 852–859. [Google Scholar] [CrossRef]
- Hurma, T.; Kose, S. XRD Raman analysis and optical properties of CuS nanostructured film. Optik 2016, 127, 6000–6006. [Google Scholar] [CrossRef]
- Santos, C.J.; Mayen, H.S.A.; Coronel, H.J.J.; Mejia, R.R.; Castanedo, P.R.; Torres, D.G.; Jimenez, S.S. Characterization of CuxS thin films obtained by CBD technique at differents annealing temperatures. Chalcogenide Lett. 2012, 9, 85–91. [Google Scholar]
- Potter, R.W. An electrochemical investigation of the system copper-sulfur. Econ. Geol. 1977, 72, 1524–1542. [Google Scholar] [CrossRef]
- Buzgar, N.; Buzatu, A.; Sanislav, I.V. The Raman study on certain sulfates. Al. I. Cuza 2009, 55, 5–23. [Google Scholar]
- Liu, Y.; Wang, A.; Freeman, J.J. Raman, MIR, and NIR spectroscopic study of calcium sulfates: Gypsum, bassanite, and anhydrite. In Mars Analogs Sulfates and Sulfides Posters, Proceedings of the 40th Lunar and Planetary Science Conference, the Woodlands, Texas, TX, USA, 23–27 March 2009; Lunar and Planetary Institute: Houston, Texas, TX, USA, 2009; p. 2128. [Google Scholar]
- White, S.N. Laser Raman spectroscopy as a technique for indentification of seafloor hydrothermal and cold seep minerals. Chem. Geol. 2008, 259, 240–252. [Google Scholar] [CrossRef]
- Kostudis, S.; Kutschke, H.M.; Pollmann, K. Micro-Raman spectroscopic imaging of copper ores. In Proceedings of the Conference on Raman and Luminescence Spectroscopy in the Earth Sciences (CORALS-2013), Vienna, Austria, 3–6 July 2013; pp. 61–62. [Google Scholar]
- Yuan, X.; Zheng, H. In situ Raman spectroscopic studies of FeS2 pyrite up to 675 K and 2100 MPa using a hydrothermal diamond anvil cell. Mineral. Mag. 2015, 79, 1–10. [Google Scholar] [CrossRef]
- Kleppe, A.; Jephcoat, A. High-pressure Raman spectroscopic studies of FeS2 pyrite. Mineral. Mag. 2004, 68, 433–441. [Google Scholar] [CrossRef]
- Kotarba, M.J.; Oszczepalski, S.; Sawłowicz, Z.; Speczik, S.; Więcław, D. Materia organiczna i jej rola w procesach złożotwórczych. In Monografia KGHM Polska Miedź S.A.; Piestrzyński, A., Ed.; KGHM Cuprum Sp. z o.o.: Lubin, Poland, 2007; pp. 138–144. (In Polish) [Google Scholar]
- Speczik, S.; Oszczepalski, S.; Nowak, G.; Karwasiecka, M. Kupferschiefer—A hunt for new reserves. Biul. Państwowego Inst. Geol. 2007, 42, 173–188, (In Polish with English Summary). [Google Scholar]
- Oszczepalski, S.; Nowak, G.J.; Bechtel, A.; Žák, K. Evidence of oxidation of the Kupferschiefer in the Lubin-Sieroszowice deposit: Implications for Cu-Ag and Au-Pt-Pd mineralization. Geol. Q. 2002, 46, 1–23. [Google Scholar]
- Kosakowski, P.; Markiewicz, A.; Kotarba, M.J.; Oszczepalski, S.; Więcław, D. The timing of ore mineraliztion based on thermal maturity modelling of Kupferschiefer strata and its relation to tectonics of southern part of Fore-Sudetic Monocline (SW Poland). Biul. Państwowego Inst. Geol. 2007, 423, 139–150, (In Polish with English Summary). [Google Scholar]
- Roseboom, E.H. An investigation of the system Cu-S and some natural copper sulfides between 25 degrees and 700 degrees C. Econ. Geol. 1966, 61, 641–672. [Google Scholar] [CrossRef]
- Mathieu, H.J.; Rickert, H. Elektrokemisch-thermodynamische Untersuchungen am System Kupfer Schwefel bei Temperaturen T = 15–90 °C. Z. Phys. Chem. 1972, 79, 315–330. [Google Scholar] [CrossRef]
- Pósfai, M.; Buseck, P. Djurleite, digenite, and chalcocite: Intergrowths and transformations. Am. Mineral. 1994, 79, 308–315. [Google Scholar]
- Zhou, X.; Soldat, A.C.; Lind, C. Phase selective synthesis of copper sulfides by nonhydrolytic sol–gel methods. RSC Adv. 2014, 4, 717–721. [Google Scholar] [CrossRef]
