Chalcopyrite and Molybdenite Flotation in Seawater: The Use of Inorganic Dispersants to Reduce the Depressing Effects of Micas
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Procedures
3. Results
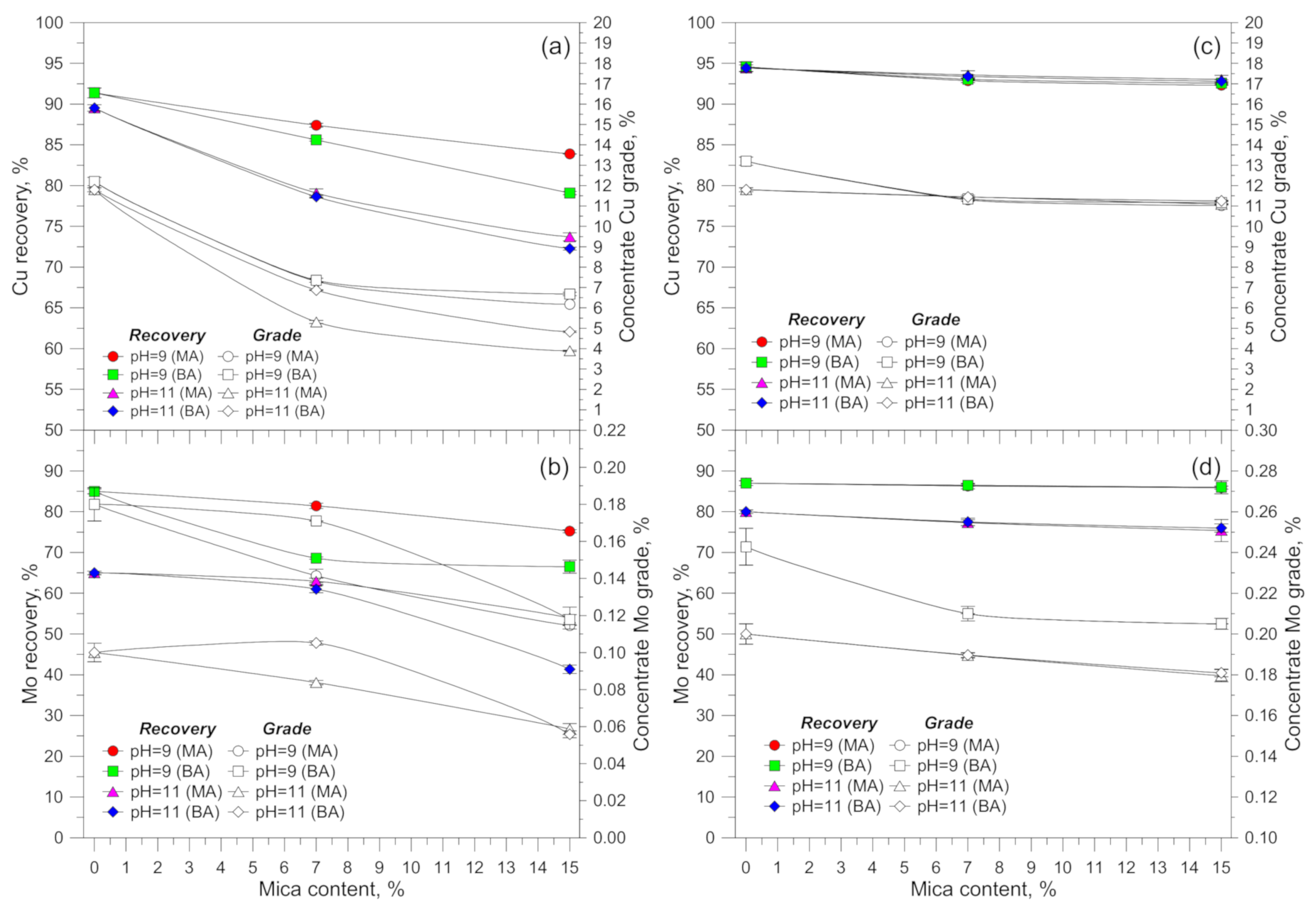
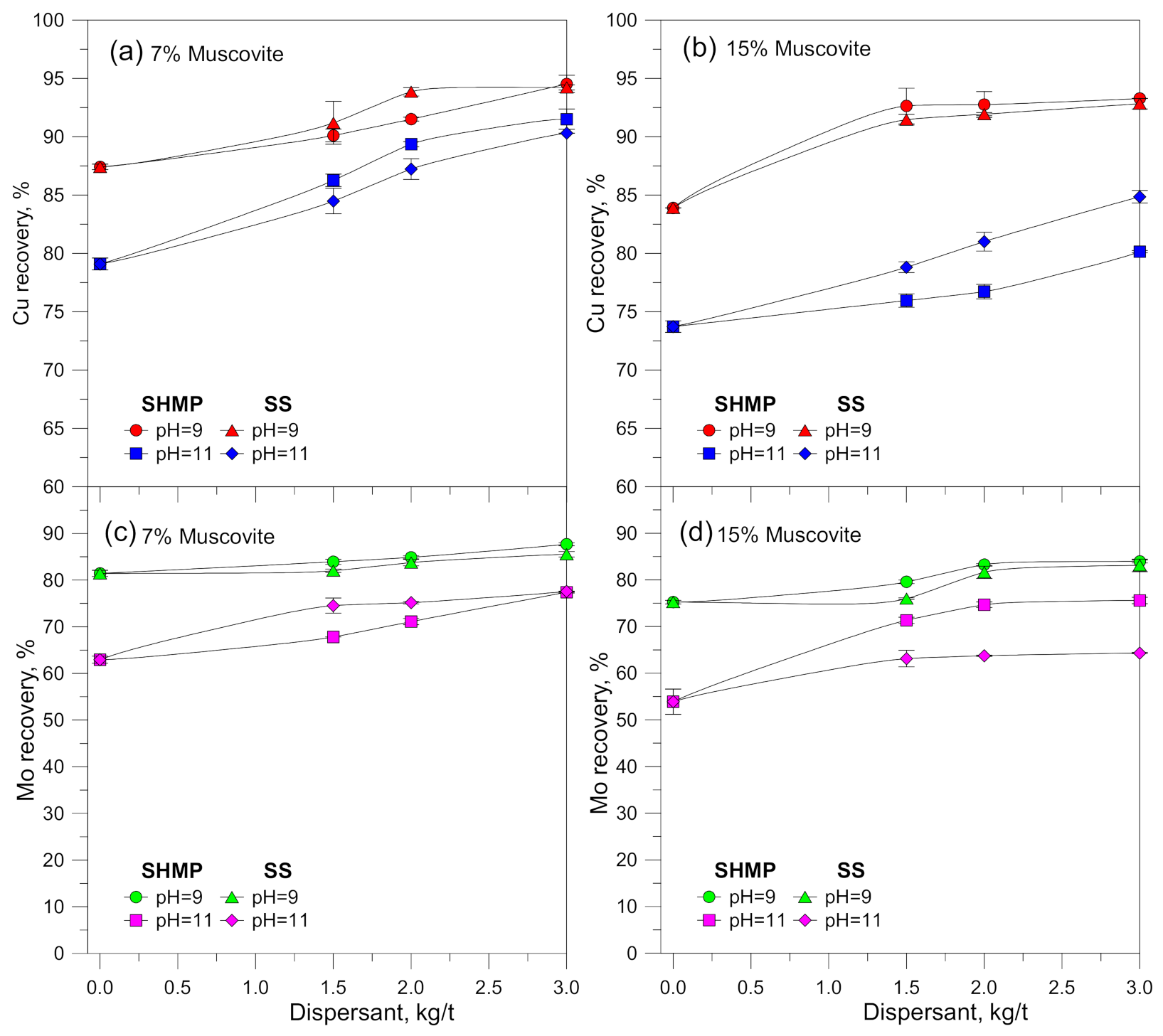
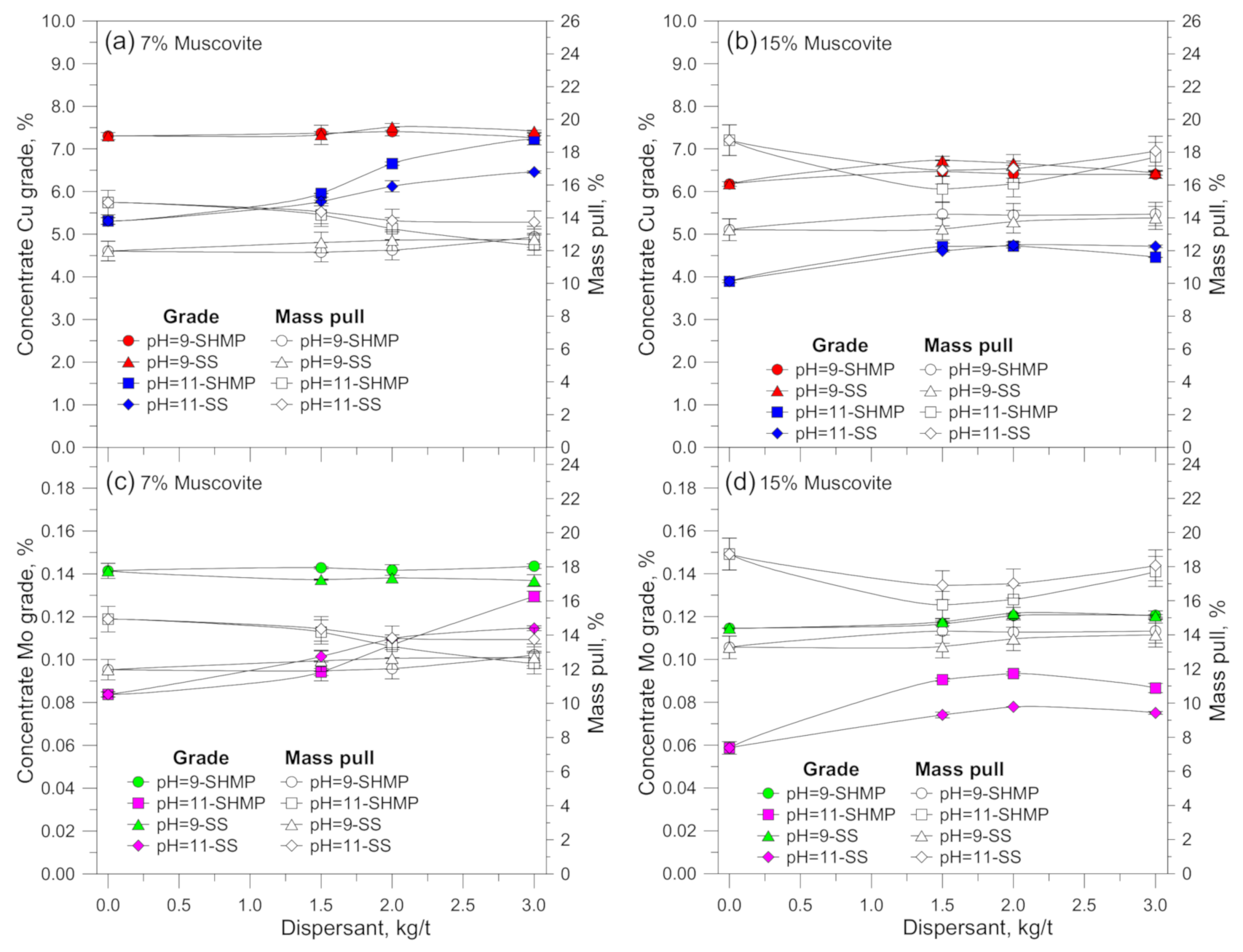
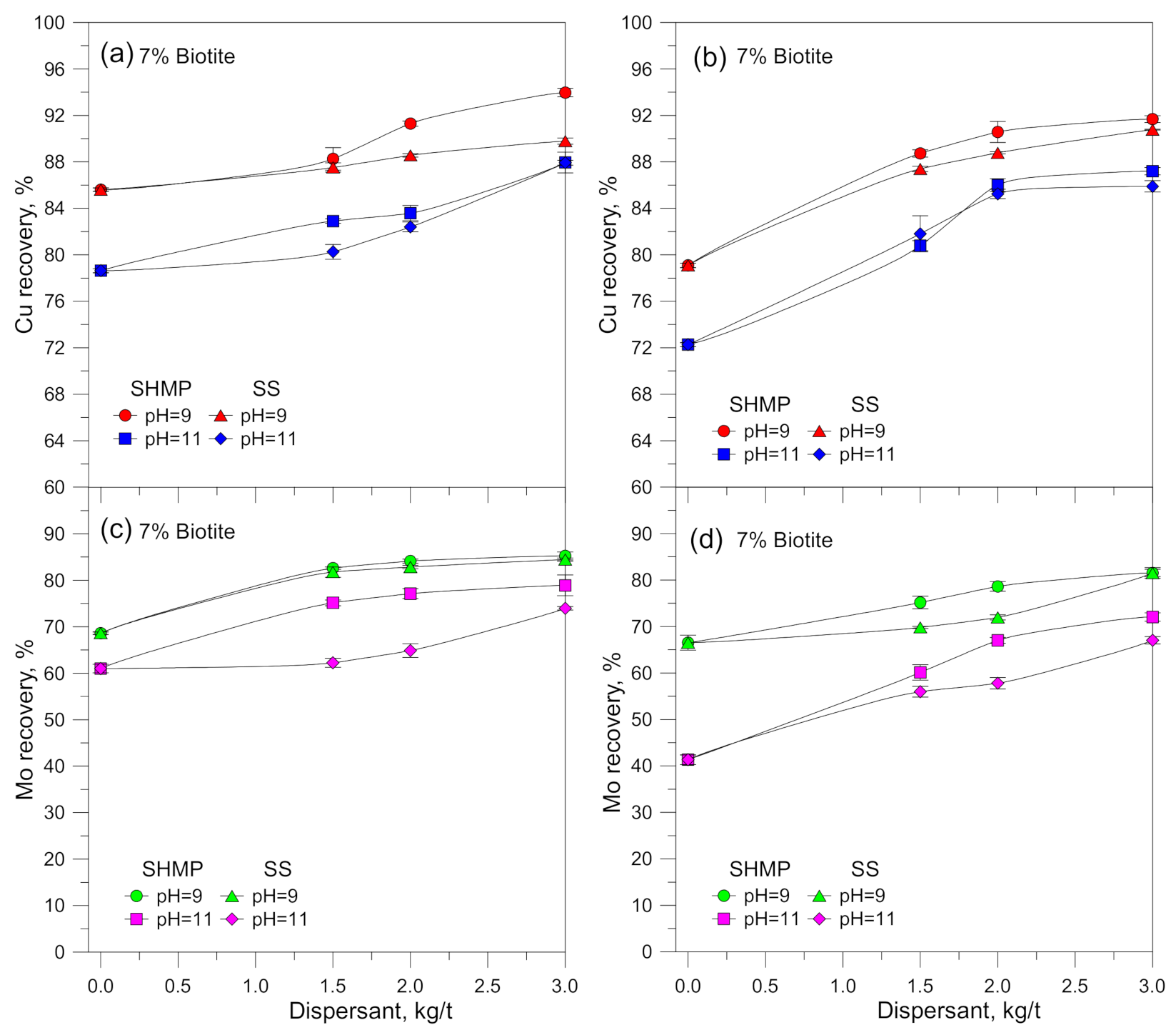
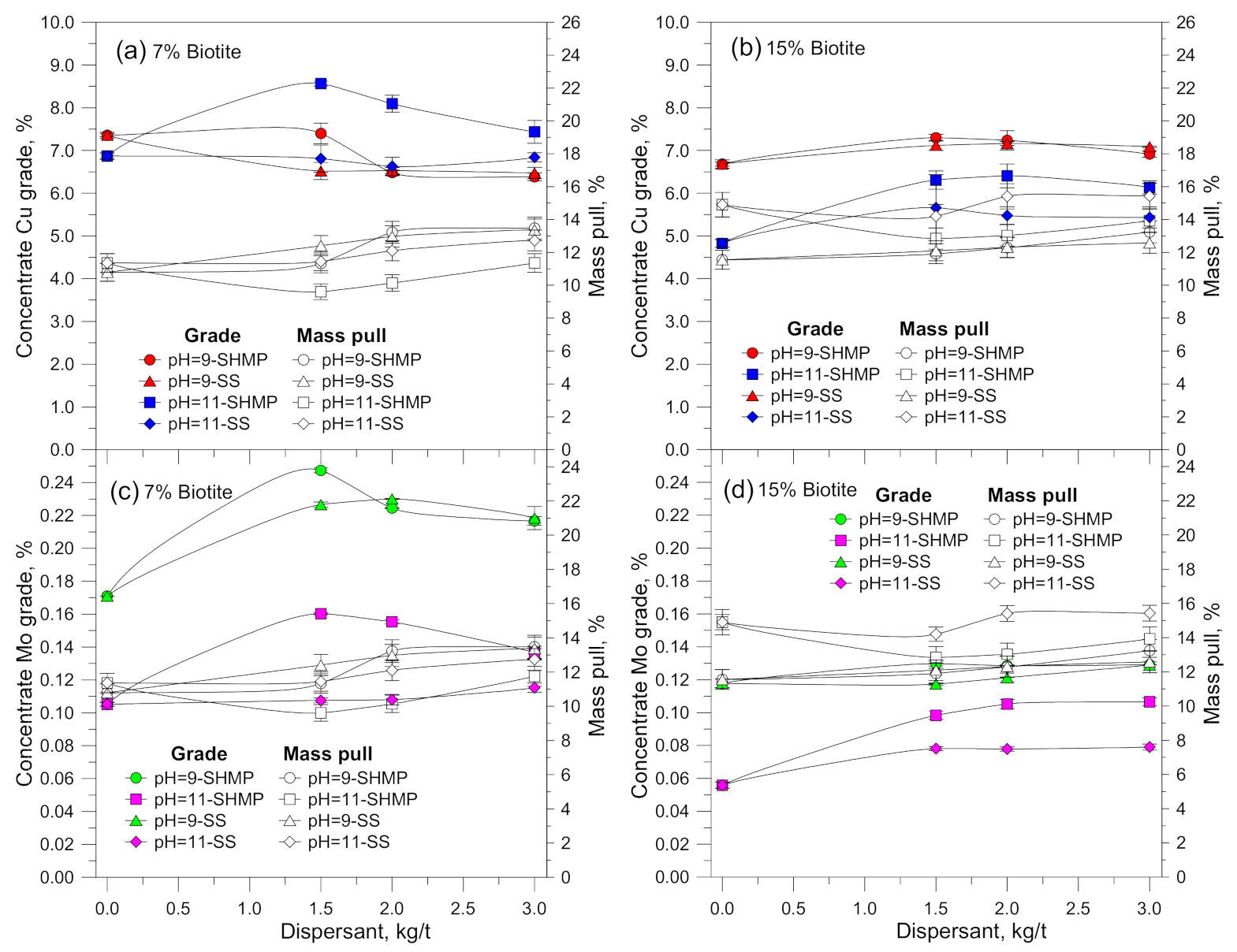
3.1. Flotation Results
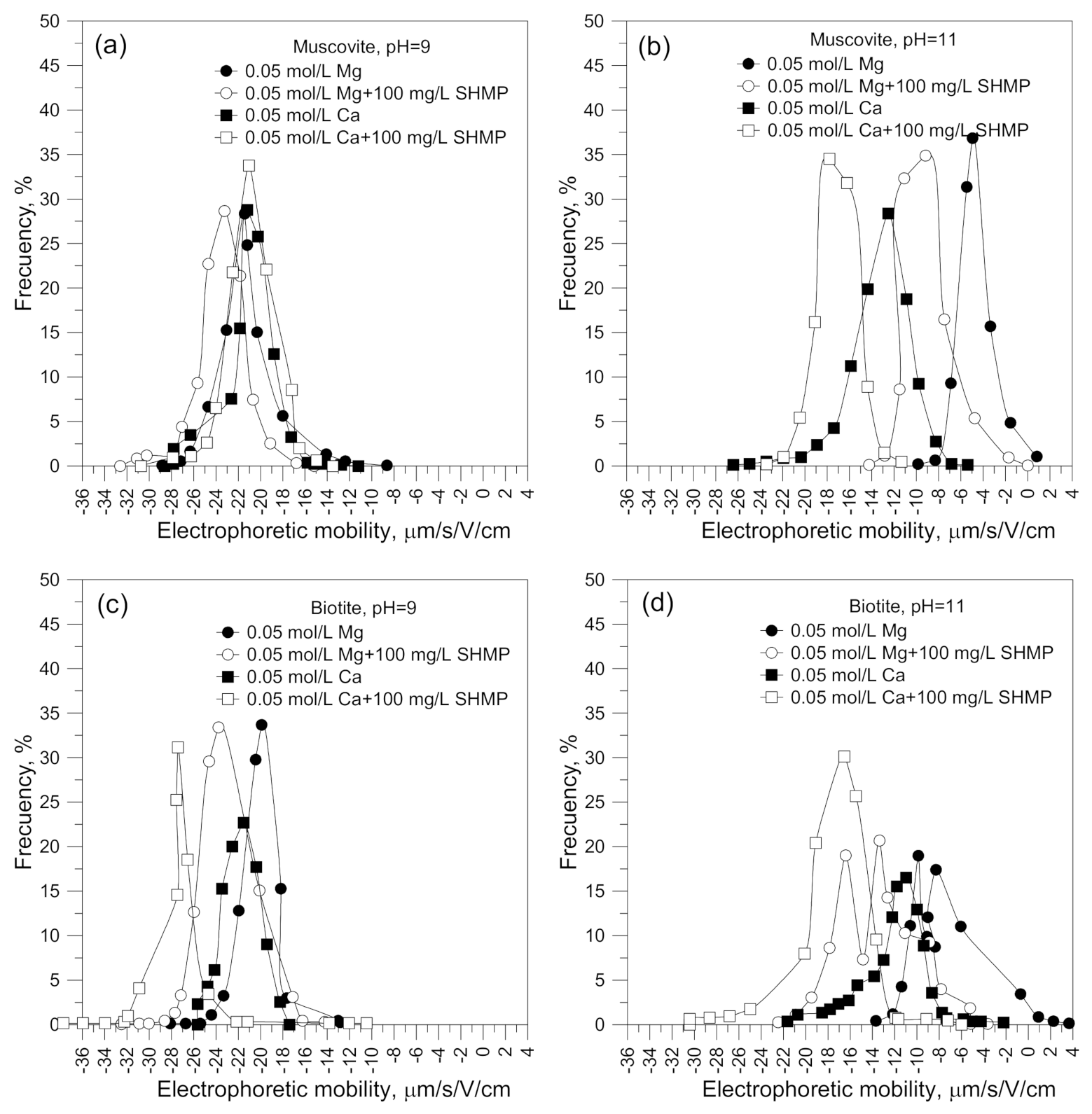
3.2. Electrophoretic Mobility
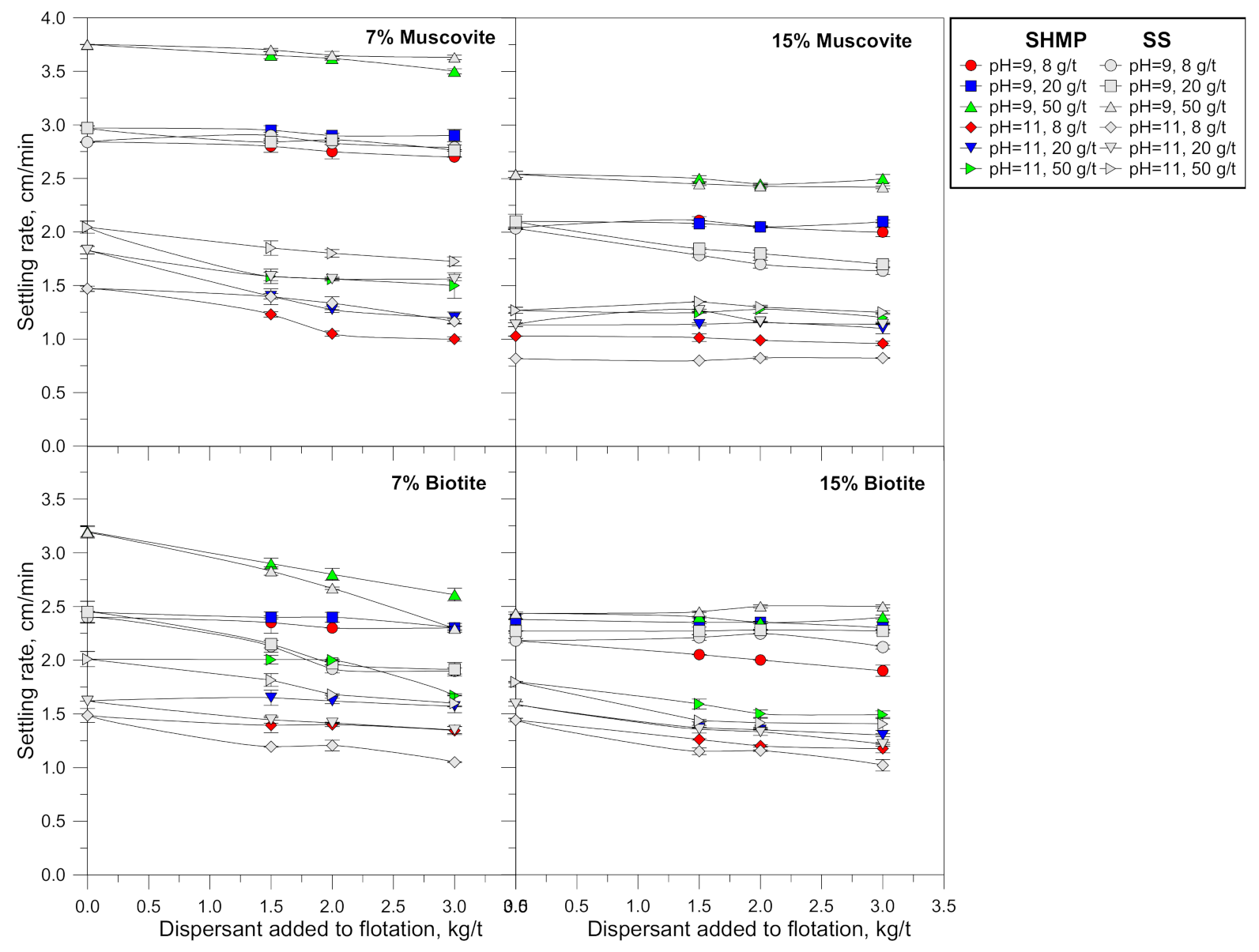
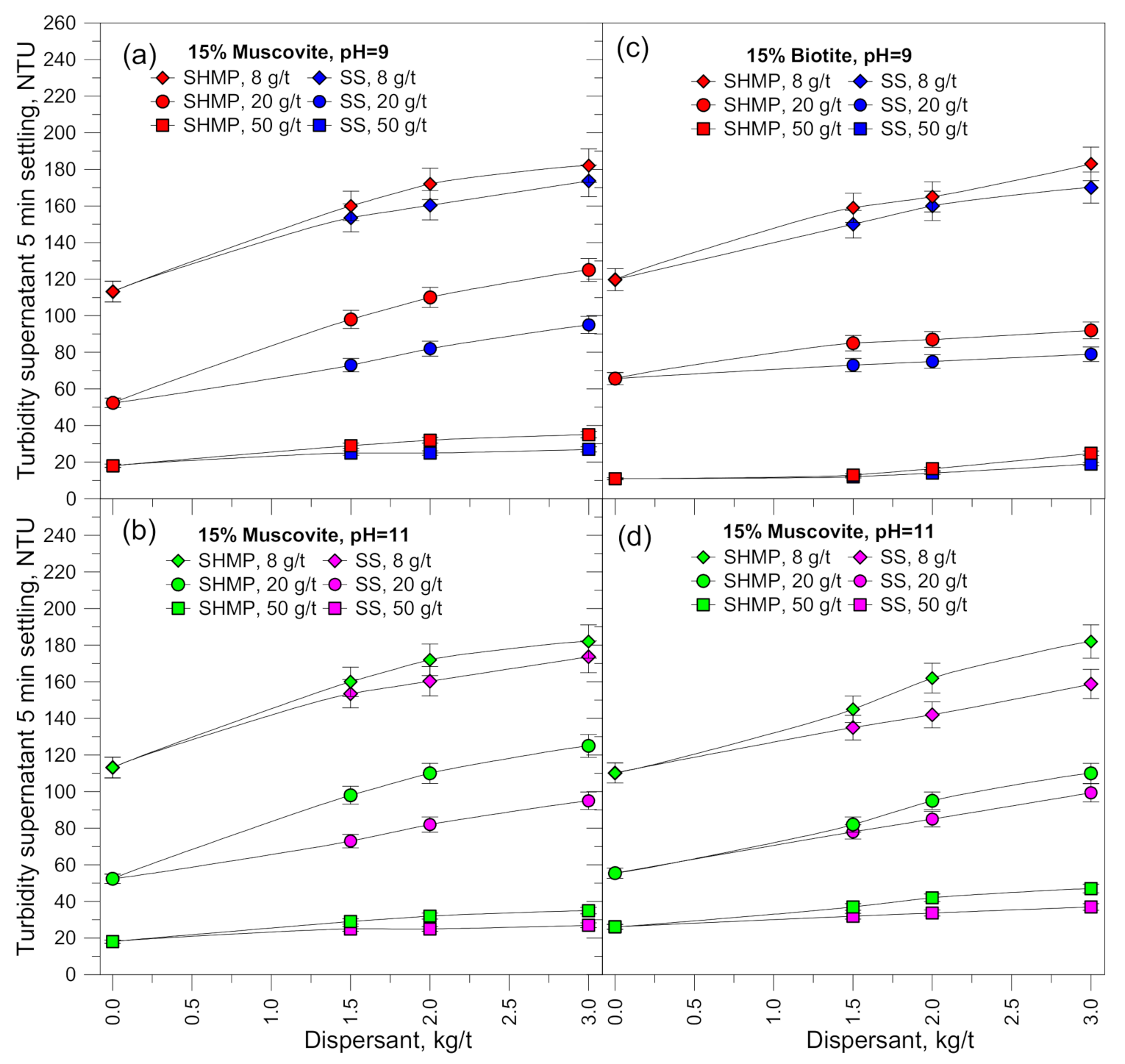
3.3. Settling Rates
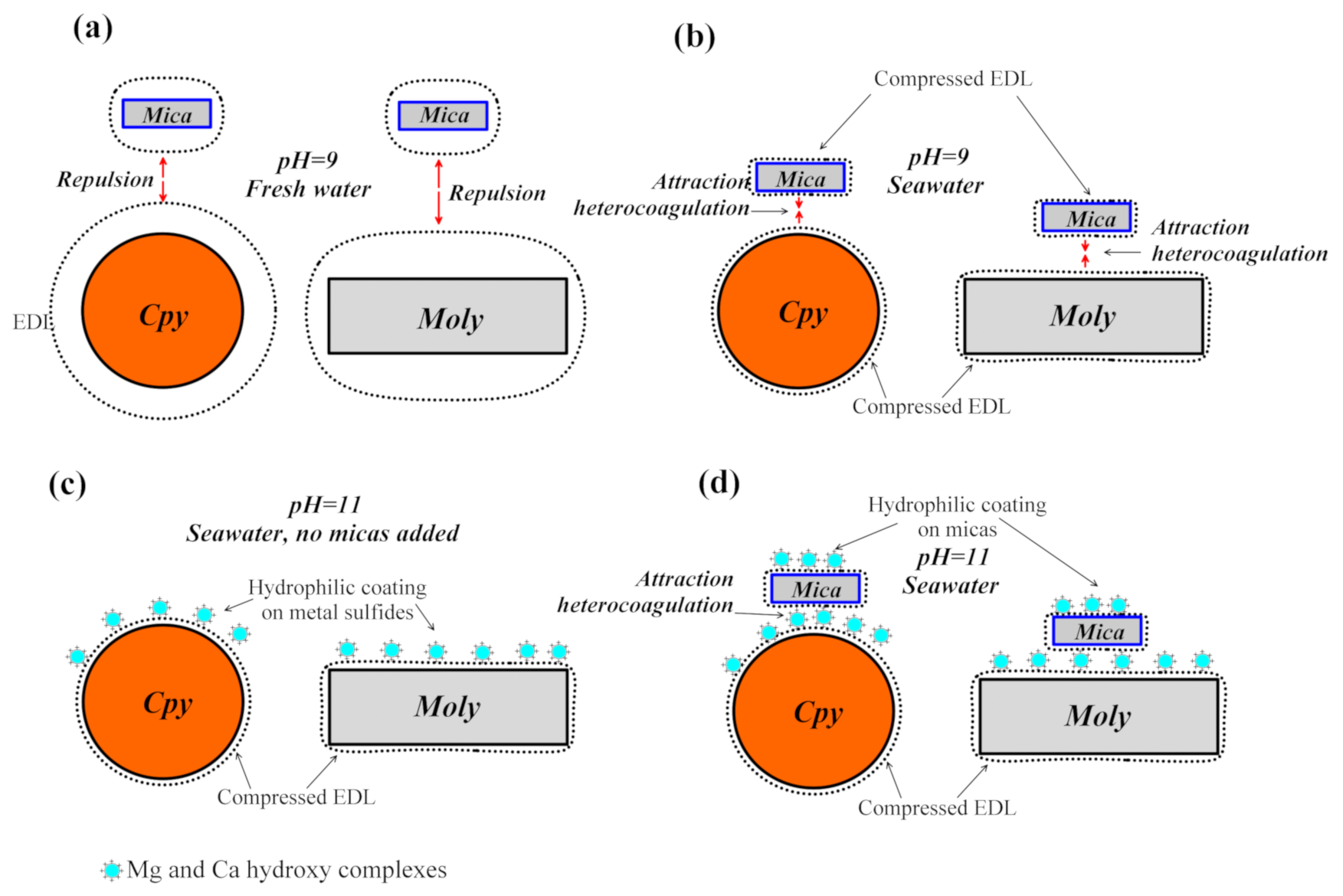
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramirez, A.; Gutierrez, L.; Laskowski, J.S. Use of oily bubbles and dispersants in flotation of molybdenite in fresh and seawater. Miner. Eng. 2020, 148, 106197. [Google Scholar] [CrossRef]
- Forbes, E.; Davey, K.J.; Smith, L. Decoupling rehology and slime coatings effect on the natural flotability of chalcopyrite in a clay-rich flotation pulp. Miner. Eng. 2014, 56, 136–144. [Google Scholar] [CrossRef]
