The Occurrence of Selected Radionuclides and Rare Earth Elements in Waste at the Mine Heap from the Polish Mining Group
Abstract
:1. Introduction
2. Research Materials and Methods
3. Results and Discussion
3.1. Radionuclides
3.2. REE
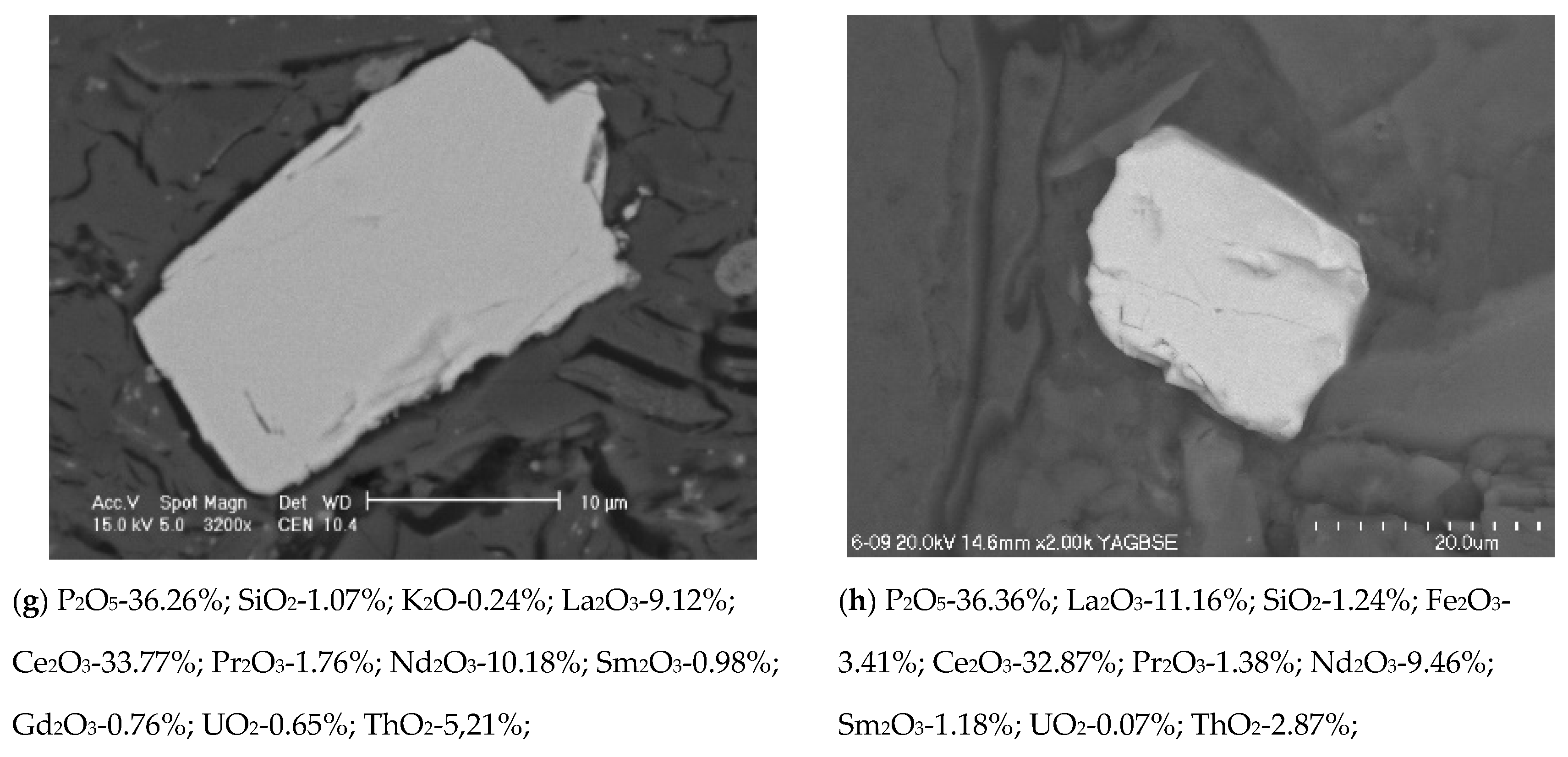
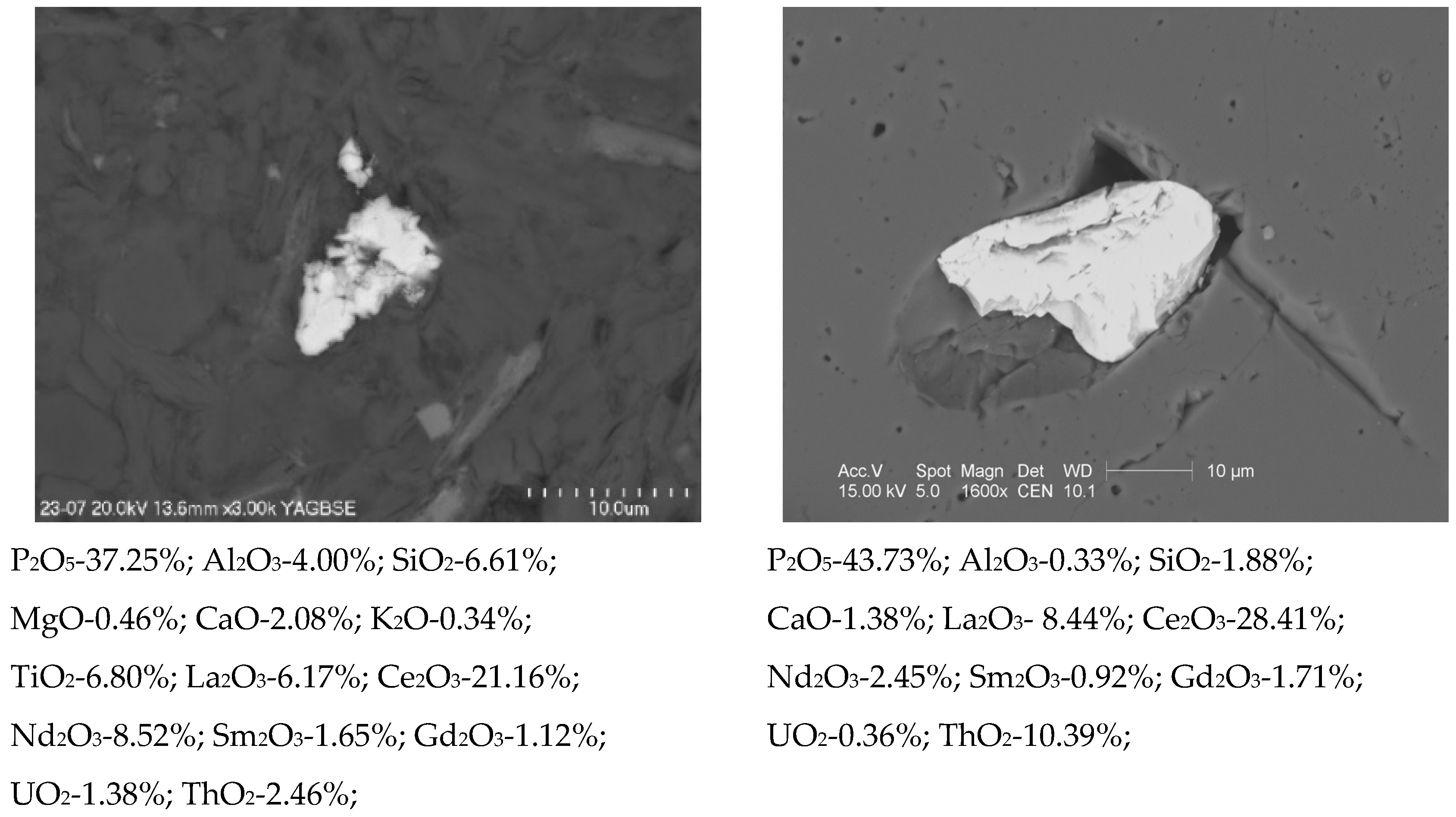
3.3. Phase Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GUS—Główny Urząd Statystyczny. Stistical Yearbook of Industry—Poland; Statistics Poland: Warszawa, Poland, 2019.
- Klojzy-Karczmarczyk, B.; Mazurek, J.; Paw, K. Możliwość zagospodarowania kruszyw i odpadów wydobywczych górnictwa węgla kamiennego ZG Janina w procesach rekultywacji wyrobisk odkrywkowych. Gospod. Surowcami Miner. MRM 2016, 32, 111–134. [Google Scholar] [CrossRef] [Green Version]
- Fabiańska, M.J.; Nádudvari, Á.; Ciesielczuk, J.; Szram, E.; Misz-Kennan, M.; Więcław, D. Organic contaminants of coal-waste dump water in the Lower- and Upper Silesian Coal Basins (Poland). Apllied Geochem. 2020, 122, 104690. [Google Scholar] [CrossRef]
- Jonczy, I.; Gawor, Ł. Coal mining and post-metallurgic dumping grounds and their connections with exploitation of raw materials in the region of Ruda Śląska. Arch. Min. Sci. 2017, 62, 301–311. [Google Scholar] [CrossRef]
- Ciesielczuk, J.; Misz-Kennan, M.; Hower, J.C.; Fabiańska, M.J. Mineralogy and geochemistry od coal wastes from the Starzykowiec coal-waste dump (Upper Silesia, Poland). Int. J. Coal G. 2014, 127, 42–55. [Google Scholar] [CrossRef]
- Marcisz, M.; Probierz, K.; Gawor, Ł. Possibilities of reclamation and using of large-surface coal mining dumping grounds in Poland. Gospod. Surowcami Miner. MRM 2020, 36, 105–122. [Google Scholar]
- Nádudvari, Á.; Fabiańska, M.J. Use of geochemical analysis and vitrinite recleftance to assess different self-heating processes in coal-waste dump (Upper Silesia, Poland). Fuel 2016, 181, 102–119. [Google Scholar] [CrossRef]
- Kruszewski, Ł.; Fabiańska, M.J.; Segit, T.; Kusy, D.; Motyliński, R.; Ciesielczuk, J.; Deput, E. Carbon nitrogen compounds, alcohols, mercaptans, monoterpenes, acetates, aldehydes, ketones, SF6, PH3, and other fire gases in coal-mninig waste heaps of Upper Silesian Coal Basin (Poland)—a re-investigation by means of in situ FTIR external database approach. Sci. Total Environ. 2020, 698, 134274. [Google Scholar]
