Lead Mobilization and Speciation in Mining Waste: Experiments and Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site, Geochemical Characterization, and ANC–BNC Tests
2.2. ANC–BNC Experiments
2.3. Geochemical Modelling
3. Results and Discussion
3.1. Preliminary Results
3.1.1. Characterization of the Mining Waste
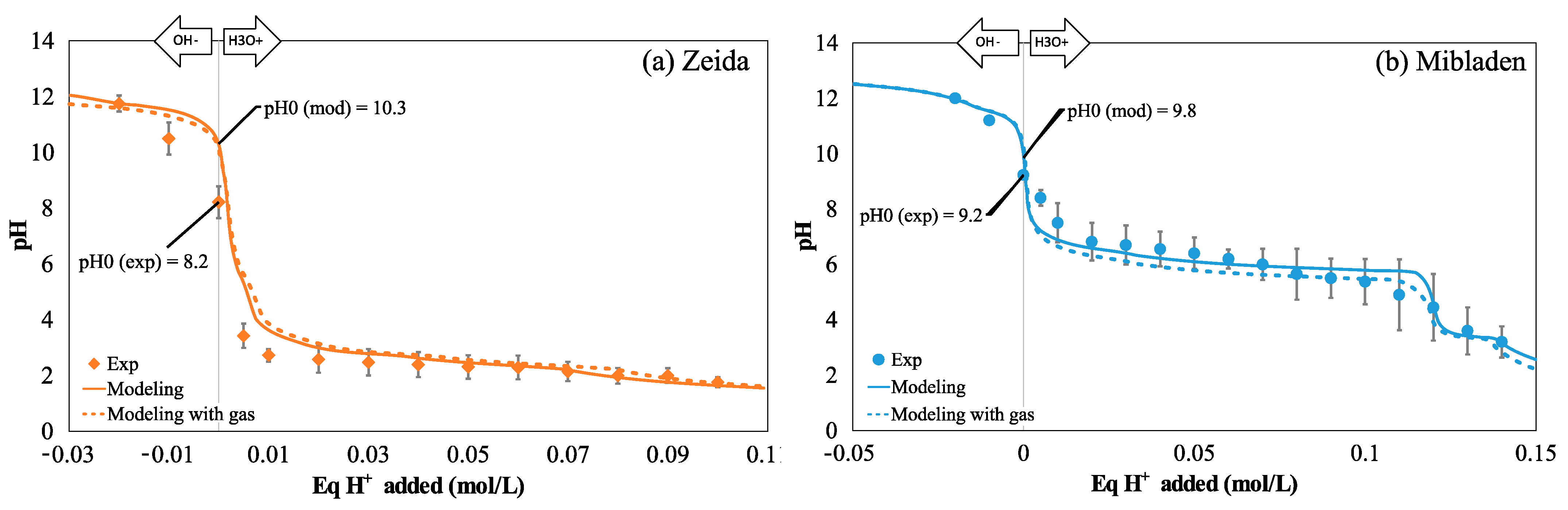
3.1.2. Modeling Approach and Validation of the Geochemical Model
3.2. Characterization of Buffer Capacity with ANC–BNC Experiments
3.2.1. Observed and Modeled Buffer Capacity
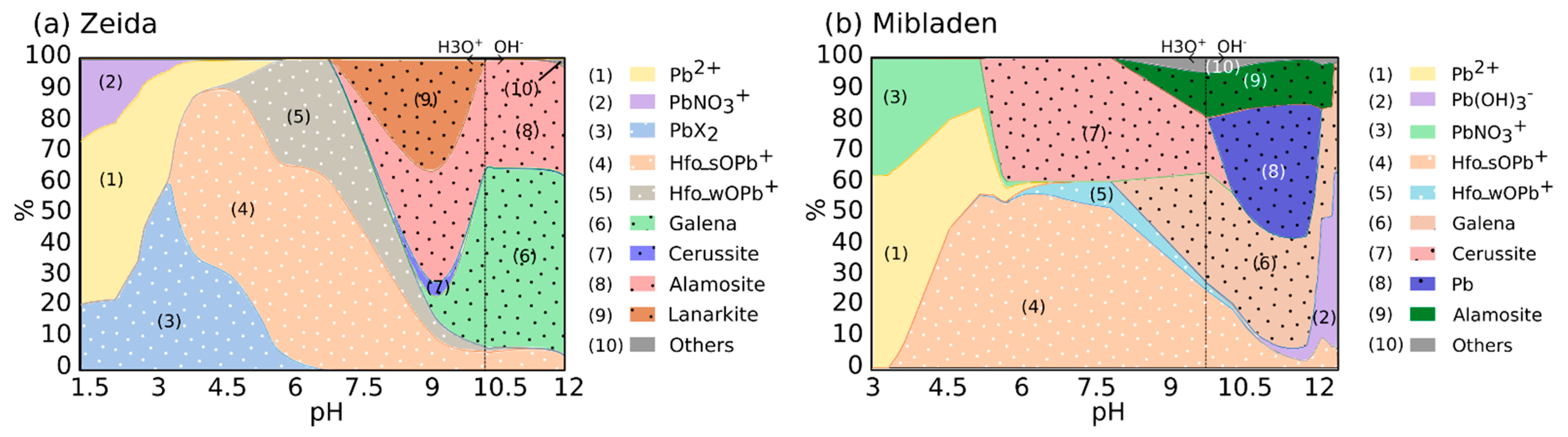
3.2.2. Identification of the Main Mineral Phases by Modeling Buffer Capacity
3.2.3. Validation of the Proposed Mineral Composition with the Tailings’ Characterization
3.2.4. Comparison of Waste from the Two Mining Sites: Two Types of Mine Drainage
3.3. Mobilization and Speciation of Lead
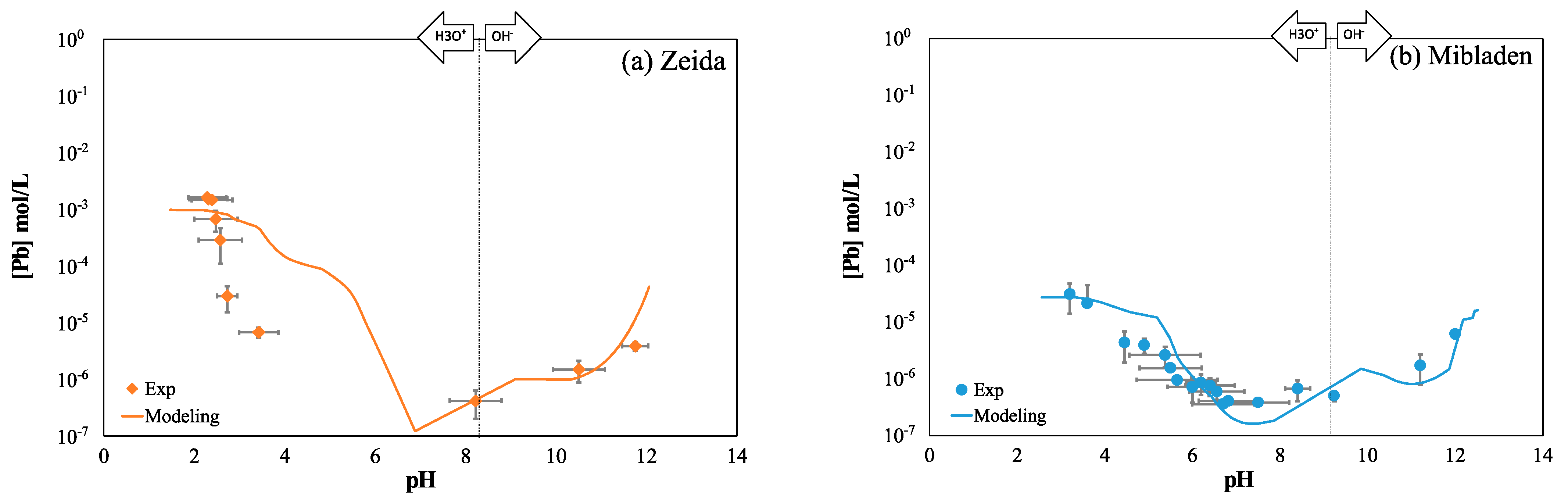
3.3.1. Mobilization of Lead as a Function of pH
3.3.2. Speciation of Lead as a Function of pH
3.4. Representativeness of ANC–BNC Experiments
- In the field, the contact with the atmosphere strongly differs between field and batch conditions, with closed conditions for batch experiments versus utterly open conditions (contact with a quasi-infinite reservoir of gas). In the case of batch reactors, only the gaseous species present in the volume of gas may interact with solutes. The consumption of oxidants (such as oxygen) may limit oxidation processes, whereas oxidants are instantaneously renewed in the case of contact with the open atmosphere. We then expect more pyrite oxidation [55] and