- Ndlovu, B.; Farrokhpay, S.; Bradshaw, D. The effect of phyllosilicate minerals on mineral processing industry. Int. J. Miner. Process. 2013, 125, 149–156. [Google Scholar] [CrossRef]
- Klein, C.; Hurlbut, C.S. Manual of Mineralogy, 21st ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Kelm, U.; Helle, S.; Jerez, O.; Pincheira, M. What Are Copper Clays? Geometallurgical Implications. In Proceedings of the Copper International Conference, Santiago, Chile, 1–4 December 2013. [Google Scholar]
- Uribe, L.; Gutierrez, L.; Jerez, O. The depressing effect of clay minerals on the floatability of chalcopyrite. Min. Proc. Ext. Met. Rev. 2016, 37, 227–235. [Google Scholar] [CrossRef]
- Uribe, L.; Gutierrez, L.; Laskowski, J.S.; Castro, S. Role of calcium and magnesium cations in the interactions between kaolinite and chalcopyrite in seawater. Physicochem. Probl. Miner. Process. 2017, 53, 737–749. [Google Scholar]
- Ramirez, A.; Rojas, A.; Gutierrez, L.; Laskowski, J.S. Sodium hexametaphosphate and sodium silicate as dispersants to reduce the negative effect of kaolinite on the flotation of chalcopyrite in seawater. Miner. Eng. 2018, 125, 10–14. [Google Scholar] [CrossRef]
