Abstract
The study of the depression effect of non-toxic depressants on the flotation separation of chalcopyrite from galena is of great importance for both industrial applications and theoretical research. The mixed depressant (DFinal) of four common inhibitors—sodium carboxymethyl cellulose, sodium silicate, sodium sulfite, and zinc sulfate—exhibited high selectivity during the separation of chalcopyrite from galena. Flotation tests on an industrial copper–lead bulk concentrate showed that using this depressant mixture can achieve highly efficient separation of chalcopyrite from galena at the natural pH of the pulp. Copper and lead concentrates were produced at grades of 21.88% (Cu) and 75.53% (Pb), with recoveries of 89.07% (Cu) and 98.26% (Pb). This showed a similar performance of DFinal with dichromate, which is a depressant that is widely used in industry, but without the environmental risks or the need for pH control. Zeta potential and Fourier transform infrared (FT-IR) results showed that interaction between the surface of the chalcopyrite and the mixed depressant was prevented by pre-treatment with a composite thiophosphate collector (CSU11), while the mixed depressant could expel/replace the composite thiophosphate on the surface of galena by chemical adsorption, depressing its flotation. This is the reason why this non-toxic depressant achieved the selective depression of galena from chalcopyrite, leading to efficient flotation separation.
1. Introduction
Copper and lead are non-ferrous metals widely used in modern industry. They are mainly extracted from chalcopyrite (CuFeS2) and galena (PbS), which are often associated with each other [1,2,3]. Due to the similar flotation behavior of chalcopyrite and galena, their separation has been always one of the difficult problems in the processing of copper-lead–zinc sulfide ores [4,5,6,7,8]. The commonlyused collectors such as xanthate and dithiophosphate show poor selectivity between chalcopyrite and galena, so it is necessary to add a depressant to achieve separation [9,10,11,12]. There are two traditional technical schemes for the separation of chalcopyrite and galena. One uses dichromate to depress galena and float chalcopyrite, and the other uses cyanide to depress chalcopyrite and float galena [13,14,15]. However, because Cr6+ ions and cyanide pose serious environmental problems, flotation processes without cyanide and Cr6+ are gradually replacing the traditional flotation processes. As a result, the development of non-toxic and environmentally friendly depressants has become the main research direction [16,17,18]. These depressants can be divided into inorganic depressants, such as sulfite [19,20,21], polyphosphate [22], sodium sulfide [23], and lime, and organic depressants such as dextrin [24], sodium humate [25], and alginate [2].
In recent years, organic depressants have been widely used depending on their environmental friendliness, availability, and selectivity [16,26,27,28]. It has been reported that sodium carboxymethyl cellulose (CMC) is an effective depressant for galena, and it can be used to separate chalcopyrite from galena due to the carboxyl substituent groups along the cellulose chain [2,17,24,29,30]. However, it also negatively impacts the floatability of chalcopyrite, leading to decreased chalcopyrite recovery.
As an inorganic depressant, sodium silicate can also be used to separate chalcopyrite from galena [8]. However, on its own, it is not effective in flotation processes. Sodium sulfite and zinc sulfate constitute a commonly used combined depressant for separating galena from sphalerite in the flotation of sulfide ores [31]. Sodium sulfite itself is a multifunctional reagent with various uses in the industrial flotation of sulfide minerals and in hydrometallurgy [21]. The zinc hydroxide precipitate is the species from the zinc sulfate solution which inhibits the flotation of the sphalerite [32].
In this study, the non-toxic, environmentally friendly mixture of sodium carboxymethyl cellulose (CMC) (an organic depressant) and sodium silicate, sodium sulfite, and zinc sulfate (all inorganic depressants) was used as a depressant for the separation of chalcopyrite from galena. For the first time, a combination of an organic depressant and inorganic depressants was used in a chalcopyrite–galena flotation system as a clean and efficient depressant of galena. High-purity specimens of chalcopyrite and galena and an industrial copper–lead bulk concentrate were selected as test samples. The effect of the mixed depressant was studied through laboratory flotation experiments on the high-purity, single-mineral samples and samples from an industrial copper–lead bulk concentrate. The interaction mechanisms between the reagents and the minerals were studied using zeta potential and Fourier transform infrared spectrum (FT-IR) measurements. Finally, a green flotation and reagent system for the separation of chalcopyrite from galena was established.
2. Materials and Methods
2.1. Samples and Reagents
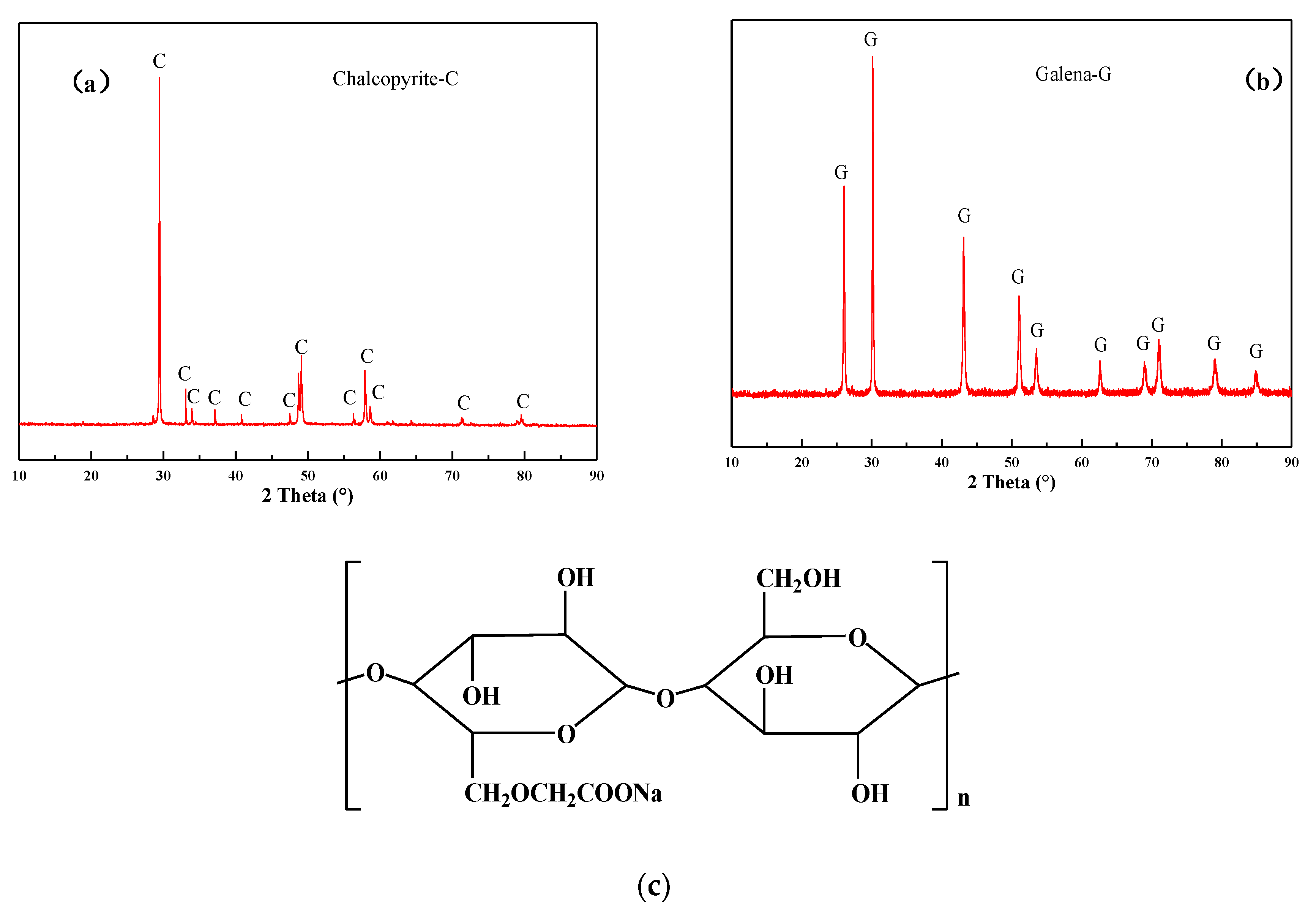
High-purity samples of chalcopyrite and galena were obtained from Beijing Shuiyuan Shanchang mineral specimen Co. LTD. Using chemical assays and X-ray diffraction (XRD, Figure 1a,b; The Netherlands, Almelo, Malvern Panalytical X Pert pro), the purities of chalcopyrite and galena were found to be 94.92% (32.86% Cu, 29.03% Fe, 33.17% S) and 97.67% (84.58% Pb, 13.09% S), respectively. The samples were dry ground and screened to produce −74 + 38 µm and −38 µm samples for the flotation tests and the interaction mechanism measurements (zeta-potential and FT-IR), respectively. The ground minerals were all sealed in the jar and put into a vacuum tank to avoid the oxidation of the sulfides surface.
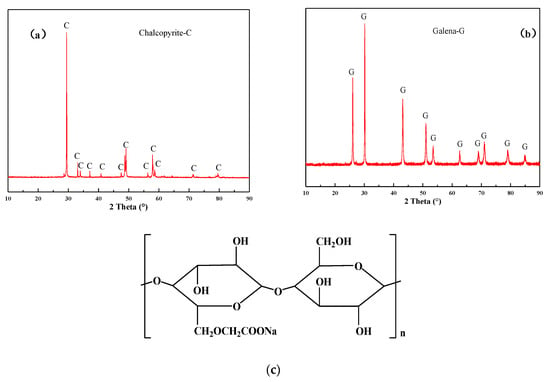
Figure 1.
XRD pattern of (a) chalcopyrite and (b) galena; (c) the structure of CMC.