induced acidification, mineral dissolution, and lead release under field conditions (no limitation of O2(g)), e.g., [2,53]. In other words, if the three phases remain the same between batch and field conditions, the gas phase does not have the same composition.
- In the field, very slow processes that are kinetically limited may govern element release for a very long period of time, whereas the contact time in batch reactors is restricted to a few days, thus selecting mostly the instantaneous mechanisms. The addition of acid or alkalinity that is performed to boost dissolution and element release may not compensate for the very long time needed for some minerals to dissolve. For instance, we expect pyrite dissolution to be kinetically limited [56,57] and thus much less important under batch conditions than under field conditions. The same expectations may be stated for calcite and dolomite dissolutions that are prone to significant kinetic limitations [58].
- The liquid/solid ratio is all but the same between batch and field conditions. Indeed, in batch reactors, the solid phase is dispersed into the liquid solution with a very high liquid/solid (L/S) ratio, in the order of 10 L/kg in our cases and in most studies [54]. In the field, assuming usual values of soil bulk and mineral densities, i.e., 1.75 g/cm3 and 2.65 g/cm3, nominal values of saturated water content and the corresponding L/S ratio can be estimated, revealing a value of 0.2 L/kg in the field versus 10 L/kg in the batch reactors. We expect that higher L/S ratios would promote solubilization and element release.