- Lauer, N.E.; Hower, J.C.; Hsu-Kim, H.; Taggart, R.K.; Vengosh, A. Naturally Occurring Radioactive Materials in Coals and Coal Combustion Residuals in the United States. Environ. Sci. Technol. 2015, 49, 11227–11233. [Google Scholar] [CrossRef]
- Kolo, M.T.; Khandaker, M.U.; Amin, Y.M.; Abdullah, W.H.B. Quantification and Radiological Risk Estimation Due to the Presence of Natural Radionuclides in Maiganga Coal, Nigeria. PLoS ONE 2016, 11, e0158100. [Google Scholar] [CrossRef]
- Kozłowska, B.; Walencik, A.; Dorda, J.; Zipper, W. Radioactivity of dumps in mining areas of The Upper Silesian Coal Basin in Poland. In EPJ Web of Conferences; EDP Sciences: Les Ulis, France, 2012; Volume 24, p. 05006. [Google Scholar]
- Singh, R.K.; Gupta, N.C.; Guha, B.K. pH dependence leaching characteristics of selected metals from coal ash and its impact on ground water quality. Int. J. Chem. Env. Enginn. 2014, 5, 218–222. [Google Scholar]
- Da Silva, E.B.; Li, S.; De Oliveira, L.M.; Gress, J.; Dong, X.; Wilkie, A.C.; Townsend, T.; Ma, L.Q. Metal leachability from coal combustion residuals under different pHs and liquid/solid ratios. J. Hazard. Mater. 2018, 341, 66–74. [Google Scholar] [CrossRef]
- Dai, S.; Li, D.; Chou, C.-L.; Zhao, L.; Zhang, Y.; Ren, D.; Ma, Y.; Sun, Y. Mineralogy and geochemistry of boehmite-rich coals: New insights from the Haerwusu Surface Mine, Jungar Coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Zhou, Y.; Hower, J.C.; Li, D.; Chen, W.; Zhu, X.; Zou, J. Chemical and mineralogical compositions of silicic, mafic, and alkali tonsteins in the late Permian coals from the Songzao Coalfield, Chongqing, Southwest China. Chem. Geol. 2011, 282, 29–44. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Blissett, R.S.; Smalley, N.; Rowson, N.A. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Howard, B.H.; Roth, E.A.; Bank, T.L.; Granite, E.J.; Soong, Y. Enrichment of rare earth elements from coal and coal by-products by physical separations. Fuel 2017, 200, 506–520. [Google Scholar] [CrossRef] [Green Version]