- Ramirez, A.; Gutierrez, L.; Vega-Garcia, D.; Reyes-Bozo, L. The Depressing Effect of Kaolinite on Molybdenite Flotation in Seawater. Minerals 2020, 10, 578. [Google Scholar] [CrossRef]
- McKeown, D.A.; Bell, M.I.; Etz, E.S. Vibrational analysis of the dioctahedral mica: 2M1 muscovite. Am. Min. 1999, 84, 1041–1048. [Google Scholar] [CrossRef]
- Maslova, M.V.; Gerasimova, L.G.; Forsling, W. Surface properties of cleaved mica. Colloid J. 2004, 66, 322–328. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock Forming Minerals, 2nd ed.; Longman Scientific and Technical: New York, NY, USA, 1992. [Google Scholar]
- Laskowski, J.S. Role of model systems in fundamental studies on particle-bubble interaction. In Separation Technologies for Minerals, Coal, and Earth Resources, 1st ed.; Luttrell, G., Young, C., Eds.; SME: Englewood, NJ, USA, 2012. [Google Scholar]
- Fuerstenau, M.C.; Lopez-Valdivieso, A.; Fuerstenau, D.W. Role of hydrolyzed cations in the natural hydrophobicity of talc. Int. J. Miner. Process. 1988, 23, 161–170. [Google Scholar] [CrossRef]
- Castro, S.; Laskowski, J.S. Froth flotation in saline water. KONA Powder Part. J. 2011, 29, 4–15. [Google Scholar] [CrossRef]