The commercial copper–lead bulk concentrate for the locked-cycle batch flotation tests was obtained from the Nanan Copper–lead–zinc Mine, Guangxi province, China. The chemical assays are shown in Table 1. Table 2 displays the chemical phase of copper and lead in the copper–lead bulk concentrate, and Table 3 displays its mineral composition.

Table 1.
Chemical analysis of the industrial copper–lead bulk concentrate (wt %).

Table 2.
Chemical phase of copper and lead in the copper–lead bulk concentrate (wt %).

Table 3.
The mineral composition of the copper–lead bulk concentrate (wt %).
In the experiments, the reagent CSU11 (a composite thiophosphate) was used as a dual-function reagent, acting as both collector and frother. In addition, sodium carboxymethyl cellulose (CMC, whose structure is illustrated in Figure 1c) [33], sodium silicate (Na2O2.7SiO2, SS), sodium sulfite (Na2SO3), zinc sulfate (ZnSO4), and potassium dichromate (K2Cr2O7) were used as depressants. All reagents used in the micro-flotation tests and mechanism measurements were of analytical (AR) grade. The reagents used in the batch flotation tests were tech grade. Deionized water was used for the micro-flotation tests, zeta potential, and FT-IR measurements. Tap water was used in the batch flotation tests.
2.2. Flotation Experiments
The micro-flotation experiments were carried out in an XFG−40 mL flotation machine (Figure 2; XFGII, Jilin Exploration Machinary Plant, Jilin, China). The impeller speed of the flotation machine was set to 1800 rpm. During each experiment, 2.0 g of sample was placed in a Plexiglass cell, which was then filled with 35 mL of distilled water. After agitating the pulp for 1 min, the collector (conditioning time = 2 min) and the depressant (conditioning time = 2 min) were added in sequence. Then, flotation was performed for 5 min. The concentrate (floated by the air bubbles) and tailings were weighed separately after filtration and drying, and the recovery was calculated based on the weight of the products. Three micro-flotation experiments were performed for each set of conditions, and the average results are reported.

Figure 2.
XFG−40 mL flotation machine with the flotation cell, dam board, and scraper.
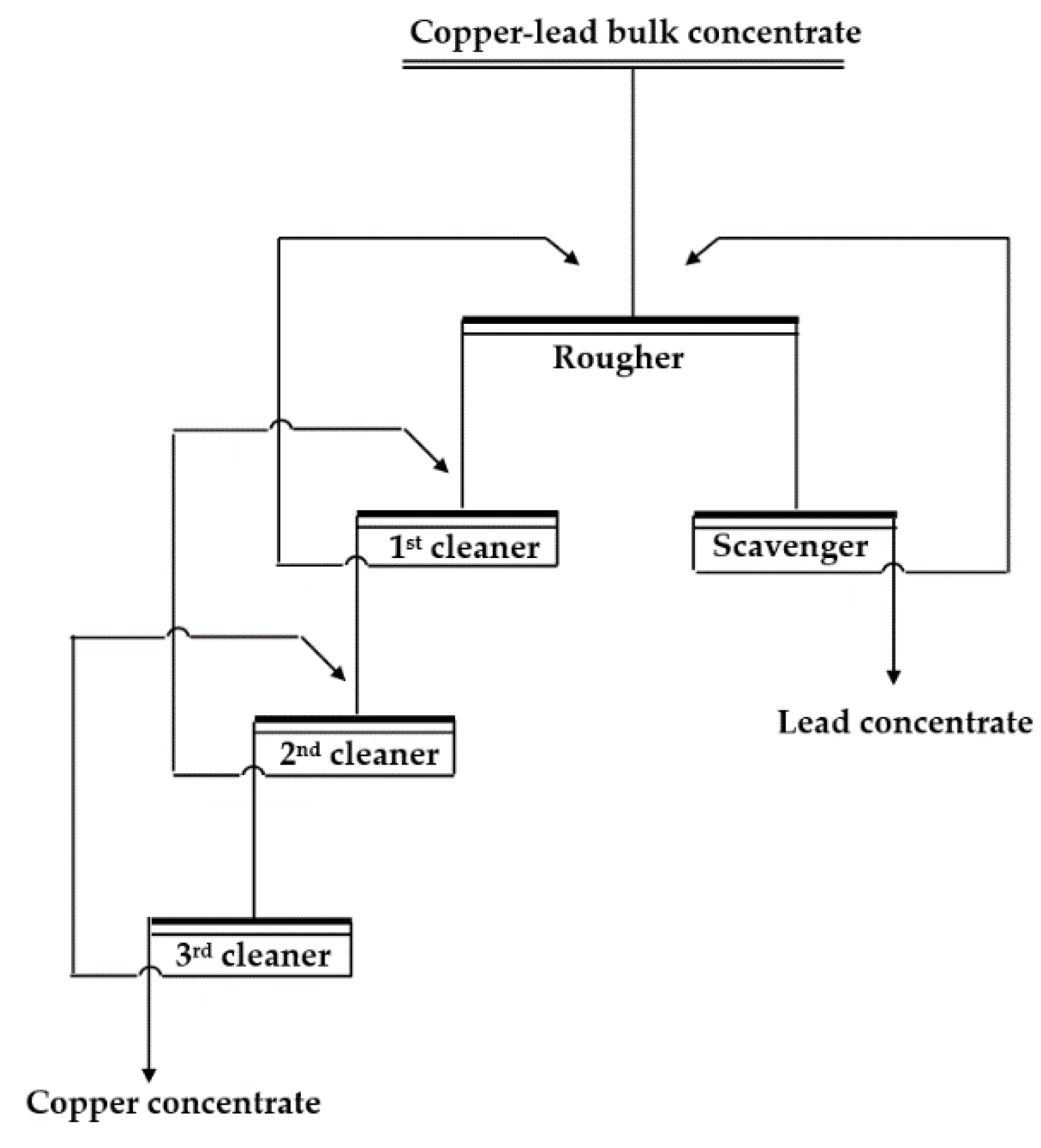
The batch flotation tests for the industrial copper–lead bulk concentrate were performed in an XFD-63 flotation cell, using a 1000 mL cell for the rougher and scavenger and a 500 mL cell for the cleaner. The impeller speed was again set to 1800 rpm. During the flotation tests, the reagents were added in sequence to the flotation cell at 2 min intervals, and then flotation was performed for 5 min. The final products of the concentrates and tailings were filtered, dried, weighed, and analyzed for Cu and Pb, respectively. The flowsheet of the batch locked-cycle tests is shown in Figure 3. The experimental system used the non-toxic depressant mixture of CMC + SS + sodium sulfite + zinc sulfate, while the baseline comparison system used potassium bichromate as the depressant. To ensure a high degree of confidence in the batch flotation tests, the calculated feed grade was compared to the head assay. If the calculated Cu feed grade did not fall within the range of 3.53 ± 0.02%, then the concentrates and tailings were re-assayed, and if the new calculated Cu feed grade remained outside this range, then those results were discarded, and that group of flotation tests was repeated.
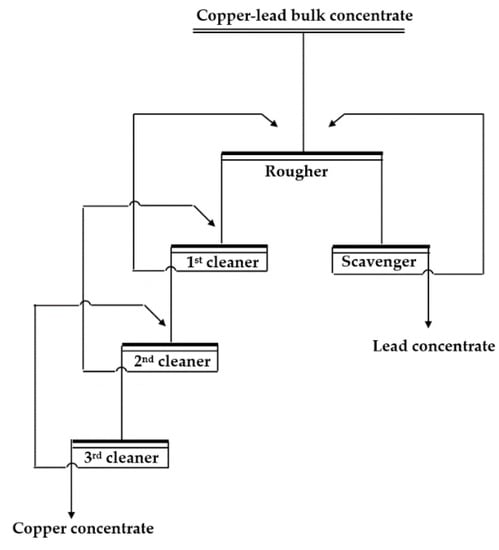
Figure 3.
Flowsheet of the batch locked-cycle tests.
2.3. Zeta Potential Tests
Zeta potential tests on chalcopyrite and galena were carried out using a Coulter DELSA440S II (Beckman Coulter & Company, Brea, CA, USA). The −38 μm fractions were first hand milled to about −5 μm using an agate mortar. Then, 2.0 g of sample was added to 40 mL of deionized water with or without the prescribed amount of reagent, and the suspension was ultrasonicated for 3 min and then magnetically stirred for 10 min. Then, the pH was adjusted by adding HCl or NaOH. The pH value was measured using a PHS-3C type pH meter. Then, the zeta potential of each sample was measured separately. The test results were found to be within 3 mV after at least three tests under each experimental condition, and the average values are reported.
2.4. FT-IR Tests
The following procedure was used for the FT-IR spectra measurements. The −38 μm fractions were first hand milled to about −5 μm using an agate mortar. The spectra of the solids were measured against a KBr background spectrum. For each experimental condition, 2.0 g of sample was added into 30 mL aqueous solution with or without the prescribed amount of reagent, followed by ultrasonication for 5 min. Then, the solution was magnetically stirred for 40 min and allowed to settle for another 40 min. After filtering and vacuum drying, the Fourier transform infrared (FT-IR) spectrum was recorded using a Nicolet FT-IR-740 spectrometer (ThemoScientific, Waltham, MA, USA).
