Elimination of Ferric Ion Effect on Separation between Kyanite and Quartz Using Citric Acid as Regulator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Microflotation Tests
2.3. Contact Angle Tests
2.4. FTIR Spectroscopy Measurement
2.5. XPS Measurement
3. Results
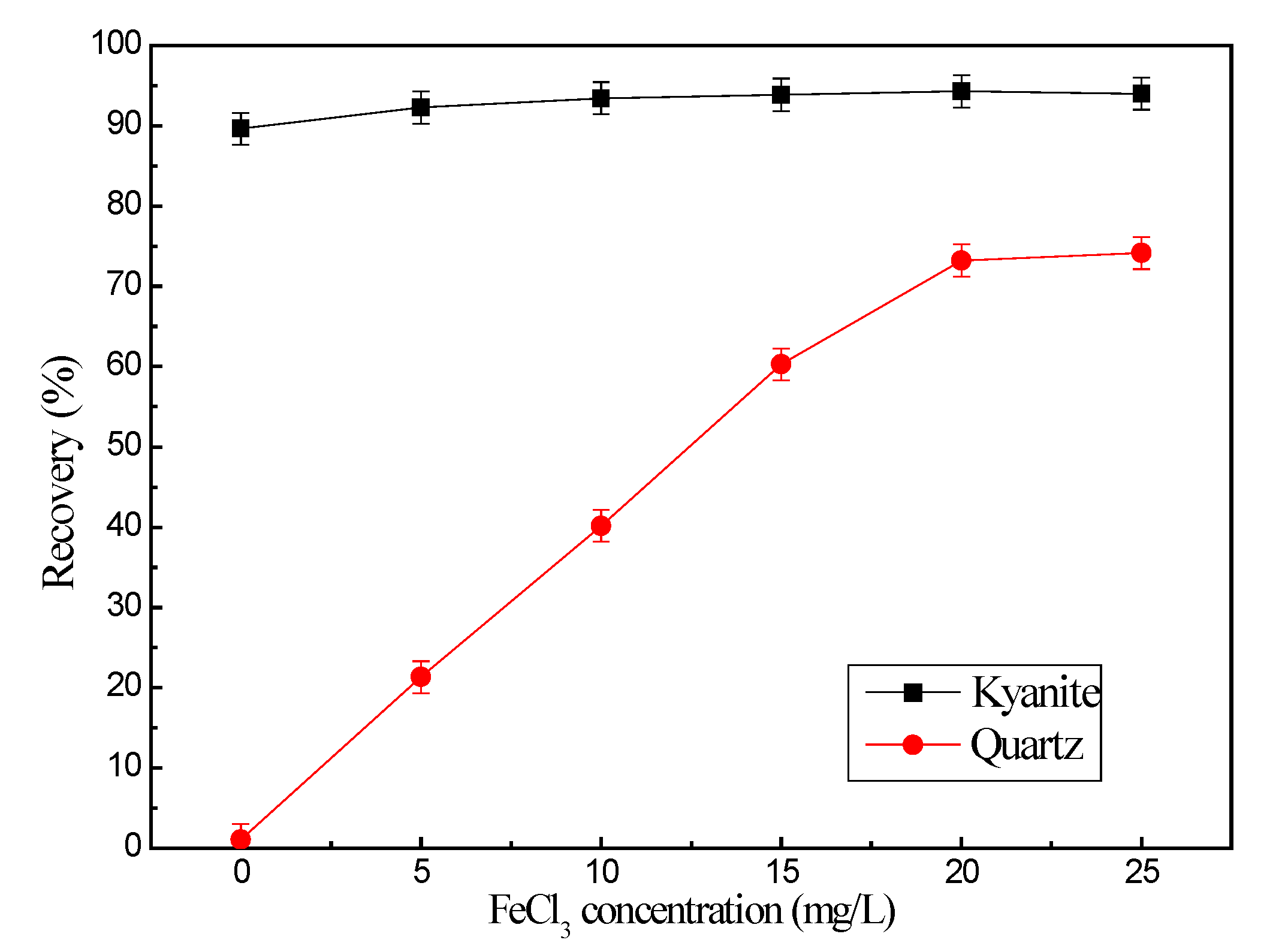
3.1. Microflotation Tests
3.2. Contact Angle Tests
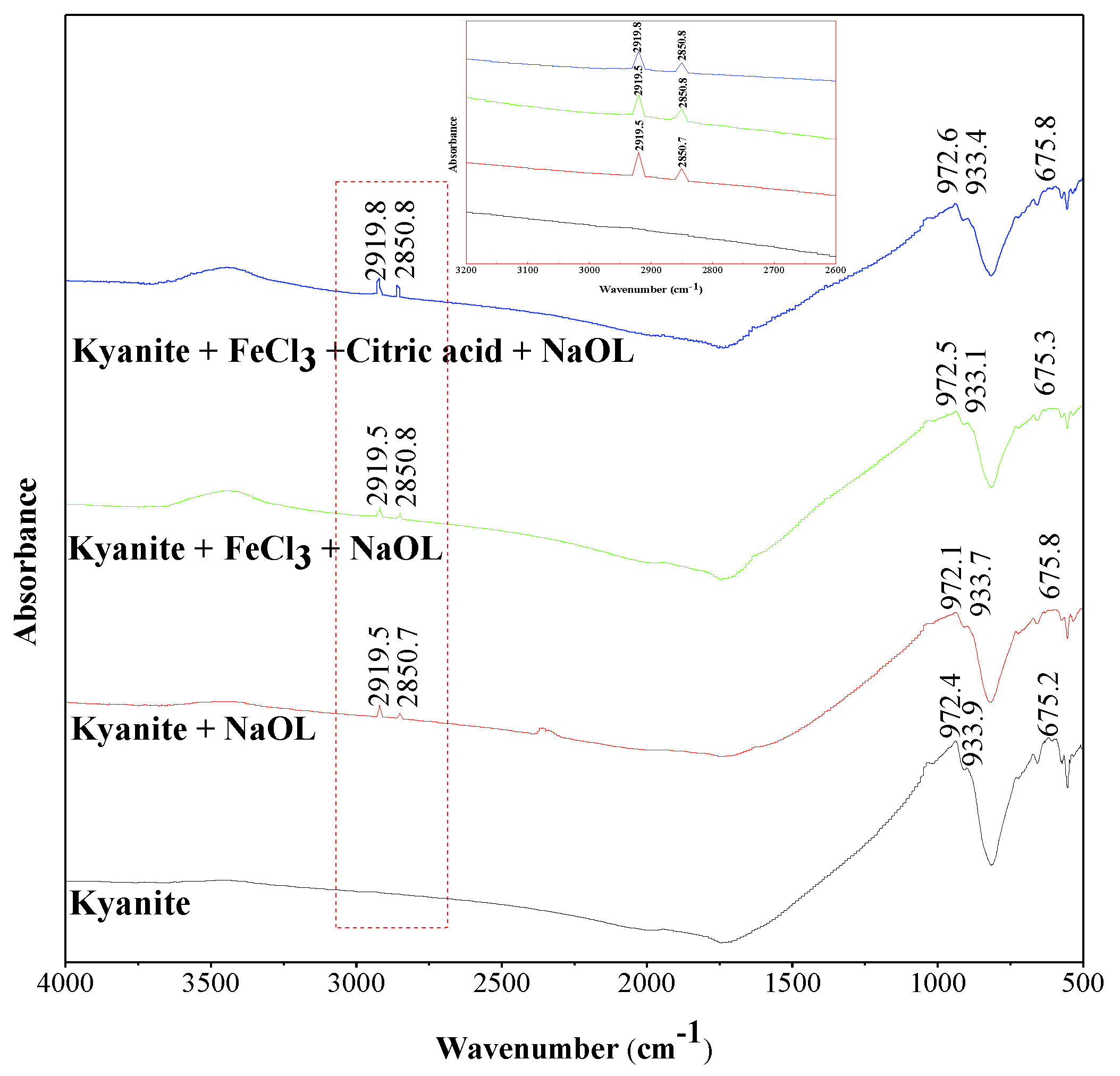
3.3. FTIR Measurements
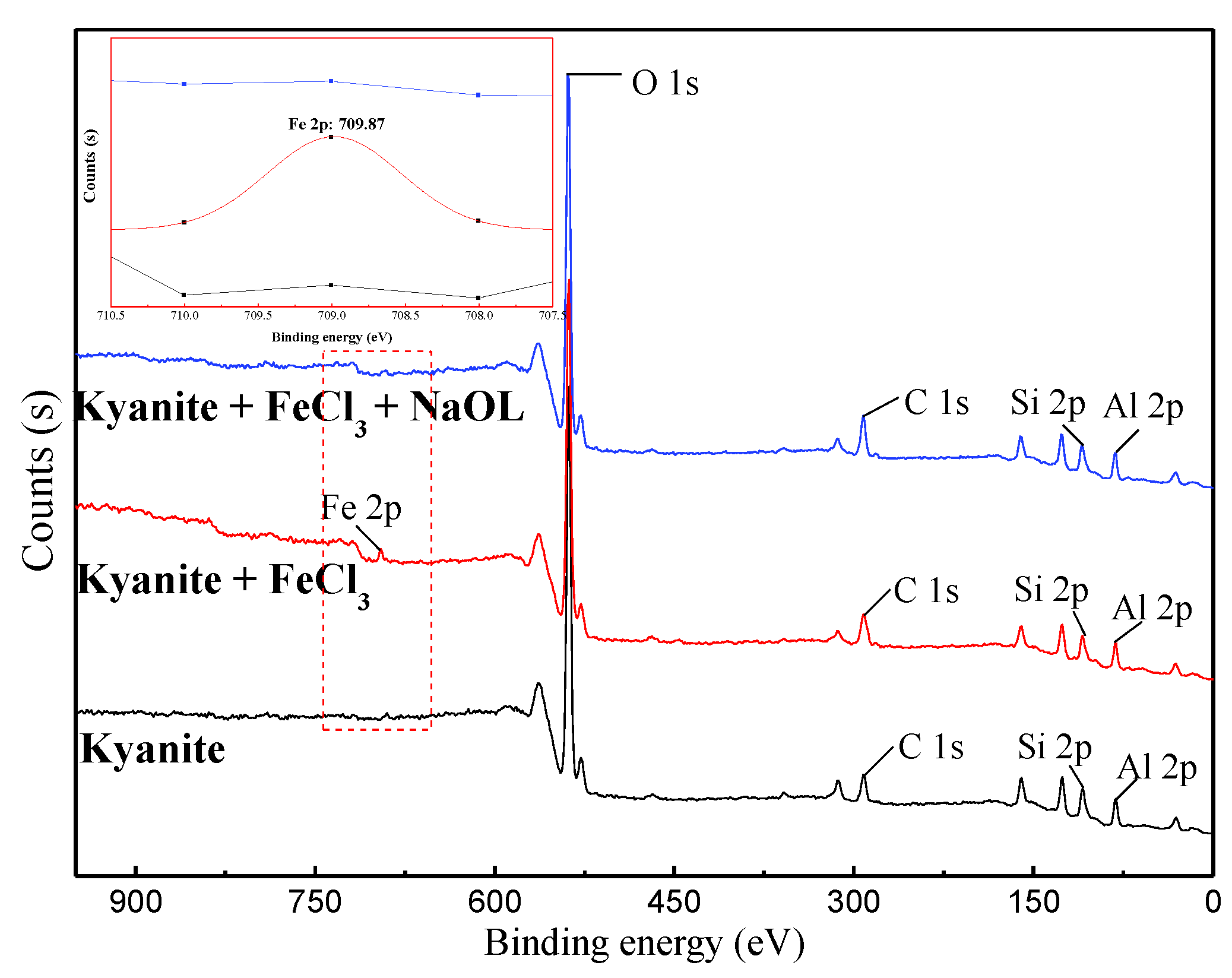
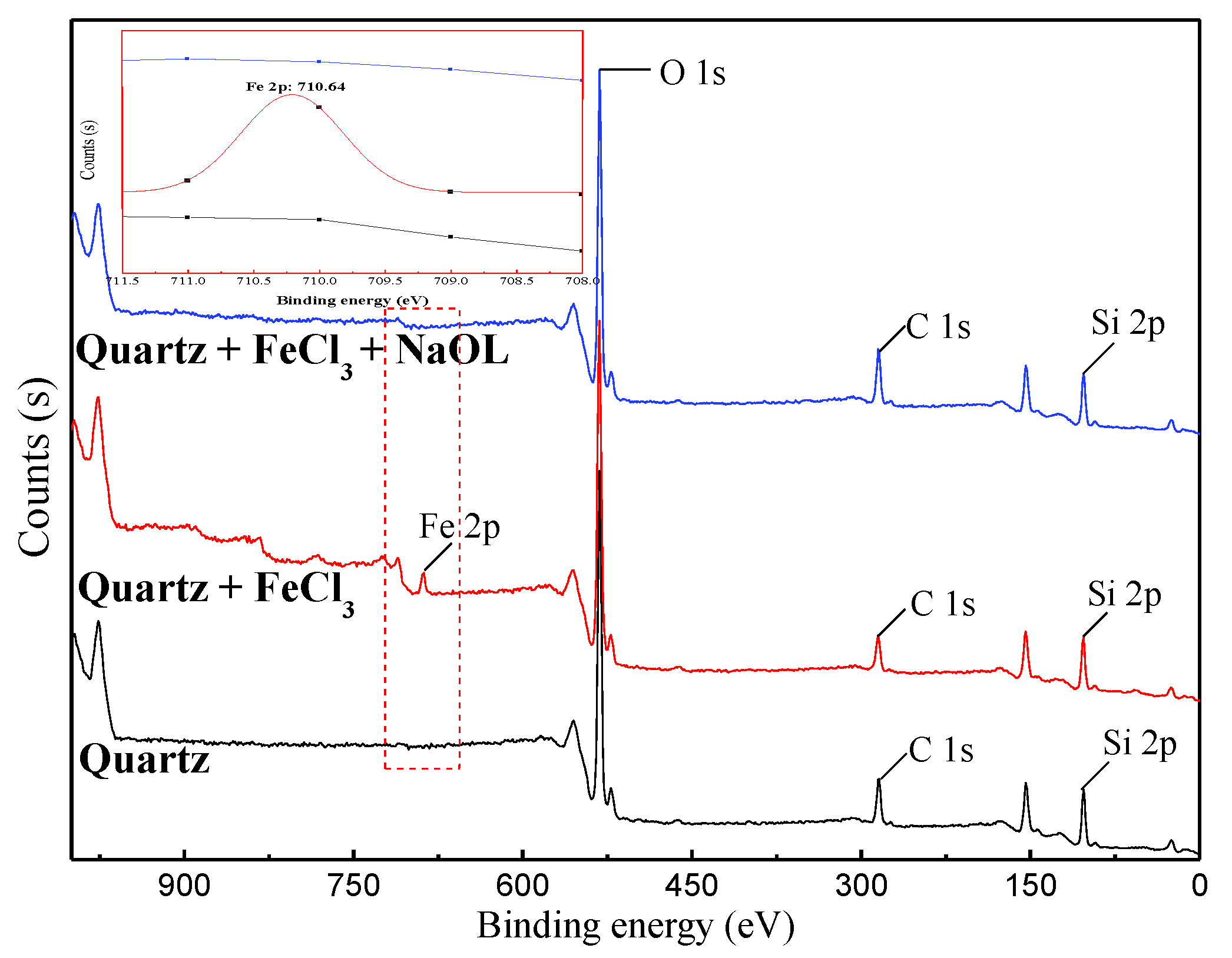
3.4. XPS Measurement
4. Conclusions
- The addition of citric acid weakened the effect of ferric ions on quartz flotation and had minimal influence on kyanite.
- In the presence of NaOL and FeCl3, the addition of citric acid made the quartz surface hydrophilic again but also slightly deceased the hydrophobicity of the kyanite. Thus, the citric acid maintained the significant recovery of kyanite.
- Citric acid could eliminate the activation of FeCl3 on the quartz, resulting in the nonadsorption of NaOL onto the quartz surface. However, the FeCl3 and citric acid had a negligible effect on NaOL adsorption onto the kyanite surface.