- Yepsen, R.; Gutierrez, L.; Laskowski, J. Flotation behavior of enargite in the process of flotation using seawater. Miner. Eng. 2019, 142, 105897. [Google Scholar] [CrossRef]
- Suyantara, G.P.; Hirajima, T.; Miki, H.; Sasaki, K. Floatability of molybdenite and chalcopyrite in artificial seawater. Miner. Eng. 2018, 115, 117–130. [Google Scholar] [CrossRef]
- Rebolledo, E.; Laskowski, J.S.; Gutierrez, L.; Castro, S. Use of dispersants in flotation of molybdenite in seawater. Miner. Eng. 2017, 100, 71–74. [Google Scholar] [CrossRef]
- Castro, P.; Huber, M. Chemical and Physical Features of Seawater and the World Ocean. In Marine Biology; McGraw-Hill Higher Education: New York, NY, USA, 2003; pp. 48–71. [Google Scholar]
- Li, C.; Somasundaran, P. Reversal of bubble charge in multivalent inorganic salt solutions—Effect of magnesium. J. Colloid Interface Sci. 1991, 146, 215–218. [Google Scholar] [CrossRef]
- Quezada, G.R.; Jeldres, M.; Toro, N.; Robles, P.; Toledo, P.G.; Jeldres, R.I. Understanding the flocculation mechanism of quartz and kaolinite with polyacrylamide in seawater: A molecular dynamics approach. Colloids Surf. A 2021, 608, 125576. [Google Scholar] [CrossRef]
