Noble Metal Speciations in Hydrothermal Sulphides
Abstract
:1. Introduction
2. Geological Framework
2.1. Gold Deposits
2.2. VMS Deposits


3. Materials and Methods
4. Noble Metal Distribution and Speciations
4.1. Gold Deposits
4.1.1. The Vorontsovka Gold Deposit
4.1.2. The Berezovsk Gold Deposit




4.1.3. The Svetlinsk Gold-Telluride Deposit
4.1.4. The Petropavlovsk Gold-Porphyry Deposit



4.1.5. The Novogodnee-Monto Iron-Gold-Skarn Deposit
4.2. The VMS Deposits of the Urals
4.2.1. The Gai Deposit
4.2.2. The Uzelga Deposit
4.2.3. The Uchaly Deposit
4.2.4. The Galka Deposit
4.3. Experimental Results
4.3.1. Geochemistry of Au in Sulphides
4.3.2. Noble Metal Speciations in Synthetic Sphalerite and Greenockite
5. Discussion
5.1. Conditions Conducive to the Formation of Au-Saturated Sphalerite
5.2. Correlation of As and Au Contents in Pyrite
5.3. Forms of Gold in Arsenopyrite
5.4. Crystal-Chemical Basics of Noble Metal Speciation in Sulphides
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Element | X-ray | Crystal | Standard | Time | Detection Limit, 3 σ(wt%) | |
|---|---|---|---|---|---|---|
| Peak | Back | |||||
| Py, Apy | ||||||
| As | Lα | TAP | GaAs | 30 | 15 | 0.05 |
| Fe | Kα | LIF | Pyrite | 10 | 5 | 0.06 |
| S | Kα | PETH | Pyrite | 10 | 5 | 0.02 |
| Ni | Kα | LIF | NiSbS | 10 | 5 | 0.04 |
| Cu | Kα | LIF | CuFeS2 | 10 | 5 | 0.07 |
| Sb | Lα | PETH | NiSbS | 10 | 5 | 0.05 |
| Co | Kα | LIF | Co | 30 | 15 | 0.06 |
| CCp | ||||||
| As | Lα | TAP | GaAs | 30 | 15 | 0.05 |
| Cu | Kα | LIF | CuFeS2 | 10 | 5 | 0.06 |
| S | Kα | PETH | CuFeS2 | 10 | 5 | 0.02 |
| Zn | Kα | LIF | ZnS | 10 | 5 | 0.08 |
| Fe | Kα | LIF | CuFeS2 | 10 | 5 | 0.06 |
| Cd | Lα | PETH | CdS | 10 | 5 | 0.05 |
| Ni | Kα | LIF | Ni | 10 | 5 | 0.06 |
| Mn | Kα | LIF | Mn | 10 | 5 | 0.06 |
| Co | Kα | LIF | Co | 30 | 15 | 0.06 |
| Sp | ||||||
| Cd | Lα | PETJ | CdS | 30 | 15 | 0.11 |
| Fe | Kα | LIF | Pyrite | 20 | 10 | 0.05 |
| S | Kα | PETH | ZnS | 10 | 5 | 0.02 |
| Zn | Kα | LIF | ZnS | 10 | 5 | 0.1 |
| In | Lα | PETJ | InSb | 10 | 5 | 0.08 |
| Mn | Kα | LIF | Mn | 20 | 10 | 0.04 |
| Ag | Lα | PETH | Ag | 10 | 5 | 0.04 |
| Cu | Kα | LIF | CuFeS2 | 20 | 10 | 0.06 |
| Hg | Mα | PETH | HgS | 20 | 10 | 0.06 |
| Sn | Lα | PETH | Sn | 20 | 10 | 0.04 |
| Tnt | ||||||
| Sb | Lα | PETJ | Sb2S3 | 10 | 5 | 0.10 |
| As | Lα | TAP | GaAs | 10 | 5 | 0.10 |
| Zn | Kα | LIF | ZnS | 10 | 5 | 0.09 |
| S | Kα | PETH | CuFeS2 | 10 | 5 | 0.02 |
| Ag | Lα | PETJ | Ag | 10 | 5 | 0.16 |
| Se | Lα | TAP | CdSe | 10 | 5 | 0.07 |
| Cu | Kα | LIF | Cu | 10 | 5 | 0.08 |
| Hg | Mα | PETH | HgS | 10 | 5 | 0.09 |
| Te | Lα | PETJ | Te | 10 | 5 | 0.11 |
| Fe | Kα | LIF | CuFeS2 | 10 | 5 | 0.06 |
| Bi | Mα | PETH | Bi2Te3 | 10 | 5 | 0.09 |
| Cd | Lβ | PETJ | CdSe | 10 | 5 | 0.20 |
| Pb | Mα | PETH | PbS | 10 | 5 | 0.09 |
| Gn | ||||||
| Pb | Mα | PETJ | PbS | 20 | 10 | 0.22 |
| Se | Lα | TAP | CdSe | 10 | 5 | 0.09 |
| Cu | Kα | LIF | CuFeS2 | 20 | 10 | 0.09 |
| S | Kα | PETH | PbS | 10 | 5 | 0.02 |
| Sb | Lα | PETJ | Sb2S3 | 20 | 10 | 0.09 |
| As | Lα | TAP | GaAs | 20 | 10 | 0.09 |
| Fe | Kα | LIF | CuFeS2 | 20 | 10 | 0.06 |
| Ag | Lα | PETH | Ag | 10 | 5 | 0.06 |
| Bi | Mβ | PETH | Bi | 30 | 15 | 0.10 |
References
- Lincoln, F.C. Certain natural associations of gold. Econ. Geol. 1911, 6, 247–302. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.M. Host minerals of native gold. Econ. Geol. 1944, 39, 371–411. [Google Scholar] [CrossRef]
- Burg, G.H. Sichtbarmachumg des Feinverteilten Goldes in Goldhoffigen Erzen und Ihre Wirtschaftliche Bedeutung. Metall Erz. 1930, 27, 333–338. [Google Scholar]
- Bortnikov, N.S.; Genkin, A.D.; Chryssoulis, S. Deposition environment of gold–bearing arsenopyrite in mesothermal deposits. In Current Research in Geology Applied to ore Deposits; SGA: Granada, Spain, 1993; pp. 419–422. [Google Scholar]
- McClenaghan, S.H.; Lentz, D.R.; Cabri, L.J. Abundance and speciation of gold in massive sulphides of the Bathurst Mining Camp, New Brunswick, Canada. Can. Mineral. 2004, 42, 851–871. [Google Scholar] [CrossRef]
- Large, R.R.; Maslennikov, V.V.; Robert, F.; Danyushevsky, L.V.; Chang, Z. Multistage sedimentary and metamorphic origin of pyrite and gold in the giant Sukhoi Log deposit, Lena gold province, Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.V.; Hollit, C.; Maslennikov, V.V.; Meffre, S.; E Gilbert, S.; Bull, S.W.; Scott, R.J.; Emsbo, P.; Thomas, H.; et al. Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and Carlin-style sediment-hosted deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Mao, J. Textural control on gold distribution in As-free pyrite from the Dongping, Huangtuliang and Hougou gold deposits, North China Craton (Hebei Province, China). Chem. Geol. 2009, 264, 101–121. [Google Scholar] [CrossRef]
- Vikent’ev, I.V.; Moloshag, V.P.; Yudovskaya, M.A. Speciation of noble metals and conditions of their concentration in massive sulphide ores of the Urals. Geol. Ore Dep. 2006, 48, 77–107. [Google Scholar] [CrossRef]
- Kreiter, V.M. Gold particle sizes in sulphide deposits as a sign of postore metamorphosis. Russ. Izv. Akad. Nauk SSSR Ser. Geol. 1948, 1, 159–162. [Google Scholar]
- Bürg, G.H. Natur des in den Pyriten nicht sichtbar enthaltenen Goldes. Zeitschr. Prakt. Geol. 1935, 43, 17–26. [Google Scholar]
- Genkin, A.D.; Bortnikov, N.S.; Cabri, L.J.; Wagner, F.E.; Stanley, C.J.; Safonov, Y.G.; McMahon, G.; Friedl, J.; Kerzin, A.L.; Gamyanin, G.N. A multidisciplinary study of invisible gold in arsenopyrite from four mesothermal gold deposits in Siberia, Russian Federation. Econ. Geol. 1998, 93, 463–487. [Google Scholar] [CrossRef]
- Tauson, V.L.; Pastushkova, T.M.; Bessarabova, O.I. On the limit and speciation of gold in hydrothermal pyrite. Russ. Geol. Geophys. 1998, 39, 924–933. [Google Scholar]
- Tauson, V.L.; Kravtsova, R.G.; Smagunov, N.V. Structural and surface–bond gold in pyrite from deposits of various genetic types. Russ. Geol. Geophys. 2014, 55, 350–369. [Google Scholar] [CrossRef]
- Vikentyev, I.V. Invisible and microscopic gold in pyrite: Methods and new data for massive sulfide ores of the Urals. Geol. Ore Depos. 2015, 57, 237–265. [Google Scholar] [CrossRef]
- Tagirov, B.R.; Dikov, Y.P.; Buleev, M.I.; Koval’chuk, E.V.; Kokh, M.A.; Borisovskii, S.E.; Abramova, V.D.; Garas’ko, M.I.; Kovalenker, V.A.; Bortnikov, N.S.; et al. “Invisible” gold in covellite (CuS): Synthesis and studies by EPMA, LA–ICP–MS, and XPS techniques. Dokl. Earth Sci. 2014, 459, 1381–1386. [Google Scholar] [CrossRef]
- Tagirov, B.R.; Trigub, A.L.; Kvashnina, K.O.; Shiryaev, A.A.; Chareev, D.A.; Nickolsky, M.S.; Abramova, V.D.; Kovalchuk, E.V. Covellite CuS as a matrix for “invisible” gold: X-ray spectroscopic study of the chemical state of Cu and Au in synthetic minerals. Geochim. Cosmochim. Acta 2016, 191, 58–69. [Google Scholar] [CrossRef]
- Kovalchuk, E.V.; Tagirov, B.R.; Vikentyev, I.V.; Chareev, D.A.; Tyukova, E.E.; Nickolsky, M.S.; Borisovsky, S.E.; Bortnikov, N.S. «Invisible» gold in synthetic and natural crystals of arsenopyrite (Vorontsovka deposit, North Ural). Geol. Ore Dep. 2019, 61, 62–83. [Google Scholar] [CrossRef]
- Trigub, A.L.; Tagirov, B.R.; Chareev, D.A.; Nickolsky, M.S.; Shiryaev, A.A.; Kovalchuk, E.V.; Mokhov, A.V.; Kvashnina, K.O.; Baranova, N.N. X–Ray spectroscopy study of the chemical state of “invisible” Au in synthetic minerals in the Fe–As–S system. Am. Mineral. 2017, 102, 1057–1065. [Google Scholar]
- Kesler, S.E. Copper, molybdenum and gold abundances in porphyry copper deposits. Econ. Geol. 1973, 68, 106–112. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Gold–rich porphyry deposits: Descriptive and genetic models and their role in exploration and discovery. Econ. Geol. Rev. 2000, 13, 315–345. [Google Scholar]
- Filimonova, O.N.; Nickolsky, M.S.; Trigub, A.L.; Chareev, D.A.; Kvashnina, K.O.; Kovalchuk, E.V.; Vikentyev, I.V.; Tagirov, B.R. The state of platinum in pyrite studied by X-ray absorption spectroscopy of synthetic crystals. Econ. Geol. 2019, 114, 1649–1663. [Google Scholar] [CrossRef]
- Filimonova, O.N.; Trigub, A.L.; Nickolsky, M.S.; Chareev, D.A.; Kvashnina, K.O.; Kovalchuk, E.V.; Vikentyev, I.V.; Tagirov, B.R. The state of platinum in pyrrhotite: X-ray absorption spectroscopy study and implications for the role of Fe sulfides as plati-num carriers. Ore Geol. Rev. 2021. in preparation. [Google Scholar]