3. Results and Discussion
3.1. Micro-Flotation Test Results
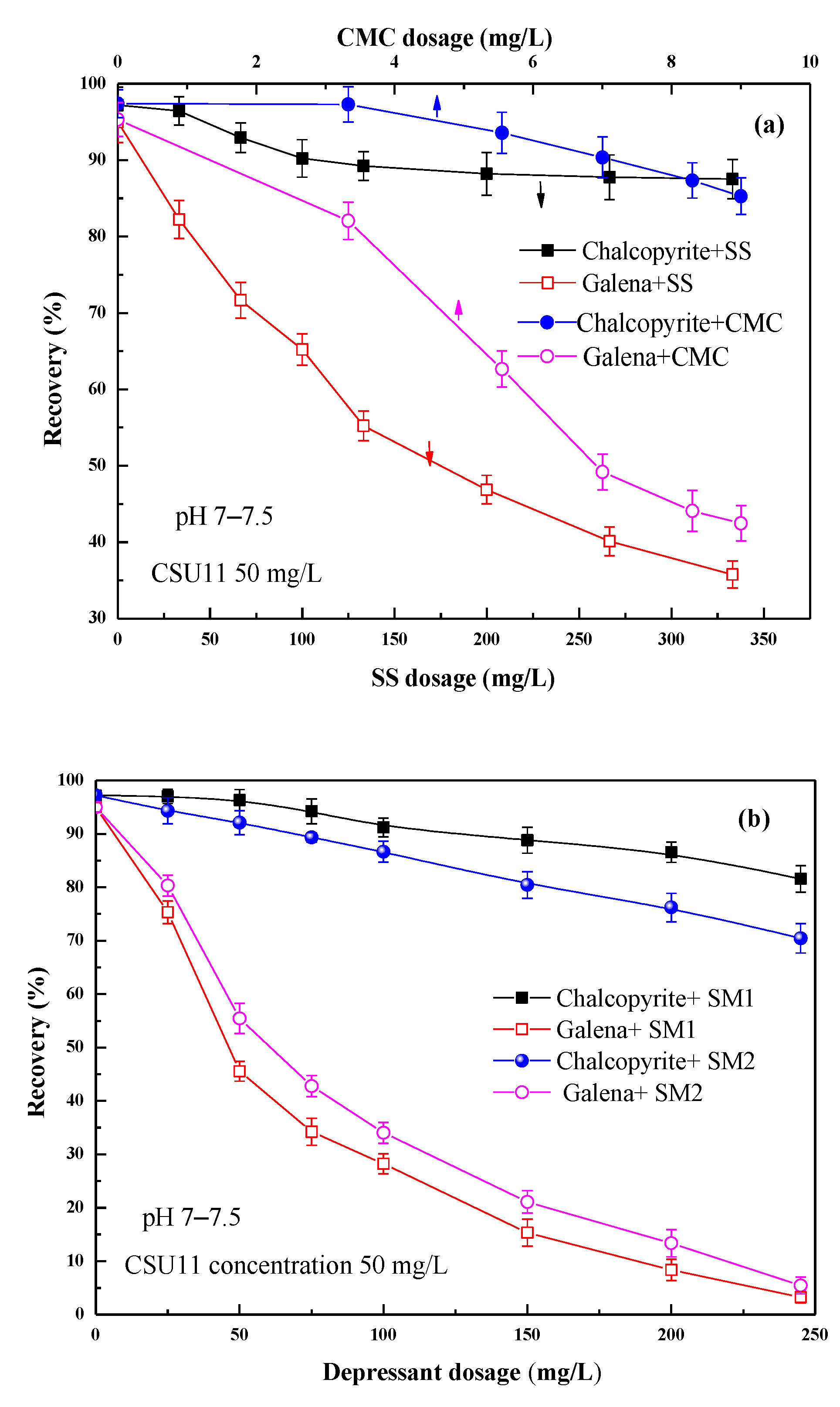
Figure 4 shows the effect of the concentration of the single SS and CMC, and their depressant mixtures on chalcopyrite and galena flotation at natural pH. During the tests, the pH was observed in the range of pH 7–7.5.
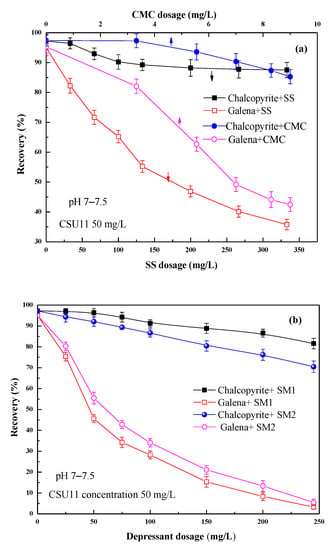
Figure 4.
Effect of (a) SS/CMC and (b) their mixtures SM1/SM2 on single mineral flotation behavior.
As shown in Figure 4a, single CMC and SS all showed some depression on the flotation of chalcopyrite and galena. However, they either inhibited both the two minerals slightly (CMC) or inhibited both of them stronger (SS); i.e., the selectivities of SS and CMC are all poor.
Two depressant mixtures were used—SM1 (with a 50:1 mass ratio of SS and CMC) and SM2 (with a 20:1 mass ratio). Although both SM1 and SM2 significantly reduced the floatability of galena, it was reduced more by SM1 than SM2. Meanwhile, SM1 significantly depressed galena, and it had a small effect on chalcopyrite. The best separation results were observed when the SM1 concentration was approximately 200 mg/L. At this concentration, the recoveries of chalcopyrite and galena were 85.5% and 8.4%, respectively. Moreover, compared with single CMC and SS, their mixtures SM1 and SM2 showed better selectivity, and the optimal depressant concentration became lower than that of SS.
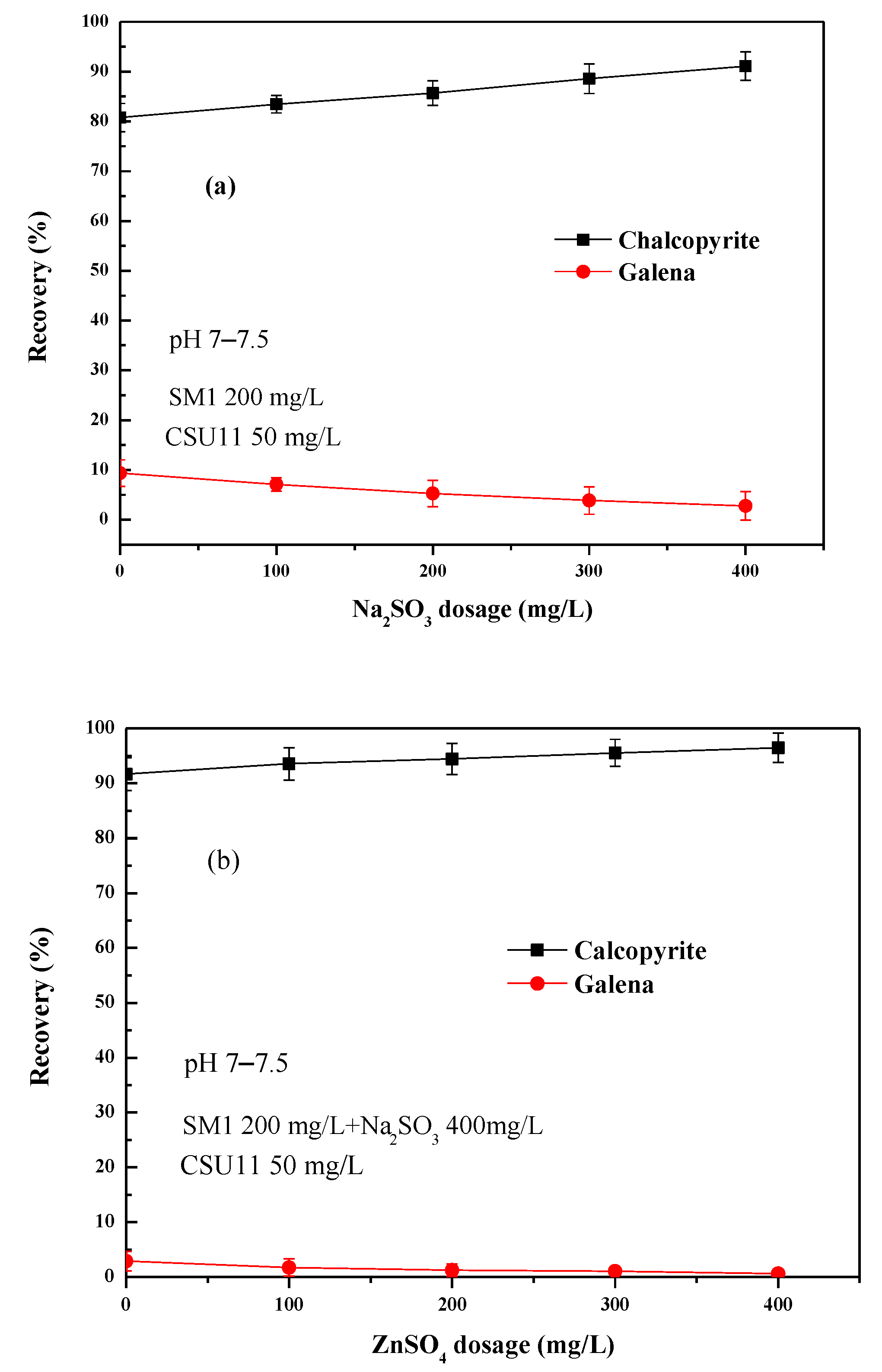
From Figure 4b, it can be found that the depression effect of SM1 varied significantly with concentration. Figure 5 shows the effect of the combined use of SM1 (fixed 200 mg/L), sodium sulfite, and zinc sulfate on chalcopyrite and galena flotation at natural pH (pH 7–7.5). As shown in Figure 5a, the addition of sodium sulfite to SM1 increased the difference in floatability between chalcopyrite and galena, and at the same time, it also had an activation effect on the flotation of chalcopyrite. When zinc sulfate was added to SM1 and sodium sulfite, the floatability difference between the two minerals further enlarged (Figure 5b). The mixed depressant (DFinal) with a mass ratio of 1:2:2 (SM1: sodium sulfite: zinc sulfate = 1:2:2) achieved the best flotation performance. Chalcopyrite had a very favorable recovery of 99.6%, and the recovery of galena was 0.7%, indicating that a highly efficient separation of chalcopyrite from galena could be achieved at natural pH using this depressant mixture.