- Arbuzov, S.; Chekryzhov, I.; Finkelman, R.; Sun, Y.; Zhao, C.; Il’Enok, S.; Blokhin, M.; Zarubina, N. Comments on the geochemistry of rare-earth elements (La, Ce, Sm, Eu, Tb, Yb, Lu) with examples from coals of north Asia (Siberia, Russian far East, North China, Mongolia, and Kazakhstan). Int. J. Coal Geol. 2019, 206, 106–120. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A.; Dołęgowska, S. Extreme enrichment of arsenic and rare earth elements in acid mine drainage: Case study of Wiśniówka mining area (south-central Poland). Environ. Pollut. 2019, 244, 898–906. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Pierwiastki Śladowe w Geo- i Biosferze; IUNG-PIB: Puławy, Poland, 2012. (In Polish) [Google Scholar]
- Rostron, P.D.; Ramsey, M.H. Cost effective, robust estimation of measurement uncertainty from sampling using unbalanced ANOVA. Accredit. Qual. Assur. 2011, 17, 7–14. [Google Scholar] [CrossRef]
- Róg, L. Natural radioactivity of hard coals and coal density fractions of differentiated petrografical and chemical construction. In Prace Naukowe GIG. Górnictwo i Środowisko/Główny Instytut Górnictwa; Main Mining Institute: Katowice, Poland, 2005; pp. 81–101. (In Polish) [Google Scholar]
- Bojakowska, I.; Lech, D.; Wołkowicz, S. Uranium and thorium in hard and brown coals from Polish deposits. Gospod. Surowcami Miner. MRM 2008, 24, 53–65. [Google Scholar]
- Parzentny, H.R.; Róg, L. The role of mineral matter in concentrating uranium and thorium in coal and combustion residues from power plant in Poland. Minerals 2019, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Huang, W.; Wan, H.; Finkelman, R.B.; Tang, X.; Zhao, Z. Distribution of Uranium in the Main Coalfields of China. Energy Explor. Exploit. 2012, 30, 819–836. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Niu, Y.; Xie, Z.; Zhang, K.; Zhou, J.; Arbuzov, S. Geochemical characteristics of elements in coal seams 41 and 42 of Heshan Coalfield, South China. Energy Explor. Exploit. 