- Rao, K.H.; Cases, J.M.; Barres, O.; Forssberg, K.S.E. Flotation, electrokinetic and FT-IR studies of mixed anionic/cationic collectors in muscovite-biotite system. In Mineral Processing: Recent Advances and Future Trends; Allied Publishers Limited: New Delhi, India, 1995; pp. 29–44. [Google Scholar]
- de Kort, E.; Minor, M.; Snoeren, T.; van Hooijdonk, T.; van der Linden, E. Calcium-biding capacity of organic and inorganic ortho- and polyphosphates. Dairy Sci. Technol. 2009, 89, 283–299. [Google Scholar] [CrossRef]
- Vujicic, I.; Batra, S.C.; Deman, J.M. Interaction of alkaline earth ions with polyphosphates and citrate in the presence and absence of casein. J. Agric. Food Chem. 1967, 15, 403–407. [Google Scholar] [CrossRef]
- Ma, M. The dispersive effect of sodium hexametaphosphate on kaolinite in saline water. Clays Clay Miner. 2012, 60, 405–410. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Wei, Z.; Xiao, Q.; Song, S. Fundamental studies of SHMP in reducing negative effects of divalent ions on molybdenite flotation. Minerals 2018, 8, 404. [Google Scholar] [CrossRef]
- Li, W.; Li, Y. Improved understanding of chalcopyrite flotation in seawater using sodium hexametaphosphate. Miner. Eng. 2019, 134, 269–274. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Fan, R.; Fan, R. Influencing mechanisms of sodium hexametaphosphate on molybdenite flotation using sea water. Physicochem. Probl. Miner. Process. 2019, 55. [Google Scholar] [CrossRef]
- Leja, J. Surface Chemistry of Froth Flotation, 1st ed.; Springer Science: New York, NY, USA, 1982. [Google Scholar]
- Lagerstrom, G.B. Equilibrium in sodium silicate solutions. Acta Chem. Scand. 1959, 13, 722–736. [Google Scholar]
- Ingri, N. Equilibrium Studies of Polyanions. 4. Silicate Ions in NaCl Medium. Acta Chem. Scand. 1959, 13, 758–775. [Google Scholar] [CrossRef]
- Ndlovu, B.; Forbes, E.; Farrokhpay, S.; Becker, M.; Bradshaw, D.; Deglon, D. A preliminary rheological classification of phyllosilicate group minerals. Miner. Eng. 2014, 55, 190–200. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Dontsova, T.A.; Korolyov, V.J. Ultra-flocculation of diluted fine disperse suspensions. Min. Proc. Extr. Metall. Rev. 2005, 26, 203–217. [Google Scholar] [CrossRef]