- Vikent’ev, I.V.; Moloshag, V.P.; Yudovskaya, M.A. Platinum group elements in ores of massive sulfide deposits of the Urals. Dokl. Earth Sci. 2002, 385, 488–492. [Google Scholar]
- Vikentyev, I.V.; Yudovskaya, M.A.; Mokhov, A.V.; Kerzin, A.L.; Tsepin, A.I. Gold and PGE in sulphide massive sulphide ore of the Uzelginsk deposit, Southern Urals, Russia. Can. Mineral. 2004, 42, 651–665. [Google Scholar] [CrossRef] [Green Version]
- Vikentyev, I.V.; Grabezhev, A.I.; Moloshag, V.P.; Novokreschenov, S.M.; Neustroeva, I.I. PGE in the ores of magnet-ite-copper-skarn deposits of the Urals. In Yearbook-2004; Zavaritsky Institute of Geology and Geochemistry, Uralian Branch, RAS: Yekaterinburg, Russia, 2005; pp. 328–331. (In Russian) [Google Scholar]
- Murzin, V.V.; Varlamov, D.A.; Vikentyev, I.V. Copper-cobalt mineralization of the Pyshminsk-Klyuchevsk deposit in the Middle Urals: Mineral composition of ore and metasomatites, stages, P-T conditions of mineral formation. Lithosphere 2011, 6, 103–122. [Google Scholar]
- Vikentyev, I.V.; Abramova, V.D.; Moloshag, V.P.; Shangguo, S. PGE in minerals of volcanogenic massive sulfide deposits of the Urals: Ore geochemistry and first LA-ICP-MS data. In Abs.12th International Platinum Symposium; Zavaritsky Institute of Geology and Geochemistry: Yekaterinburg, Russia, 2014; pp. 326–327. [Google Scholar]
- Mansur, E.T.; Barnes, S.-J.; Duran, C.J. An overview of chalcophile element contents of pyrrhotite, pentlandite, chalcopyrite, and pyrite from magmatic Ni-Cu-PGE sulfide deposits. Miner. Depos. 2021, 56, 179–204. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Franchini, M.; McFarlane, C.; Maydagán, L.; Reich, M.; Lentz, D.R.; Meinert, L.; Bouhier, V. Trace metals in pyrite and mar-casite from the Agua Rica porphyry–high sulphidation epithermal deposit, Catamarca, Argentina: Textural features and metal zoning at the porphyry to epithermal transition. Ore Geol. Rev. 2015, 66, 366–387. [Google Scholar] [CrossRef]
- Fleet, M.E.; Chryssoulis, S.L.; MacLean, P.J.; Davidson, R.; Weisener, C.G. Arsenian pyrite from gold deposits; Au and As distribution investigated by SIMS and EMP, and color staining and surface oxidation by XPS and LIMS. Can. Mineral. 1993, 31, 1–17. [Google Scholar]
- Leistel, J.M.; Marcoux, E.; Deschamps, Y.; Joubert, M. Antithetic behavior of gold in the volcanogenic massive sulphide de-posits of the Iberian Pyrite Belt. Mineral. Dep. 1997, 33, 82–97. [Google Scholar] [CrossRef]
- Bi, S.-J.; Li, J.-W.; Zhou, M.-F.; Li, Z.-K. Gold distribution in As-deficient pyrite and telluride mineralogy of the Yangzhaiyu gold deposit, Xiaoqinling district, southern North China craton. Miner. Depos. 2011, 46, 925–941. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Li, J.; Hofstra, A.H.; Liu, Y.; Koenig, A.E. Extreme variation of sulphur isotopic compositions in pyrite from the Qiuling sediment–hosted gold deposit, West Qinling orogen, central China: An in situ SIMS study with implications for the source of sulphur. Mineral. Dep. 2015, 50, 643–656. [Google Scholar] [CrossRef]
- Vikentyev, I.V. Invisible and microscopic gold in pyrite: New data for volcanogenic massive sulphide ores of the Urals. In Mineral Resources in a Sustainable World; Andre-Mayer, A.S., Ed.; SGA: Nancy, France, 2015; pp. 2113–2116. [Google Scholar]
- Tseluyko, A.S.; Maslennikov, V.V.; Ayupova, N.R.; Maslennikova, S.P.; Danyushevsky, L.V. Tellurium–bearing minerals in clastic ores of Ybileynoe massive sulphide deposit (South Urals). Geol. Ore Dep. 2019, 61, 133–161. [Google Scholar] [CrossRef]
- Ivanova, J.N.; Tykova, E.E.; Abramova, V.D.; Kovalchuk, E.V.; Vikentyev, I.V. Ores mineralogy and first data about “in-visible” form of Au in pyrite of the Novogodnee–Monto deposit (the Polar Urals, Russia). In Mineral Resources in a Sustainable World; Andre–Mayer, A.S., Ed.; SGA: Nancy, France, 2015; pp. 121–125. [Google Scholar]
- Vikentiev, I.V.; Abramova, V.D.; Ivanova, Y.N.; Tyukova, E.E.; Kovalchuk, E.V.; Bortnikov, N.S. Trace elements in pyrite from the Petropavlovsk gold–porphyry deposit (Polar Urals): Results of LA-ICP-MS analysis. Dokl. Earth Sci. 2016, 470, 977–981. [Google Scholar] [CrossRef]
- Vikentyev, I.V.; Mansurov, R.K.; Ivanova, Y.N.; Tyukova, E.E.; Sobolev, I.D.; Abramova, V.D.; Vykhristenko, R.I.; Trofimov, A.P.; Khubanov, V.B.; Groznova, E.O.; et al. Porphyry-style Petropavlovskoe gold deposit, the Polar Urals: Geological position, mineralogy, and formation conditions. Geol. Ore Depos. 2017, 59, 482–520. [Google Scholar] [CrossRef]
- Vikent’eva, O.V.; Bortnikov, N.S. The large Svetlinsk Au-Te deposit, South Urals: Telluride mineralization for genetic recon-structions. In Mineral Resources in a Sustainable World; Andre-Mayer, A.S., Ed.; SGA: Nancy, France, 2015; pp. 851–854. [Google Scholar]
- Vikent’eva, O.; Vikentev, I. Occurrence modes of As, Sb, Te, Bi, Ag in sulphide assemblages of gold deposits of the Urals. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 123, pp. 1–4. [Google Scholar] [CrossRef]
- Kampmann, T.C.; Jansson, N.F.; Stephens, M.B.; Olin, P.H.; Gilbert, S.; Wanhainen, C. Syn-tectonic sulphide remobilization and trace element redistribution at the Falun pyritic Zn-Pb-Cu-(Au-Ag) sulphide deposit, Bergslagen, Sweden. Ore Geol. Rev. 2018, 96, 48–71. [Google Scholar] [CrossRef]
- Chareev, D.A. General principles of the synthesis of chalcogenides and pnictides in salt melts using a steady-state temperature gradient. Crystallogr. Rep. 2016, 61, 506–511. [Google Scholar] [CrossRef]
- Chareev, D.A.; Volkova, O.S.; Geringer, N.V.; Koshelev, A.V.; Nekrasov, A.N.; Osadchii, V.O.; Osadchii, E.G.; Filimonova, O.N. Synthesis of chalcogenide and pnictide crystals in salt melts using a steady-state temperature gradient. Crystallogr. Rep. 2016, 61, 682–691. [Google Scholar] [CrossRef]
- Widler, A.M.; Seward, T.M. The adsorption of gold(I) hydrosulphide complexes by iron sulphide surfaces. Geochim. Cosmochim. Acta 2002, 66, 383–402. [Google Scholar] [CrossRef]
- Laptev, Y.V.; Rozov, K.B. Interaction of gold with sulphide surface as a factor of its concentration in hydrothermal ore formation. Dokl. Earth Sci. 2006, 411, 1229–1232. [Google Scholar] [CrossRef]
- Cardile, C.M.; Cashion, J.D.; McGrath, A.C.; Renders, P.; Seward, T.M. 197Au Mössbauer study of Au2S and gold adsorbed onto As2S3 and Sb2S3 substrates. Geochim. Cosmochim. Acta 1993, 57, 2481–2486. [Google Scholar] [CrossRef]
- Kozerenko, S.V.; Wagner, F.E.; Friedl, J.; Fadeev, V.V. Gold in pyrite formation processes: 3. Mössbauer study of synthetic gold–bearing iron sulphides. Geochem. Intern. 2001, 39, S167–S172. [Google Scholar]
- Simon, G.; Huang, H.; Penner–Hahn, J.E.; Kesler, S.; Kao, L.S. Oxidation state of gold and arsenic in gold–bearing arsenian pyrite. Am. Mineral. 1999, 84, 1071–1079. [Google Scholar] [CrossRef]
- Cabri, L.J.; Newville, M.; Gordon, R.A.; Crozier, E.D.; Sutton, S.R.; McMahon, G.; Jiang, D.-T. Chemical Speciation of Gold in Arsenopyrite. Can. Miner. 2000, 38, 1265–1281. [Google Scholar] [CrossRef] [Green Version]
- Filimonova, O.N.; Tagirov, B.R.; Trigub, A.L.; Nickolsky, M.S.; Rovezzi, M.; Belogub, E.V.; Reukov, V.L.; Vikentyev, I.V. The state of Au and as in pyrite studied by X-ray absorption spectroscopy of natural minerals and synthetic phases. Ore Geol. Rev. 2020, 121, 103475. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific: Oxford, UK, 1985; pp. 1–190. [Google Scholar]
- Sillitoe, R.H. Gold deposit types: An overview. In Geology of the World’s Major Gold Deposits and Provinces; Spec. Publ. No. 23; Society of Economic Geologists Inc.: Littleton, CO, USA, 2020; pp. 1–28. [Google Scholar]
- Sazonov, V.N.; Van Herk, A.H.; de Boorder, H. Spatial and temporal distribution of gold deposits in the Urals. Econ. Geol. 2001, 96, 685–703. [Google Scholar] [CrossRef]
- Prokin, V.A.; Buslaev, F.P. Massive copper–zinc sulphide deposits in the Urals. Ore Geol. Rev. 1998, 14, 1–69. [Google Scholar] [CrossRef]
- Vikentyev, I.V.; Belogub, E.V.; Novoselov, K.A.; Moloshag, V.P. Metamorphism of volcanogenic massive sulphide deposits in the Urals. Ore geology. Ore Geol. Rev. 2017, 85, 30–63. [Google Scholar] [CrossRef]
- Herrington, R.; Zaykov, V.V.; Maslennikov, V.V.; Brown, D.; Puchkov, V.N. Mineral deposits of the Urals and links to geo-dynamic evolution. Econ. Geol. 2005, 100, 1069–1095. [Google Scholar]
- Bortnikov, N.S.; Vikentyev, I.V. Endogenous metallogeny of the Urals. In Mineral Deposit Research for a High-Tech World Proceedings of the 12th Biennial SGA Meeting; Jonsson, E., Ed.; SGA: Uppsala, Sweden, 2013; pp. 1508–1511. [Google Scholar]