2019, 38, 137–157. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Song, W. Mineralogical and Chemical Characteristics of Coal Ashes from Two High-Sulfur Coal-Fired Power Plants in Wuhai, Inner Mongolia, China. Minerals 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Ndhlalose, M.; Malumbazo, N.; Wagner, N. Coal quality and uranium distribution in Springbok Flats Coalfield samples. J. South. Afr. Inst. Min. Met. 2015, 115, 1167–1174. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Walencik-Łata, A.; Smołka-Danielowska, D. 234U, 238U, 226Ra, 228Ra and 40K concentrations in feed coal and its combustion products during technological processes in the Upper Silesian Industrial Region, Poland. Environ. Pollut. 2020, 267, 115462. [Google Scholar] [CrossRef]
- Lu, X.; Li, L.Y.; Wang, F.; Wang, L.; Zhang, X. Radiological hazards of coal and ash samples collected from Xi’an coal-fired pow-er plants of China. Environ. Earth Sci. 2012, 66, 1925–1932. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Q.; Sun, R.; Liu, G. Radioactivity of Natural Nuclides (40K, 238U, 232Th, 226Ra) in Coals from Eastern Yunnan, China. Minerals 2015, 5, 637–646. [Google Scholar] [CrossRef]
- You, M.; Hu, Y.; Lu, J.; Li, C. Evaluation of the radiological characterization in a coal-fired power plant, China. Environ. Prog. Sustain. Energy 2015, 34, 1080–1084. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radia-tion: Sources; United Nations Publications: New York, NY, USA, 2000. [Google Scholar]
- Calusz-Moszko, J.; Białecka, B. Analysis of the possibilities of rare earth elements obtaining from coal and fly ash. Gospod. Surowcami Miner. MRM 2013, 29, 67–80. [Google Scholar]
- Żelazny, S.; Świnder, H.; Jarosiński, A.; Białecka, B. The recovery of rare-earth e metals from fly ash using alkali pre-treatment with sodium hydroxide. Gospod. Surowcami Miner. MRM 2020, 36, 127–144. [Google Scholar]
- Ramakrishna, C.; Thenepalli, T.; Nam, S.Y.; Kim, C.; Ahn, J.W. The brief review on coal origin and distribution of rare earth elements in various coal ash samples. J. Energy Eng. 2018, 27, 61–69. [Google Scholar]