- Quezada, G.R.; Jeldres, R.I.; Fawell, P.D.; Toledo, P.G. Use of molecular dynamics to study the conformation of an anionic polyelectrolyte in saline medium and its adsorption on a quartz surface. Miner. Eng. 2018, 129, 102–105. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Piceros, E.C.; Leiva, W.H.; Toledo, P.G.; Herrera, N. Viscoelasticity and yielding properties of flocculated kaolinite sediments in saline water. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 1009–1015. [Google Scholar] [CrossRef]

| Element | Concentration, mg/L |
|---|---|
| Cl− | 19,345 |
| Na+ | 10,752 |
| SO42− | 2701 |
| Mg2+ | 1295 |
| Ca2+ | 416 |
| K+ | 390 |
| HCO3− | 154 |
| Br− | 66 |
| BO33− | 27 |
| Sr2+ | 13 |
| F− | 1 |
| Others | <1 |
| Muscovite, % | %−38 μm | Biotite, % | %−38 μm |
|---|---|---|---|
| 0 | 38 | 0 | 38 |
| 7 | 41 | 7 | 40 |
| 15 | 42 | 15 | 43 |
| 0.05 mol/L Mg | 0.05 mol/L Ca | ||||||
|---|---|---|---|---|---|---|---|
| pH | 0.01 M NaCl | No Dispersant | 100 mg/L SHMP | 100 mg/L SS | No Dispersant | 100 mg/L SHMP | 100 mg/L SS |
| 9 | −29 | −6.1 | −7.5 | −6.3 | −13.2 | −27.1 | −20.5 |