- Puchkov, V.N. General features relating to the occurrence of mineral deposits in the Urals: What, where, when and why. Ore Geol. Rev. 2017, 85, 4–29. [Google Scholar] [CrossRef]
- Puchkov, V.N. Geology of Urals and Cis–Urals (Actual Problems of Stratigraphy, Tectonics, Geodynamics and Metallogeny); Design Poligraph Service Publ.: Ufa, Russia, 2010. (In Russian) [Google Scholar]
- Sazonov, V.N.; Murzin, V.V.; Grigor’ev, N.A. Vorontsovsk gold deposit: An example of Carlin–type mineralization in the Urals, Russia. Geol. Ore Dep. 1998, 40, 139–151. [Google Scholar]
- Vikentyev, I.V.; Tyukova, E.E.; Vikent’eva, O.V.; Chugaev, A.V.; Dubinina, E.O.; Prokofiev, V.Y.; Murzin, V.V. Vorontsovka Carlin–style gold deposit in the north Urals: Mineralogy, fluid inclusion and isotope data for genetic model. Chem. Geol. 2019, 508, 144–166. [Google Scholar] [CrossRef]
- Vikent’eva, O.V.; Bortnikov, N.S.; Vikentyev, I.V.; Groznova, E.O.; Lyubimtseva, N.G.; Murzin, V.V. The Berezovsk giant intrusion-related gold-quartz deposit, Urals, Russia: Evidence for multiple magmatic and metamorphic fluid reservoirs. Ore Geol. Rev. 2017, 91, 837–863. [Google Scholar] [CrossRef]
- Sazonov, V.N.; Popov, V.A.; Grigoryev, N.A.; Murzin, V.V.; Metsner, E.I. Crust–Mantle Mineralisation in the Salic Blocks of Eugeosyncline; UrO USSR AN: Sverdlovsk, Russia, 1989. (In Russian) [Google Scholar]
- Vikent’eva, O.; Prokofiev, V.; Borovikov, A.; Kryazhev, S.; Groznova, E.; Pritchin, M.; Vikentyev, I.; Bortnikov, N. Contrasting fluids in the Svetlinsk gold-telluride hydrothermal system, South Urals. Minerals 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Vikentyev, I.V. Precious metal and telluride mineralogy of large volcanic–hosted massive sulphide deposits in the Urals. Mineral. Petrol. 2006, 87, 305–326. [Google Scholar] [CrossRef]
- Seravkin, I.B. Correlation of compositions of ore and host rocks in volcanogenic massive sulphide deposits (on the samples of South Urals). Geol. Ore Dep. 2013, 55, 68–83. [Google Scholar] [CrossRef]
- Karpukhina, V.S.; Naumov, V.B.; Vikent’ev, I.V. Genesis of massive sulphide deposits in the Verkhneural’sk ore district, the South Urals, Russia: Evidence for magmatic contribution of metals and fluids. Geol. Ore Dep. 2013, 55, 125–143. [Google Scholar] [CrossRef]
- Seravkin, I.B.; Kosarev, A.M.; Puchkov, V.N. Geodynamic conditions of formation of massive sulfide deposits in the Magnitogorsk Megazone, Southern Urals, and prospection criteria. Geol. Ore Depos. 2017, 59, 227–243. [Google Scholar] [CrossRef]
- Vikentyev, I.V.; Simonov, V.A.; Borisova, A.Y.; Karpukhina, V.S.; Naumov, V.B. Volcanic–hosted massive sulphide deposits of the Urals, Russia: Evidence for a magmatic contribution of metals and fluid. In Mineral Deposit Research for a High–Tech World; Jonsson, E., Ed.; SGA: Uppsala, Sweden, 2013; pp. 1526–1529. [Google Scholar]
- Vikentev, I. Selenium, tellurium and precious metal mineralogy in Uchalinsk copper-zinc-pyritic district, the Urals. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 123, p. 012027. [Google Scholar]
- Prokin, V.A.; Buslaev, F.P.; Vinogradov, A.M.; Moloshag, V.P.; Kuznetsov, S.I. Gaisk Mining and Processing Company: Geology of Gaisk and Podolsk Copper-Zinc Pyritic Deposits on the Urals; Nauka: Yekaterinburg, Russia, 2004; 148p. (In Russian) [Google Scholar]
- Vikentyev, I.; Seravkin, I.; Moloshag, V.; Skuratov, V.; Yudovskaya, M.; Mokhov, A.; Kerzin, A.; Tsepin, A. Au and Ag in ores of the Gaisk giant VHMS deposit, South Urals. Extended Abs.12th IAGOD Symp. In Understanding the Genesis of Ore Deposits to Meet the Demands of the 21st Century; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1305–1308. [Google Scholar]
- Pshenichny, G.N. Gai Copper–VMS Deposit of the South Urals; Nauka: Moscow, Russia, 1975; 188p. (In Russian) [Google Scholar]
- Vikentyev, I.V. Formation Conditions and Metamorphism of Pyrite Ores; Nauch: Moscow, Russia, 2004; 344p. (In Russian) [Google Scholar]
- Yakubovich, O.V.; Podolskaya, M.M.; Vikentyev, I.V.; Fokina, E.L.; Kotov, A.B. U-Th-He geochronology of pyrite from the Uzelga VMS deposit (South Urals)—new perspectives for direct dating of the ore-forming processes. Minerals 2020, 10, 629. [Google Scholar] [CrossRef]
- Vikentyev, I.; Chugaev, A.; Karpukhina, V.; Nosik, L.; Rimskaya–Korsakova, M. Origin of Uzelginsk Zn–Cu–Ag VHMS deposit, Southern Urals. Extended abs. In: 12th IAGOD Symp. In Understanding the Genesis of ore Deposits to Meet the Demands of the 21st Century; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1233–1236. [Google Scholar]
- Vikentyev, I.V.; Karpukhina, V.S. Uzelginsk Zn–Cu–Ag VMS deposit, South Urals: Genetic aspect. In Applied Mineralogy; Rammlmair, Ed.; Balkema: Rotterdam, The Netherlands, 2000; pp. 455–459. [Google Scholar]
- Vikentyev, I.V.; Tyukova, E.E.; Murzin, V.V.; Vikent’eva, O.V.; Pavlov, L.G. Vorontsovka Gold Deposit. Geology, Gold Modes, Genesis; FortDialogIset Publ.: Ekaterinburg, Russia, 2016; 204p. (In Russian) [Google Scholar]
- Akinfiev, N.N.; Vikentyev, I.V. Physicochemical modeling of ore formation at the gold and volcanogenic massive sulfide deposits of the Northern Urals. Geochem. Int. 2020, 58, 1437–1442. [Google Scholar] [CrossRef]
- Zhukhlistov, A.P.; Vikent’ev, I.V.; Rusinova, O.V. Electron diffraction study of 1 M illites with interstratified trans- and cis-vacant 2:1 layers. Crystallogr. Rep. 2012, 57, 270–276. [Google Scholar] [CrossRef]
- Udachin, V.; Williamson, B.J.; Purvis, O.W.; Spiro, B.; Dubbin, W.; Brooks, S.; Coste, B.; Herrington, R.; Mikhailova, I. As-sessment of environmental impacts of active smelter operations and abandoned mines in Karabash, Ural Mountains, Russia. J. Sustain. Dev. 2003, 11, 133–142. [Google Scholar] [CrossRef]
- Mercier–Langevin, P.; Hannington, M.D.; Dubé, B.; Bécu, V. The gold content of volcanogenic massive sulphide deposits. Mineral. Dep. 2011, 46, 509–539. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.A.; Ridley, I.; Koenig, A.E. Development of sulphide calibration standards for the laser ablation inductively–coupled plasma mass spectrometry technique. J. Anal. At. Spec. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Wohlgemuth–Ueberwasser, C.C.; Ballhaus, C.; Berndt, J.; Stotter née Paliulionyte, V.; Meisel, T. Synthesis of PGE sulphide standards for laser ablation inductivelycoupled plasma mass spectrometry (LA-ICP-MS). Contrib. Mineral. Petrol. 2007, 154, 607–617. [Google Scholar] [CrossRef]
- Vinokurov, S.F.; Vikentyev, I.V. Ionic mode of gold in sulphide ores. Dokl. Earth Sci. 2008, 422, 1062–1064. [Google Scholar] [CrossRef]
- Vinokurov, S.F.; Vikent’ev, I.V. Quantifying the ionic form of gold in sulfide ores. Her. Russ. Acad. Sci. 2009, 79, 462–467. [Google Scholar] [CrossRef]
- Vinokurov, S.F.; Vikent’ev, I.V.; Sychkova, V.A. Determining ionic gold species in massive sulphide ores. Geochem. Intern. 2010, 48, 510–516. [Google Scholar] [CrossRef]
- Chareev, D.A.; Osadchii, V.O.; Shiryaev, A.A.; Nekrasov, A.N.; Koshelev, A.V.; Osadchii, E.G. Single-crystal Fe-bearing sphalerite: Synthesis, lattice parameter, thermal expansion coefficient and microhardness. Phys. Chem. Miner. 2017, 44, 287–296. [Google Scholar] [CrossRef]
- Filimonova, O.N.; Minervina, E.A.; Kovalchuk, E.V.; Abramova, V.D.; Vikent’iev, I.; Tagirov, B.R.; Chareev, D.A.; Chvosti-cov, V.A. An experimental study of noble and base metals (Au, Ag, Pt, Pd, Zn) distribution in pyrite and pyrrhotite. In Mineral Resources in a Sustainable World; Andre–Mayer, A.S., Ed.; SGA: Nancy, France, 2015. [Google Scholar]
- Filimonova, O.N.; Trigub, A.L.; Tonkacheev, D.E.; Nickolsky, M.S.; Kvashnina, K.O.; Chareev, D.A.; Chaplygin, I.V.; Kovalchuk, E.V.; Lafuerza, S.; Tagirov, B.R. Substitution mechanisms in In-, Au-, and Cu-bearing sphalerites studied by X-ray absorption spectroscopy of synthetic compounds and natural minerals. Miner. Mag. 2019, 83, 435–451. [Google Scholar] [CrossRef]
- Vikentyev, I.V.; Vikentyeva, O.V. Precious metal minerals and «invisible» gold in sulfide ores of Urals. Mater. of XII Int. sci. conf. «Advanced technologies, equipment and analytical systems for materials and nano-materials». Ust-Kamenogorsk East-Kazakhstan State Tech. Univ. 2015, 3, 33–41. [Google Scholar]