- Zou, J.; Cheng, L.; Guo, Y.; Wang, Z.; Tian, H.; Li, T. Mineralogical and Geochemical Characteristics of Lithium and Rare Earth Elements in High-Sulfur Coal from the Donggou Mine, Chongqing, Southwestern China. Minerals 2020, 10, 627. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Hower, M.N.; Johnston, W.; Song, W.; Wang, P.; Zhang, S. Petrology, mineralogy and chemistry od size-fractioned fly ash from the Jungar power plant, Inner Mongolia, China, with emphasis on the distribution of rare earth elements. Energy Fuels 2014, 28, 1502–1514. [Google Scholar] [CrossRef]
- Masto, R.E.; Ram, L.C.; George, J.; Vetrivel, A.; Selvi, A.K.; Sinha, S.K.; Verma, T.K.; Rout, P.; Prabal, P. Impacts of opencast coal mine and mine fire on the trace elements’ content of the surrounding soil vis-a-vis human health risk. Toxicol. Env. Chem. 2011, 93, 223–237. [Google Scholar] [CrossRef]
- Kokowska-Pawłowska, M. Pierwiastki ziem rzadkich (REE) w iłowcach z wybranych pokładów węgla kamiennego serii mułowcowej i piaskowcowej Górnośląskiego Zagłębia Węglowego. Gospod. Surowcami Miner. MRM 2016, 32, 39–66. [Google Scholar] [CrossRef]
- Nowak, J.; Kokowska-Pawłowska, M. Zmiany koncentracji wybranych pierwiastków ziem rzadkich (REEs) w odpadach węglowych (Changes in the concentration of some rare earth elements in coal waste). Arch. Min. Sci. 2017, 62, 495–507. [Google Scholar]
- Migaszewski, Z.; Gałuszka, A. Pierwiastki ziem rzadkich w kwaśnych wodach kopalnianych—zarys problematyki. Przegląd Geol. 2019, 67, 105–114. [Google Scholar] [CrossRef]
- Grawunder, A.; Merten, D.; Büchel, G. Origin of middle rare earth element enrichment in acid mine drainage-impacted areas. Environ. Sci. Pollut. Res. 2014, 21, 6812–6823. [Google Scholar] [CrossRef]
- Dai, S.; Graham, I.T.; Ward, C.R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- Nádudvari, Á.; Abramowicz, A.; Fabiańska, M.; Misz-Kennan, M.; Ciesielczuk, J. Classification of fires in coal waste dumps based on Landsat, Aster thermal bands and thermal camera in Polish and Ukrainian mining regions. Int. J. Coal Sci. Technol. 2020, 1, 1–16. [Google Scholar] [CrossRef]