- Citric acid decreased the ferric ion content adsorbed onto the kyanite and quartz surfaces.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shackelford, J.F.; Doremus, R.H. Ceramic and Glass Materials: Structure, Properties and Processing, 3rd ed.; Springer: New York, NY, USA, 2008; p. 41. [Google Scholar]
- Andrey, D.K.; Oleg, G.P.; Leonid, A.S.; Sergey, N.V. Kyanite ore processing by carbochlorination. Int. J. Miner. Process. 2014, 126, 70–75. [Google Scholar]
- Jin, J.; Gao, H.; Chen, X.; Peng, Y. The separation of kyanite from quartz by flotation at acidic pH. Miner. Eng. 2016, 92, 221–228. [Google Scholar] [CrossRef]
- Zhang, W.; Meng, Q.; Dai, W. Research on application of kyanite in plastic refractory. Chin. J. Geochem. 2013, 32, 326–330. [Google Scholar] [CrossRef]
- Skoog, A.; Moore, R. Refractory of the past for the future: Mullite and its use as a bonding phasse. Am. Ceram. Soc. Bull. 1988, 67, 1180–1185. [Google Scholar]
- Brandao, P.R.G.; Mendes, S.I.C. Kyanite from Minas Gerais, Brasil: Characterization for use in ceramic. Innovations in Mineral and Coal Processing. In Proceedings of the 7th International Mineral Processing Symposium, Istanbul, Turkey, 29 April 1998. [Google Scholar]
- Jamerson, H.; Dixon, G.; Brown, J.J. Mineralogy of the kyanite beneficiation process. In Proceedings of the 7th United International Technical Conference on Refractories, Cancun, Mexico; 2001. [Google Scholar]
- Guanghuan, W. Chinese resources and processing technology for kyanite minerals. Ind. Miner. 1990, 95–98. [Google Scholar]
- Bulut, G.; Yurtsever, C. Flotation behaviour of Bitlis kyanite ore. Int. J. Miner. Process. 2004, 73, 29–36. [Google Scholar] [CrossRef]
- Belogurova, O.A.; Grishin, N.N. Carbidized heat insulation materials from kyanite ore. Refract. Ind. Ceram. 2012, 53, 26–30. [Google Scholar] [CrossRef]
- Amanullah, S.; Rao, G.M.; Satyanarayana, K. Beneficiation of mica-quartzbearing kyanite. Ind. Miner. 1990, 271, 24–29. [Google Scholar]
- Wildner, M.; Beran, A.; Koller, F. Spectroscopic characterisation and crystal field calculations of varicoloured kyanites from Loliondo. Tanzan. Mineral. Pet. 2013, 107, 289–310. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Evans, D.G.; Duan, X. Surface modification of layered double hydroxides and incorporation of hydrophobic organic compounds. Mater. Chem. Phys. 2005, 92, 190–196. [Google Scholar] [CrossRef]
- Wang, F. Experimental Research on Low Grade Refractory Kyanite in Jiangsu Province. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2010. [Google Scholar]
- Abramov, A.A. Flotation Methods of Mineral Processing; Nedra: Moscow, Russia, 1993. [Google Scholar]