- Rogozhnikov, D.A.; Shoppert, A.A.; Dizer, O.A.; Karimov, K.A.; Rusalev, R.E. Leaching kinetics of sulfides from refractory gold concentrates by nitric acid. Metals 2019, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Cabri, L.J.; Chryssoulis, S.L.; De Villiers, J.P.R.; Laflamme, J.H.G.; Buseck, P.R. The nature of “invisible” gold in arsenopyrite. Can. Mineral. 1989, 27, 353–362. [Google Scholar]
- Fleet, M.E.; Mumin, A.H. Gold-bearing arsenian pyrite and marcasite and arsenopyrite from Carlin Trend gold deposits and laboratory synthesis. Am. Miner. 1997, 82, 182–193. [Google Scholar] [CrossRef]
- Cepedal, A.; Fuertes–Fuente, M.; Martin-Izard, A.; González-Nistal, S.; Barrero, M. Gold–bearing As–rich pyrite and arsenopy-rite from the El Valle gold deposit, Asturias. Northwest Spain. Can. Mineral. 2008, 46, 233–247. [Google Scholar] [CrossRef]
- Murzin, V.V.; Naumov, E.A.; Azovskova, O.B.; Varlamov, D.A.; Rovnushkin, M.Y.; Pirajno, F. The Vorontsovskoe Au-Hg-As ore deposit (Northern Urals, Russia): Geological setting, ore mineralogy, geochemistry, geochronology and genetic model. Ore Geol. Rev. 2017, 85, 271–298. [Google Scholar] [CrossRef]
- Sidorova, N.V.; Vikent’ev, I.V.; Abramova, V.D.; Koval’chuk, E.V. Gold and other impurity elements in pyrite from the Berezovskoe deposit in the Middle Urals. Lithosphere 2019, 2, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.L.; Cook, N.J.; Pring, A.; Brugger, J.; Danyushevsky, L.V.; Shimizu, M. ‘Invisible gold’ in bismuth chalcogenides. Geochim. Cosmochim. Acta 2009, 73, 1970–1999. [Google Scholar] [CrossRef]
- Steadman, J.A.; Large, R.R. Synsedimentary, diagenetic, and metamorphic pyrite, pyrrhotite, and marcasite at the Homestake BIF-Hosted gold deposit, South Dakota, USA: Insights on Au-As ore genesis from textural and LA-ICP-MS trace element studies. Econ. Geol. 2016, 111, 1731–1752. [Google Scholar] [CrossRef]
- Tauson, V.L.; Babkin, D.N.; Pastushkova, T.M.; Krasnoshchekova, T.S.; Lustenberg, E.E.; Belozerova, O.Y. Dualistic distribution coefficients of elements in the system mineral-hydrothermal solution. I. Gold accumulation in pyrite. Geochem. Int. 2011, 49, 568–577. [Google Scholar] [CrossRef]
- Vikent’ev, I.V. Composition of native gold in massive sulphide ores of the Urals. Dokl. Earth Sci. 2003, 393, 1284–1288. [Google Scholar]
- Maslennikov, V.V.; Lein, A.Y.; Maslennikova, S.P.; Bogdanov, Y.A. Phanerozoic “black smokers” as indicator of host rocks composition. Lithosphere 2010, 10, 153–162. [Google Scholar]
- Zaykov, V.V.; Melekestseva, I.Y. Gold and silver minerals in the ore facies from gold–polymetallic deposits of Baimak ore district, the South Urals. Lithosphere 2011, 11, 71–90. [Google Scholar]
- Petrovskaya, N.V. Native Gold; Nauka Publ.: Moscow, Russia, 1973. (In Russian) [Google Scholar]
- Boyle, R.W. The Geochemistry of Gold and Its Deposits. Bull. Geol. Surv. Can. 1979, 280, 584. [Google Scholar]
- Trofimov, N.D.; Trigub, A.L.; Tagirov, B.R.; Filimonova, O.N.; Evstigneeva, P.V.; Chareev, D.A.; Kvashnina, K.O.; Nickolsky, M.S. The state of trace elements (In, Cu, Ag) in sphalerite studied by X-ray absorption spectroscopy of synthetic minerals. Minerals 2020, 10, 640. [Google Scholar] [CrossRef]
- Pring, A.; Wade, B.; McFadden, A.; Lenehan, C.E.; Cook, N.J. Coupled substitutions of minor and trace elements in co-existing sphalerite and wurtzite. Minerals 2020, 10, 147. [Google Scholar] [CrossRef] [Green Version]
- Tonkacheev, D.E.; Chareev, D.A.; Abramova, V.D.; Yudovskaya, M.A.; Minervina, E.A.; Tagirov, B.R. Sphalerite as a matrix for noble, non–ferrous metals and semimetals: A EPMA and LA–ICP–MS study of synthetic crystals. In Mineral Resources in a Sustainable World; Andre–Mayer, A.S., Ed.; SGA: Nancy, France, 2015; pp. 847–850. [Google Scholar]
- Tonkacheev, D.E.; Chareev, D.A.; Abramova, V.D.; Trofimov, N.D.; Tagirov, B.R. “Invisible” gold and PGE elements in synthetic crystals of sphalerite, greenockite and covellite: A EPMA, LA–ICP–MS and XAFS study. In Proceedings of the Fifteenth International Symposium on Experimental Mineralogy, Petrology and Geochemistry (EMPG-XV), ETH Zurich, Switzerland, 5–8 June 2016. [Google Scholar]
- Palyanova, G.A. Gold and silver minerals in sulfide ore. Geol. Ore Deposits 2020, 62, 383–406. [Google Scholar] [CrossRef]
- Safonov, Y.G. Topical issues of the theory of gold deposit formation. Geol. Ore Deposits 2010, 52, 438–458. [Google Scholar] [CrossRef]
- Simmons, S.F.; Tutolo, B.M.; Barker, S.L.L.; Goldfarb, R.J.; Robert, F. Hydrothermal gold deposition in epithermal, Carlin, and orogenic deposits. In Geology of the World’s Major Gold Deposits and Provinces; Spec. Publ. No. 23; Society of Economic Geologists Inc.: Littleton, CO, USA, 2020; pp. 823–845. [Google Scholar]
- Vikent’ev, I.V.; Belen’kaya, Y.A.; Ageev, B.I. The Aleksandrinsk polymetallic massive sulphide deposit (the Urals, Russia). Geol. Ore Dep. 2000, 42, 221–246. [Google Scholar]
- Ganzhenko, G.D.; Yudovskaya, M.A.; Vikentyev, I.V. Gold–polymetallic mineralization of the Ridder–Sokolnoye deposit in the Rudny Altai (Eastern Kazakhstan). Mineralogy 2018, 4, 8–34. [Google Scholar]
- Bortnikov, N.S.; Cabri, L.; Vikent’ev, I.V.; McMahon, G.; Bogdanov, Y.A. Invisible gold in sulphides from recent submarine hydrothermal mounds. Dokl. Earth Sci. 2000, 373, 863–866. [Google Scholar]
- Bortnikov, N.S.; Cabri, L.J.; Vikentiev, I.V.; Tagirov, B.R.; Mc Mahon, G.; Bogdanov, Y.A.; Stavrova, O.O. Invisible gold in sulphides from seafloor massive sulphide edifices. Geol. Ore Dep. 2003, 45, 201–212. [Google Scholar]
- Bortnikov, N.S.; Vikent’ev, I.V. Modern base metal sulphide mineral formation in the world ocean. Geol. Ore Dep. 2005, 47, 13–44. [Google Scholar]
- Sayab, M.; Suuronen, J.-P.; Molnar, F.; Villanova, J.; Kallonen, A.; O’Brien, H.; Lahtinen, R.; Lehtonen, M. Three-dimensional textural and quantitative analyses of orogenic gold at the nanoscale. Geology 2016, 44, 739–742. [Google Scholar] [CrossRef]
- Chryssoulis, S.L.; McMullen, J. Mineralogical investigation of gold ores. Dev. Miner. Process. 2005, 15, 21–71. [Google Scholar]
- Tauson, V.L.; Lipko, S.V.; Smagunov, N.V.; Kravtsova, R.G. Trace element partitioning dualism under mineral–fluid interaction: Origin and geochemical significance. Minerals 2018, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Novgorodova, M.I. Native Metals in Hydrothermal Ores; Nauka Publ.: Moscow, Russia, 1983. (In Russian) [Google Scholar]
- Nekrasov, I.Y. Geochemistry, Mineralogy, and Origin of Gold Ore Deposits; Nauka Publ.: Moscow, Russia, 1991; 304p. (In Russian) [Google Scholar]
- Pal’yanova, G.; Mikhlin, Y.; Kokh, K.; Karmanov, N.; Seryotkin, Y. Experimental constraints on gold and silver solubility in iron sulfides. J. Alloys Compd. 2015, 649, 67–75. [Google Scholar] [CrossRef]
- Pal’yanova, G.A.; Mikhlin, Y.L.; Karmanov, N.S.; Kokh, K.A.; Seryotkin, Y.V. Visible and “invisible” forms of gold and silver in the products of melt crystallization in the Fe–S–Ag–Au system: Experimental data. Dokl. Earth Sci. 2017, 474, 636–640. [Google Scholar] [CrossRef]
- Large, R.R.; Maslennikov, V.V. Invisible gold paragenesis and geochemistry in pyrite from orogenic and sediment-hosted gold deposits. Minerals 2020, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Lipko, S.; Tauson, V.; Bychinskii, V. Gold partitioning in a model multiphase mineral-hydrothermal fluid system: Distribution coefficients, speciation and segregation. Minerals 2020, 10, 890. [Google Scholar] [CrossRef]
- Johan, Z. Indium and germanium in the structure of sphalerite: An example of coupled substitution with Copper. Miner. Pet. 1988, 39, 211–229. [Google Scholar] [CrossRef]
- Murakami, H.; Ishihara, S. Trace elements of Indium-bearing sphalerite from tin-polymetallic deposits in Bolivia, China and Japan: A femto-second LA-ICPMS study. Ore Geol. Rev. 2013, 53, 223–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, F.; Li, Z.; Dong, Y.; Wang, H.; Li, X.; Long, J. Gold enrichment in hydrothermal sulfides from the Okinawa Trough: An in situ LA-ICP-MS study. Ore Geol. Rev. 2020, 116, 103255. [Google Scholar] [CrossRef]
- Wohlgemuth-Ueberwasser, C.C.; Viljoen, F.; Petersen, S.; Vorster, C. Distribution and solubility limits of trace elements in hydrothermal black smoker sulfides: An in-situ LA-ICP-MS study. Geochim. Cosmochim. Acta 2015, 159, 16–41. [Google Scholar] [CrossRef]
- Osadchii, V.O. Trivalent iron in the structure of sphalerite. In Lomonosov Reading–2016; Moscow State University: Moscow, Russia, 2016; Available online: https://istina.msu.ru/conferences/presentations/19830602/ (accessed on 26 April 2021). (In Russian)