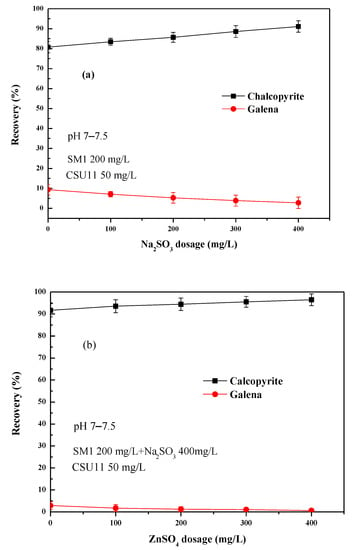
Figure 5.
(a) Effect of Na2SO3 dosage on single mineral flotation behavior; (b) Effect of ZnSO4 dosage on single mineral flotation behavior.
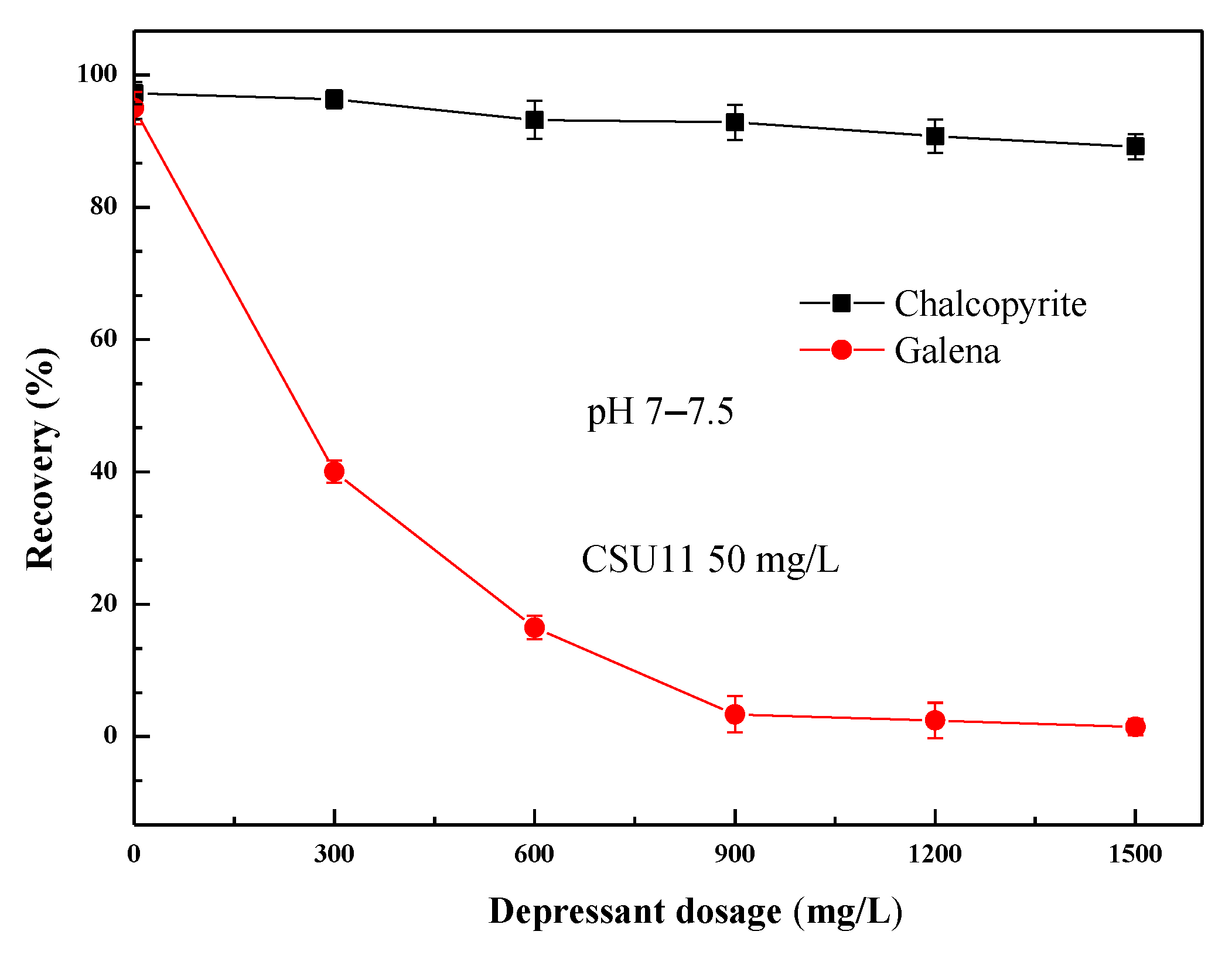
Figure 6 shows the effect of mixed depressant (SM1: sodium sulfite: zinc sulfate = 1:2:2, DFinal) concentration on chalcopyrite and galena flotation at natural pH (pH 7–7.5). The mixed depressant DFinal selectively depressed galena. The effect was significant: galena recovery rapidly decreased with increasing depressant concentration until 900 mg/L (at which the recovery of galena was 3.3%), with little effect on chalcopyrite flotation (the recovery of chalcopyrite was 93%). As the concentration of mixed depressant DFinal was increased further, the recovery of chalcopyrite decreased to some degree.

Figure 6.
Effect of depressant mixture DFinal concentration on single mineral flotation behavior.
3.2. Batch Flotation Test Results
Based on the results of the micro-flotation tests and the subsequent flotation condition tests, batch locked-cycle testing was carried out using SM1 + sodium sulfite + zinc sulfate (mass ratio of 1:2:2) as a mixed depressant (DFinal) at natural pH (experimental system). The comparison test was conducted using the industry-standard depressant potassium dichromate.
The closed flotation circuit included one roughing, one scavenging, and three cleaning steps (shown in Figure 3). The flotation conditions are shown in Table 4. The best flotation result achieved is shown in Table 5. Concentrate grades of 22.8% Cu and 75.34% Pb at recoveries of 90% Cu and 98.49% Pb were achieved using the industry standard depressant potassium dichromate. Concentrate grades of 21.88% Cu and 75.53% Pb at recoveries of 89.07% Cu and 98.26% Pb were achieved using the depressant mixture DFinal. This matched the performance of the industry standard depressant but without the environmental risks or the need for pH control. This illustrates that the 1:2:2 depressant mixture SM1 + sodium sulfite + zinc sulfate has great potential as an effective, non-toxic depressant for industrial application in the flotation of copper–lead bulk concentrate.

Table 4.
Flotation conditions of the batch locked-cycle tests.

Table 5.
Results of the batch locked-cycle tests.
3.3. Selective Adsorption Mechanism of the Mixed Depressant
Zeta potential and FT-IR measurements were conducted to investigate the selective depression mechanism of SM1 (the 50:1 mixing mass ratio of SS and CMC, the key part of the mixed depressant DFinal) in the separation of galena from chalcopyrite.