- Jin, J.; Gao, H.; Chen, X. The flotation of aluminosilicate polymorphic minerals with anionic and cationic collectors. Miner. Eng. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Alekseev, V.S.; Morozov, G.G. Rate of flotation of kyanite, quartz, and muscovite. Sov. Min. 1975, 11, 62–64. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y. Andalusite flotation using alkyl benzene sulfonate as the collector. Min. Procext. Met. Rev. 2011, 32, 267–277. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Z.; Ali, M.; Gu, X.; Danish, M.; Sui, Q.; Lyu, S. Degradation of trichloroethene by citric acid chelated Fe(II) catalyzing sodium percarbonate in the environment of sodium dodecyl sulfate aqueous solution. Chemosphere 2021, 281, 130798. [Google Scholar] [CrossRef]
- Yu, S.; Gu, X.; Lu, S.; Xue, Y.; Zhang, X.; Xu, M.; Qiu, Z.; Sui, Q. Degradation of phenanthrene in aqueous solution by a persulfate/percarbonate system activated with CA chelated-Fe(II). Chem. Eng. J. 2017, 333, 122–131. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, X.; Lu, S.; Miao, Z.; Xu, M.; Fu, X.; Danish, M.; Brusseau, M.; Qiu, Z.; Sui, Q. Enhanced degradation of trichloroethene by calcium peroxide activated with Fe(III) in the presence of citric acid. Front. Environ. Sci. Eng. 2016, 10, 502–512. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, C.; Yin, W.; Zhang, X.; Xu, H.; Zhang, X. Selective flotation separation of andalusite and quartz and its mechanism. Int. J. Min. Met. Mater. 2019, 26, 1059. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Zhu, Z.; Yin, W.; Sheng, Q.; Hou, Y.; Yao, J. New insights into selective-depression mechanism of novel depressant EDTMPS on magnesite and quartz surfaces: Adsorption mechanism, DFT calculations, and adsorption model. Miner. Eng. 2021, 160, 106660. [Google Scholar] [CrossRef]
- Yang, B.; Yin, W.; Zhu, Z.; Sun, H.; Sheng, Q.; Fu, Y.; Yao, J.; Zhao, K. Differential adsorption of hydrolytic polymaleic anhydride as an eco-friendly depressant for the selective flotation of apatite from dolomite. Sep. Purif. Technol. 2021, 256, 117803. [Google Scholar] [CrossRef]
- Yao, J.; Sun, H.; Yang, B.; Zhou, Y.; Yin, W.; Zhu, Z. Selective co-adsorption of a novel mixed collector onto magnesite surface to improve the flotation separation of magnesite from dolomite. Powder Technol. 2020, 371, 180–189. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, W.; Wang, D.; Sun, H.; Chen, K.; Yang, B. The role of surface roughness in the wettability and floatability of quartz particles. Appl. Surf. Sci. 2020, 527, 146799. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H.; Liu, Y. Flotation separation of smithsonite from calcite using 2-phosphonobutane-1,2,4-tricarboxylic acid as a depressant. Powder Technol. 2019, 352, 11–15. [Google Scholar] [CrossRef]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Ning, J.; He, J.; Lu, L.; Wang, Y.; Sun, L. Relation between brine-crude oil-quartz contact angle formed on flat quartz slides and in capillaries with brine composition: Implications for low-salinity waterflooding. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 660–667. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, D.; Yang, B.; Yin, W.; Ardakanib, M.S.; Yao, J.; Drelich, J.W. Effect of nano-sized roughness on the flotation of magnesite particles and particle-bubble interactions. Miner. Eng. 2020, 151, 106340. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Wang, B.; Zhao, Q.; Duan, H.; Chen, X. Molecular-level insights into the adsorption of a hydroxy-containing tertiary amine collector on the surface of magnesite ore. Powder Technol. 2019, 355, 700–707. [Google Scholar] [CrossRef]