- Chang, C.; Li, F.; Liu, C.; Gao, J.; Tong, H.; Chen, M. Fractionation characteristics of rare earth elements (REEs) linked with secondary Fe, Mn, and Al minerals in soils. Acta Geochim. 2016, 35, 329–339. [Google Scholar] [CrossRef]
- Lefticariu, L.; Klitzing, K.L.; Kolker, A. Rare Earth Elements and Yttrium (REY) in coal mine drainage from the Illinois Basin, USA. Int. J. Coal Geol. 2020, 217, 103327. [Google Scholar] [CrossRef]
- Dai, S.; Jiang, Y.; Ward, C.R.; Gu, L.; Seredin, V.V.; Liu, H.; Zhou, D.; Wang, X.; Sun, Y.; Zou, J.; et al. Mineralogical and geochemical compositions of the coal in the Guanbanwusu Mine, Inner Mongolia, China: Further evidence for the existence of an Al (Ga and REE) ore deposit in the Jungar Coalfield. Int. J. Coal Geol. 2012, 98, 10–40. [Google Scholar] [CrossRef]
- Hower, J.C.; Qian, D.; Briot, N.J.; Santillan-Jimenez, E.; Hood, M.M.; Taggart, R.K.; Hsu-Kim, H. Nano-Scale Rare Earth Distribution in Fly Ash Derived from the Combustion of the Fire Clay Coal, Kentucky. Minerals 2019, 9, 206. [Google Scholar] [CrossRef] [Green Version]
- Hower, J.C.; Groppo, J.G.; Joshi, P.; Preda, D.V.; Gamliel, D.P.; Mohler, D.T.; Wiseman, J.D.; Hopps, S.D.; Morgan, T.D.; Beers, T.; et al. Distribution of Lanthanides, Yttrium, and Scandium in the Pilot-Scale Beneficiation of Fly Ashes Derived from Eastern Kentucky Coals. Minerals 2020, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Pluta, I.; Pindel, T.; Janeczek, J. Aktywność izotopów radu w wodach kopalń Górnośląskiego Zagłębia Węglowego. Wiad. Gor. 2012, 6, 351–357. [Google Scholar]

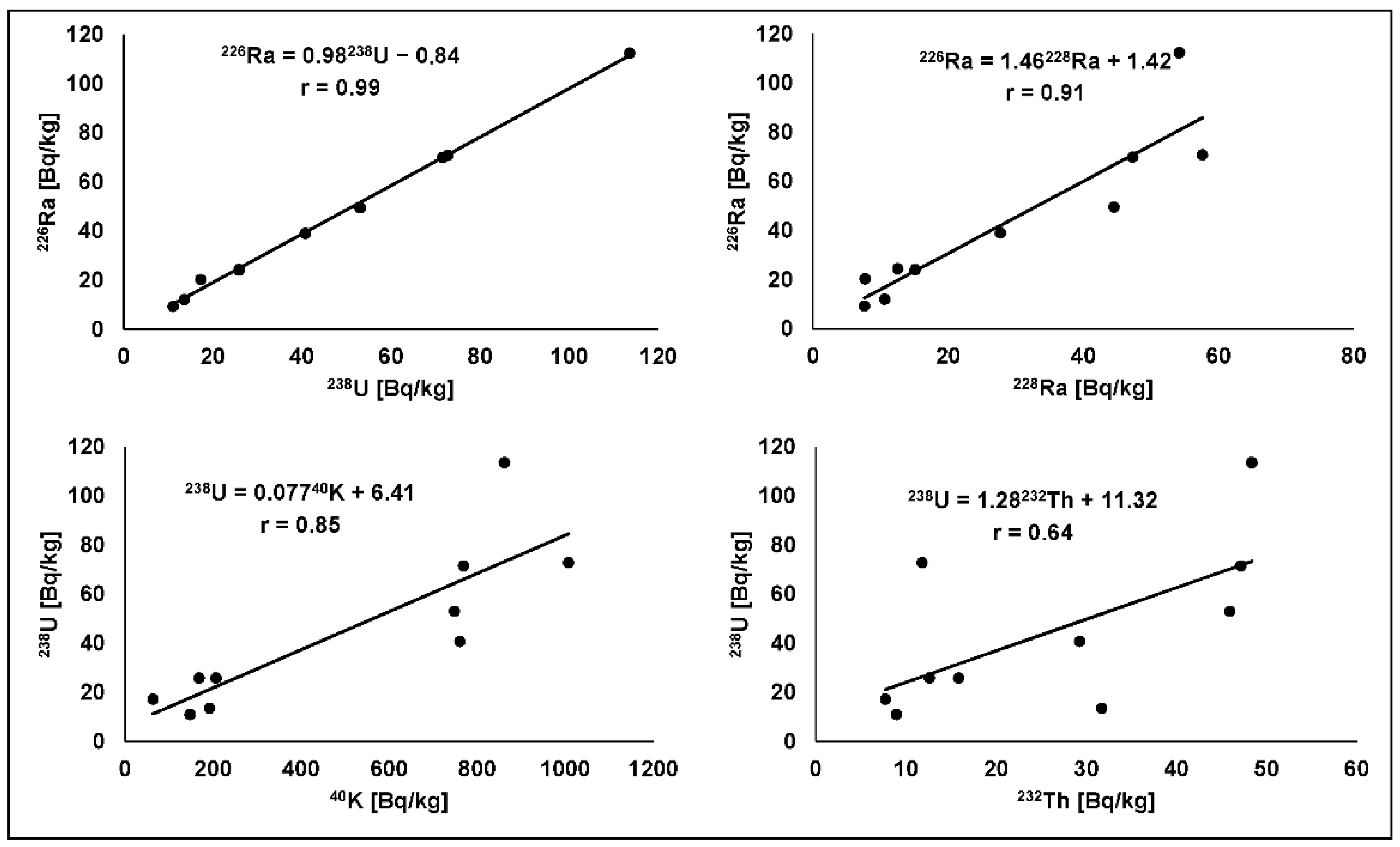
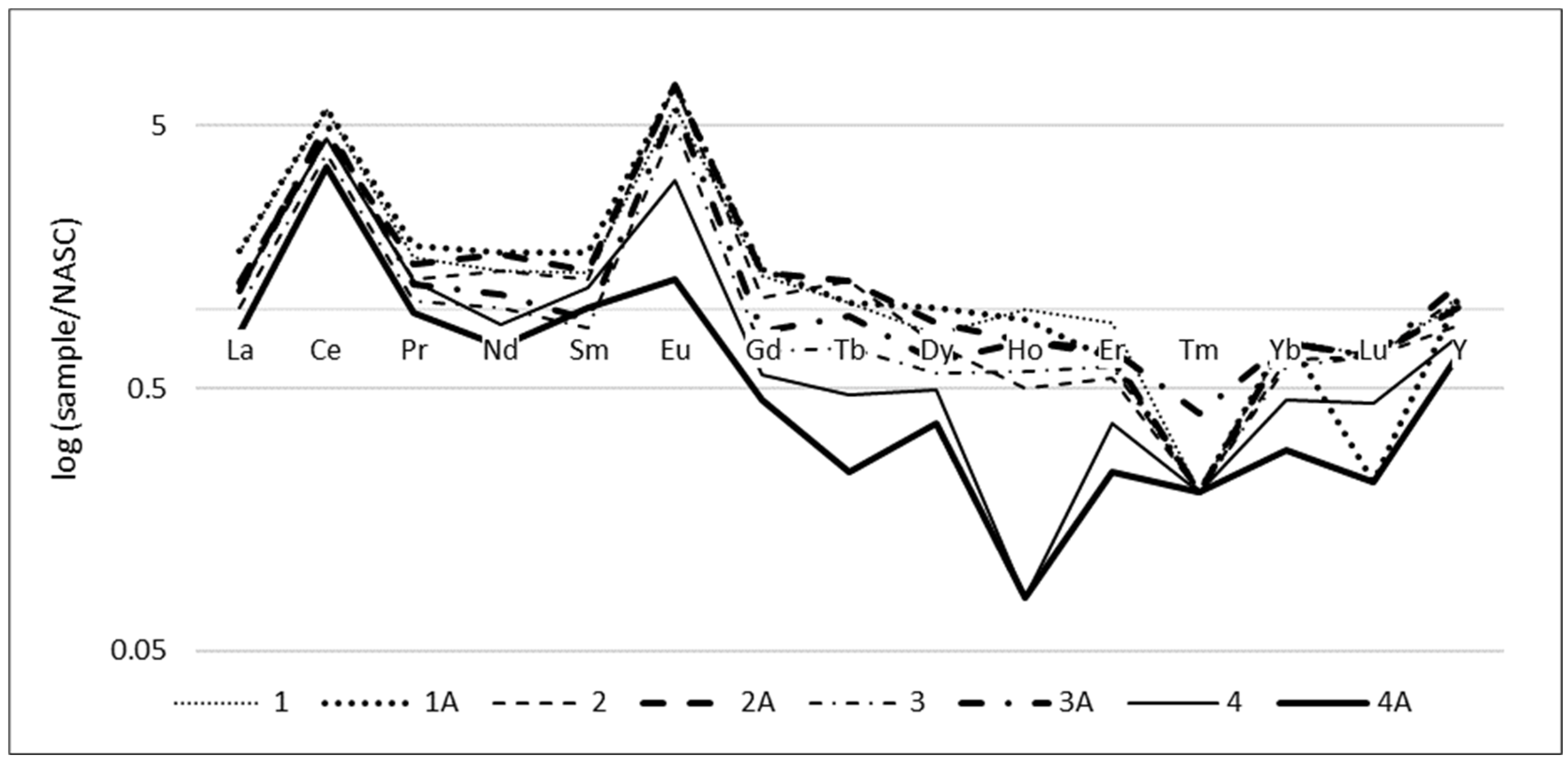

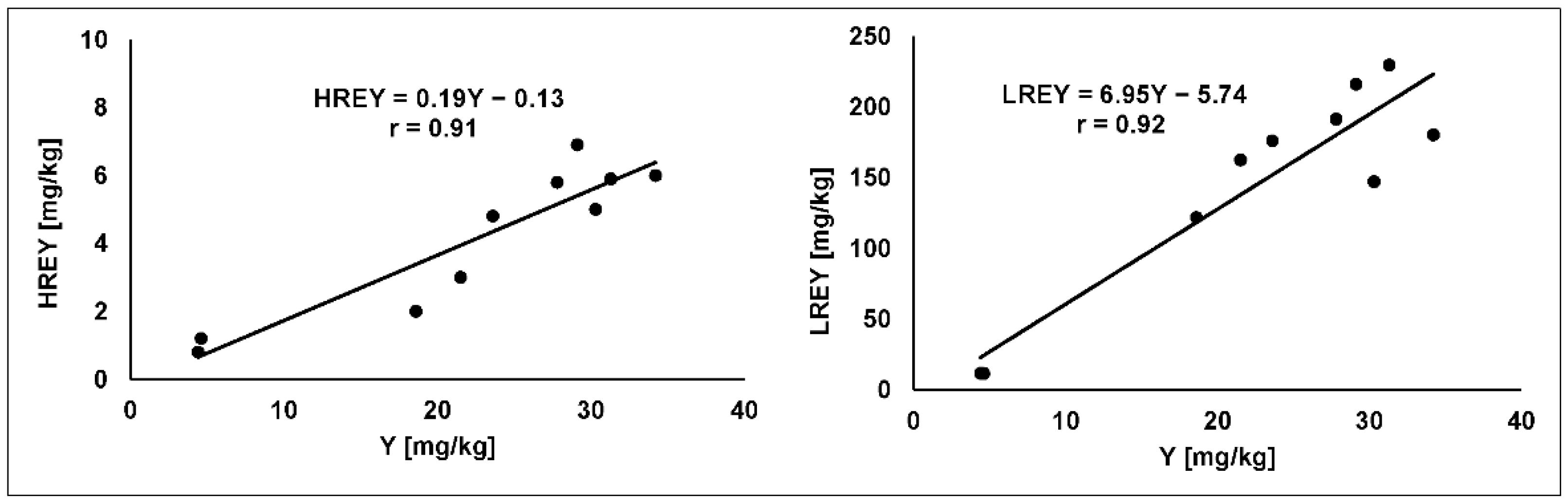
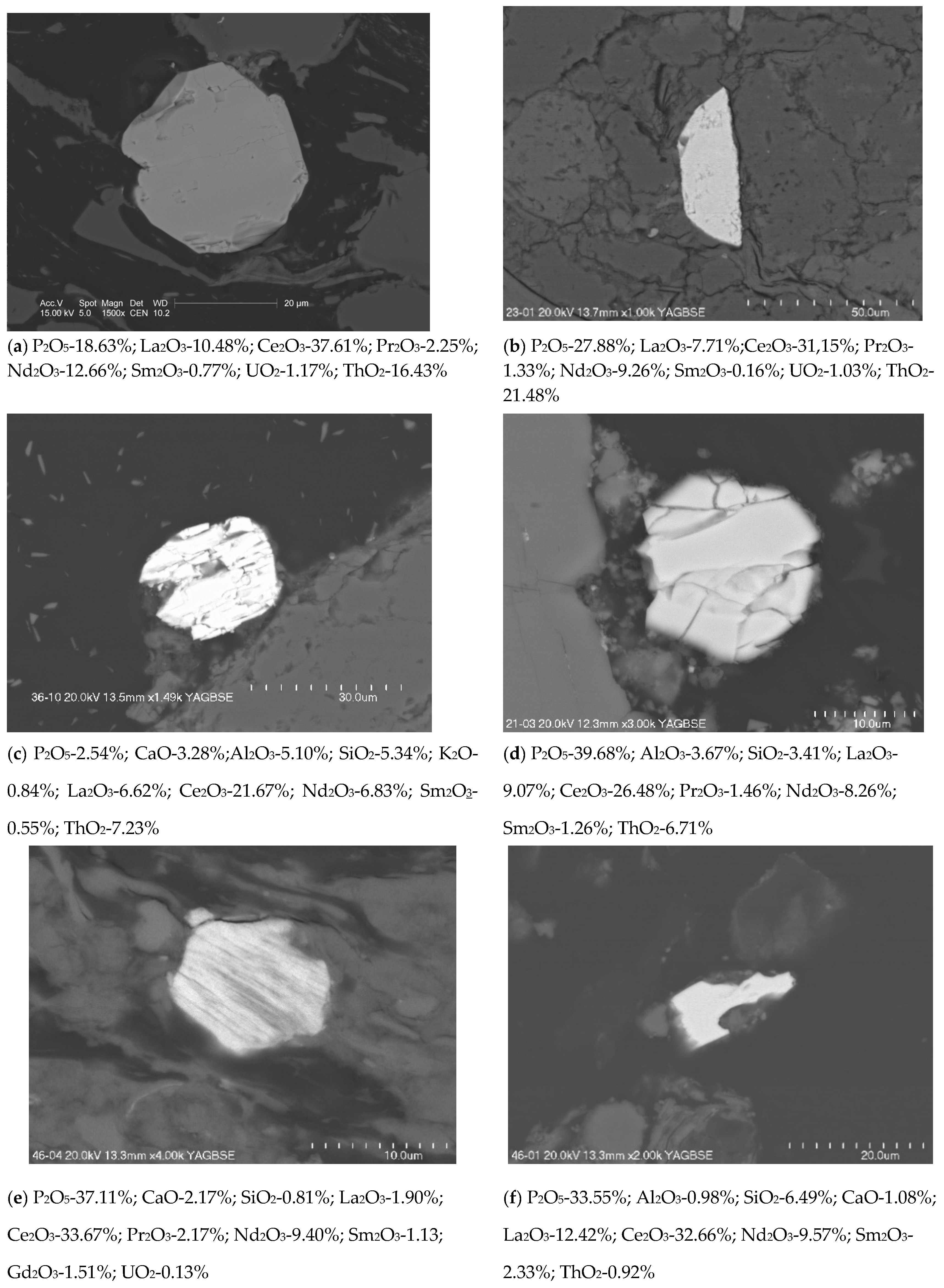

| Sample Number | 226Ra [Bq/kg] | 228Ra(228Ac) [Bq/kg] | 40K [Bq/kg] | U [mg/kg] | Th [mg/kg] | |
|---|---|---|---|---|---|---|
| Silstone | 1 | 49.5 ± 1.7 | 44.5 ± 1.0 | 749 ± 12 | 4.3 | 11.3 |
| 1A | 112.3 ± 4.1 | 54.2 ± 1.0 | 862 ± 13 | 9.2 | 11.9 | |
| Sandstone | 2 | 9.3 ± 0.3 | 7.6 ± 0.2 | 148 ± 3 | 0.9 | 2.2 |
| 2A | 24.4 ± 0.9 | 12.5 ± 0.4 | 207 ± 4 | 2.1 | 3.1 | |