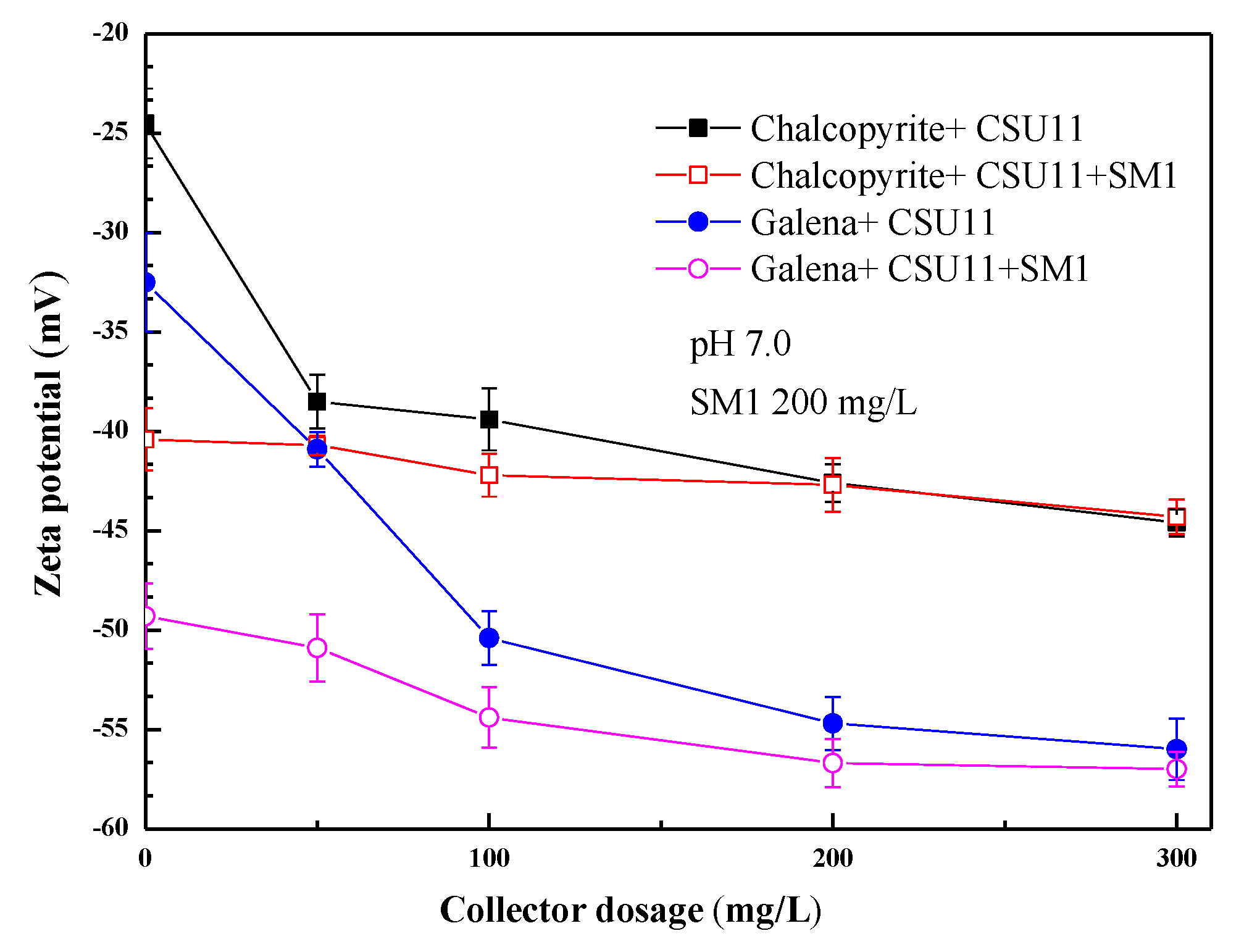
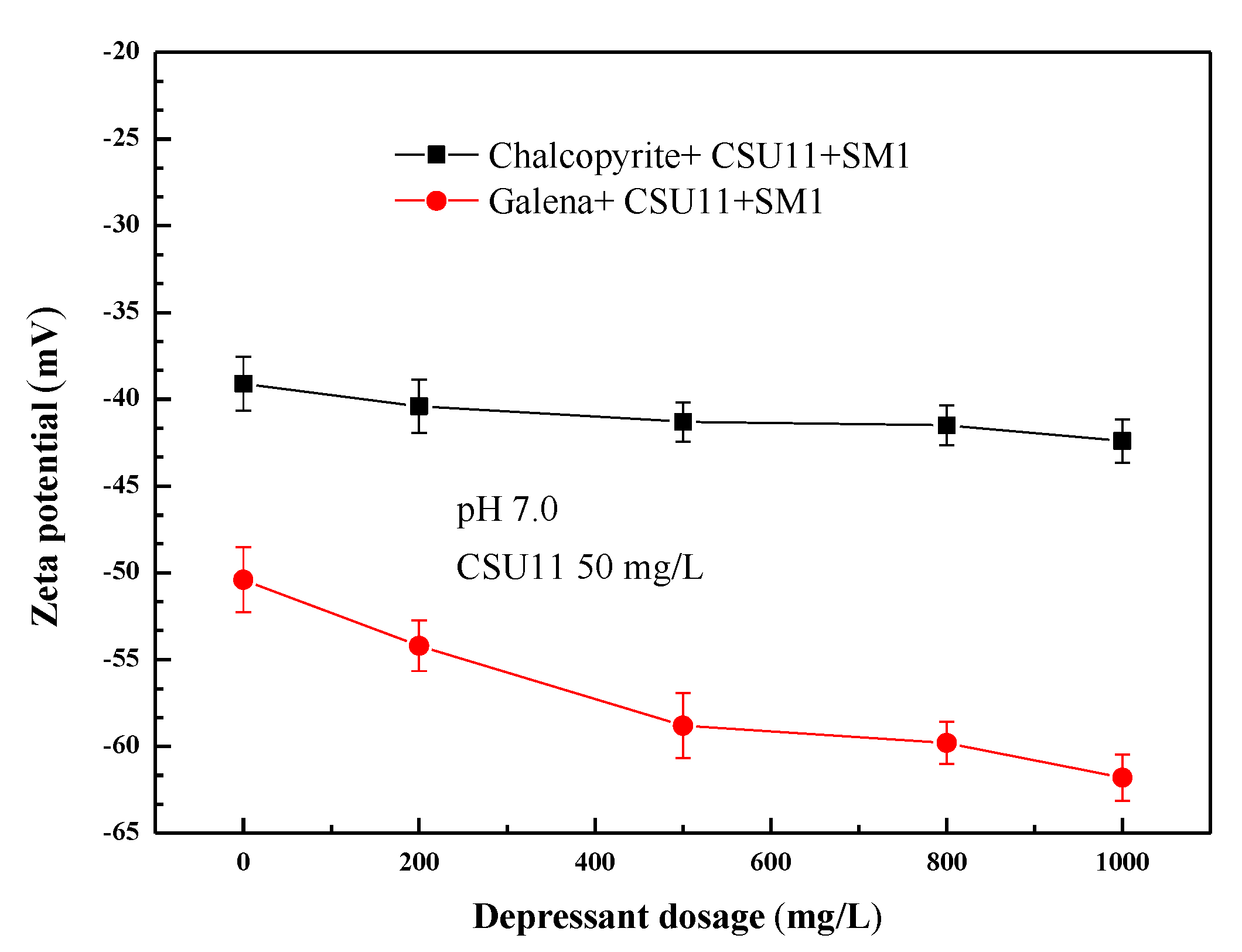
Figure 7 shows the effect of the concentration of collector CSU11 on the zeta potentials of chalcopyrite and galena at pH 7.0. Figure 8 shows the effect of the concentration of the depressant SM1 on the zeta potentials of chalcopyrite and galena, also at pH 7.0. Before interacting with any reagent, zeta potentials of −24.3 mV and −32.2 mV were obtained for chalcopyrite and galena, respectively. These values are consistent with the previous work of Chen [2]. After the two minerals were treated with anionic CSU11, the zeta potentials decreased, indicating that CSU11 had adsorbed onto the bare mineral surface [34]. As the concentration of collector CSU11 or the depressant SM1 increased, the zeta potential of the chalcopyrite with pre-adsorbed CSU11 (labeled “chalcopyrite + CSU11 + SM1”) remained nearly unchanged, implying that the interaction between the surface of the chalcopyrite and SM1 had been prevented by the CSU11 pre-treatment. However, a remarkable decrease in the zeta potential of galena with pre-adsorbed CSU11 (labeled “galena + CSU11 + SM1”) was observed as the concentration of CSU11 or SM1 increased, the drop indicated that the anionic SM1 still strongly interacted with the surface of the galena with CSU11 pre-treatment (“galena + CSU11”).

Figure 7.
Effect of collector CSU11 concentration on the zeta potentials of mineral samples.

Figure 8.
Effect of SM1 concentration on the zeta potentials of the mineral samples.
These results illustrate that SM1 can preferentially adsorb onto the surface of “galena + CSU11”, while the addition of SM1 has negligible effect on the adsorption of CSU11 onto the surface of the chalcopyrite. In other words, SM1 can expel/replace or cover the adsorbed CSU11 on the galena surface, but fails this on chalcopyrite surface. The difference in SM1 adsorption onto the surfaces of “chalcopyrite + CSU11” and “galena + CSU11” explains the high copper recovery and strong depression of galena when using the “SM1 + sodium sulfite + zinc sulfate” (DFinal) reagent scheme. The results are of great importance in explaining the selective depression effect of SM1 on galena and in defining the depression mechanism of SM1 in the separation process. These results are consistent with the flotation experiments.
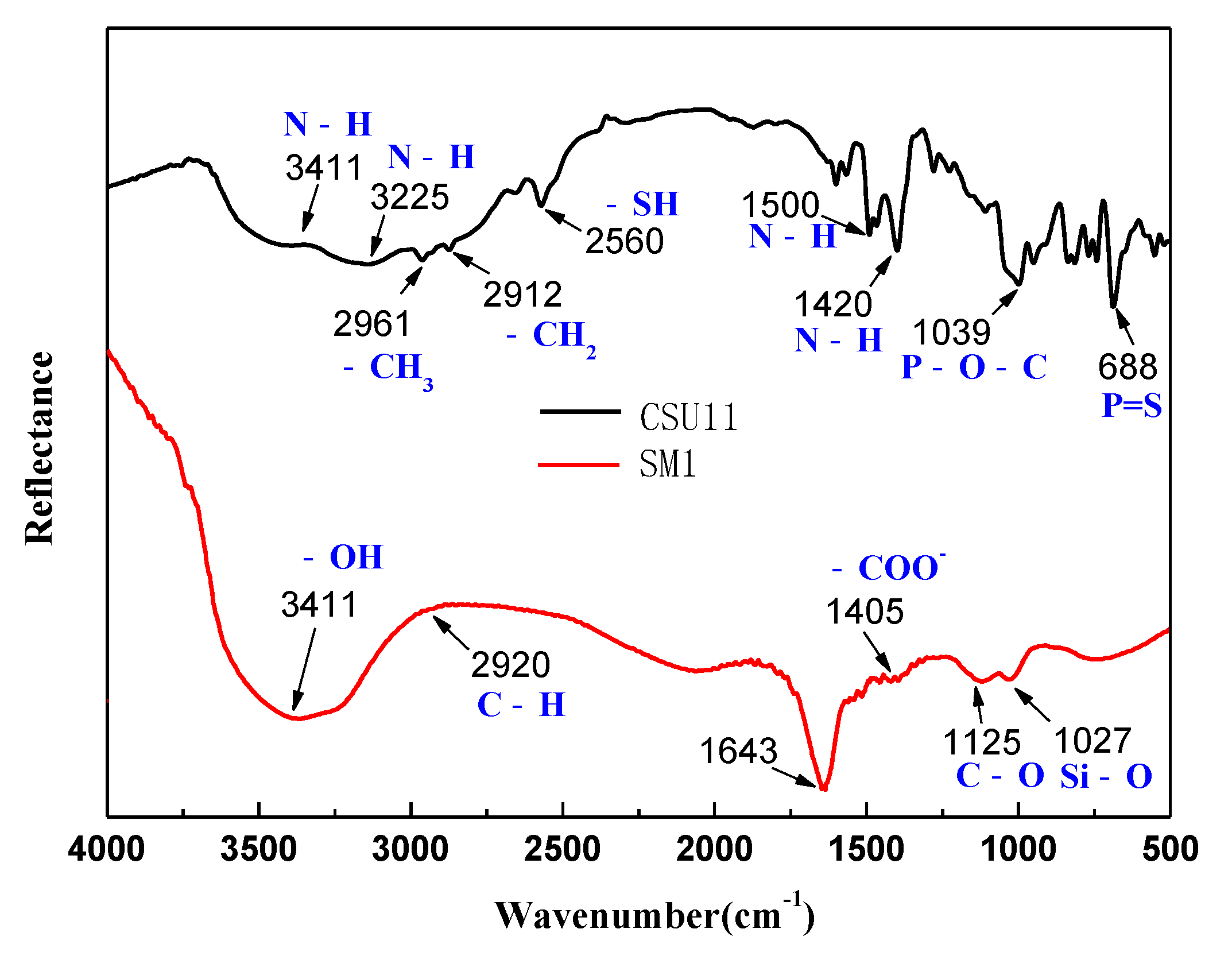
Figure 9 shows the FT-IR spectra of the collector CSU11 and the depressant SM1. In the FT-IR spectrum of CSU11, the bands at 3411 cm−1 and 3225 cm−1 were attributed to the stretching vibration of -NH [35]. The bands at 2560 cm−1 and 1039 cm−1 corresponded to -SH and P-O-C stretching vibrations, respectively. The results confirmed the main functional groups of the CSU11 molecule (-NH, -SH, and P-O-C). In the FT-IR spectrum of SM1, the characteristic bands at 3411 cm−1, 2920 cm−1, 1405 cm−1, and 1027 cm−1 correspond to the stretching vibrations of –OH, C-H, COO-, and Si-O, respectively [25,36,37].