| 11 | −39 | 10.1 | −3.5 | −8.4 | −10.3 | −20.2 | −12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepsen, R.; Roa, J.; Toledo, P.G.; Gutiérrez, L. Chalcopyrite and Molybdenite Flotation in Seawater: The Use of Inorganic Dispersants to Reduce the Depressing Effects of Micas. Minerals 2021, 11, 539. https://doi.org/10.3390/min11050539
Yepsen R, Roa J, Toledo PG, Gutiérrez L. Chalcopyrite and Molybdenite Flotation in Seawater: The Use of Inorganic Dispersants to Reduce the Depressing Effects of Micas. Minerals. 2021; 11(5):539. https://doi.org/10.3390/min11050539
Chicago/Turabian StyleYepsen, Rodrigo, Joaquín Roa, Pedro G. Toledo, and Leopoldo Gutiérrez. 2021. "Chalcopyrite and Molybdenite Flotation in Seawater: The Use of Inorganic Dispersants to Reduce the Depressing Effects of Micas" Minerals 11, no. 5: 539. https://doi.org/10.3390/min11050539
APA StyleYepsen, R., Roa, J., Toledo, P. G., & Gutiérrez, L. (2021). Chalcopyrite and Molybdenite Flotation in Seawater: The Use of Inorganic Dispersants to Reduce the Depressing Effects of Micas. Minerals, 11(5), 539. https://doi.org/10.3390/min11050539