- In addition to the difference in the liquid/solid ratio, field conditions are characterized by dynamic conditions (flow-induced solute transport), whereas batch reactors simulate static conditions with optimal contact between the solid grains and solutes. Numerous previous works have already demonstrated that the elements’ fate significantly differs between static and dynamic conditions [59,60,61]. In columns, the local hydrodynamic conditions make the access of solutes to sorption sites more difficult and thus may derive from optimal sorption [62]. In addition, the soil structure may be prone to preferential flows, in particular for soils with aggregates and macropores, e.g., [63,64], which may, in turn, reduce the access of solutes to the reactive particles and decrease solute sorption [65,66,67].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Argane, R.; El Adnani, M.; Benzaazoua, M.; Bouzahzah, H.; Khalil, A.; Hakkou, R.; Taha, Y. Geochemical Behavior and Environmental Risks Related to the Use of Abandoned Base-Metal Tailings as Construction Material in the Upper-Moulouya District, Morocco. Environ. Sci. Pollut. Res. 2016, 23, 598–611. [Google Scholar] [CrossRef]
- Dold, B. Evolution of Acid Mine Drainage Formation in Sulphidic Mine Tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef]
- Bussiere, B. Colloquium 2004: Hydrogeotechnical Properties of Hard Rock Tailings from Metal Mines and Emerging Geoenvironmental Disposal Approaches. Can. Geotech. J. 2007, 44, 1019–1052. [Google Scholar] [CrossRef]
- Coussy, S. Stabilisation de Rejets Miniers Pollués à l’arsenic à l’aide de Sous-Produits Cimentaires: Étude de l’influence de La Cristallochimie Sur Le Risque de Mobilisation des Polluants; INSA de Lyon: Lyon, France, 2011. [Google Scholar]
- Benzaazoua, M.; Bussière, B.; Dagenais, A.-M.; Archambault, M. Kinetic Tests Comparison and Interpretation for Prediction of the Joutel Tailings Acid Generation Potential. Environ. Geol. 2004, 46, 1086–1101. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Acid Mine Drainage at the Abandoned Kettara Mine (Morocco): 2. Mine Waste Geochemical Behavior. Mine Water Environ. 2008, 27, 160–170. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Hakkou, R.; Bouamrane, A. A Comparative Study on the Practical Use of Low Sulfide Base-Metal Tailings as Aggregates for Rendering and Masonry Mortars. J. Clean. Prod. 2016, 112, 914–925. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Hakkou, R.; Bouamrane, A. Reuse of Base-Metal Tailings as Aggregates for Rendering Mortars: Assessment of Immobilization Performances and Environmental Behavior. Constr. Build. Mater. 2015, 96, 296–306. [Google Scholar] [CrossRef]
- Baghdad, B.; Naimi, M.; Bouabdli, A.; Sonnet, P.; Lutts, S. Heavy Metals in Tailings, Soils and Vegetation. Ecol. Mediterr. 2006, 32, 86. [Google Scholar]
- Bouabdli, A.; Saidi, N.; M’rabet, S.; Escarre, J.; Leblanc, J.M. Heavy Metal Transport by the Moulouya River (Morocco). Rev. Sci. Eau 2005, 18, 199–213. [Google Scholar]
- El Hachimi, M.; El Founti, L.; Bouabdli, A.; Saidi, N.; Fekhoui, M.; Tassé, N. Pb et As Dans Des Eaux Alcalines Minières: Contamination, Comportement et Risques (Mine Abandonnée de Zeïda, Maroc). Rev. Sci. Eau J. Water Sci. 2007, 20, 1–13. [Google Scholar] [CrossRef]
- El Kheir, S.B.; Oubbih, J.; Saidi, N.; Bouabdli, A. Uptake and Fixation of Zn, Pb, and Cd by Thlaspi Caerulescens: Application in the Cases of Old Mines of Mibladen and Zaida (West of Morocco). Arab. J. Geosci. 2008, 1, 87–95. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A Review of Soil Heavy Metal Pollution from Mines in China: Pollution and Health Risk Assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Sheoran, A.S.; Sheoran, V. Heavy Metal Removal Mechanism of Acid Mine Drainage in Wetlands: A Critical Review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy Metal Pollution from Gold Mines: Environmental Effects and Bacterial Strategies for Resistance. Int. J. Environ. Res. Public. Health 2016, 13, 1047. [Google Scholar] [CrossRef]
- Sigg, L.; Stumm, W.; Behra, P. Chimie des Milieux Aquatiques; Dunot: Paris, France, 2000. [Google Scholar]