Figure 9.
FT-IR spectra of the collector CSU11 and the depressant SM1 (CSU11 concentration 50 mg/L, SM1 concentration 200 mg/L).
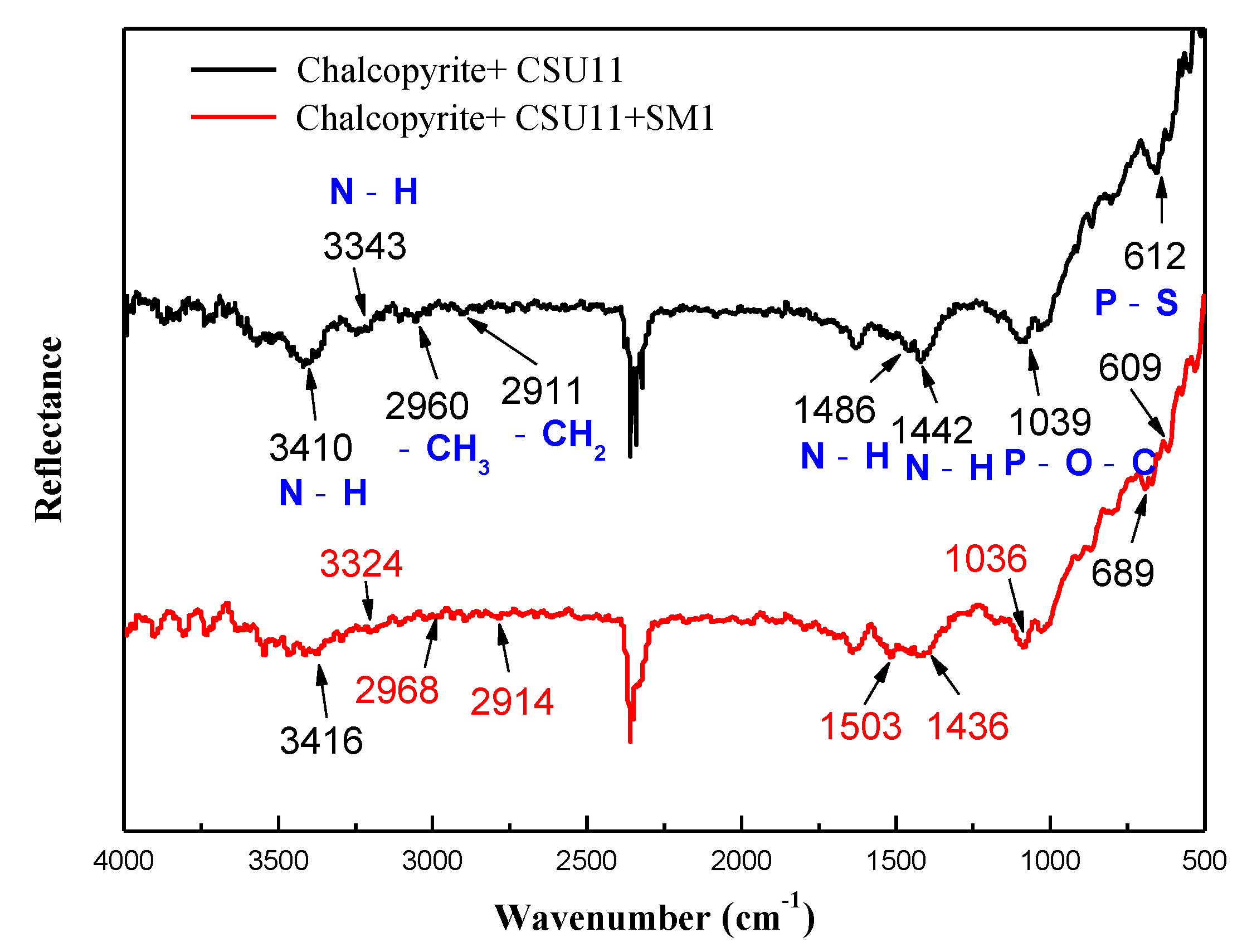
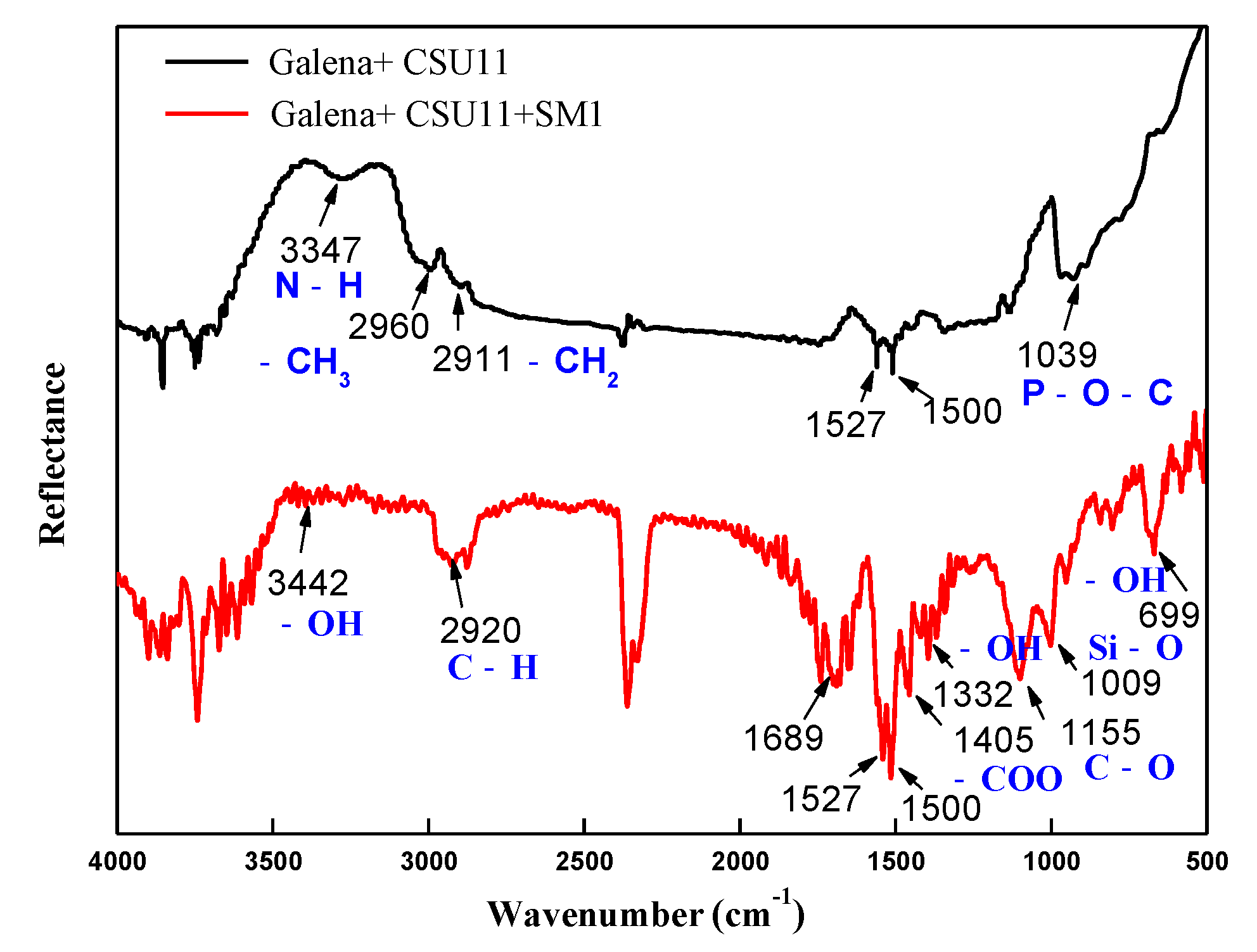
Figure 10 and Figure 11 show the FT-IR spectra of chalcopyrite and galena after being treated with the collector CSU11 and the depressant SM1. After the two minerals were treated with CSU11, new bands appeared at 3410 cm−1, 3347 cm−1, and 1039 cm−1 corresponding to -NH, -NH, and P-O-C stretching vibrations, respectively [2,38]. This suggests that CSU11 had strongly adsorbed onto the mineral surfaces.