- Cook, N.J.; Ciobanu, C.L.; Brugger, J.; Etschmann, B.; Howard, D.L.; De Jonge, M.D.; Ryan, C.; Paterson, D. Determination of the oxidation state of Cu in substituted Cu-In-Fe-bearing sphalerite via XANES spectroscopy. Am. Miner. 2012, 97, 476–479. [Google Scholar] [CrossRef]
- Belissont, R.; Muñoz, M.; Boiron, M.-C.; Luais, B.; Mathon, O. Distribution and oxidation state of Ge, Cu and Fe in sphalerite by μ-XRF and K-edge μ-XANES: Insights into Ge incorporation, partitioning and isotopic fractionation. Geochim. Cosmochim. Acta 2016, 177, 298–314. [Google Scholar] [CrossRef]
- Tonkacheev, D.E.; Chareev, D.A.; Abramova, V.D.; Kovalchuk, E.V.; Vikentyev, I.V.; Tagirov, B.R. The substitution mechanism of Au in In–, Fe– and In–Fe–bearing synthetic crystals of sphalerite, based on the data from EPMA and LA–ICP–MS study. Lithosphere 2019, 19, 148–161. [Google Scholar] [CrossRef]
- Koelmans, H. Association and dissociation of centres in luminescent ZnS-In. J. Phys. Chem. Solids 1960, 17, 69–79. [Google Scholar] [CrossRef]
- Pattrick, R.A.D.; Mosselmans, J.F.W.; Charnock, J.M. An X-ray absorption study of doped sphalerites. Eur. J. Miner. 1998, 10, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Di Benedetto, F.; Bernardini, G.P.; Costagliola, P.; Plant, D.; Vaughan, D.J. Compositional zoning in sphalerite crystals. Am. Miner. 2005, 90, 1384–1392. [Google Scholar] [CrossRef]
- Lin, Y.; Cook, N.J.; Ciobanu, C.L.; Liu, Y.P.; Zhang, Q.; Liu, T.G.; Gao, W.; Yang, Y.L.; Danyushevskiy, L. Trace and minor elements in sphalerite from base metal deposits in South China: A LA-ICPMS study. Ore Geol. Rev. 2011, 39, 188–217. [Google Scholar]
- Pokrovski, G.S.; Akinfiev, N.N.; Borisova, A.Y.; Zotov, A.V.; Kouzmanov, K. Gold speciation and transport in geological fluids: Insights from experiments and physical-chemical modelling. Geol. Soc. Lond. Spéc. Publ. 2014, 402, 9–70. [Google Scholar] [CrossRef]
- Tauson, V.L.; Mironov, A.G.; Smagunov, N.V.; Bugaeva, B.G.; Akimov, V.V. Gold in sulphides: State of the art of occurrence and horizons of experimental studies. Rus. Geol. Geophys. 1996, 37, 1–11. [Google Scholar]
- Pals, D.W.; Spry, P.G.; Chryssoulis, S. Invisible gold and tellurium in arsenic-rich pyrite from the Emperor gold deposit, Fiji: Implications for gold distribution and deposition. Econ. Geol. 2003, 98, 479–493. [Google Scholar] [CrossRef]
- McClenaghan, S.H.; Lentz, D.R.; Martin, J.; Diegor, J. Gold in the Brunswick No. 12 volcanogenic massive sulphide deposit, Bathurst Mining Camp, Canada: Evidence from bulk–ore analysis and laser–ablation ICP–MS–data on sulphide phases. Mineral. Dep. 2009, 44, 523–557. [Google Scholar] [CrossRef]
- Tauson, V.L.; Lustenberg, E.K. Quantitative determination of modes of gold occurrence in minerals by the statistical analysis of analytical data samplings. Geochem. Int. 2008, 46, 423–428. [Google Scholar] [CrossRef]
- Economou–Eliopoulos, M.; Eliopoulos, D.G.; Chryssoulis, S. A comparison of high–Au massive sulphide ores hosted in ophiolite complexes of the Balkan Peninsula with modern analogues: Genetic significance. Ore Geol. Rev. 2008, 33, 81–100. [Google Scholar] [CrossRef]
- Clark, L.A. The Fe-As-S system; phase relations and applications. Econ. Geol. 1960, 55, 1631–1652. [Google Scholar] [CrossRef]
- Arehart, G.B.; Chryssoulis, S.L.; Kesler, S.E. Gold and arsenic in iron sulfides from sediment-hosted disseminated gold deposits; implications for depositional processes. Econ. Geol. 1993, 88, 171–185. [Google Scholar] [CrossRef]
- Palenik, C.S.; Utsunomiya, S.I.; Reich, M.; Kesler, S.E.; Wang, L.; Ewing, R.C. “Invisible” gold revealed: Direct imaging of gold nanoparticles in a Carlin-type deposit. Am. Miner. 2004, 89, 1359–1366. [Google Scholar] [CrossRef]
- Griffin, W.L.; Ashley, P.M.; Ryan, C.G.; Sie, S.H.; Suter, G.F. Pyrite geochemistry in the North Arm Epitermal Ag–Au deposit, Queensland, Australia. A Proton microprobe study. Can. Mineral. 1991, 29, 185–198. [Google Scholar]
- Aylmore, M.G. Distribution and Agglomeration of Gold in Arsenopyrite and Pyrite. Ph.D. Thesis, Curtin University of Technology, Perth, Australia, 1995; 209p. [Google Scholar]
- Su, W.; Zhang, H.; Hu, R.; Ge, X.; Xia, B.; Chen, Y.; Zhu, C. Mineralogy and geochemistry of gold–bearing arsenian pyrite from the Shuiyindong Carlin–type gold deposit. Guizhou. China: Implications for gold depositional processes. Mineral. Dep. 2012, 47, 653–662. [Google Scholar] [CrossRef]
- Sack, P.J.; Large, R.R.; Gregory, D.D. Geochemistry of shale and sedimentary pyrite as a proxy for gold fertility in the Selwyn basin area, Yukon. Miner. Depos. 2018, 53, 997–1018. [Google Scholar] [CrossRef]
- Cathelineau, M.; Boiron, M.-C.; Holliger, P.; Marion, P.; Denis, M. Gold in arsenopyrites: Crystal chemistry, location and state, physical and chemical conditions of deposition. Econ. Geol. Monogr. 1989, 6, 328–341. [Google Scholar]
- Cook, N.; Chryssoulis, S. Concentration of “invisible” gold in the common sulphides. Can. Mineral. 1990, 28, 1–16. [Google Scholar]
- Vaughan, J.P.; Kyin, A. Refractory gold ores in Archaean greenstones, Western Australia: Mineralogy, gold paragenesis, metallurgical characterization and classification. Miner. Mag. 2004, 68, 255–277. [Google Scholar] [CrossRef]
- Kovalev, K.R.; Kalinin, Y.A.; Naumov, E.A.; Kolesnikova, M.K.; Korolyuk, V.N. Gold–bearing arsenopyrite in eastern Kazakhstan gold–sulfide deposits. Russ. Geol. Geophys. 2011, 52, 178–192. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Marion, P.; Robaut, F.; Pinto, A. Gold–bearing arsenopyrite and pyrite in refractory ores: Analytical refinements and new understanding of gold mineralogy. Mineral. Mag. 2007, 71, 123–142. [Google Scholar] [CrossRef]
- Mumin, A.H.; Fleet, M.E.; Chryssoulis, S.L. Gold mineralization in As-rich mesothermal gold ores of the Bogosu-Prestea mining district of the Ashanti gold belt, Ghana: Remobilization of ‘invisible’ gold. Mineral. Dep. 1994, 29, 445–460. [Google Scholar] [CrossRef]
- Yang, S.; Blum, N.; Rahders, E.; Zhang, Z. The nature of invisible gold in sulfides from the Xiangxi Au-Sb-W ore deposit in North-western Hunan, People’s republic of China. Can. Mineral. 1998, 36, 136l–1372. [Google Scholar]
- Sung, Y.-H.; Brugger, J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Nugus, M. Invisible gold in arsenian pyrite and arsenopyrite from a multistage Archaean gold deposit: Sunrise Dam, Eastern Goldfields province, Western Australia. Miner. Depos. 2009, 44, 765–791. [Google Scholar] [CrossRef]
- Goldmann, S.; Junge, M.; Wirth, R.; Schreiber, A. Distribution of trace elements in sphalerite and arsenopyrite on the nano-metre scale — Discrete phases versus solid solution. Europ. J. Miner. 2019, 31, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Belov, N.V.; Godovikov, A.A.; Bakakin, V.V. Ocherki po Teoreticheskoy Mineralogii (Essays on Theoretical Mineralogy); Publ. House Nauka: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- Makovicky, E. Crystal structures of sulphides and other chalcogenides. Rev. Mineral. Geochem. 2006, 61, 7–125. [Google Scholar] [CrossRef]
- Rieder, M.; Crelling, J.C.; Šustai, O.; Drábek, M.; Weiss, Z.; Klementová, M. Arsenic in iron disulphides in a brown coal from the North Bohemian Basin, Czech Republic. Inter. J. Coal Geol. 2007, 71, 115–121. [Google Scholar] [CrossRef]
- Bindi, L.; Moelo, Y.; Léone, P.; Suchaud, M. Stoichiometric arsenopyrite, FeAsS, from La Roche–Balue Quarry, Loire–Atlantique, France: Crystal structure and Mössbauer study. Can. Mineral. 2012, 50, 471–479. [Google Scholar] [CrossRef]
- Lutz, H.D.; Jung, M.; Wäschenbach, G. Kristallstrukturen des Löllingits FeAs2 und des Pyrits RuTe2. Z. Anorg. Allg. Chem. 1987, 554, 87–91. [Google Scholar] [CrossRef]
- Slipukhina, I.V.; Bercha, D.M. Elementary energy bands in isovalent IV–VI orthorhombic and cubic crystals and their solid solutions. Phys. Status Solidi 2007, 244, 650–668. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Heaton, L.; Mueller, M.H. Neutron diffraction techniques and their applications to some problems in physics. J. Appl. Phys. 1959, 30, 1323. [Google Scholar] [CrossRef]
- Kratz, T.; Fuess, H. Simultane Strukturbestimmung von Kupferkies und Bornit an einem Kristall. Z. Krist. 1989, 186, 167. [Google Scholar]
- Sowa, H. On the mechanism of the pressure–induced würtzite–to NaCl–type phase transition in CdS: An X–ray diffraction study. Solid State Sci. 2005, 7, 73–78. [Google Scholar] [CrossRef]