- Chatain, V.; Benzaazoua, M.; Cazalet, M.L.; Bouzahzah, H.; Delolme, C.; Gautier, M.; Blanc, D.; de Brauer, C. Mineralogical Study and Leaching Behavior of a Stabilized Harbor Sediment with Hydraulic Binder. Environ. Sci. Pollut. Res. 2013, 20, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, C.; Delolme, C.; Chatain, V.; Gautier, M.; Blanc, D.; Benzaazoua, M.; Lassabatere, L. Spatial and Temporal Stability of Major and Trace Element Leaching in Urban Stormwater Sediments. Open J. Soil Sci. 2017, 7, 19. [Google Scholar] [CrossRef][Green Version]
- Benvenuti, M.; Mascaro, I.; Corsini, F.; Lattanzi, P.; Parrini, P.; Tanelli, G. Mine Waste Dumps and Heavy Metal Pollution in Abandoned Mining District of Boccheggiano (Southern Tuscany, Italy). Environ. Geol. 1997, 30, 238–243. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Tang, C.; Ke, Z.; Wang, J.; Shimizu, Y.; Zhu, A. Distribution, Migration and Potential Risk of Heavy Metals in the Shima River Catchment Area, South China. Environ. Sci. Process. Impacts 2015, 17, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Tye, A.M.; Chenery, S.R. Lability, Solubility and Speciation of Cd, Pb and Zn in Alluvial Soils of the River Trent Catchment UK. Environ. Sci. Process. Impacts 2013, 15, 1844–1858. [Google Scholar] [CrossRef]
- Marsalek, J.; Watt, W.E.; Anderson, B.C. Trace Metal Levels in Sediments Deposited in Urban Stormwater Management Facilities. Water Sci. Technol. 2006, 53, 175–183. [Google Scholar] [CrossRef]
- Roessler, J.G.; Townsend, T.G.; Ferraro, C.C. Use of Leaching Tests to Quantify Trace Element Release from Waste to Energy Bottom Ash Amended Pavements. J. Hazard. Mater. 2015, 300, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.S.; Ye, Z.H.; Lan, C.Y.; Zhang, Z.Q.; Wong, M.H. Acidification of Lead/Zinc Mine Tailings and Its Effect on Heavy Metal Mobility. Environ. Int. 2001, 26, 389–394. [Google Scholar] [CrossRef]
- Zuo, X.; Fu, D.; Li, H. Speciation Distribution and Mass Balance of Copper and Zinc in Urban Rain, Sediments, and Road Runoff. Environ. Sci. Pollut. Res. 2012, 19, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy Metals in Soils. Blackie Academic and Professional; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Barceloux, D.G.; Barceloux, D. Chromium. J. Toxicol. Clin. Toxicol. 1999, 37, 173–194. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Van der Sloot, H.A.; van Zomeren, A.; Dijkstra, J.J.; Meeussen, J.C.L.; Comans, R.N.J.; Scharff, H. Prediction of the Leaching Behaviour of Waste Mixtures by Chemical Speciation Modelling Based on a Limited Set of Key Parameters. In Proceedings of the Tenth International Waste Management and Landfill Symposium, Sardinia, Italy, 3–7 October 2005. [Google Scholar]
- Parkhurst, D.L.; Appelo, C. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. 1999. Water-Resour. Investig. Rep. 1999, 99, 312. [Google Scholar]
- Gidlow, D.A. Lead Toxicity. Occup. Med. 2004, 54, 76–81. [Google Scholar] [CrossRef]
- Argane, R. Valorisation Des Rejets Miniers à Faible Teneur En Sulfures Comme Granulats Pour Mortiers. Ph.D. Thesis, INSA, Lyon, France, 2015. [Google Scholar]
- El Azhari, A.; Rhoujjati, A.; El Hachimi, M.L.; Ambrosi, J. Pollution and Ecological Risk Assessment of Heavy Metals in the Soil-Plant System and the Sediment-Water Column around a Former Pb/Zn-Mining Area in NE Morocco. Ecotoxicol. Environ. Saf. 2017, 144, 464–474. [Google Scholar] [CrossRef]
- Combe, M.; Simonot, M. La Haute Moulouya, Le Sillon d’Itzer-Enjil et Le Massif de Bou-Mia-Aouli. Notes Mém. Serv. Géol. Domaines Rif. Maroc. Orient. Ed. Serv. Géol. Maroc. 1971, 231, 193–201. [Google Scholar]
- Coussy, S.; Benzaazoua, M.; Blanc, D.; Moszkowicz, P.; Bussière, B. Arsenic Stability in Arsenopyrite-Rich Cemented Paste Backfills: A Leaching Test-Based Assessment. J. Hazard. Mater. 2011, 185, 1467–1476. [Google Scholar] [CrossRef]
- Glass, G.; Buenfeld, N. Differential Acid Neutralisation Analysis. Cem. Concr. Res. 1999, 29, 1681–1684. [Google Scholar] [CrossRef]
- Peyronnard, O.; Blanc, D.; Benzaazoua, M.; Moszkowicz, P. Study of Mineralogy and Leaching Behavior of Stabilized/Solidified Sludge Using Differential Acid Neutralization Analysis: Part II: Use of Numerical Simulation as an Aid Tool for Cementitious Hydrates Identification. Cem. Concr. Res. 2009, 39, 501–509. [Google Scholar] [CrossRef]
- AFNOR CEN/TS 14429 Characterization of Waste. Leaching Behavior Tests. Influence of PH on Leaching with Initial Acid/Base Addition. 2015. Available online: http://www.coal-ash.co.il/research/Yoetz_Deutsch_2008_full.pdf (accessed on 1 May 2021).