Figure 10.
FT-IR spectra of chalcopyrite treated with the collector CSU11 and the depressant SM1 at natural pH (CSU11 concentration 50 mg/L, SM1 concentration 200 mg/L).
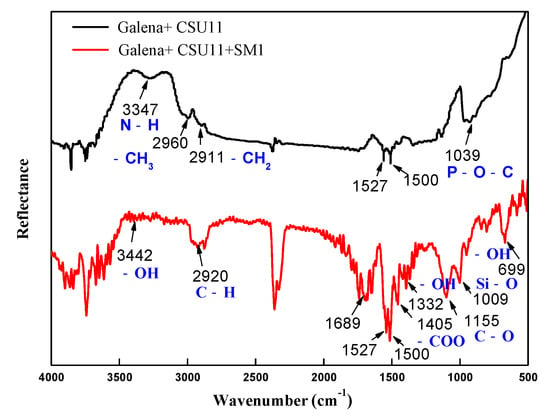
Figure 11.
FT-IR spectra of galena treated with the collector CSU11 and the depressant SM1 at natural pH (CSU11 concentration 50 mg/L, SM1 concentration 200 mg/L).
In the FT-IR spectrum of chalcopyrite with pre-adsorbed CSU11 (labeled “chalcopyrite + CSU11 + SM1”), no new absorption bands that could be attributed to the functional groups of SM1 were observed, indicating that there was no SM1 adsorption. However, in the FT-IR spectrum of galena with pre-adsorbed CSU11 (labeled “galena + CSU11 + SM1”), new bands emerged at 3442 cm−1, 2920 cm−1, 1405 cm−1, and 1009 cm−1, corresponding to -OH, C-H, COO—, and Si-O stretching vibrations, respectively [39]. The new bands correspond to the stretching vibrations of the polar groups in the SM1 molecule and clearly demonstrate the intense chemisorption of SM1 onto the surface of galena with CSU11 pre-treatment, which was consistent with in the results of the zeta potential measurements.
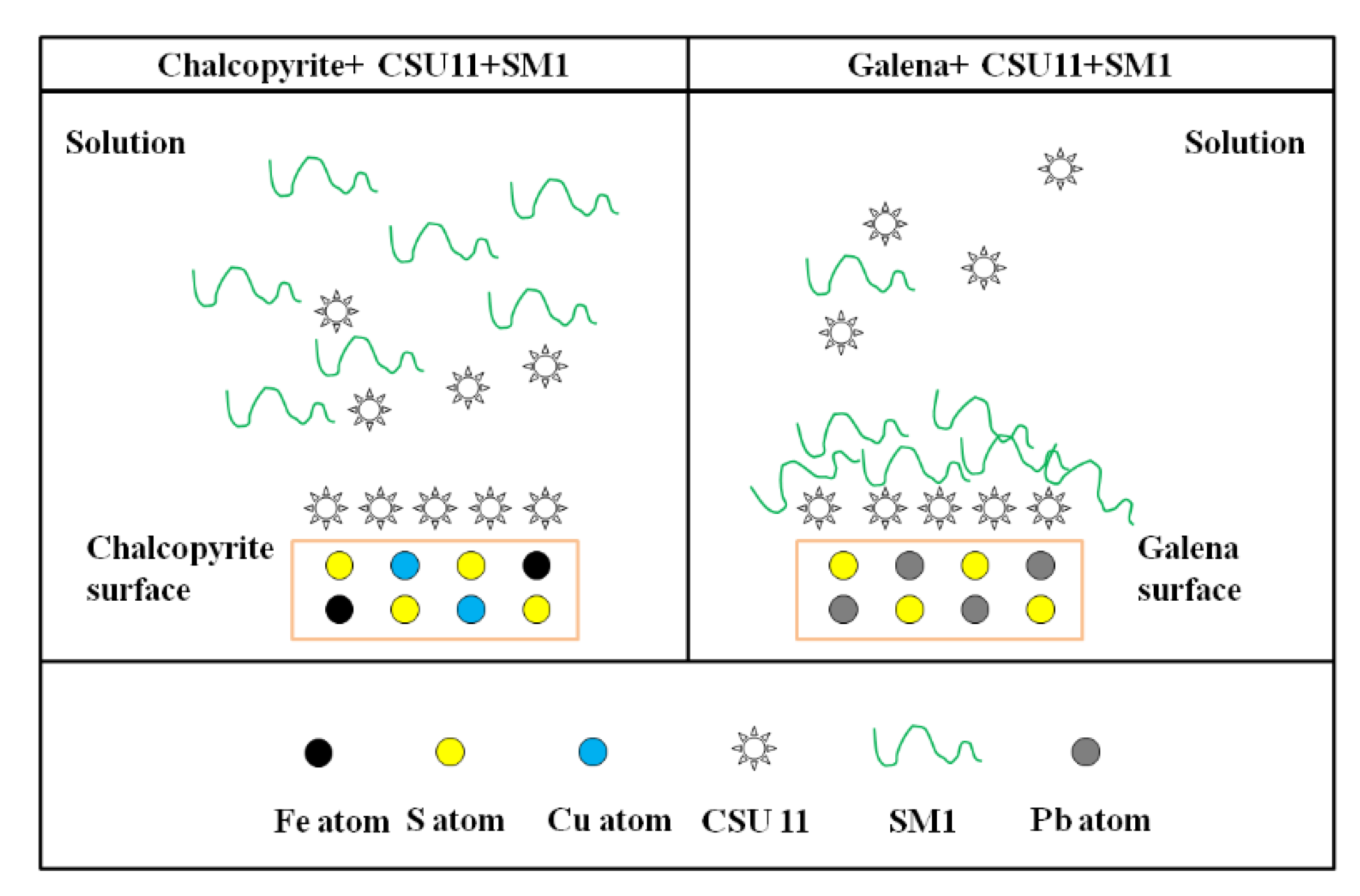
Based on the flotation results and the zeta potential and FT-IR measurements, it can be concluded that SM1 selectively adsorbs onto the surface of galena pre-treated with CSU11. A possible adsorption model of the SM1 and CSU11 onto the mineral surfaces is simplified in Figure 12. The collector CSU11 can adsorb onto the bare mineral surfaces and make them hydrophobic. The depressant SM1 has almost no interaction with chalcopyrite. The interaction between the surface of the chalcopyrite and SM1 is prevented by the CSU11 pre-treatment, while SM1 can cover the CSU11 on the surface of galena by chemical adsorption, making it hydrophilic. SM1 can expel/replace or cover the adsorbed CSU11 on the galena surface, but it fails this on chalcopyrite surface. This is the reason why the non-toxic depressant has a strong depression effect on galena but does not affect the flotation of chalcopyrite.

Figure 12.
Schematic diagram of the adsorption model of the collector CSU11 and the depressant SM1 onto the mineral surfaces.
4. Conclusions
The mixed depressant (DFinal) of sodium carboxymethyl cellulose, sodium silicate, sodium sulfite, and zinc sulfate exhibit high efficiency for selectively separating chalcopyrite from galena. The suggested mechanism is that the interaction between the surface of the chalcopyrite surface and the 50:1 depressant mixture of sodium silicate and sodium carboxymethyl cellulose (SM1) has been prevented by pre-treatment with the composite thiophosphate CSU11. However, SM1 can favorably adsorb onto the surface of galena after pre-treatment with CSU11 by chemisorption. Based on the key component SM1, the 1:2:2 depressant mixture of SM1 + sodium sulfite + zinc sulfate shows great potential as an effective, non-toxic depressant for industrial application in separating galena from chalcopyrite in the flotation of copper–lead bulk concentrate.