- Jamieson, J.C.; Demarest, H.H., Jr. A note on the compression of cubic ZnS. J. Phys. Chem. Solids 1980, 41, 963–964. [Google Scholar] [CrossRef]
- Kisi, E.H.; Elcombe, M.M. U parameters for the wurtzite structure of ZnS and ZnO using powder neutron diffraction. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1989, 45, 1867–1870. [Google Scholar] [CrossRef]
- Ishikawa, K.; Isonaga, T.; Wakita, S.; Suzuki, Y. Structure and electrical properties of Au2S. Solid State Ionics 1995, 79, 60–66. [Google Scholar] [CrossRef]
- Sadanaga, R.; Sueno, S. X–ray study on the α–β transition of Ag2S. Mineral. J. 1967, 5, 124–143. [Google Scholar] [CrossRef] [Green Version]
- Kjekshus, A.; Skansen, T.; Ehrenberg, L.; Brunvoll, J.; Bunnenberg, E.; Djerassi, C.; Records, R. On the homogeneity range of the PtS phase. Acta Chem. Scand. 1966, 20, 577–579. [Google Scholar] [CrossRef]
- Furuseth, S.; Selte, K.; Kjekshus, A.; Gronowitz, S.; Hoffman, R.A.; Westerdahl, A. Redetermined crystal structures of NiTe2, PdTe2, PtS2, PtSe2, and PtTe2. Acta Chem. Scand. 1965, 19, 257–258. [Google Scholar] [CrossRef]
- Brese, N.E.; Squattrito, P.J.; Ibers, J.A. Reinvestigation of the structure of PdS. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1985, 41, 1829–1830. [Google Scholar] [CrossRef]
- Grønvold, F.; Røst, E. The crystal structure of PdSe2 and PdS2. Acta Crystallogr. 1957, 10, 329–331. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]

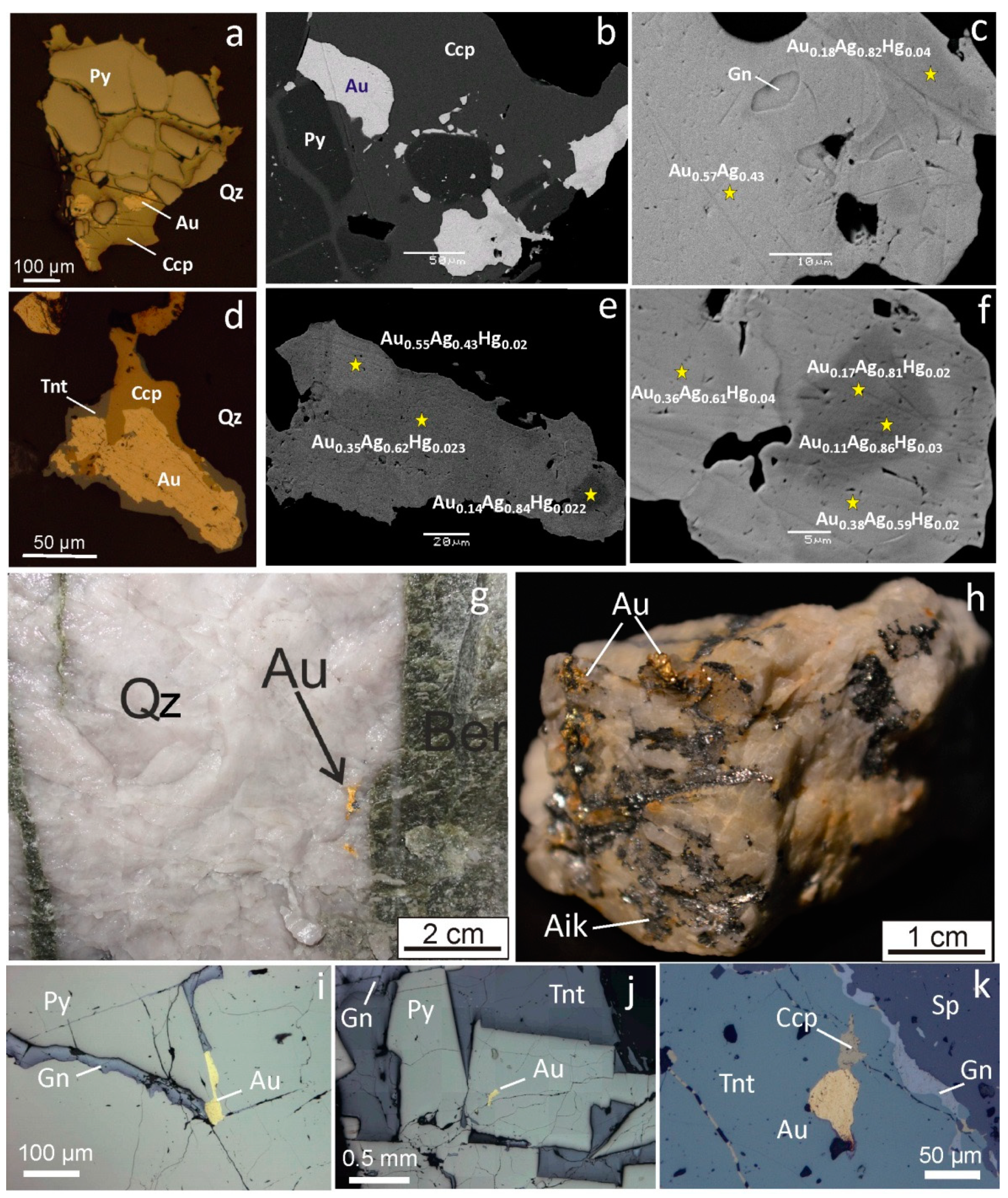
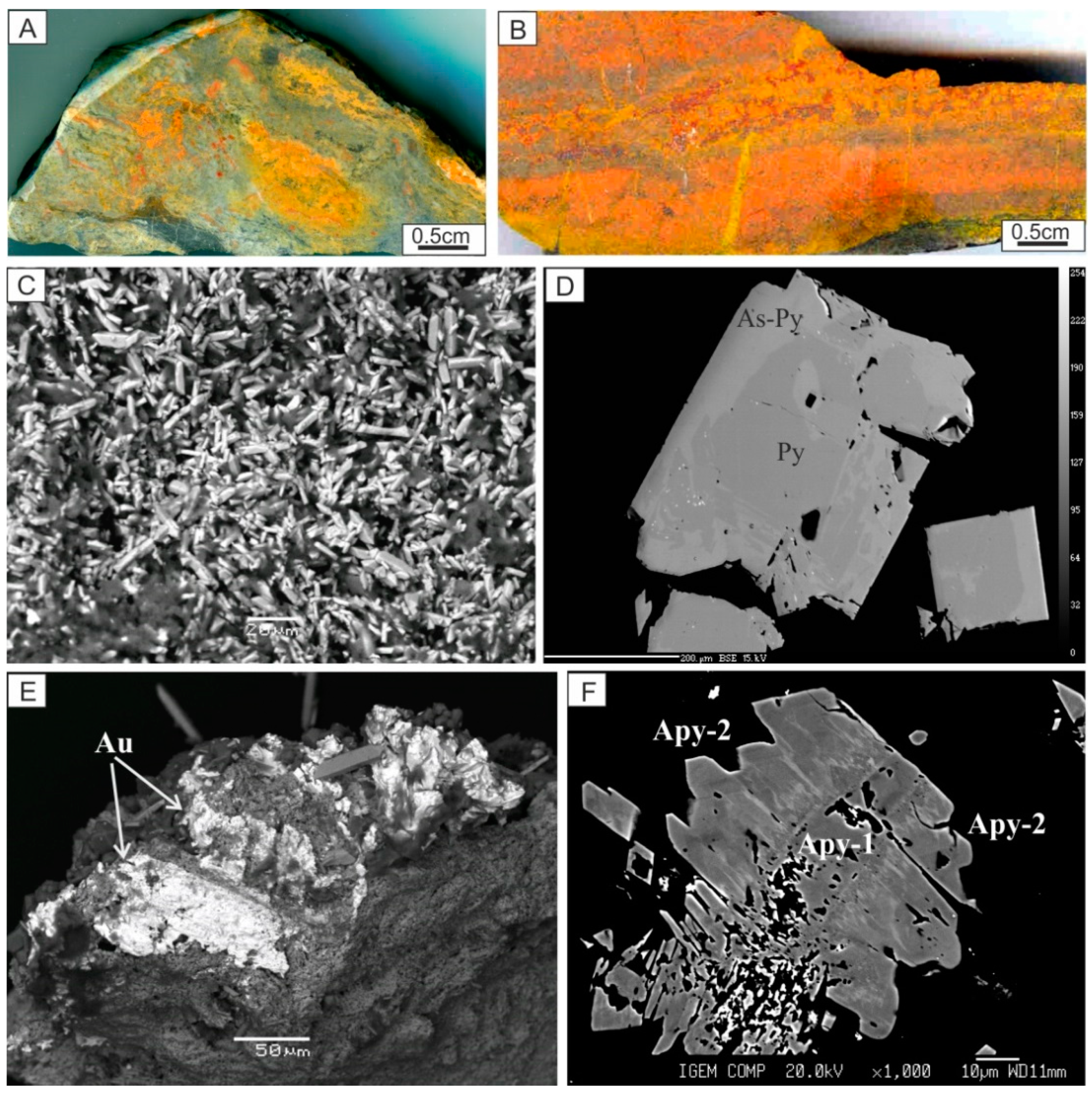




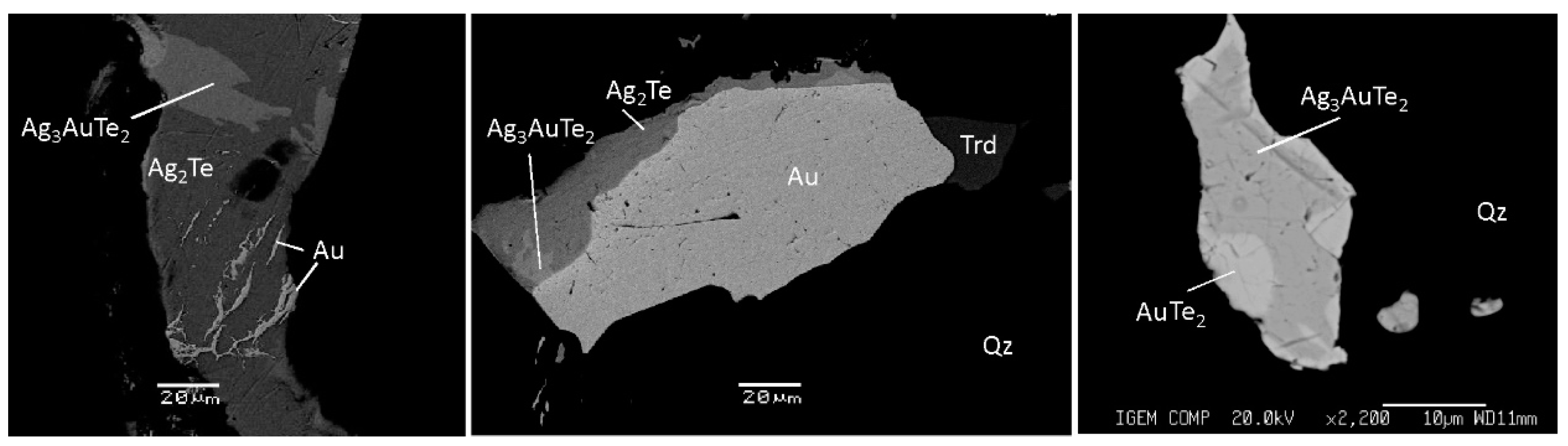
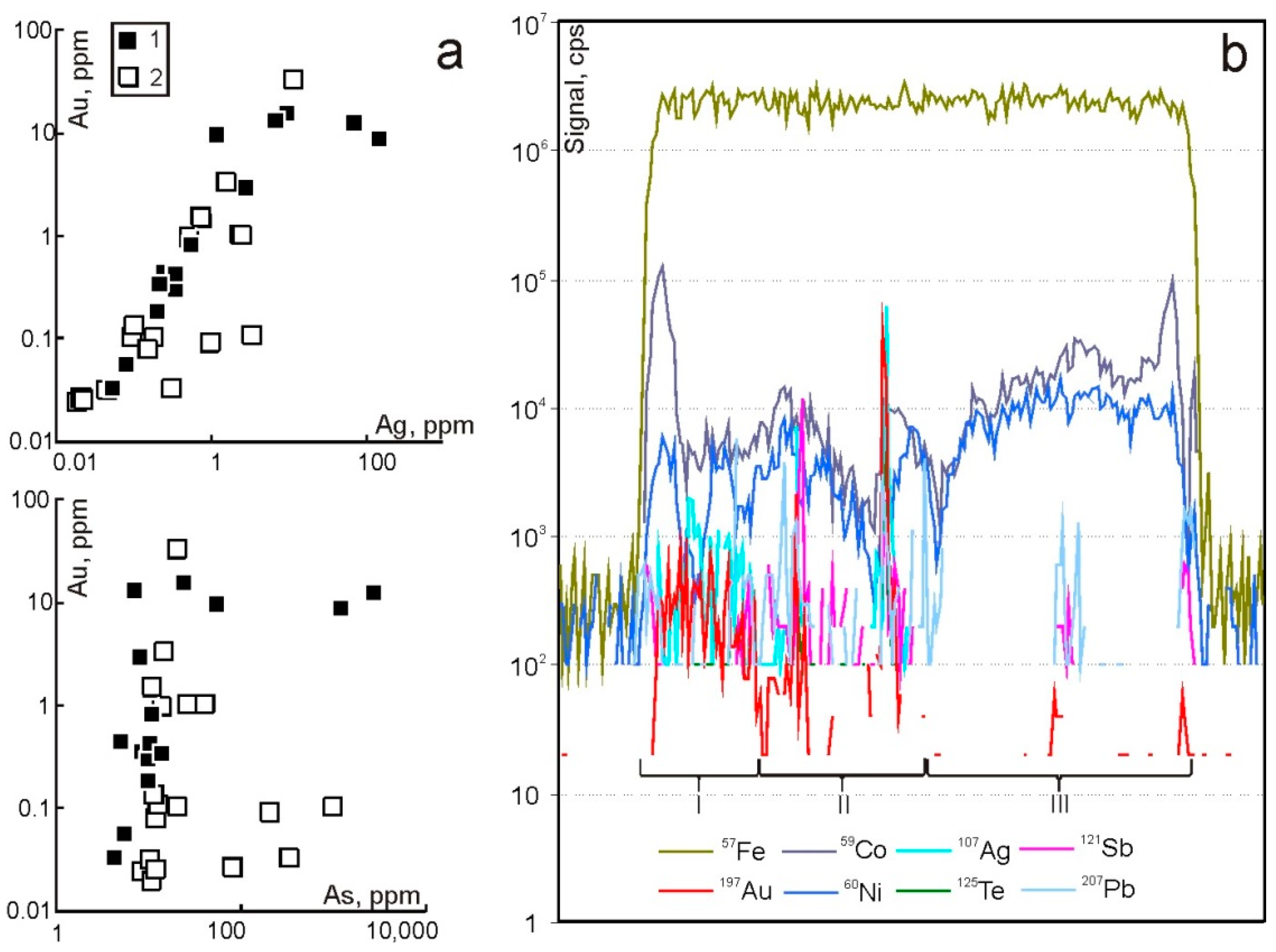









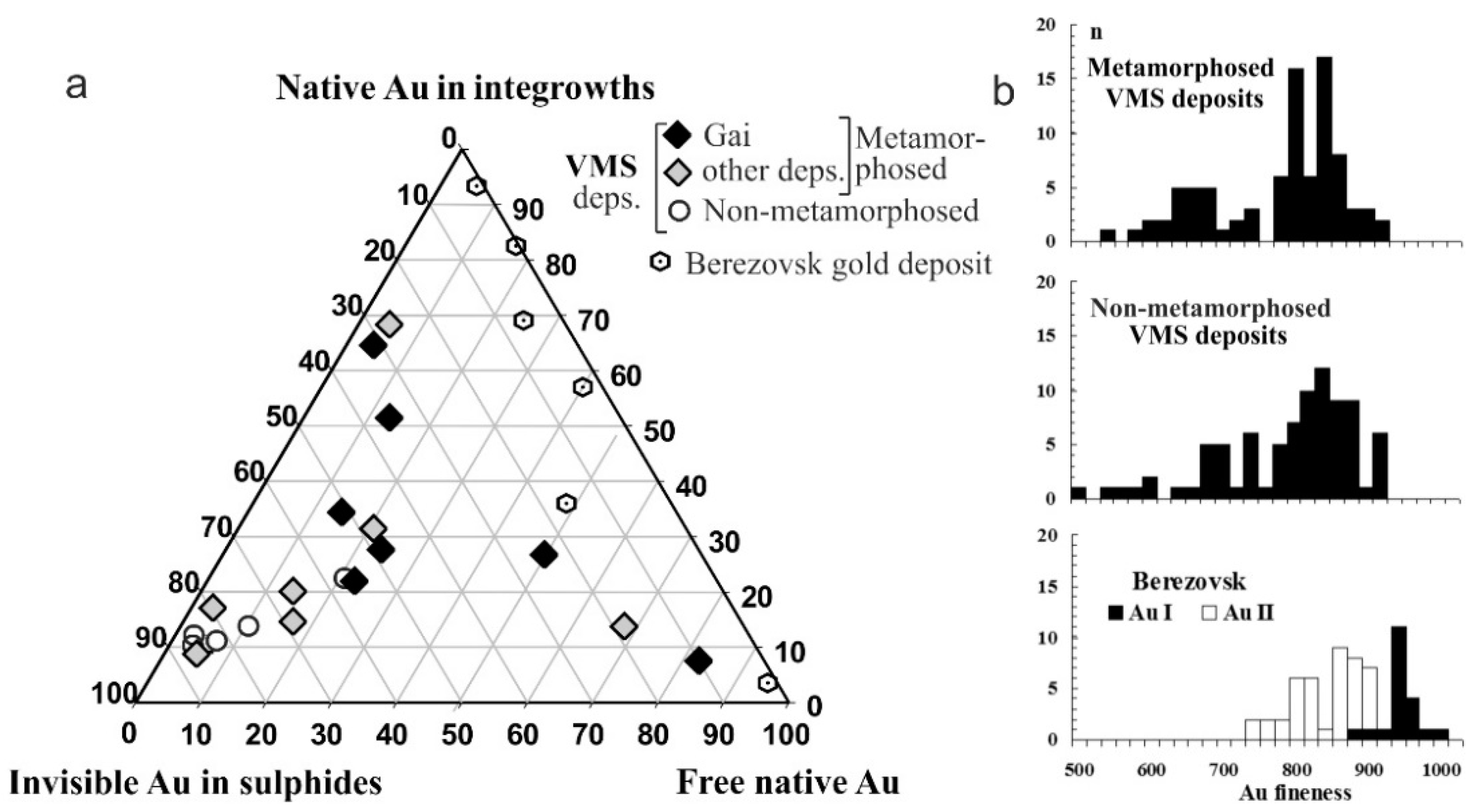
















| Geodynamic Environments | Ore Deposit Type | Ore-Bearing Magmatic Complexes | Main Ore Elements | Examples of Ore Deposits | |
|---|---|---|---|---|---|
| Oceanic spreading O1–2 | Co-Ni-sulphide | Ultramafic, tholeiite-basalt | Co, Ni (As, Au) | Ivanovka, Dergamysh | |
| Island arc (O3-D1) | primitive | Cu VMS (Dombarovsk) | Tholeiite-basalt | Cu (Zn, Co) | Mauk, Letnee, Buribai, Koktau |
| Cu-Zn VMS (Uralian) | Sodium rhyolite-basalt | Cu, Zn (Au) | Gai, Safyanovka, Yubileynoe, Priorskoe | ||
| Sodium basalt-rhyolite | Zn, Cu (Au, Ag, Se, Te) | Uchaly, Novo-Uchaly, Sibai, Uzelga, Degtyarsk, Podolsk | |||
| Cu-barite-Cu-Zn VMS (Baimak) | Potassium-sodium andesite-dacite | Cu, Zn, Au, Ba (Pb, Ag) | Bakr-Tau, Balta-Tau, Maiskoe, Tash-Tau, Uvarjazh, Galka | ||
| Cu-titanomagnetite-apatite | Gabbro-norite | Cu, Fe (Au, Pd, Pt, Ti, V, P) | Volkovskoe | ||
| mature | Cu-porphyry | Andesite-diorite | Cu | Tominskoe | |
| Au-porphyry | Plagiogranite | Au, Cu | Yubileinoe (Au) | ||
| Au-epithermal | Andesite-diorite | Au, Cu (Pb, Zn, Se, Te) | Bereznyakovskoe | ||
| Cu-skarn (porphyry) | Rhyolite-basalt, gabbro-diorite | Cu, Fe (Au) | Gumeshki | ||
| Au-polymetallic | Andesite-dacite | Au, Ag (Pb, Zn) | Murtykty | ||
| Arc-continent collision and active margin of continent (D3-C1) | Skarn-magnetite | Sodium andesite-basalt, gabbro-diorite | Fe (Cu, Au) | Sokolovskoe, Sarbay | |
| Potassium-sodium andesite-basalt, gabbro-diorite-granite | Fe (Cu, Co, Au) | Vysokogorsk, Goroblagodat | |||
| Cu-magnetite skarn | Cu, Fe (Au, Co) | Tur’insk group | |||
| Au-sulphide-realgar | Au, Ag (Hg, Sb,Tl) | Vorontsovka | |||
| Au-magnetite-skarn | Potassium-sodium andesite-basalt, gabbro-diorite | Fe, Au (Cu, Mo, Co, Ag) | Novogodnee-Monto | ||
| Au-porphyry | Au (Ag, Te, W) | Petropavlovsk | |||
| Au-skarn (porhyry) | Diorite-granodiorite | Au, Cu | Varvarinskoe | ||
| Cu-porphyry (Mo) | Cu (Mo, Au, Re) | Mikheevskoe | |||
| Cu-porphyry | Cu (Mo, Au) | Benkala | |||
| The main collision (C/P) | Au-sulphide-quartz | Tonalite-granodiorite | Au(Cu,Pb,Zn,Ag) | Berezovsk | |
| Au (As) | Kochkar’ | ||||
| Au-telluride | Gabbro-diabase, plagiogranite | Au (Te, Ag) | Svetlinsk | ||
| Deposit, Region | Svetlinsk, South Urals | Berezovsk, Middle Urals | Vorontsovka, North Urals | Petropavlovsk, Polar Urals |
|---|---|---|---|---|
| Host rocks | Metamorphosed volcaniclastic (D-C) | Volcaniclastic (O-S), gabbro, serpentinite, granite | Volcaniclastic (S2-D1) | Volcaniclastic (S2-D1) |
| Geochemical type | Au-Te | Au, Ag (W, Bi) | Au-As-Sb-Hg-Tl | Au (Ag, Te, Bi, W) |
| Ore bodies | Vein-disseminated zones; veins | Suits of veins usually occurred inside of dykes | Vein-disseminated zones; rare veinlets | Stockwork, vein-disseminated zones |
| Wall rock alteration | Quartz-biotite (with amphibole), quartz ± biotite-sericite | Beresite, listvenite | Propylitic, quartz-sericite, argillic, jasperoid | Silicification, albitisation, chloritisation, sericitisation |
| Stage of mineral formation | Quartz-pyrite→ Au-Te-polymetalic→ Quartz-carbonate-sulphide | Ankerite-quartz → Pyrite-quartz → Polymetalic → Carbonate | Arsenopyrite-pyrite → Pyrite-realgar → Sulphosalt- polymetalic → Polymetalic | Pyrite-magnetite → Pyrite (± chalcopyrite, sphalerite, pyrrhotite) → Gold–telluride → Quartz–carbonate |
| Au reserves (CAu) | ~135 t (1.8–2.8 g/t) | 490 t (2.4 g/t) | ~101 t (2.8 g/t) | 26 t (1.4 g/t) |
| № | Mineral Ore Type | Ore Mineral Assemblage | t, °C | P, kbar | log f S2 |
|---|---|---|---|---|---|
| I | impregnated gold-polymetallic | arsenopyrite-sulphosalts- polymetallic | 400–270 | 0.6–0.2 | −7 to −9 |
| II | finely disseminated gold-pyrite-realgar | arsenic-löllingite- arsenopyrite | 370–250 | 0.2–0.15 | −12 to −17 |
| Ore Deposit | Mineral Assemblage (and Gold Mineralisation) | Thom, °C | Salt Composition | Csalt, wt%-eq. NaCl |
|---|---|---|---|---|
| Novogodnee-Monto Fe-Au-skarn | Chalcopyrite-pyrite-quartz, early stage | 430–300 | (Na, Mg)Cl | 10–12.9 |
| Pyrite-chalcopyrite-quartz (with Au) | 315–270 | (Na, K)Cl | 4.5–12.2 | |
| Chalcopyrite-pyrite-magnetite (with Co and Au) | 230–215 | NaCl | 3.4–9.2 | |
| Pyrite-chalcopyrite-telluride (with Au) | 210–180 | NaCl | 10.5–13.9 | |
| Polysulphide-quartz-carbonate | 170–140 | (Na, K)Cl | 6.0–8.0 | |
| Petropavlovsk Au-porphyry | Chalcopyrite-pyrite-magnetite (with Au) | 250 * | no data | |
| Polysulphide-quartz (with Au) | 260–245 | no phase transitions were observed | ||
| Pyrite-chalcopyrite-telluride (with Au) | 200 | (Na, K)Cl | 11 | |
| Polysulphide-quartz-carbonate (with Au) | 160–150 | (Na, K)Cl | 14 | |
| Deposit, Region | Gai | Uchaly, Novo-Uchaly | Uzelga | Galka, North Urals |
|---|---|---|---|---|
| South Urals | ||||
| Host rocks | Bimodal-mafic (with minor chert) | Bimodal-felsic (with minor chert) | Bimodal-felsic (with minor chert, limestone, andesite and dacite) | Bimodal-felsic (with minor andesite and dacite) |
| Geological age | Emsian | Mid-Eifelian | Late Eifelian–Early Givetian | Late Ordovician–Early Llandoverian |
| Geochemical type | Zn-Cu (Au, Ag) | Cu-Zn (Au, Ag) | Cu-Zn (Au, Ag) | Zn-(Cu-Pb-Ag-Au) |
| Metamorphic grade (t, °C) | Greenschist, 250–450 | Prehnite-pumpellyite, 150–350 (locally up to 400) | Prehnite-pumpellyite, 180–350 (locally up to 450) | Zeolite, 100–200 |
| Dominant ore-host structures | Steeply dipping to vertical, pseudomonoclinal shear-related structures | Steeply dipping to vertical; limb of large anticlinal fold | Gentle doms and trenchs | Gentle doms and trenchs |
| Ore bodies | Platelike, podiform | Lensoid, antiform | Lensoid | Stockwork, vein-disseminated zones; (minor lensoid) |
| Wall rock alteration | Albitisation, silicification, chloritisation, sericitisation (±pyrophyllite) | Silicification, sericitisation, albitisation, chloritisation | Silicification, sericitisation, carbonation, albitisation, chloritisation | Argillic, silicification, sericitisation |
| Ore reserves | 450 Mt * | 230.4 (116 + 114.4) Mt * | 80.7 Mt * | 4.3 Mt |
| Cu + Pb + Zn, wt% | 2.2 | 4.3 (4.8 and 3.6) | 4.2 | 3.3 |
| Au reserves | 520 t * | 344 (180 + 164) t * | 136.6 t * | 6 t |
| CAu, g/t | 1.2 | 1.5 | 1.7 | 1.35 |
| Ag reserves | 6,300 t * | 4,381 t * | 2,495 t * | 200 t |
| CAg, g/t | 14 | 19 | 31 | 46.5 |
| Vorontsovka | Berezovsk | Svetlinsk | Petropavlovsk |
|---|---|---|---|
| Hessite Ag2Te, native gold, küstelite, Ag-tetrahedrite, freibergite (Ag,Cu)12Sb4S13 | Native silver, native gold, freibergite (Ag,Cu)12Sb4S13, matildite AgBiS2, hessite Ag2Te, acanthite Ag2S | Native gold, calaverite AuTe2, aurostibite AuSb2, montbrayite (Au,Sb)2Te3, krennerite (Au,Ag)Te2, sylvanite AuAgTe4, petzite Ag3AuTe2, volynskite AgBiTe2, maldonite Au2Bi, hessite Ag2Te, γ-hessite Ag1.9Te, acanthite Ag2S | Native gold, hessite Ag2Te, petzite Ag3AuTe2, calaverite AuTe2, sylvanite AuAgTe4 |