- Drapeau, C.; Delolme, C.; Vézin, C.; Blanc, D.; Baumgartl, T.; Edraki, M.; Lassabatère, L. Anc–Bnc Titrations and Geochemical Modeling for Characterizing Calcareous and Siliceous Mining Waste. Minerals 2021, 11, 257. [Google Scholar] [CrossRef]
- Engelsen, C.J.; van der Sloot, H.A.; Wibetoe, G.; Petkovic, G.; Stoltenberg-Hansson, E.; Lund, W. Release of Major Elements from Recycled Concrete Aggregates and Geochemical Modelling. Cem. Concr. Res. 2009, 39, 446–459. [Google Scholar] [CrossRef]
- Halim, C.E.; Short, S.A.; Scott, J.A.; Amal, R.; Low, G. Modelling the Leaching of Pb, Cd, As, and Cr from Cementitious Waste Using PHREEQC. J. Hazard. Mater. 2005, 125, 45–61. [Google Scholar] [CrossRef]
- Biondi, D.; Freni, G.; Iacobellis, V.; Mascaro, G.; Montanari, A. Validation of Hydrological Models: Conceptual Basis, Methodological Approaches and a Proposal for a Code of Practice. Phys. Chem. Earth Parts ABC 2012, 42, 70–76. [Google Scholar] [CrossRef]
- Pollacco, J.A.P.; Nasta, P.; Soria-Ugalde, J.M.; Angulo-Jaramillo, R.; Lassabatere, L.; Mohanty, B.P.; Romano, N. Reduction of Feasible Parameter Space of the Inverted Soil Hydraulic Parameter Sets for Kosugi Model. Soil Sci. 2013, 178, 267–280. [Google Scholar] [CrossRef]
- Sposito, G. The Chemistry of Soils; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Blowes, D.; Ptacek, C.; Jambor, J.; Weisener, C. The Geochemistry of Acid Mine. Environ. Geochem. 2005, 9, 149. [Google Scholar]
- Lapakko, K.A.; Antonson, D.A.; Wagner, J.R. Mixing of Limestone with Finely-Crushed Acid Producing Rock. In Proceedings of the the Fourth International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; pp. 1345–1360. [Google Scholar]
- Singer, P.C.; Stumm, W. Acidic Mine Drainage: The Rate-Determining Step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef]
- Ramos, L.; Hernandez, L.M.; Gonzalez, M.J. Sequential Fractionation of Copper, Lead, Cadmium and Zinc in Soils from or near Doñana National Park. J. Environ. Qual. 1994, 23, 50–57. [Google Scholar] [CrossRef]
- Marguı, E.; Salvadó, V.; Queralt, I.; Hidalgo, M. Comparison of Three-Stage Sequential Extraction and Toxicity Characteristic Leaching Tests to Evaluate Metal Mobility in Mining Wastes. Anal. Chim. Acta 2004, 524, 151–159. [Google Scholar] [CrossRef]
- Othmani, M.A.; Souissi, F.; Bouzahzah, H.; Bussière, B.; Da Silva, E.F.; Benzaazoua, M. The Flotation Tailings of the Former Pb-Zn Mine of Touiref (NW Tunisia): Mineralogy, Mine Drainage Prediction, Base-Metal Speciation Assessment and Geochemical Modeling. Environ. Sci. Pollut. Res. 2015, 22, 2877–2890. [Google Scholar] [CrossRef] [PubMed]