| Mudstone | 3 | 39.0 ± 1.3 | 27.7 ± 0.6 | 761 ± 12 | 3.3 | 7.2 |
| 3A | 70.8 ± 2.6 | 57.6 ± 1.2 | 1008 ± 16 | 5.9 | 2.9 | |
| Burnt coal shale | 4 | 69.9 ± 2.3 | 47.3 ± 0.9 | 769 ± 12 | 5.8 | 11.6 |
| 4A | 12.0 ± 0.5 | 10.6 ± 0.3 | 192 ± 4 | 1.1 | 7.8 | |
| Hard coal | 5 | 20.3 ± 0.7 | 7.7 ± 0.3 | 64 ± 2 | 1.4 | 1.9 |
| 5A | 24.1 ± 0.9 | 15.1 ± 0.4 | 168 ± 4 | 2.1 | 3.9 | |
| Sample Number | Raeq [Bq/kg] | Hex | D [nGy/h] | ED [mSv/y] | |
|---|---|---|---|---|---|
| Silstone | 1 | 173 | 0.5 | 83.5 | 0.10 |
| 1A | 248 | 0.7 | 117.0 | 0.14 | |
| Sandstone | 2 | 33 | 0.1 | 16.7 | 0.02 |
| 2A | 58 | 0.2 | 28.2 | 0.03 | |
| Mudstone | 3 | 139 | 0.4 | 68.2 | 0.08 |
| 3A | 165 | 0.4 | 82.8 | 0.10 | |
| Burnt coal shale | 4 | 197 | 0.5 | 93.6 | 0.11 |
| 4A | 72 | 0.2 | 33.4 | 0.04 | |
| Hard coal | 5 | 36 | 0.1 | 15.3 | 0.02 |
| 5A | 60 | 0.2 | 28.6 | 0.04 | |
| Element | Sample Number | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Silstone | Sandstone | Mudstone | Burnt Coal Shale | Hard Coal | ||||||
| 1 | 1A | 2 | 2A | 3 | 3A | 4 | 4A | 5 | 5A | |
| La | 50.3 | 52.2 | 36.4 | 39.1 | 31.6 | 36.8 | 37.7 | 25.2 | 1.9 | 2.1 |
| Ce | 111.0 | 114.0 | 87.2 | 91.2 | 76.5 | 99.2 | 88.2 | 67.3 | 5.5 | 5.3 |
| Pr | 12.5 | 13.8 | 10.3 | 11.8 | 8.5 | 9.9 | 10.2 | 7.7 | 0.8 | 0.8 |
| Nd | 42.5 | 49.8 | 42.4 | 49.5 | 30.8 | 34.6 | 26.7 | 21.8 | 3.6 | 3.5 |
| Sm | 8.2 | 9.8 | 7.8 | 8.4 | 5.1 | 5.5 | 7.2 | 6.1 | 0.8 | 0.7 |
| Eu | 1.3 | 1.6 | 1.6 | 1.6 | 1.1 | 1.3 | 0.7 | 0.3 | 0.8 | 0.6 |
| Gd | 7.3 | 7.8 | 6.1 | 7.6 | 3.8 | 4.5 | 3.1 | 2.5 | 0.7 | 0.7 |
| Tb | 0.9 | 0.9 | 1.1 | 1.1 | 0.6 | 0.8 | 0.4 | 0.2 | 0.1 | 0.1 |
| Dy | 4.6 | 5.8 | 4.2 | 5.1 | 3.3 | 3.6 | 2.8 | 2.1 | 0.6 | 0.7 |
| Ho | 1.2 | 1.1 | 0.6 | 0.9 | 0.7 | 0.9 | 0.1 | 0.1 | 0.1 | 0.1 |
| Er | 2.9 | 2.1 | 1.8 | 2.2 | 2.3 | 2.0 | 1.2 | 0.8 | 0.4 | 0.4 |
| Tm | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 |
| Yb | 2.4 | 2.5 | 2.0 | 2.3 | 1.9 | 2.3 | 1.4 | 0.9 | 0.3 | 0.5 |
| Lu | 0.3 | 0.1 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.1 | 0 | 0.1 |
| Y | 29.1 | 31.3 | 23.6 | 27.8 | 30.3 | 34.2 | 21.5 | 18.6 | 4.4 | 4.6 |