| n | Concentration | Co | Ni | As | Se | Ag | Au | Sn | Te | Bi |
|---|---|---|---|---|---|---|---|---|---|---|
| Pyrite-1 (skarn-magnetite assemblage) | ||||||||||
| 25 | min | 3.4 | 8 | 18 | 1.6 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 |
| max | 17,141 | 3738 | 1944 | 42 | 16 | 13 | 0.4 | 66 | 3 | |
| geom. mean | 250 | 52 | 55 | 15 | 0.3 | 0.2 | 0.14 | 8 | 0.4 | |
| Pyrite-2 (gold-sulphide assemblage) | ||||||||||
| 15 | min | 2 | 4.5 | 11 | 2 | 14 | 2 | 0.03 | 65 | 0.03 |
| max | 75 | 37 | 211 | 26 | 105 | 80 | 0.4 | 650 | 116 | |
| geom. mean | 18 | 10 | 40 | 11 | 47 | 18 | 0.1 | 120 | 0.34 | |
| Pyrite-3 (gold-telluride assemblage) | ||||||||||
| 70 | min | 0.2 | 0.02 | 9.6 | 3.2 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 |
| max | 2234 | 3791 | 7048 | 223 | 111 | 66 | 4.4 | 137 | 5 | |
| geom. mean | 17 | 12 | 56 | 13 | 1.3 | 0.6 | 0.1 | 6 | 0.5 | |
| Pyrite-4 (quartz-carbonate assemblage) | ||||||||||
| 30 | min | 0.07 | 3 | 5 | 1.4 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| max | 737 | 70 | 417 | 40 | 4.2 | 1.7 | 1.6 | 28 | 2.3 | |
| geom. mean | 27 | 21 | 37 | 14 | 0.5 | 0.3 | 0.3 | 4 | 0.3 | |
| Mineral | Main Trace Elements | Minor Elements (<50 ppm) | Mineral Inclusions | ||
|---|---|---|---|---|---|
| Common | Rare | Submicroscopic * | |||
| Py-1 | Cu, Co, As, Ni, Zn, Te | Sn, Bi, Se, Ag, Au, Pb | Sp, Mt | Hem, Rt | |
| Py-2 | Pb, Te, Bi, Au, Co, Zn, As, Ag | Se, Ni, Sn, Cu | Ccp, Sp | Mt, Po | Au |
| Py-3 | Ni, As, Zn, Se, Ag, Sn, Cu, Au, Te, Pb | Bi | Ccp, Gn, Hs, Pz, Alt, Cal, Syl, Au, Sp | Po | Ks, Cal, Pz, Hs |
| Py-4 | Co, Ni | Se, Ag, Au, Sn, Te, Bi, Zn, Pb, Cu | Ccp | ||
| Chemical Element | Zones | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 197Au | 0.52 | 0.06 | 2.7 | 0.03 | 1 |
| 107Ag | 2.5 | 0.6 | 8.6 | 1.14 | 2.4 |
| 124Te | 0.88 | 0.7 | 0.23 | 0.19 | 0.3 |
| n | Concentration | Co | Ni | As | Se | Ag | Au | Sn | Te | Bi |
|---|---|---|---|---|---|---|---|---|---|---|
| Chalcopyrite-1 (gold-sulphide assemblage) | ||||||||||
| 2 | min | 0.02 | 0.03 | 4 | 14 | 0.7 | 0.01 | 0.07 | 0.02 | 6 |
| max | 143 | 272 | 80 | 194 | 63 | 2.5 | 4 | 68 | 8 | |
| geom. mean | 0.4 | 15 | 30 | 43 | 7 | 0.3 | 1 | 0.8 | 7 | |
| Chalcopyrite-2 (gold-telluride assemblage) | ||||||||||
| 21 | min | 0.08 | 0.03 | 26 | 28 | 870 | 6 | 1 | 870 | 0.05 |
| max | 0.14 | 57 | 44 | 32 | 7600 | 25 | 1.8 | 4200 | 11 | |
| geom. mean | 0.11 | 1.3 | 33.8 | 30 | 2571 | 12.3 | 1.4 | 1912 | 0.3 | |
| Mineral | Main trace Elements | Minor Elements (<50 ppm) | Mineral Inclusions | ||
|---|---|---|---|---|---|
| Main | Rare | Submicroscopic | |||
| Ccp-1 | Co, Ni, As, Se, Sn, Ag, Te | Bi, Au | Sp, Py | Cbt, Fhl | |
| Ccp-2 | Ag, Au, Te, Bi Ni | As, Se, Sn | Sp | Hs, Au | Alt, Pz, Au, Hs, Cal |
| n | Concentration | Co | Ni | As | Se | Ag | Au | Sn | Te | Bi |
|---|---|---|---|---|---|---|---|---|---|---|
| 37 | Galena-1 (gold-sulphide assemblage) | |||||||||
| min | 0.02 | 0.02 | 0.8 | 1.2 | 0.7 | 0.09 | 0.03 | 0.03 | 0.01 | |
| max | 1060 | 670 | 93 | 407 | 42 | 5 | 0.8 | 730 | 2.7 | |
| geom. mean | 0.6 | 3 | 10 | 19 | 7 | 0.6 | 0.3 | 11.4 | 0.2 | |
| 6 | Galena-2 (gold-telluride assemblage) | |||||||||
| min | 0.13 | 3.1 | 2.5 | 2.4 | 23 | 13 | 0.13 | 56 | 0.01 | |
| max | 1030 | 252 | 73 | 58 | 2050 | 980 | 0.4 | 1770 | 62 | |
| geom. mean | 200 | 70 | 31 | 16 | 263 | 74 | 0.2 | 252 | 1 | |
| Mineral | Main Trace Elements | Minor Elements (<50 ppm) | Mineral Inclusions | ||
|---|---|---|---|---|---|
| Common | Rare | Submicroscopic | |||
| Gn-1 | Co, Ni, As, Se, Sn, Te | Ag, Au, Bi | Sp, Py, Ccp | Hs, Em | |
| Gn-2 | Ag, Au, Te, Bi, Co, Ni, As, Se | Sn | Alt | Pz, Au, Hs | |
| n | Concentration | Co | Ni | As | Sb | Ag | Au | Sn | Te | Bi |
|---|---|---|---|---|---|---|---|---|---|---|
| Pyrite-1 (magnetite-pyrite assemblage) | ||||||||||
| 14 | min | 144 | 3.3 | 6 | 0.06 | 0.02 | 0.05 | 0.08 | 0.02 | 0.02 |
| max | 3530 | 774 | 11,050 | 15 | 64 | 12 | 0.3 | 89 | 4.5 | |
| geom. mean | 1938 | 34 | 1625 | 0.7 | 0.8 | 0.6 | 0.2 | 7 | 0.15 | |
| Pyrite-2 (polymetallic assemblage) | ||||||||||
| 12 | min | 0.8 | 0.02 | 0.6 | 0.05 | 0.16 | 0.04 | 0.09 | 1.3 | 0.02 |
| max | 922 | 2 | 5287 | 60 | 14 | 1.3 | 0.4 | 33 | 5 | |
| geom. mean | 288 | 79 | 735 | 13 | 6 | 0.44 | 0.2 | 11 | 0.8 | |
| Pyrite-3 (gold-sulphide-magnetite assemblage in skarns) | ||||||||||
| 7 | min | 14 | 5 | 53 | 0.05 | 0.2 | 0.16 | 0.13 | 0.5 | 0.03 |
| max | 592 | 18 | 4168 | 0.3 | 2 | 1.2 | 0.3 | 12 | 0.6 | |
| geom. mean | 90 | 11 | 70 | 0.2 | 0.6 | 0.4 | 0.2 | 3.5 | 0.1 | |
| Pyrite-4 (gold-sulphide and gold-telluride assemblage) | ||||||||||
| 5 | min | 146 | 11 | 60 | 0.08 | 0.04 | 0.3 | 0.12 | 26 | 0.03 |
| max | 707 | 416 | 455 | 0.65 | 159 | 2 | 0.3 | 141 | 15 | |
| geom. mean | 378 | 57 | 142 | 0.2 | 4 | 0.6 | 0.23 | 50 | 0.51 | |
| Pyrite-5 (quartz-carbonate assemblage) | ||||||||||
| 7 | min | 0.8 | 0.3 | 0.6 | 0.02 | 0.03 | 0.07 | 0.2 | 1.2 | 0.02 |
| max | 920 | 251 | 3830 | 98 | 2.3 | 0.6 | 0.36 | 39 | 0.8 | |
| geom. mean | 177 | 14 | 284 | 0.4 | 0.3 | 0.3 | 0.2 | 7.7 | 0.1 | |
| Mineral | Main Trace Elements * | Minor Elements (<50 ppm) | Minerals Inclusions | |||
|---|---|---|---|---|---|---|
| Maximum, ppm | High Values, ppm | Main | Rare | Submicroscopic | ||
| Py-1 | Co, Ni, As, Au, Zn, Cu | Ag, Te | Sb, Sn, Bi | Mt, Ccp | Cbt | Cbt, As |
| Py-2 | Sb, Sn | Co, As, Cu | Ni, Ag, Au, Te, Bi, Zn | Ccp, Sp, Gn | Hem, Cbt, Apy, Po | |
| Py-3 | Co, As | Ni, Sb, Sn, Au, Ag, Te, Bi, Cu, Zn | Ccp | Cbt, Ag | ||
| Py-4 | Ag, Te, Bi | Co, Ni, As, Cu | Au, Sb, Sn, Zn | Pt, Hs, Alt | Au, Ag | Pz, Clc, Gt, Ccp, Dg |
| Py-5 | Sb | Co, Ni, As, Cu | Ag, Au, Sn, Te, Bi, Zn | Bn, Cv, Clc, Gt, Dg, Cu | ||
| n | Concentration | Co | Ni | As | Sb | Ag | Au | Sn | Te | Bi |
|---|---|---|---|---|---|---|---|---|---|---|
| 22 | Chalcopyrite-1 (polymetallic assemblage) | |||||||||
| min | 0.02 | 0.02 | 2.8 | 0.04 | 0.2 | 0.02 | 0.06 | 0.07 | 0.02 | |
| max | 887 | 8.8 | 3230 | 37 | 88 | 13 | 2.14 | 9.1 | 0.4 | |
| geom. mean | 1.3 | 0.31 | 73 | 0.8 | 2.34 | 0.22 | 0.15 | 0.5 | 0.04 | |
| Chalcopyrite-2 (gold-sulphide-magnetite assemblage in skarns) | ||||||||||
| 4 | min | 0.02 | 0.1 | 0.9 | 0.03 | 0.03 | 0.06 | 0.2 | 5.7 | 1.03 |
| max | 50 | 5.7 | 23 | 3.5 | 11.3 | 1 | 0.6 | 15 | 3 | |
| geom. mean | 1.3 | 2 | 5 | 0.75 | 1.7 | 0.4 | 0.4 | 9 | 1.8 | |
| Mineral | Main Trace Elements | Minor Elements (<50 ppm) | Mineral Inclusions | |||
|---|---|---|---|---|---|---|
| Maximum, ppm | High Values, ppm | Main | Rare | Submicroscopic | ||
| Ccp-1 | Co, Ni, As, Sb, Ag, Au, Sn | Te, Bi | Po, Sp, Gn | Cal, Alt, Au, Hs, Pz | ||
| Ccp-2 | Te, Bi | Co | Ni, As, Sb, Ag, Au, Sn | Au | ||
| Highly Metamorphosed | Weakly Metamorphosed | Non-Metamorphosed | |
|---|---|---|---|
| Gai | Uchaly | Uzelga | Galka |
| Hessite Ag2Te, native gold (710–980), native silver (100–0), calaverite AuTe2, sylvanite AuAgTe4, krennerite (Au,Ag)Te2, petzite Ag3AuTe2, montbrayite (Au,Sb)2Te3, muthmannite AuAgTe2, acanthite Ag2S, Ag-betekhtenite (Ag,Cu,Fe)21Pb2S15 | Hessite Ag2Te, empressite AgTe, electrum (340–690), freibergite Ag12Sb4S13, petrovskaite AuAg(S,Se), acanthite Ag2S, pearceite [Ag9CuS4][(Ag,Cu)6(As,Sb)2S7], polybasite [(Ag,Cu)6(Sb,As)2S7][Ag9CuS4], ytenbogaardtite AuAg3S2 | Hessite Ag2Te, stützite Ag5Te3, native gold (770–870), native silver, acanthite Ag2S, sylvanite AuAgTe4, petzite Ag3AuTe2 | Native gold (700–1000), electrum (250–700), acanthite Ag2S, freibergite Ag12Sb4S13, argentotetrahedrite, pyrargyrite Ag3SbS3, stephanite Ag5SbS4, proustite Ag3AsS3, polybasite [(Ag,Cu)6(Sb,As)2S7][Ag9CuS4], petrovskaite AuAg(S,Se), ytenbogaardtite AuAg3S2, kurilite Ag8Te3Se |
| Deposit | n | Ci | Au | Ag | Se | Te | As |
|---|---|---|---|---|---|---|---|
| Gai | 17 | min | 1.4 | 3.8 (8) | 7 | 11 (8) | 25 |
| max | 31 | 47.5 | 267 | 115 | 1901 | ||
| geom. mean | 3.8 | 8 | 61 | 24 | 226 | ||
| k (Au) | 0.27 | −0.05 | 0.2 | 0.64 | |||
| Uzelga | 67 | min | 0.01 | 0.3 | 2 (66) | 1 (19) | 0.2 (66) |
| max | 20 | 204 | 657 | 197 | 5599 | ||
| geom. mean | 0.5 | 15 | 83 | 22 | 114 | ||
| k (Au) | 0.57 | 0.02 | 0.96 | −0.13 | |||
| Galka | 27 | min | 0.01 | 2.2 | 0.5 (13) | 0.8 (10) | 14 (26) |
| max | 9 | 934 | 32 | 392 | 15,486 | ||
| geom. mean | 1.2 | 57 | 3.5 | 19 | 450 | ||
| k (Au) | 0.17 | 0.49 | 0.23 | 0.13 | |||