- Asavapisit, S.; Fowler, G.; Cheeseman, C. Solution Chemistry during Cement Hydration in the Presence of Metal Hydroxide Wastes. Cem. Concr. Res. 1997, 27, 1249–1260. [Google Scholar] [CrossRef]
- Fanfani, L.; Zuddas, P.; Chessa, A. Heavy Metals Speciation Analysis as a Tool for Studying Mine Tailings Weathering. J. Geochem. Explor. 1997, 58, 241–248. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption Isotherms: A Review on Physical Bases, Modeling and Measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Long, H.; Dixon, D.G. Pressure Oxidation of Pyrite in Sulfuric Acid Media: A Kinetic Study. Hydrometallurgy 2004, 73, 335–349. [Google Scholar] [CrossRef]
- Williamson, M.A.; Rimstidt, J.D. The Kinetics and Electrochemical Rate-Determining Step of Aqueous Pyrite Oxidation. Geochim. Cosmochim. Acta 1994, 58, 5443–5454. [Google Scholar] [CrossRef]
- Bierens de Haan, S. A Review of the Rate of Pyrite Oxidation in Aqueous Systems at Low Temperature. Earth-Sci. Rev. 1991, 31, 1–10. [Google Scholar] [CrossRef]
- Sjöberg, E.L.; Rickard, D.T. Temperature Dependence of Calcite Dissolution Kinetics between 1 and 62 °C at PH 2.7 to 8.4 in Aqueous Solutions. Geochim. Cosmochim. Acta 1984, 48, 485–493. [Google Scholar] [CrossRef]
- Lassabatere, L.; Winiarski, T.; Galvez Cloutier, R. Retention of Three Heavy Metals (Zn, Pb, and Cd) in a Calcareous Soil Controlled by the Modification of Flow with Geotextiles. Environ. Sci. Technol. 2004, 38, 4215–4221. [Google Scholar] [CrossRef]
- Kuechler, R.; Noack, K. Comparison of the Solution Behaviour of a Pyrite–Calcite Mixture in Batch and Unsaturated Sand Column. J. Contam. Hydrol. 2007, 90, 203–220. [Google Scholar] [CrossRef]
- Zhou, L.; Martin, S.; Cheng, W.; Lassabatere, L.; Boily, J.-F.; Hanna, K. Water Flow Variability Affects Adsorption and Oxidation of Ciprofloxacin onto Hematite. Environ. Sci. Technol. 2019, 53, 10102–10109. [Google Scholar] [CrossRef]
- Bai, H.J.; Lassabatere, L.; Lamy, E. Colloid Transport in Aggregated Porous Media with Intra- and Interaggregate Porosities. Ind. Eng. Chem. Res. 2018, 57, 6553–6567. [Google Scholar] [CrossRef]
- Lassabatere, L.; Yilmaz, D.; Peyrard, X.; Peyneau, P.E.; Lenoir, T.; Šimůnek, J.; Angulo-Jaramillo, R. New Analytical Model for Cumulative Infiltration into Dual-Permeability Soils. Vadose Zone J. 2014, 13, 1–15. [Google Scholar] [CrossRef]
- Lassabatere, L.; Di Prima, S.; Bouarafa, S.; Iovino, M.; Bagarello, V.; Angulo-Jaramillo, R. BEST-2K Method for Characterizing Dual-Permeability Unsaturated Soils with Ponded and Tension Infiltrometers. Vadose Zone J. 2019, 18. [Google Scholar] [CrossRef]