| ∑REE | 245.5 | 261.6 | 201.9 | 221.2 | 166.3 | 201.9 | 180.0 | 135.2 | 15.6 | 15.7 |
| LREY | 224.5 | 239.6 | 184.1 | 200.0 | 152.5 | 186.0 | 170.0 | 128.1 | 12.6 | 12.4 |
| MREY | 43.2 | 47.4 | 36.6 | 43.2 | 39.1 | 44.4 | 28.5 | 23.7 | 6.6 | 6.4 |
| HREY | 6.9 | 5.9 | 4.8 | 5.8 | 5.3 | 5.7 | 3.0 | 2.0 | 0.9 | 1.2 |
| Anomaly Coefficient | Sample Number | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Silstone | Sandstone | Mudstone | Burnt Coal Shale | Hard Coal | ||||||
| 1 | 1A | 2 | 2A | 3 | 3A | 4 | 4A | 5 | 5A | |
| Ce/CeNASC | 5.7 | 5.8 | 4.5 | 4.7 | 3.9 | 5.1 | 4.5 | 3.5 | 0.6 | 0.3 |
| La/YbNASC | 16.2 | 16.8 | 11.7 | 12.6 | 10.2 | 11.8 | 12.1 | 8.1 | 0.6 | 0.4 |
| La/SmNASC | 8.4 | 8.7 | 6.1 | 6.5 | 5.3 | 6.2 | 6.3 | 4.2 | 0.5 | 0.6 |
| Sm/YbNASC | 2.6 | 3.2 | 2.5 | 2.7 | 1.6 | 1.8 | 2.3 | 2.0 | 1.4 | 0.7 |
| Eu/EuNASC | 5.9 | 7.2 | 7.2 | 7.2 | 5.0 | 5.9 | 3.1 | 1.3 | 3.6 | 2.7 |
| REE+Y [mg/kg] Concentrations in Shales (NASC) 1 | ||||||||||
| Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho |
| 27 | 31.1 | 67.033 | 7.9 | 30.4 | 5.98 | 1.25333 | 5.5 | 0.85 | 5.75 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smołka-Danielowska, D.; Walencik-Łata, A. The Occurrence of Selected Radionuclides and Rare Earth Elements in Waste at the Mine Heap from the Polish Mining Group. Minerals 2021, 11, 504. https://doi.org/10.3390/min11050504
Smołka-Danielowska D, Walencik-Łata A. The Occurrence of Selected Radionuclides and Rare Earth Elements in Waste at the Mine Heap from the Polish Mining Group. Minerals. 2021; 11(5):504. https://doi.org/10.3390/min11050504
Chicago/Turabian StyleSmołka-Danielowska, Danuta, and Agata Walencik-Łata. 2021. "The Occurrence of Selected Radionuclides and Rare Earth Elements in Waste at the Mine Heap from the Polish Mining Group" Minerals 11, no. 5: 504. https://doi.org/10.3390/min11050504
APA StyleSmołka-Danielowska, D., & Walencik-Łata, A. (2021). The Occurrence of Selected Radionuclides and Rare Earth Elements in Waste at the Mine Heap from the Polish Mining Group. Minerals, 11(5), 504. https://doi.org/10.3390/min11050504