Author Contributions
Methodology, G.G.; formal analysis, Z.G. and G.G.; investigation, K.Z. and C.M.; writing—original draft preparation, K.Z. and C.M.; writing—review and editing, Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support of the Key Program for International S & T Cooperation Projects of China (2021YFE0106800), the National Natural Science Foundation of China (No. 52074358, U2067201), the Science Fund for Distinguished Young Scholars of Hunan Province (2020JJ2044), the Young Elite Scientists Sponsorship Program of Hunan province, China (2018RS3011), the Open Foundation of State Key Laboratory of Mineral Processing (BGRIMM-KJSKL-2021-20), and the Geological Survey project of the Geological Survey of China (DD20211235).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hayes, R.A.; Ralston, J. The collectorless flotation and separation of sulphide minerals by Eh control. Int. J. Miner. Process. 1988, 23, 55–84. [Google Scholar] [CrossRef]
- Chen, W.; Chen, T.; Bu, X.Z.; Chen, F.F.; Ding, Y.H.; Zhang, C.H.; Deng, S.; Song, Y.H. The selective flotation of chalcopyrite against galena using alginate as a depressant. Miner. Eng. 2019, 141, 105848. [Google Scholar] [CrossRef]
- Lotter, N.O.; Bradshaw, D.J.; Barnes, A.R. Classification of the Major Copper Sulphides into semiconductor types, and associated flotation characteristics. Miner. Eng. 2016, 96, 177–184. [Google Scholar] [CrossRef]
- Bu, Y.J.; Hu, Y.H.; Sun, W.; Gao, Z.Y.; Liu, R.Q. Fundamental Flotation Behaviors of Chalcopyrite and Galena Using O-Isopropyl-N-Ethyl Thionocarbamate as a Collector. Minerals 2018, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.W.; Han, Y.X.; Zhu, Y.M.; Li, Y.J.; Liu, H. Flotation behaviors and mechanisms of chalcopyrite and galena after cyanide treatment. Trans. Nonferrous Met. Soc. 2016, 26, 3245–3252. [Google Scholar] [CrossRef]
- Öztürk, Y.; Bıçak, Ö.; Özdemir, E.; Ekmekçi, Z. Mitigation negative effects of thiosulfate on flotation performance of a Cu-Pb-Zn sulfide ore. Miner. Eng. 2018, 122, 142–147. [Google Scholar] [CrossRef]
- Sehlotho, N.; Sindane, Z.; Bryson, M.; Lindvelt, L. Flowsheet development for selective Cu-Pb-Zn recovery at Rosh Pinah concentrator. Miner. Eng. 2018, 122, 10–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.Q.; Sun, W.; Wang, L.; Dong, Y.H.; Wang, C.T. Electrochemical mechanism and flotation of chalcopyrite and galena in the presence of sodium silicate and sodium sulfite. Trans. Nonferrous Met. Soc. 2020, 30, 1091–1101. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. An electrochemical and electron spectroscopic investigation of the galena/diethyl dithiophosphate system. Colloid Surf. 1991, 59, 307–319. [Google Scholar] [CrossRef]
- Güler, T.; Hiçyilmaz, C.; Gökagˇaç, G.; Ekmeçi, Z. Adsorption of dithiophosphate and dithiophosphinate on chalcopyrite. Miner. Eng. 2005, 19, 62–71. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Wang, Y.M.; Liu, G.Y.; Liu, S.; Liu, J.; Yang, X.L. Separation of chalcopyrite from galena with 3-amyl-4-amino-1, 2, 4-triazole-5-thione collector: Flotation behavior and mechanism. J. Ind. Eng. Chem. 2020, 92, 210–217. [Google Scholar] [CrossRef]
- Da Silva, G.R.; Waters, K.E. The effects of microwave irradiation on the floatability of chalcopyrite, pentlandite and pyrrhotite. Adv. Powder Technol. 2018, 29, 3049–3061. [Google Scholar] [CrossRef]
- Prestidge, C.A.; Ralston, J.; Smart, R.S.C. The role of cyanide in the interaction of ethyl xanthate with galena. Colloid Surf. A-Physicochem. Eng. Asp. 1993, 81, 103–119. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice. Flotation of Tantalum/Niobium Ores; Elsevier: Amsterdam, The Netherlands, 2007; pp. 127–149. [Google Scholar]
- Kocabağ, D.; Güler, T. Two-liquid flotation of sulphides: An electrochemical approach. Miner Eng. 2007, 20, 1246–1254. [Google Scholar] [CrossRef]
- Huang, P.; Wang, L.; Liu, Q. Depressant function of high molecular weight polyacrylamide in the xanthate flotation of chalcopyrite and galena. Int. J. Miner. Process. 2014, 128, 6–15. [Google Scholar] [CrossRef]
- Qiu, X.M.; Yang, H.Y.; Chen, G.B.; Zhong, S.P.; Cai, C.K.; Lan, B.B. Inhibited mechanism of carboxymethyl cellulose as a galena depressant in chalcopyrite and galena separation flotation. Miner. Eng. 2020, 150, 106273. [Google Scholar]
- Zhang, X.R.; Qian, Z.B.; Zheng, G.B.; Zhu, Y.G.; Wu, W.G. The design of a macromolecular depressant for galena based on DFT studies and its application. Miner. Eng. 2017, 112, 50–56. [Google Scholar] [CrossRef]
- Houot, R.; Duhamet, D. The use of sodium sulphite to improve the flotation selectivity between chalcopyrite and galena in a complex sulphide ore. Miner. Eng. 1992, 5, 343–355. [Google Scholar] [CrossRef]
- Sui, C.; Finch, J.A.; Nesset, J.E.; Kim, J.; Lajoie, S. Characterisation of the surfaces of galena and sphalerite in the presence of dithionite. Dev. Miner. Process. 2000, 13, C8b–15–C8b–22. [Google Scholar]
- Multani, R.S.; Waters, K.E. Pyrrhotite depression studies with DETA and SMBS on a Ni-Cu sulphide ore. Can. J. Chem. Eng. 2019, 97, 2121–2130. [Google Scholar] [CrossRef]
- Qin, W.Q.; Wei, Q.; Jiao, F.; Wang, L.; Wang, P.P.; Ke, L.F. Effect of sodium pyrophosphate on the flotation separation of chalcopyrite from galena. Int. J. Min. Sci. Technol. 2012, 22, 345–349. [Google Scholar] [CrossRef]
- Yoon, R.H. Collectorless flotation of chalcopyrite and sphalerite ores by using sodium sulfide. Int. J. Miner. Process. 1981, 8, 31–48. [Google Scholar] [CrossRef]
- Qin, W.Q.; Wei, Q.; Jiao, F.; Yang, C.G.; Liu, R.Z.; Wang, P.P.; Ke, L.F. Utilization of polysaccharides as depressants for the flotation separation of copper/lead concentrate. Int. J. Min. Sci. Technol. 2013, 23, 191–198. [Google Scholar] [CrossRef]
- Liu, R.Z.; Qin, W.Q.; Jiao, F.; Wang, X.J.; Pei, B.; Yang, Y.J.; Lai, C.H. Flotation separation of chalcopyrite from galena by sodium humate and ammonium persulfate. Trans. Nonferrous Met. Soc. 2016, 26, 265–271. [Google Scholar] [CrossRef]
- Li, J.M.; Song, K.W.; Liu, D.W.; Zhang, X.L.; Lan, Z.Y.; Sun, Y.L.; Wen, S.M. Hydrolyzation and adsorption behaviors of SPH and SCT used as combined depressants in the selective flotation of galena from sphalerite. J. Mol. Liq. 2017, 231, 485–490. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.H. Effect of calcium ions and citric acid on the flotation separation of chalcopyrite from galena using dextrin. Miner. Eng. 2000, 13, 1405–1416. [Google Scholar] [CrossRef]
- Qi, L.; Laskowski, J.S. The role of metal hydroxides at mineral surfaces in dextrin adsorption, II. Chalcopyrite-galena separations in the presence of dextrin. Int. J. Miner. Process. 1989, 27, 147–155. [Google Scholar]
- Jin, S.Z.; Shi, Q.; Li, Q.; Ou, L.M.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Liu, M.F.; Zhang, C.Y.; Hu, B.; Sun, Z.M.; Xu, Q.H.; Wen, J.; Xiao, J.; Dong, Y.H.; Gan, M.; Sun, W.; et al. Enhancing flotation separation of chalcopyrite and galena by the surface synergism between sodium sulfite and sodium lignosulfonate. Appl. Surf. Sci. 2020, 507, 145042. [Google Scholar] [CrossRef]
- Gonzalez, M.S.; Fornasiero, D. Understanding the effect of sulphate in mining-process water on sulphide flotation. Miner. Eng. 2021, 165, 106865. [Google Scholar] [CrossRef]
- Jin, S.Z.; Zhang, P.Y.; Ou, L.M. Study on the depression mechanism of zinc sulfate on talc in chalcopyrite flotation. Colloid Surf. A-Phys. Eng. 2020. [Google Scholar] [CrossRef]
- Zhao, K.L.; Wang, X.H.; Yan, W.; Gu, G.H.; Wang, C.Q.; Wang, Z.; Xu, L.H.; Peng, T.F. Depression mechanism of pyrophyllite by a novel polysaccharide xanthan gum. Miner. Eng. 2019, 132, 134–141. [Google Scholar] [CrossRef]
- Zhang, W.J.; Sun, W.; Hu, Y.H.; Cao, J.; Gao, Z.Y. Selective Flotation of Pyrite from Galena Using Chitosan with Different Molecular Weights. Minerals 2019, 9, 549. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Huang, X.P.; Huang, K.H.; Wang, S.; Cao, Z.F.; Zhong, H. Synthesis, flotation performance and adsorption mechanism of 3-(ethylamino)-N-phenyl-3-thioxopropanamide onto galena/sphalerite surfaces. J. Ind. Eng. Chem. 2019, 77, 416–425. [Google Scholar] [CrossRef]
- Xiao, J.J.; Liu, G.Y.; Zhong, H. The adsorption mechanism of N -butoxypropyl- S -[2-(hydroxyimino) propyl] dithiocarbamate ester to copper minerals flotation. Int. J. Miner. Process. 2017, 166, 53–61. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Y.; Zhong, H.; Wang, S.; Liu, G.Y.; Zhao, G. A novel surfactant S-benzoyl-N, N-diethyldithiocarbamate synthesis and its flotation performance to galena. Appl. Surf. Sci. 2016, 365, 342–351. [Google Scholar] [CrossRef]
- Zhang, W.J.; Feng, Z.T.; Yang, Y.H.; Sun, W.; Pooley, S.; Cao, J.; Gao, Z.Y. Bi-functional hydrogen and coordination bonding surfactant: A novel and promising collector for improving the separation of calcium minerals. J. Colloid Inter. Sci. 2021, 585, 787–799. [Google Scholar] [CrossRef]
- Zhang, W.J.; Feng, Z.T.; Mulenga, H.; Sun, W.; Cao, J.; Gao, Z.Y. Synthesis of a novel collector based on selective nitrogen coordination for improved separation of galena and sphalerite against pyrite. Chem. Eng. Sci. 2020, 226, 115860. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).