- Lassabatere, L.; Spadini, L.; Delolme, C.; Février, L.; Galvez Cloutier, R.; Winiarski, T. Concomitant Zn-Cd and Pb Retention in a Carbonated Fluvio-Glacial Deposit under Both Static and Dynamic Conditions. Chemosphere 2007, 69, 1499–1508. [Google Scholar] [CrossRef]
- Batany, S.; Peyneau, P.-E.; Lassabatère, L.; Béchet, B.; Faure, P.; Dangla, P. Interplay between Molecular Diffusion and Advection during Solute Transport in Macroporous Media. Vadose Zone J. 2019, 18. [Google Scholar] [CrossRef]
- Raimbault, J.; Peyneau, P.-E.; Courtier-Murias, D.; Bigot, T.; Gil Roca, J.; Béchet, B.; Lassabatère, L. Investigating the Impact of Exit Effects on Solute Transport in Macropored Porous Media. Hydrol. Earth Syst. Sci. Discuss. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-Thinking Mining Waste through an Integrative Approach Led by Circular Economy Aspirations. Minerals 2019, 9, 286. [Google Scholar] [CrossRef]
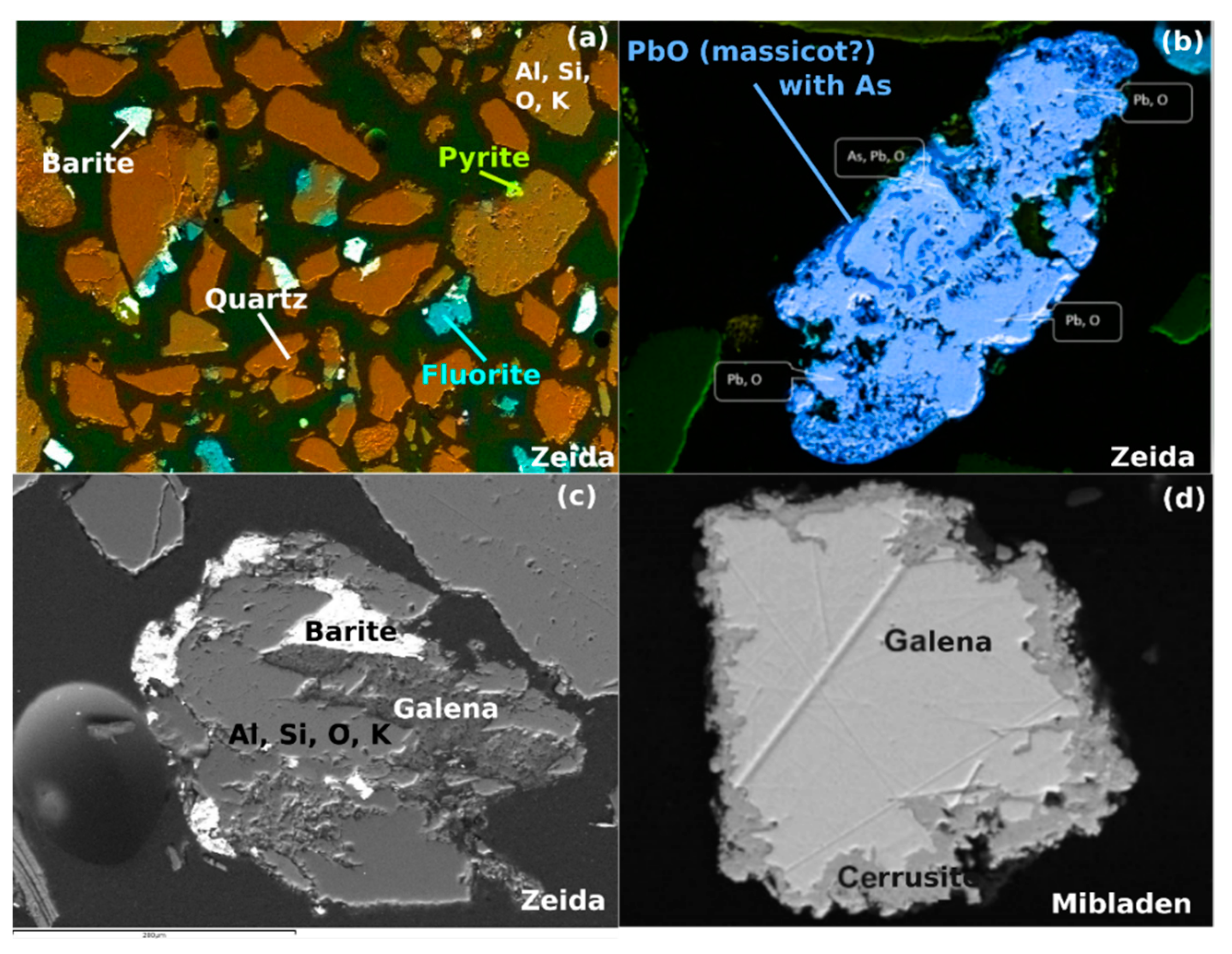

| Mineral Phase | Formula | % Zeida | % Mibladen | |
|---|---|---|---|---|
| Silicates | Quartz | SiO2 | 48.8 | 7.16 |
| Orthoclase | KAlSi3O8 | 31.6 | - | |
| Chamosite | Fe2Al2SiO5(OH)4 | 2.6 | 2.72 | |
| Albite | NaAlSi3O8 | 5.5 | - | |
| Kaolinite | Al2Si2O5(OH)4 | 1.5 | - | |
| Carbonates | Dolomite | CaMg(CO3)2 | - | 51.35 |
| Calcite | CaCO3 | - | 22.2 | |
| Others | Barite | BaSO4 | 7.9 | 16.57 |
| Fluorite | CaF2 | 2.1 | 3 | |
| Phases | Chemical Reactions | Equilibrium Constants (pK) | Initial Concentrations (mol/L) | |
|---|---|---|---|---|
| Zeida | Mibladen | |||
| Main Mineral Phases | ||||
| Carbonate | ||||
| Calcite | CaCO3(s) + H+ ↔ Ca2+ + HCO3− | 1.8487 | - | 0.014326 (MC) |
| Content of Pb in inclusion | - | 0.001188 (SEM/EDS) | ||
| Dolomite | CaMg(CO3)2(s) + 2 H+ ↔ Ca2+ + Mg2+ + 2 HCO3− | 2.5135 | - | 0.0222198 (MC) |
| Silicate | ||||
| Albite (DO) | NaAlSi3O8(s) + 4H+ ↔ Al3+ + Na+ + 2H2O + 3SiO2 | 2.7645 | 0.0003013 (MC) | - |
| Chamosite (DO) | Fe2Al2SiO5(OH)4(s) + 10H+ ↔ SiO2 + 2Al3+ + 2Fe2+ + 7 H2O | 32.8416 | 0.0002005 (MC) | 0.0003767 (MC) |
| Kaolinite (DO) | Al2Si2O5(OH)4(s) + 6H+ ↔ 2 Al3+ + 2 SiO2 + 5 H2O | 6.8101 | 0.001822 (MC) | - |
| Quartz (DO) | SiO2(s) ↔ SiO2 | −3.9993 | 0.0129952 (MC) | 0.008307 (MC) |
| Orthoclase (DO) | KAlSi3O8(s) + 4H+ ↔ Al3+ + K+ + 2H2O + 3SiO2 | −0.2753 | 0.00908 (MC) | - |
| Others | ||||
| Pyrite | FeS2(s) + H2O ↔ 0.25H+ + 0.25SO42− + Fe2+ + 1.75HS− | −24.6534 | 0.0001 (SEM/EDS) | 0.000025 (SEM/EDS) |
| Barite | BaSO4(s) ↔ Ba2+ + SO42− | −9.9711 | 0.003385 (MC) | 0.00284 (MC) |
| Fluorite | CaF2(s) ↔ Ca2+ + 2 F− | −10.0370 | 0.000269 (MC) | 0.00457 (MC) |
| Iron hydroxydes | Fe(OH)3(s) +3 H+ ↔ Fe3+ + 3 H2O | 5.6556 | 0.001 (SEM/EDS) | 0.00743 (SEM/EDS) |
| Hematite (DO) | Fe2O3(s) + 6 H+ ↔ 2 Fe3+ + 3 H2O | 0.1086 | 0.0001 (IM) | 0.001 (IM) |