- Yao, J.; Sun, H.; Han, F.; Yin, W.; Hong, J.; Wang, Y.; Won, C.; Du, L. Enhancing selectivity of modifier on magnesite and dolomite surfaces by pH control. Powder Technol. 2020, 362, 698–706. [Google Scholar] [CrossRef]
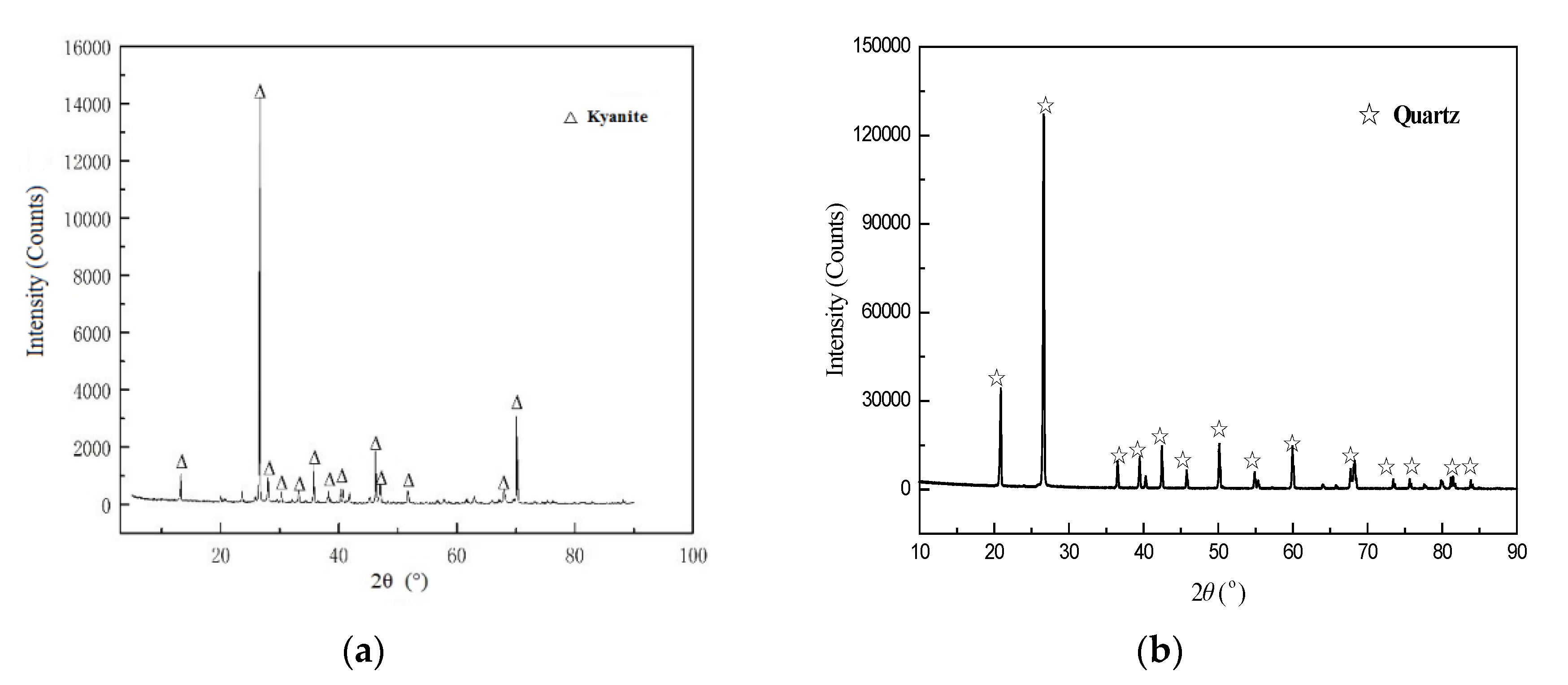



| Minerals | SiO2 | Al2O3 | TFe | MgO | CaO | TiO2 | Mn | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|
| Kyanite | 36.52 | 60.40 | 0.81 | 0.18 | — | 0.060 | — | 0.51 |
| Quartz | 99.31 | — | 0.08 | — | — | — | — | — |
| Flotation Conditions | Product | Yield (%) | Grade (%) | Recovery (wt%) | ||
|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | Kyanite | Quartz | |||
| NaOL: 150 mg/L; pH: 7.5; FeCl3: 20 mg/L. | Concentrate | 81.50 | 35.50 | 64.46 | 92.00 | 71.00 |
| Tailings | 18.50 | 13.60 | 86.39 | 8.00 | 29.00 | |
| Feed | 100.00 | 31.45 | 59.26 | 100.00 | 100.00 | |
| NaOL: 150 mg/L; pH: 7.5; FeCl3: 20 mg/L; Citric acid: 8 mg/L. | Concentrate | 51.62 | 52.51 | 47.43 | 86.11 | 16.89 |
| Tailings | 48.38 | 9.08 | 90.91 | 13.89 | 83.11 | |
| Feed | 100.00 | 31.45 | 59.26 | 100.00 | 100.00 | |
| Test Conditions | Contact Angles of Minerals (°) | |
|---|---|---|
| Kyanite | Quartz | |
| Natural minerals | 23 | 14 |
| NaOL: 150 mg/L | 92 | 17 |
| FeCl3: 20 mg/L; NaOL: 150 mg/L | 97 | 72 |
| FeCl3: 20 mg/L; Citric acid: 8 mg/L; NaOL: 150 mg/L | 91 | 22 |
| Minerals | Reagent | Content of Element (%) | ||||
|---|---|---|---|---|---|---|
| C 1s | O 1s | Si 2p | Al 2p | Fe 2p | ||
| Kyanite | None | 14.51 | 56.34 | 12.92 | 16.24 | — |
| FeCl3 | 17.20 | 53.48 | 12.01 | 15.73 | 1.57 | |
| FeCl3 + Citric acid | 17.94 | 53.14 | 12.23 | 16.57 | 0.12 | |
| Quartz | None | 12.18 | 55.35 | 32.48 | — | |
| FeCl3 | 18.34 | 52.67 | 26.97 | 2.02 | ||
| FeCl3 + Citric acid | 22.13 | 49.28 | 28.50 | 0.09 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Li, Y.; Sun, H.; Sun, C.; Yin, W.; Xu, H. Elimination of Ferric Ion Effect on Separation between Kyanite and Quartz Using Citric Acid as Regulator. Minerals 2021, 11, 599. https://doi.org/10.3390/min11060599
Niu Y, Li Y, Sun H, Sun C, Yin W, Xu H. Elimination of Ferric Ion Effect on Separation between Kyanite and Quartz Using Citric Acid as Regulator. Minerals. 2021; 11(6):599. https://doi.org/10.3390/min11060599
Chicago/Turabian StyleNiu, Yanping, Ya Li, Haoran Sun, Chuanyao Sun, Wanzhong Yin, and Hongfeng Xu. 2021. "Elimination of Ferric Ion Effect on Separation between Kyanite and Quartz Using Citric Acid as Regulator" Minerals 11, no. 6: 599. https://doi.org/10.3390/min11060599