- Large, D.; MacQuaker, J.; Vaughan, D.; Sawłowicz, Z.; Gize, A.P. Evidence for Low-Temperature Alteration of Sulfides in the Kupfersehiefer Copper Deposits of Southwestern Poland. Econ. Geol. 1995, 90, 2143–2155. [Google Scholar] [CrossRef]

| d-Spacing (A) | Relative X-ray Intensity (%) | d-Spacing (A) | Relative X-ray Intensity (%) |
|---|---|---|---|
| 6.0497 | 1.5 | 2.5197 | 12.0 |
| 5.6502 | 1.2 | 2.4820 | 9.7 |
| 5.0709 | 1.2 | 2.4196 | 12.3 |
| 4.7772 | 1.8 | 2.3919 | 64.1 |
| 4.5970 | 2.1 | 2.3202 | 6.3 |
| 4.4589 | 2.0 | 2.2923 | 6.2 |
| 4.2910 | 5.3 | 2.2853 | 6.7 |
| 4.1279 | 1.2 | 2.2674 | 4.8 |
| 3.9051 | 7.3 | 2.2348 | 3.7 |
| 3.7649 | 11.8 | 2.1934 | 4.5 |
| 3.6496 | 4.0 | 2.1443 | 6.7 |
| 3.5910 | 7.4 | 2.1111 | 4.4 |
| 3.4780 | 3.3 | 2.0721 | 6.6 |
| 3.3960 | 29.0 | 2.0659 | 6.4 |
| 3.2854 | 12.3 | 2.0481 | 6.4 |
| 3.1967 | 14.6 | 1.9896 | 5.7 |
| 3.1080 | 11.8 | 1.9590 | 78.6 |
| 3.0407 | 15.4 | 1.9372 | 7.1 |
| 3.0154 | 15.8 | 1.8730 | 100.0 |
| 2.9448 | 6.7 | 1.8324 | 2.8 |
| 2.8953 | 13.6 | 1.7809 | 3.7 |
| 2.8713 | 12.4 | 1.7681 | 2.7 |
| 2.8380 | 13.7 | 1.7541 | 1.8 |
| 2.8165 | 11.3 | 1.7214 | 2.0 |
| 2.7878 | 6.4 | 1.6930 | 21.8 |
| 2.7378 | 6.0 | 1.6829 | 14.4 |
| 2.7103 | 9.2 | 1.6470 | 9.1 |
| 2.6925 | 10.5 | 1.6460 | 8.1 |
| 2.6581 | 16.5 | 1.5779 | 0.2 |
| 2.6236 | 3.9 | 1.5398 | 0.9 |
| 2.6012 | 8.3 | 1.5166 | 5.3 |
| 2.5596 | 11.9 | 1.4654 | 1.8 |
| Compound | an.1 | an.2 | an.3 | an.4 | an.5 | an.6 | an.7 | an.8 | an.9 | an.10 | an.11 | an.12 | an.13 | an.14 | an.15 | an.16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag (wt.%) | 0.49 | 0.42 | 0.44 | 0.47 | 0.42 | 0.41 | 0.36 | 0.48 | 0.41 | 0.37 | 0.45 | 0.55 | 0.42 | 0.38 | 0.47 | 0.45 |
| Fe | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.23 | 0.19 | 0.31 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.19 |
| Cu | 79.78 | 79.90 | 80.51 | 79.80 | 76.61 | 79.47 | 79.90 | 79.57 | 79.83 | 80.28 | 78.83 | 79.15 | 79.48 | 79.74 | 80.21 | 78.40 |
| S | 20.93 | 20.86 | 21.00 | 20.84 | 20.01 | 20.80 | 20.82 | 20.85 | 20.93 | 21.04 | 20.99 | 20.71 | 20.77 | 20.81 | 20.96 | 20.55 |
| Total | 101.21 | 101.18 | 101.95 | 101.11 | 97.04 | 100.69 | 101.08 | 101.12 | 101.35 | 101.99 | 100.26 | 100.40 | 100.67 | 100.93 | 101.64 | 99.60 |
| Ag (apfu) | 0.11 | 0.10 | 0.10 | 0.11 | 0.10 | 0.09 | 0.08 | 0.11 | 0.09 | 0.08 | 0.10 | 0.13 | 0.10 | 0.09 | 0.11 | 0.10 |
| Fe | - | - | - | - | - | - | - | 0.10 | 0.08 | 0.13 | - | - | - | - | - | 0.09 |
| Cu | 30.78 | 30.92 | 30.96 | 30.91 | 30.91 | 30.85 | 30.98 | 30.82 | 30.80 | 30.81 | 30.33 | 30.86 | 30.89 | 30.94 | 30.90 | 30.80 |
| S | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szopa, K.; Krzykawski, T.; Banasik, K.; Król, P.; Skreczko, S.; Mounteanou, S.A.; Koziarska, M. EMPA, XRD, and Raman Characterization of Ag-Bearing Djurleite from the Lubin Mine, Lower Silesia, Poland. Minerals 2021, 11, 454. https://doi.org/10.3390/min11050454
Szopa K, Krzykawski T, Banasik K, Król P, Skreczko S, Mounteanou SA, Koziarska M. EMPA, XRD, and Raman Characterization of Ag-Bearing Djurleite from the Lubin Mine, Lower Silesia, Poland. Minerals. 2021; 11(5):454. https://doi.org/10.3390/min11050454
Chicago/Turabian StyleSzopa, Krzysztof, Tomasz Krzykawski, Kamila Banasik, Piotr Król, Sylwia Skreczko, Stefania Andriopoulou Mounteanou, and Marta Koziarska. 2021. "EMPA, XRD, and Raman Characterization of Ag-Bearing Djurleite from the Lubin Mine, Lower Silesia, Poland" Minerals 11, no. 5: 454. https://doi.org/10.3390/min11050454