| Fluorapatite | Ca5(PO4)3F(s) + 3 H+ ↔ F− + 3 HPO42− + 5 Ca2+ | −24.9940 | 0.0001 (IM) | - |
| Gas phases | ||||
| CO2(g) | CO2(g) + H2O ↔ H+ + HCO3− | Vol_Air_fix * = 2.5 P_Air_0 ** = 1 | In equilibrium with neutral batch solution | |
| O2(g) | NO2(g) + 0.5H2O + 0.25O2(g) ↔ H+ + NO3− | |||
| Mineral phases bearing lead | ||||
| Alamosite (DO) | PbSiO3(s) + 2 H+ ↔ H2O + Pb2+ + SiO2 | 5.6733 | 0.00043 (IM) | 0.00000396 (IM) |
| Cerussite (DO) | PbCO3(s) + H+ ↔ HCO3− + Pb2+ | −3.2091 | 0.00009 (SEM/EDS) | 1.098 × 10−5 (SEM/EDS) |
| Galena (DO) | PbS(s) + H+ ↔ HS− + Pb2+ | −14.8544 | 0.00071 (SEM/EDS) | 9.794 × 10−6 (SEM/EDS) |
| Pb (DO) | P + 2 H+ + 0.5 O2 ↔ H2O + Pb2+ | 47.1871 | - | 2.9 × 10−6 (SEM/EDS) |
| Lanarkite | Pb2(SO4)O(s) + 2 H+ ↔ H2O + SO42+ + 2 Pb2+ | −0.4692 | 0.000001 (IM) | - |
| Surface complexation site for sorbing lead | ||||
| ≡Hfo_sOH | ≡Hfo_sOH + Pb2+ ↔ ≡Hfo_sOPb+ + H+ | 4.65 | 0.5 | 0.002 |
| ≡Hfo_wOH | ≡Hfo_wOH + Pb2+ ↔ ≡Hfo_wOPb+ + H+ | 0.3 | 1.3 | 0.0015 |
| X (ion exchange) sorption site for lead | ||||
| X− | Pb2+ + 2 X− ↔ PbX2 | 1.05 | 0.009 | 0.0003 |
| Figure Number | Modeled data | NRMSE-Normalization Root Mean Square Error | R2 Coefficient of Determination |
|---|---|---|---|
| Figure 2a | pH = f(Eq H+ added) for Zeida tails | 21.6% | 0.965 |
| Figure 2b | pH = f(Eq H+ added) for Mibladen tails | 7.50% | 0.963 |
| Figure 4a | Pb = f(pH) for Zeida tails | 18.6% | 0.729 |
| Figure 4b | Pb = f(pH) for Mibladen tails | 7.11% | 0.825 |
| Zeida Tails Mean | 20.1% | 0.847 | |
| Mibladen Tails Mean | 7.30% | 0.894 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drapeau, C.; Argane, R.; Delolme, C.; Blanc, D.; Benzaazoua, M.; Hakkou, R.; Baumgartl, T.; Edraki, M.; Lassabatere, L. Lead Mobilization and Speciation in Mining Waste: Experiments and Modeling. Minerals 2021, 11, 606. https://doi.org/10.3390/min11060606
Drapeau C, Argane R, Delolme C, Blanc D, Benzaazoua M, Hakkou R, Baumgartl T, Edraki M, Lassabatere L. Lead Mobilization and Speciation in Mining Waste: Experiments and Modeling. Minerals. 2021; 11(6):606. https://doi.org/10.3390/min11060606
Chicago/Turabian StyleDrapeau, Clémentine, Rabei Argane, Cécile Delolme, Denise Blanc, Mostafa Benzaazoua, Rachid Hakkou, Thomas Baumgartl, Mansour Edraki, and Laurent Lassabatere. 2021. "Lead Mobilization and Speciation in Mining Waste: Experiments and Modeling" Minerals 11, no. 6: 606. https://doi.org/10.3390/min11060606
APA StyleDrapeau, C., Argane, R., Delolme, C., Blanc, D., Benzaazoua, M., Hakkou, R., Baumgartl, T., Edraki, M., & Lassabatere, L. (2021). Lead Mobilization and Speciation in Mining Waste: Experiments and Modeling. Minerals, 11(6), 606. https://doi.org/10.3390/min11060606