| Uchaly | 79 | min | 0.01 | 0.01 | 3 | 3 (42) | 34 |
| max | 29 | 326 | 1800 | 2500 | 5370 | ||
| geom. mean | 1.6 | 13 | 54 | 54 | 1311 | ||
| k (Au) | 0.67 | −0.26 | −0.11 | 0.3 | |||
| Deposit | n | Minerals | Au, ppm | Ag, ppm | ||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | geom. mean | Min | Max | geom. mean | |||
| Gai | 17 | pyrite | 1.4 | 31 | 3.8 | 3.8 | 47.5 | 8 |
| 11 | chalcopyrite | 0.01 | 1.2 | 0.06 | 0.19 | 18 | 1.2 | |
| 4 | sphalerite | 0.01 | 0.03 | 0.016 | 2.4 | 5.5 | 3.2 | |
| 10 | bornite | 0.01 | 0.99 | 0.04 | 5.3 | 1250 | 223 | |
| 16 | fahlore | 0.01 | 1.4 | 0.05 | 132 | 432 | 285 | |
| Uzelga | 67 | pyrite | 0.01 | 20 | 0.5 | 0.3 | 204 | 15 |
| 17 | chalcopyrite | 0.01 | 19 | 0.2 | 0.3 | 511 | 7.4 | |
| 14 | sphalerite | 0.01 | 11 | 0.4 | 1.3 | 207 | 21.5 | |
| Galka | 27 | pyrite | 0.01 | 9 | 1.2 | 2.2 | 934 | 57 |
| 4 | chalcopyrite | 1.2 | 3.7 | 1.9 | 32 | 127 | 76 | |
| 21 | sphalerite | 0.01 | 32 | 0.64 | 1.2 | 167 | 51 | |
| 5 | marcasite | 0.02 | 5 | 0.6 | 7 | 60 | 22 | |
| 1 | galena | 0.05 | 153 | |||||
| Uchaly | 79 | pyrite | 0.01 | 29 | 1.6 | 0.01 | 326 | 13 |
| 3 | chalcopyrite | 1 | 6 | 3 | 0.04 | 0.15 | 0.07 | |
| Deposit | n | Ci | Au | Ag | Se | Te | As |
|---|---|---|---|---|---|---|---|
| Gai | 11 | min | 0.01 | 0.19 | 0.01 (10) | 0.01 (10) | 6 |
| max | 1.2 | 18 | 167 | 22.05 | 312 | ||
| geom. mean | 0.06 | 1.2 | 19 | 0.02 | 15 | ||
| k (Au) | 0.96 | 0.7 | 0.97 | −0.05 | |||
| Uzelga | 17 | min | 0.01 | 0.3 | 39 | 0.01 (14) | 9 (7) |
| max | 19 | 511 | 714 | 314 | 1530 | ||
| geom. mean | 0.2 | 7.4 | 217 | 4.7 | 109 | ||
| k (Au) | 0.87 | −0.52 | 0.9 | −0.48 | |||
| Galka | 4 | min | 1.2 | 32 | - | 0.01 | 556 |
| max | 3.7 | 127 | - | 326 | 4058 | ||
| geom. mean | 1.9 | 76 | - | 5 | 1615 | ||
| Uchaly | 3 | min | 1 | 0.04 | 7 | 2 | 14 |
| max | 6 | 0.15 | 73 | 4 | 45 | ||
| geom. mean | 3 | 0.07 | 25 | 2.9 | 22 | ||
| Deposit | n | Ci | Au | Ag | Se | Te | As |
|---|---|---|---|---|---|---|---|
| Gai | 4 | min | 0.01 | 2.4 | 0.01 | 0.01 | 2 |
| max | 0.03 | 5.5 | 2.3 | 0.03 | 3.6 | ||
| geom. mean | 0.016 | 3.2 | 0.4 | 0.19 | 2.8 | ||
| Uzelga | 14 | min | 0.01 | 1.3 | 0.01 | 1.1 (9) | 2.8 (8) |
| max | 11 | 207 | 470 | 1367 | 1195 | ||
| geom. mean | 0.4 | 21.5 | 13 | 47 | 81 | ||
| k (Au) | 0.8 | 0.1 | 0.99 | 0.7 | |||
| Galka | 21 | min | 0.01 | 1.2 | 0.3 (22) | 0.4 (15) | 1.2 (20) |
| max | 32 | 167 | 94 | 258 | 5426 | ||
| geom. mean | 0.64 | 51 | 11 | 15 | 139 | ||
| k (Au) | 0.89 | −0.6 | 0.38 | 0.1 | |||
| Deposit | Minerals | Pt | Pd | Rh | Ru | Os | Ir |
|---|---|---|---|---|---|---|---|
| Gai | fahlore | - | - | 12.6 | 0.062 | 0.005 | 0.01–0.07 |
| sphalerite | 0.016 | - | - | - | - | - | |
| Uchaly | pyrite | - | 0.03–0.2 | 0.04–0.26 | - | - | - |
| Sample | 2027 | 2032 |
|---|---|---|
| CIn | 86 ± 5 | 5400 ± 200 |
| CAu | 5 ± 3 | 84 ± 10 |
| Samples | 2289 | 2290 | 2291 | 2292 |
|---|---|---|---|---|
| CIn | 64 ± 14 | 1913 ± 113 | 6914 ± 212 | 7012 ± 201 |
| CAu | 6 ± 1 | 3314 ± 112 | 5142 ± 224 | 6033 ± 511 |
| f S2, bar | 0.1 1 | 0.23 | 2.26 | 8.72 |
| FeS, mol% | wt% ± 2σ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Zn | S | Fe | Mn | Cd | Se | In | Au | Total | |
| 2.80 | 63.95 ± 0.51 | 33.73 ± 0.32 | 1.62 ± 0.13 | 0.24 ± 0.05 | 0.48 ± 0.11 | 0.13 ± 0.07 | 0.32 ± 0.03 | 0.30 ± 0.05 | 100.77 |
| Sample (color 1) | FeS, mol.% | EPMA, wt% ± 2σ | Total | Formula | LA-ICPMS, wt% ± 2σ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | S | Fe | Mn | Cd | Se | In | Au | |||||
| 1660 (blue) | 3.70 | 64.15 (0.50) | 33.72 (0.11) | 2.15 (0.07) | 0.12 (0.02) | 0.50 (0.04) | 0.25 (0.03) | 0.17 (0.02) | 0.20 (0.01) | 101.26 | Zn0.94Fe0.04S1.01 | 0.1892 (0.0112) |
| 1661 (grey) | 3.24 | 65.08 (0.43) | 33.82 (0.49) | 1.89 (0.06) | - | - | - | 0.22 (0.03) | 0.20 (0.01) | 101.21 | Zn0.95Fe0.03S1.01 | 0.2092 (0.0082) |
| 1662 (black) | 1.67 | 64.77 (1.61) | 33.37 (0.85) | 0.96 (0.01) | - | - | - | - | - | 99.66 | Zn0.96Fe0.02 Cu0.02S1.01 | 0.0077 (0.0046) |
| 1663 (green) | 4.20 | 64.17 (0.89) | 33.51 (0.39) | 2.44 (0.08) | 0.50 (0.10) | - | - | - | - | 100.62 | Zn0.94Fe0.04S1.01 | 0.0094 (0.0012) |
| 1665 2 (red) | 4.33 | 63.29 (1.45) | 33.33 (0.71) | 2.48 (0.22) | - | - | - | - | - | 99.10 | Zn0.94Fe0.04S1.01 | 0.0096 (0.0046) |
| 1666 (yellow) | - | 67.09 (0.64) | 33.19 (0.23) | - | - | - | 0.94 (0.06) | - | - | 101.22 | Zn0.99SSe0.01 | 0.0014 (0.0007) |
| Sample | EPMA, wt% (±2σ) | LA-ICPMS Au, wt% (±2σ) | |||||
|---|---|---|---|---|---|---|---|
| Cd | S | Fe | In | Au | Total | ||
| 5457 | 78.18 (0.07) | 22.23 (0.17) | - | - | bdl | 100.41 (0.24) | 0.0015 (±0.0005) |
| 5458 | 74.78 (0.22) | 22.84 (0.29) | 2.84 (0.04) | - | bdl | 100.46 (0.55) | 0.0410 (±0.0005) |
| 5459 | 77.76 (0.62) | 22.27 (0.23) | - | 0.73 (0.03) | bdl | 100.77 (0.88) | 0.0311 (±0.0020) |
| Mineral | Formula | Space Group | Lattice Constants (Å) | Structure Type | Me-Me Distance | Me-S Distance | CNMe | CPMe | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Pyrite | FeS2 | Pa-3 | a = 5.416 | NaCl | 3.830 | 2.263 | 6 | Octahedron | [165] |
| Marcasite | FeS2 | Pnnm | a = 4.445 b = 5.425 c = 3.386 | Rutile | 3.386 | 2 × 2.239 4 × 2.252 | 6 | Octahedron | [165] |
| Arsenopyrite | FeAsS | P21/c | a = 5.761 b = 5.684 c = 5.767 β = 111.7210° | Rutile | 2.734 | S: 2.229, 2.230, 2.233 As: 2.370, 2.409, 2.412 | 6 | Octahedron | [166] |
| Lollengite | FeAs2 | Pnnm | a = 5.300 b = 5.984 c = 2.882 | Rutile | 2.882 | 2 × 2.361 4 × 2.388 | 6 | Octahedron | [167] |
| Galena | PbS | Fm-3m | a = 5.805 | NaCl | 4.105 | 2.903 | 6 | Octahedron | [168] |
| Pyrrhotite | FenSn+1 | P63/mmc | a = 3.446 c = 5.743 | NiAs | 2.871 | 2.453 | 6 | Octahedron | [169] |
| Chalcopyrite | CuFeS2 | I-42d | a = 5.277 c = 10.441 | ZnS | 3.712 | Cu: 2.295 Fe: 2.259 | 4 | Tetrahedron | [170] |
| Greenockite | CdS | P63mc | a = b = 4.136 c = 6.716 | ZnO | 4.121 | 3 × 2.527 1 × 2.532 | 4 | Tetrahedron | [171] |
| Sphalerite | ZnS | F-43m | a = b = c = 5.410 | ZnS | 3.826 | 2.343 | 4 | Tetrahedron | [172] |
| Würtzite | ZnS | P63mc | a = b = 3.823 c = 6.261 | ZnO | 3.823 | 3 × 2.342 1 × 2.347 | 4 | Tetrahedron | [173] |
| CN | Type of Hybridisation | Elements |
|---|---|---|
| 2 | sp; p2 | Cu+, Cu2+, Ag+, Au+ |
| 4tetr | sp3 | Cu+, Ag+, Au+, Zn2+, Fe2+ |
| 4sq | dsp2 | Pd2+, Pt2+, Cu2+, Fe3+ |
| 6 | d2sp3 | Pt4+, Fe2+ |
| Mineral | Formula | Space Group | Lattice Constants (Å) | Structure Type | Me-Me Distance | Me-S Distance | CNMe | CPMe | Ref |
|---|---|---|---|---|---|---|---|---|---|
| n/a | Au2S | Pn-3m | 5.0206 | Cu2O | 3.550 | 2.174 | 2 | Dumbbell | [174] |
| Acanthite | α-Ag2S | P121/c1 | a = 4.231 b = 6.930 c = 8.293 β = 110.71° | Ag2S | 3.084 | AgI: 2.475, 2.511 AgII: 2.547, 2.563, 2.699 | AgI: 2 AgII: 3 | AgI: dumbbell AgII: coplanar triangle | [175] |
| Cooperite | PtS | P42/mmc | a = 3.4701 c = 6.1092 | PtS | 3.470 | 2.311 | 4 | Square | [176] |
| n/a | PtS2 | P-3m1 | a = 3.5432 c = 5.0388 | CdI2 | 3.543 | 2.421 | 6 | Octahedron | [177] |
| n/a | PdS | P42/m | a = 6.429 c = 6.611 | PdS | PdI-PdI: 3.305 PdII-PdIII: 3.389 PdIII-PdIII: 2.337 | PdI: 2.341 PdII: 2.318 PdIII: 2 × 2.337, 2.346 | 4 | Square | [178] |
| n/a | PdS2 | Pbca | a = 5.460 b = 5.541 c = 7.531 | PdSe2 | 3.889 | PdI: 2 × 2.298, 2 × 2.304, 2 × 3.312 | 6 | Octahedron | [179] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikentyev, I.; Vikent’eva, O.; Tyukova, E.; Nikolsky, M.; Ivanova, J.; Sidorova, N.; Tonkacheev, D.; Abramova, V.; Blokov, V.; Spirina, A.; et al. Noble Metal Speciations in Hydrothermal Sulphides. Minerals 2021, 11, 488. https://doi.org/10.3390/min11050488
Vikentyev I, Vikent’eva O, Tyukova E, Nikolsky M, Ivanova J, Sidorova N, Tonkacheev D, Abramova V, Blokov V, Spirina A, et al. Noble Metal Speciations in Hydrothermal Sulphides. Minerals. 2021; 11(5):488. https://doi.org/10.3390/min11050488
Chicago/Turabian StyleVikentyev, Ilya, Olga Vikent’eva, Eugenia Tyukova, Maximilian Nikolsky, Julia Ivanova, Nina Sidorova, Dmitry Tonkacheev, Vera Abramova, Vyacheslav Blokov, Adelina Spirina, and et al. 2021. "Noble Metal Speciations in Hydrothermal Sulphides" Minerals 11, no. 5: 488. https://doi.org/10.3390/min11050488
APA StyleVikentyev, I., Vikent’eva, O., Tyukova, E., Nikolsky, M., Ivanova, J., Sidorova, N., Tonkacheev, D., Abramova, V., Blokov, V., Spirina, A., Borisova, D., & Palyanova, G. (2021). Noble Metal Speciations in Hydrothermal Sulphides. Minerals, 11(5), 488. https://doi.org/10.3390/min11050488






